Abstract
Background and purpose
Impaired ambulation is a prominent disabling symptom of multiple sclerosis and can lead to reduced quality of life. Whether natalizumab, a monoclonal antibody shown to reduce disease activity in relapsing−remitting multiple sclerosis, could impact ambulation performance was examined.
Methods
A prospective open-label study, TIMER, was conducted in natalizumab-naive patients (n = 215). The timed 25-foot walk (T25FW) and timed 100-m walk (T100MW) were assessed at baseline and at weeks 24 and 48 of natalizumab therapy, together with Expanded Disability Status Scale scores. The effects of natalizumab on T25FW performance were also examined in a retrospective analysis of natalizumab-treated patients (n = 627) and placebo control patients (n = 315) from the AFFIRM study.
Results
In TIMER, a significant increase from baseline in T25FW speed was seen at week 24 (P = 0.0074) and in T100MW speed at weeks 24 and 48 (both P < 0.001). A greater proportion of patients showed clinically meaningful increases (≥20%) in walking speed on the T100MW (25%) than on the T25FW (13%) at week 48 (P = 0.032). In AFFIRM, natalizumab increased the proportion of patients with ≥20% confirmed improvement in T25FW speed at year 2 by 78% versus placebo (P = 0.0133).
Conclusions
Natalizumab increased walking speed in patients with relapsing−remitting multiple sclerosis. The T100MW may be more sensitive to changes in ambulation capacity than the T25FW, and both tests appear to detect clinically meaningful improvements in ambulatory function.
Keywords: ambulation, Expanded Disability Status Scale, natalizumab, quality of life, relapsing−remitting multiple sclerosis, timed 100-meter walk, timed 25-foot walk, walking
Introduction
Mobility problems are reported in up to 58% of patients in the first year following diagnosis of multiple sclerosis (MS) and in up to 93% of patients within 10 years of diagnosis 1. This loss of mobility leads to an increased use of healthcare resources and reduced quality of life 2,3. In this respect, 70% of patients with walking difficulty state that it is the most challenging aspect of their MS 4.
Accurate assessment of ambulation is essential for estimating disease burden in MS and for evaluating the efficacy of disease-modifying therapies. The Expanded Disability Status Scale (EDSS), which is widely used to assess disability in MS, relies on measures or estimates of maximum walking distance (MWD) to quantify the walking component of physical disability. Ambulation can also be measured objectively with tests such as the timed 25-foot walk (T25FW) 5,6 or the timed 100-m walk (T100MW) 7.
Natalizumab (Tysabri, Biogen Idec, Cambridge, MA, USA) is an α4-integrin antagonist that interferes with the migration of immune cells into the central nervous system 8. In the phase III AFFIRM trial in relapsing−remitting MS (RRMS), natalizumab monotherapy reduced annualized relapse rates by 68% and also reduced the risk of disability progression, as measured by EDSS, by 42%–54% over 2 years versus placebo 9. Natalizumab has also been shown to improve disability 10 and to increase the proportion of patients with no evidence of clinical or magnetic resonance imaging disease activity 11.
The findings from an open-label, non-randomized, prospective study that was performed to determine whether natalizumab improves ambulatory measures in RRMS (TIMER) are reported. Changes in walking capacity were measured using the T25FW and T100MW and by asking patients to estimate their MWD, over 48 weeks of treatment with natalizumab. The findings from a post hoc analysis of ambulation data from the randomized, placebo-controlled, AFFIRM trial are also reported.
Methods
This report includes analysis of patients from the TIMER (ClinicalTrials.gov NCT00871780) and AFFIRM (ClinicalTrials.gov NCT00027300) studies, as detailed below. Approval of the studies was obtained from an ethics committee or institutional review board at each site; both studies were performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines; and all patients provided written informed consent.
TIMER: prospective analysis
Participants and design
TIMER was an international, multicenter, open-label, single-arm, prospective study in which patients received natalizumab (300 mg iv) every 4 weeks for 48 weeks.
All participants had a documented diagnosis of RRMS as defined by the revised McDonald Committee criteria 12, had a magnetic resonance imaging scan within the previous 3 months, had experienced ≥1 relapse in the previous year, and satisfied the locally approved therapeutic indications for natalizumab. Patients were natalizumab-naive men and women aged 18–60 years inclusive, with an EDSS score ≤5.5 at baseline, and who were able to walk at least 100 m without assistive devices. Symptoms had to be stable for ≥30 days prior to enrollment.
Exclusion criteria included the onset of a relapse within 50 days prior to the first natalizumab infusion and the presence of walking impairment due to any cause other than MS. Patients with a history of malignancy, human immunodeficiency virus infection, organ transplantation or a clinically significant infectious disease were excluded, as were patients who had been treated with immunosuppressant medications within 6 months prior to screening.
Assessments
Neurological evaluation, MWD (reported), EDSS, T25FW (completed twice) and T100MW were performed in that order (with a 5-min rest after the T25FW) at baseline, week 24 and week 48. The T100MW consisted of a 25-m distance walked four times with three U-turns 7. EDSS scores were also assessed at least 50 days after the onset of a relapse. A follow-up assessment was conducted by telephone at week 52. Patients who withdrew from the study early were assessed at a premature treatment withdrawal visit.
The frequency of relapses was also measured. A relapse was defined as new or recurrent neurological symptoms, confirmed by the investigator, not associated with fever, lasting ≥24 h and following a period of improvement or stabilization of symptoms of ≥30 days.
Statistical analysis
Analyses included patients who received ≥1 infusion of natalizumab and completed ≥1 on-treatment evaluation. The primary outcome was the effect of natalizumab on ambulation performance, as measured by T100MW, T25FW, MWD and EDSS. Changes from baseline at weeks 24 and 48 were presented as summary statistics. Data that were approximately normally distributed were analyzed with a paired t-test; data that were not normally distributed were analyzed with a non-parametric Wilcoxon signed-rank test. Changes from baseline in T100MW, T25FW and MWD were categorized as improved, stable or worsened at weeks 24 and 48 based on a threshold of ≥20% change, which is generally regarded as clinically meaningful 13.
Changes from baseline in EDSS scores were categorized as improved, stable or worsened at weeks 24 and 48 based on a threshold of change in EDSS score of ≥1.0 point.
Correlations were evaluated using the Pearson correlation coefficient. Paired categorical responses were analyzed using the McNemar test.
The proportion of patients with no evidence of clinical disease activity, defined as no relapses and no sustained EDSS progression, was determined at week 48. Sustained EDSS progression was defined as ≥1.0-point increase in EDSS score sustained for 6 months.
Post hoc subgroup analyses were performed evaluating the proportion of patients with improvement (i.e. ≥20% increase in walking speed) in T100MW and T25FW in patients stratified by baseline EDSS score (<3.0; 3.0–4.0; 4.5–5.5) and by baseline T25FW time (<6 s; ≥6 s and <8 s; ≥8 s).
Data were analyzed using SAS 9.3 software (SAS Institute Inc., Cary, NC, USA). All tests of significance were two-sided with a significance level of 0.05.
AFFIRM: post hoc analysis
Participants and design
AFFIRM was a randomized, placebo-controlled, double-blind, phase III study of patients with RRMS who received an iv infusion of either natalizumab 300 mg (n = 627) or placebo (n = 315) every 4 weeks for up to 116 weeks. Details of the study methods and participants have been published previously 9.
Assessments
In AFFIRM, T25FW was evaluated according to the Multiple Sclerosis Functional Composite 14 at baseline and every 12 weeks for 30 months. The proportion of patients with a confirmed improvement in T25FW speed (defined as ≥20% increase in walking speed from baseline confirmed 12 weeks later) was retrospectively compared in the natalizumab and placebo groups.
Quality of life was evaluated using the validated Short Form 36 (SF-36) health survey, which was completed at baseline and at weeks 24, 52 and 104 15. In our analyses, SF-36 Physical Component Summary (SF36-PCS) scores were compared in patients with and without a confirmed improvement in T25FW speed.
Statistical analysis
Proportions of patients with confirmed improvement in T25FW speed in the natalizumab and placebo groups were compared using Kaplan–Meier analyses. Patients were also stratified by baseline EDSS scores and by baseline T25FW times. A Wilcoxon rank-sum test was used to evaluate the SF36-PCS scores in patients with and without confirmed ambulation improvement.
Results
TIMER
Patient disposition and baseline characteristics
A total of 224 patients were enrolled in TIMER, of whom 215 received ≥1 infusion of natalizumab and completed ≥1 post-baseline evaluation. Baseline characteristics of patients are shown in Table1. The mean EDSS score at baseline was 3.7. In the year prior to natalizumab treatment, 57.7% (124/215) had ≥2 relapses, and the mean relapse rate was 1.77.
Table 1.
Baseline and demographic characteristics of patients in TIMER, N = 215
| Characteristic | |
|---|---|
| Female, n (%) | 137 (63.7) |
| Age, mean (SD), years | 35.1 (9.56) |
| Years since first MS symptom, mean (SD) | 9.1 (6.14) |
| Annualized relapse rate, mean (95% CI) | 1.77 (1.60–1.96) |
| Number of relapses in last 12 months, n (%) | |
| 1 | 91 (42.3) |
| ≥2 | 124 (57.7) |
| EDSS score | |
| Mean (SD) | 3.7 (1.36) |
| Median (range) | 4.0 (2.5–5.0) |
| Number of prior DMTs used, n (%) | |
| 0 | 83 (38.6) |
| ≥1 | 132 (61.4) |
MS, multiple sclerosis; CI, confidence interval; EDSS, Expanded Disability Status Scale; DMT, disease-modifying therapy.
Clinical disease activity
The annualized relapse rate after 48 weeks of natalizumab therapy was 0.21. At week 48, 98.6% of patients were free from sustained EDSS progression, 85.1% were free from relapse and 84.2% had no evidence of clinical disease activity. The majority of patients remained stable (change of <1.0 point) or showed improvements (reductions of ≥1.0 point) in EDSS score from baseline to week 24 (96.1%) and to week 48 (96.5%).
Ambulation performance over time
Performances on the T25FW and T100MW were significantly correlated at baseline and at weeks 24 and 48 (Pearson correlation coefficients of 0.924, 0.907 and 0.892, respectively; all P < 0.0001). Changes in performance on measures of ambulation are summarized in Table2. Mean T100MW speed was significantly increased with natalizumab therapy at both weeks 24 and 48 relative to baseline (both P ≤ 0.0001). The increase in mean T25FW speed relative to baseline was statistically significant at week 24 (P = 0.0074) but not at week 48 (P = 0.1643). Patients’ self-reported MWD increased at both weeks 24 and 48, but these differences were not significant (Table2). Walking speed was stable (change of <20% from baseline) or improved (increase of ≥20% from baseline) in the majority of patients at weeks 24 and 48 (Fig.1).
Table 2.
Changes in disability and walking capacity in TIMER
| Baseline (N = 215) | Week 24 (n = 207) | Week 48 (n = 199) | |
|---|---|---|---|
| EDSS score | |||
| Mean (SD) | 3.7 (1.36) | 3.6 (1.37)a | 3.5 (1.39)a |
| Median (range) | 4.0 (2.5–5.0) | 4.0 (2.0–4.5) | 4.0 (2.0–4.5) |
| T25FW, s | |||
| Mean (SD) | 9.0 (6.76) | 8.9 (6.66) | 8.9 (6.43) |
| Median (range) | 6.6 (3.2–53.2) | 6.6 (3.2–54.1) | 6.5 (3.2–45.0) |
| T25FW speed, m/s | |||
| Mean (SD) | 1.11 (0.47) | 1.14 (0.49)b | 1.14 (0.48)c |
| Median (range) | 1.16 (0.14–2.38) | 1.16 (0.14–2.38) | 1.18 (0.17–2.38) |
| T100MW, s | |||
| Mean (SD) | 112.5 (83.02) | 108.2 (87.46) | 106.2 (85.22) |
| Median (range) | 86.0 (30.7–636.0) | 83.1 (31.2–750.0) | 83.2 (31.7–718.0) |
| T100MW speed, m/s | |||
| Mean (SD) | 1.19 (0.55) | 1.27 (0.58)a | 1.29 (0.59)d |
| Median (range) | 1.16 (0.16–3.26) | 1.20 (0.13–3.21) | 1.20 (0.14–3.16) |
| MWD, m | |||
| Mean (SD) | 651.9 (691.63)e | 674.5 (665.17)f | 701.2 (739.63)g |
| Median (range) | 350.0 (10.0–3000.0)e | 400.0 (20.0–3000.0)f | 450.0 (20.0–3500.0)g |
EDSS, Expanded Disability Status Scale; T25FW, timed 25-foot walk; T100MW, timed 100-m walk; MWD, maximum walking distance. aP < 0.0001 vs. baseline; bP = 0.0074 vs. baseline; cP = 0.1643 vs. baseline; dP = 0.0001 vs. baseline; en = 143; fn = 136; gn = 129.
Figure 1.
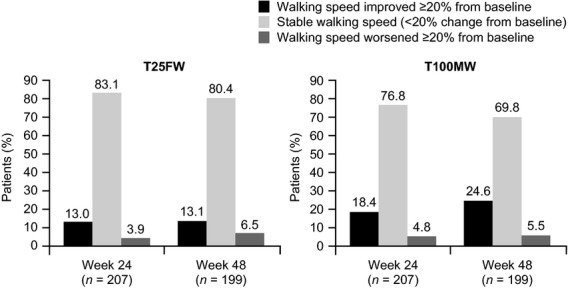
Changes in walking performance over time in TIMER.
Relation between T25FW and T100MW
At week 48, a greater proportion of patients had clinically meaningful (≥20%) increases in walking speed on the T100MW than on the T25FW (T100MW 24.6%; T25FW 13.1%; P = 0.0032). This was also true for improvements sustained at both week 24 and week 48 (T100MW 15.7%; T25FW 6.6%; P = 0.0040; Fig.2).
Figure 2.
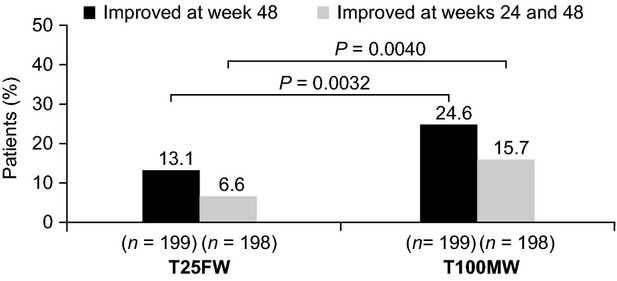
Clinically meaningful improvements in ambulation in TIMER.
In subgroup analyses of patients stratified by baseline EDSS score (Fig.3a) and by baseline T25FW time (Fig.3b), the proportions of patients with ≥20% improvement in walking speed at week 48 or sustained at both week 24 and week 48 were significantly higher for T100MW than for T25FW in patients with higher EDSS scores (4.5–5.5) at baseline. Subgroup analyses also revealed that 17.3% of patients with low EDSS scores (<3.0) and 16.9% of patients with presumably normal baseline T25FW performance (<6 s) 16 experienced a clinically meaningful improvement (≥20% increase) in T100MW speed at week 48.
Figure 3.
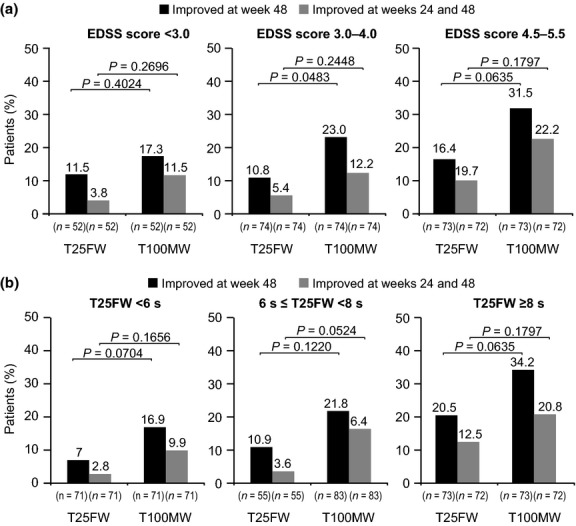
Improvements in ambulation in patients stratified by (a) baseline EDSS score and (b) baseline T25FW performance in TIMER.
Patients with a clinically meaningful improvement (≥20% increase) in T25FW speed from baseline to week 24 or week 48 had a similar level of improvement in T100MW speed, with a median increase of 22.6% at week 24 (P < 0.0001) and 22.8% at week 48 (P =0.0036). However, amongst patients with a clinically meaningful improvement (≥20% increase) in T100MW speed from baseline to week 24 or week 48, there were slightly lower median increases in T25FW speed of 14.5% at week 24 (P < 0.0001) and 7.5% at week 48 (P < 0.0001).
Relation between EDSS and measurements of ambulation (T25FW, T100MW)
Performance on each ambulation test was significantly correlated with EDSS at baseline and at weeks 24 and 48 (all P < 0.0001). Amongst patients with a stable EDSS score at week 48 (change from baseline <1.0 point), a greater proportion showed a ≥20% improvement on the T100MW than on the T25FW (23.0% vs. 10.3%; P = 0.0025; Fig.4). Amongst patients with improved EDSS scores (decrease from baseline ≥1.0 point), rates of improvement on the two walking tests were similar (35.0% with each test; Fig.4).
Figure 4.
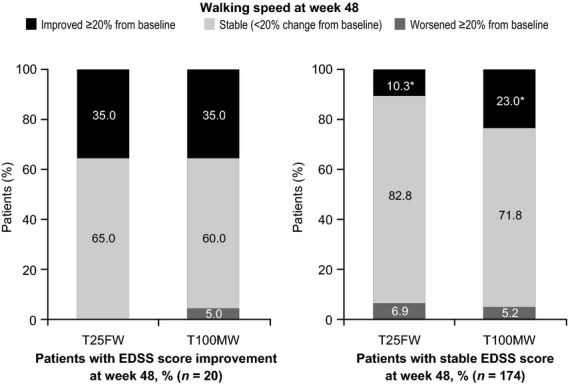
Ambulation status in patients with improved or stable EDSS scores in TIMER. *P = 0.0025.
Of the 26 patients in this study who had ≥20% improvement in T25FW walking speed at week 48, 18 (69.2%) had stable EDSS scores and seven (26.9%) had improvement in EDSS scores of ≥1.0 point. Of the 49 patients who had ≥20% improvement in T100MW speed at week 48, 40 (81.6%) had stable EDSS scores and seven (14.3%) had improvement in EDSS scores of ≥1.0 point.
AFFIRM
In AFFIRM, natalizumab increased the proportion of patients with a ≥20% improvement in T25FW speed at year 2 by 78% versus placebo (12.3% vs. 6.9%; P = 0.0133).
When the treatment groups were combined, patients with a ≥20% confirmed improvement in T25FW speed at year 2 showed a mean increase in SF36-PCS of 2.70 (improvement), whereas patients without this improvement in walking speed had a mean change of −0.30 (worsening). Amongst patients with a ≥20% confirmed improvement in T25FW speed at year 2, a confirmed EDSS improvement of ≥1.0 point was seen in 25% and 20% of participants in the natalizumab and placebo treatment groups, respectively. Treatment effects in AFFIRM were larger and occurred earlier in patients with higher baseline disability, but were significant even in patients with lower disability (data not shown).
Discussion
Natalizumab was associated with clinically meaningful (≥20%) improvements in T100MW speeds in approximately one out of four RRMS patients at 48 weeks in the TIMER study. Natalizumab also produced clinically meaningful and consistent improvements in T25FW speeds in 13% of patients in TIMER at 48 weeks and in 12% of patients in a post hoc analysis of AFFIRM at 2 years. These improvements in T25FW speeds in AFFIRM represented a statistically significant 78% improvement versus placebo at 2 years. Furthermore, the improvements in T25FW speeds in AFFIRM were associated with significant improvement in the physical components of quality of life.
Our finding that natalizumab can improve walking performance in some patients is generally consistent with previous post hoc analyses and retrospective observational studies. Natalizumab, either alone or in combination with interferon beta (IFN-beta), was shown to increase the number of T25FW responders – defined as patients who walked faster in the majority of post-baseline evaluations than in their best baseline performance – in the AFFIRM and SENTINEL trials 17. In that analysis, approximately 15%–25% of natalizumab-treated patients were classified as T25FW responders, although the beneficial effects of natalizumab only reached significance in patients with higher levels of disability. In a retrospective observational study of 45 patients who began natalizumab therapy after experiencing ≥1 relapse with IFN-beta or glatiramer acetate, 38% showed clinically significant improvement on the T25FW, and 23% on the T100MW, after 11 months of follow-up 18. Natalizumab also significantly impro-ved the performance of MS patients in an alternative measure of walking ability, the 6-min walk test 19.
A significant correlation was found between performances on the T25FW and T100MW. However, in the TIMER study, the T100MW was more sensitive than the T25FW to the effects of natalizumab; the mean increase in walking speed at week 48 was significant only for T100MW with a greater proportion of patients showing ≥20% improvement in this test. Additionally, the T100MW revealed clinically meaningful improvements in walking in a greater proportion of patients with unchanged EDSS scores than the T25FW, suggesting that the T100MW may be more sensitive to improvements in walking capacity that do not result in changes in EDSS. Moreover, a greater proportion of patients with baseline EDSS scores of 4.5–5.5 showed a clinically meaningful improvement on the T100MW than on the T25FW. These findings are similar to results obtained in an earlier study in which the T100MW was compared with T25FW in 141 MS patients and 104 healthy control patients 7. Small differences were seen in variability, reliability and correlation with MWD, with the differences favoring the T100MW 7.
An improvement in performance of ≥20% on the T25FW has been shown to be clinically meaningful 13. The fact that many patients with this level of improvement did not show a corresponding reduction in EDSS score in TIMER or AFFIRM suggests that the T25FW may be more sensitive to clinically meaningful changes in ambulatory functioning than EDSS scores alone. This has been suggested previously in progressive MS 20, and indeed early changes in T25FW performance were also better than early changes in EDSS score at predicting later self-reported disease impact in patients with progressive disease 21.
A limitation of TIMER is that it was an open-label study, which may limit the interpretation of the results. However, reassuringly, similar results were demonstrated in a post hoc analysis of data from the placebo-controlled AFFIRM trial. The TIMER and AFFIRM studies were also relatively short term, and further data are needed to determine whether the beneficial effects observed with natalizumab would be maintained over the longer term.
In summary, TIMER revealed a significant improvement in ambulation, as measured by the T25FW and the T100MW, in natalizumab-treated patients with RRMS. A significant improvement relative to placebo was also seen in natalizumab-treated patients in the pivotal AFFIRM trial. The results of TIMER suggest that the T100MW may represent a more sensitive test of walking capacity than the T25FW, whilst both tests may be able to detect clinically meaningful improvements in functioning not reflected by changes in EDSS score.
Acknowledgments
Editorial support was funded by Biogen Idec Inc.; Ryan Woodrow and Susan Denner (Infusion Communications) provided writing support, and Jackie Cannon (Infusion Communications) copyedited and styled the manuscript. Biogen Idec Inc. reviewed the manuscript and provided feedback to the authors, who had full editorial control and approved all final content. This study was supported by Biogen Idec Inc.
Disclosure of conflicts of interest
NV has received honoraria from Biogen Idec for serving as a TIMER study investigator. EH has received consulting fees and honoraria from Bayer HealthCare, Biogen Idec, Merck Serono, Novartis, Sanofi Genzyme and Teva. MH served on a medical advisory board for the CONFIRM study (BG00012) for Biogen Idec, serves on the editorial board of the Multiple Sclerosis Journal, has received speaker's honoraria from Novartis, Biogen Idec and Bayer Schering, and receives research support from Dystonia Ireland, the Health Research Board of Ireland and the Foundation for Dystonia Research. TN has received honoraria from Biogen Idec for serving as a TIMER study investigator. XY, SB, CH and DP are employees of Biogen Idec.
References
- van Asch P. Impact of mobility impairment in multiple sclerosis 2 − patients' perspectives. Eur Neurol Rev. 2011;6:115–120. [Google Scholar]
- Heesen C, Böhm J, Reich C, Kasper J, Goebel M, Gold SM. Patient perception of bodily functions in multiple sclerosis: gait and visual function are the most valuable. Mult Scler. 2008;14:988–991. doi: 10.1177/1352458508088916. [DOI] [PubMed] [Google Scholar]
- Pike J, Jones E, Rajagopalan K, Piercy J, Anderson P. Social and economic burden of walking and mobility problems in multiple sclerosis. BMC Neurol. 2012;12:94. doi: 10.1186/1471-2377-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocca NG. Impact of walking impairment in multiple sclerosis: perspectives of patients and care partners. Patient. 2011;4:189–201. doi: 10.2165/11591150-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Kaufman M, Moyer D, Norton J. The significant change for the Timed 25-foot Walk in the multiple sclerosis functional composite. Mult Scler. 2000;6:286–290. doi: 10.1177/135245850000600411. [DOI] [PubMed] [Google Scholar]
- Kieseier BC, Pozzilli C. Assessing walking disability in multiple sclerosis. Mult Scler. 2012;18:914–924. doi: 10.1177/1352458512444498. [DOI] [PubMed] [Google Scholar]
- Phan-Ba R, Pace A, Calay P, et al. Comparison of the timed 25-foot and the 100-meter walk as performance measures in multiple sclerosis. Neurorehabil Neural Repair. 2011;25:672–679. doi: 10.1177/1545968310397204. [DOI] [PubMed] [Google Scholar]
- Rudick RA, Sandrock A. Natalizumab: alpha 4-integrin antagonist selective adhesion molecule inhibitors for MS. Expert Rev Neurother. 2004;4:571–580. doi: 10.1586/14737175.4.4.571. [DOI] [PubMed] [Google Scholar]
- Polman CH, O'Connor PW, Havrdová E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- Phillips JT, Giovannoni G, Lublin FD, et al. Sustained improvement in Expanded Disability Status Scale as a new efficacy measure of neurological change in multiple sclerosis: treatment effects with natalizumab in patients with relapsing multiple sclerosis. Mult Scler. 2011;17:970–979. doi: 10.1177/1352458511399611. [DOI] [PubMed] [Google Scholar]
- Havrdová E, Galetta S, Hutchinson M, et al. Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: a retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing−Remitting Multiple Sclerosis (AFFIRM) study. Lancet Neurol. 2009;8:254–260. doi: 10.1016/S1474-4422(09)70021-3. [DOI] [PubMed] [Google Scholar]
- Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the ‘McDonald Criteria’. Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- Hobart J, Blight AR, Goodman A, Lynn F, Putzki N. Timed 25-foot walk: direct evidence that improving 20% or greater is clinically meaningful in MS. Neurology. 2013;80:1509–1517. doi: 10.1212/WNL.0b013e31828cf7f3. [DOI] [PubMed] [Google Scholar]
- Cutter GR, Baier ML, Rudick RA, et al. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain. 1999;122:871–882. doi: 10.1093/brain/122.5.871. [DOI] [PubMed] [Google Scholar]
- Rudick RA, Miller D, Hass S, et al. Health-related quality of life in multiple sclerosis: effects of natalizumab. Ann Neurol. 2007;62:335–346. doi: 10.1002/ana.21163. [DOI] [PubMed] [Google Scholar]
- Goldman MD, Motl RW, Scagnelli J, et al. Clinically meaningful performance benchmarks in MS: timed 25-foot walk and the real world. Neurology. 2013;81:1856–1863. doi: 10.1212/01.wnl.0000436065.97642.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadavid D, Jurgensen S, Lee S. Impact of natalizumab on ambulatory improvement in secondary progressive and disabled relapsing−remitting multiple sclerosis. PLoS One. 2013;8:e53297. doi: 10.1371/journal.pone.0053297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belachew S, Phan-Ba R, Bartholome E, et al. Natalizumab induces a rapid improvement of disability status and ambulation after failure of previous therapy in relapsing−remitting multiple sclerosis. Eur J Neurol. 2011;18:240–245. doi: 10.1111/j.1468-1331.2010.03112.x. [DOI] [PubMed] [Google Scholar]
- Svenningsson A, Falk E, Celius EG, et al. Natalizumab treatment reduces fatigue in multiple sclerosis. Results from the TYNERGY trial; a study in the real life setting. PLoS One. 2013;8:e58643. doi: 10.1371/journal.pone.0058643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadavid D, Tang Y, O'Neill G. Responsiveness of the Expanded Disability Status Scale (EDSS) to disease progression and therapeutic intervention in progressive forms of multiple sclerosis. Rev Neurol. 2010;51:321–329. [Article in Spanish] [PubMed] [Google Scholar]
- Bosma L, Kragt JJ, Polman CH, Uitdehaag BM. Walking speed, rather than Expanded Disability Status Scale, relates to long-term patient-reported impact in progressive MS. Mult Scler. 2013;19:326–333. doi: 10.1177/1352458512454346. [DOI] [PubMed] [Google Scholar]


