Abstract
Background
Visceral pleural invasion (VPI) is used as an indicator of adverse prognosis in non-small cell lung carcinoma (NSCLC). The purpose of this retrospective study was to evaluate the impact of VPI on disease-free survival (DFS) and overall survival (OS) in patients with node-negative NSCLC.
Methods
Between 1998 and 2009, 1166 patients with pathological N0M0 NSCLC underwent surgical resection by lobectomy. 214 patients with VPI were compared to 952 without.
Results
Median follow-up was 59 months. In multivariate analysis, VPI, larger tumor size, older age, female gender, and poor performance status were significantly associated with decreased OS. In contrast, larger tumor size, female gender, and poor performance, but notably not VPI, were associated with decreased DFS. After examining interactive effects of VPI and T subgroups, we found that VPI did not significantly affect either OS or DFS in the subgroups of patients with smaller tumor sizes T1a, T1b, or T2a. In contrast, a deleterious effect of VPI on DFS was seen for tumors > 5cm, T2b and T3, with the VPI-T3 interaction effect on DFS being statistically significant but not for OS.
Conclusions
The effect of VPI on survival in NSCLC varies greatly with tumor size, with VPI not strongly associated with OS or DFS in tumors less than 5cm, but showing large negative effects on DFS for T2b and T3 tumors. Using VPI to upstage T1 tumors to a higher T category is not warranted, since it would misrepresent these VPI-T subgroup effects.
Introduction
The leading cause of cancer-related mortality in the United States is lung cancer [1]. Visceral pleural invasion (VPI) has been recognized for the past several decades as an indicator of adverse prognosis in non-small cell lung cancer (NSCLC), and it has been included in the TNM staging systems as a factor that should upstage the T factor [2-5]. However, standard definitions and pathologic evaluations of VPI have made it difficult to characterize, and its role in the T factor remains controversial [5]. The use of elastic stains has been recommended to uniformly define VPI, along with the adoption of a standard system for classification of VPI based on the Hammar system [5-7], but currently these practices are not widely used.
In the International Association for the Study of Lung Cancer's (IASLC) 7th edition of the TNM classification system for lung cancer, 18,198 patients comprised the cohort for the T component. However, there were insufficient patient numbers, inconsistent clinical and pathologic results, and a lack of validation to include VPI and other T descriptors in the analysis [3]. The identification of survival differences at tumor size cut points 2, 3, 5, and 7cm was the basis for the categorization of tumor size into T1a, T1b, T2a and T2b, but because of the aforementioned inability to sufficiently analyze VPI in this cohort, the presence of VPI continues to upstage tumors ≤ 3cm to T2 [3]. In addition to its association the T factor, VPI is thought to be strongly associated with lymph node metastases [8-10], but it has no role in the N factor of the TNM staging systems.
The purpose of the study reported here was to evaluate the impact of VPI on survival in patients with node-negative NSCLC using a North American patient population. In order to focus on the relationship between VPI and the T factor, we chose to exclude patients with lymph node metastases. Excluding nodal disease as well as atelectasis, lobar consolidation and tumors within 2 cm of the carina allowed us to eliminate other confounders of prognosis [11].
Patients and Methods
We retrospectively reviewed a prospectively maintained database for patients undergoing thoracic surgery in our center. The study was approved by the MD Anderson Institutional Review Board, with individual consent waived owing to the retrospective nature of the study.
From 1998 to 2009, 1166 patients with pathological N0M0 NSCLC underwent surgical resection at our center. No patients received neoadjuvant chemotherapy or radiation therapy. All resections were R0 and included lobectomy only. Patients with previous lung cancer were excluded. The histologic diagnoses included Adenocarcinoma, Adenosquamous, Squamous cell carcinoma, Bronchioalveolar carcinoma (BAC), and other NSCLC.
Patient demographics, performance status, pathology reports, and imaging findings were gathered from this database and other institutional records. Overall survival (OS) was calculated from the date of operation to the last known date of follow-up. Disease-free survival (DFS) was calculated from the date of operation to the date of diagnosis of recurrence and was further classified as local, regional or distant. One patient was lost to follow up shortly after surgery. In 38 patients, recurrence data were not available.
VPI was defined by any distortion of the Pleural elastic layer by malignant cells and we did not differentiate between the degree of invasion (PL1 vs. 2).
Statistical Methods
Association of pleural invasion with categorical variables was assessed by the Fisher Exact test and its generalizations [12]; and with continuous variables by the Wilcoxon rank sum test [13]. Unadjusted distributions for OS and DFS were estimated using the method of Kaplan and Meier [14] and compared between subgroups using the log rank test [15]. Several distributional forms for regression of OS and DFS on covariates were assessed, including the Weibull, lognormal, log-logistic, exponential, and gamma. Goodness-of-fit was assessed using smoothed Martingale residual plots on age and tumor size, the Grambsch-Therneau test [16], and the Bayes Information Criterion [17]. Because the Kaplan-Meier plots appear to suggest that the event (death or progression) rate (Figure 1) and the death rate (Figure 2) decreased over time, and that these decreases may have been different depending on tumor size and VPI, we also fit Weibull regression models to assess this, allowing the Weibull shape parameter to vary with (tumor size, VPI). Values of a Weibull shape parameter >1, =1, or < 1 correspond, respectively, to an increasing, constant (exponential distribution), and decreasing hazard over time. While the shape parameter estimates went from values > 1 for small tumors to values < 1 for large tumors, most of these estimates did not differ significantly from 1. Consequently, we did not adopt this more complex model, but instead concluded that regression models with exponentially distributed OS time and DFS time fit the data best. All event time regression models were fit using the servreg function of the CRAN survival package in R [18]. Patients were categorized into tumor size groups based on the pathologic tumor size of the AJCC 7th edition staging system for lung cancer [3].
Figure 1. Kaplan Meier survival plots for disease free survival for each T group.
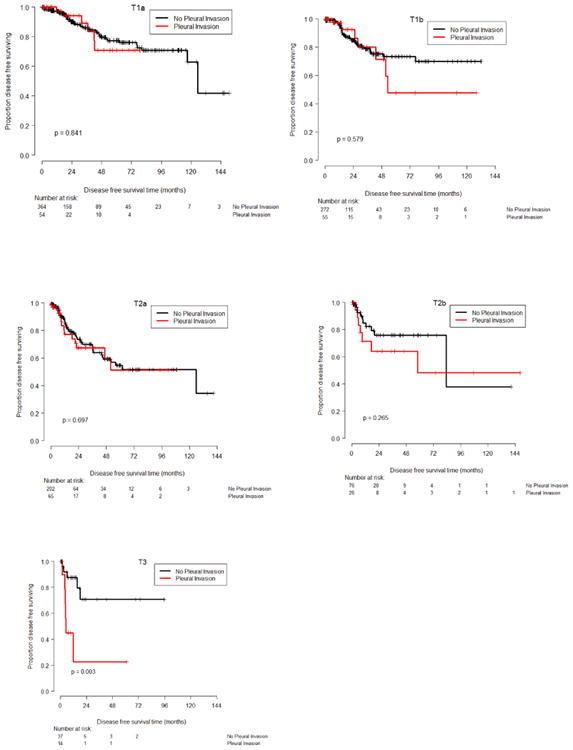
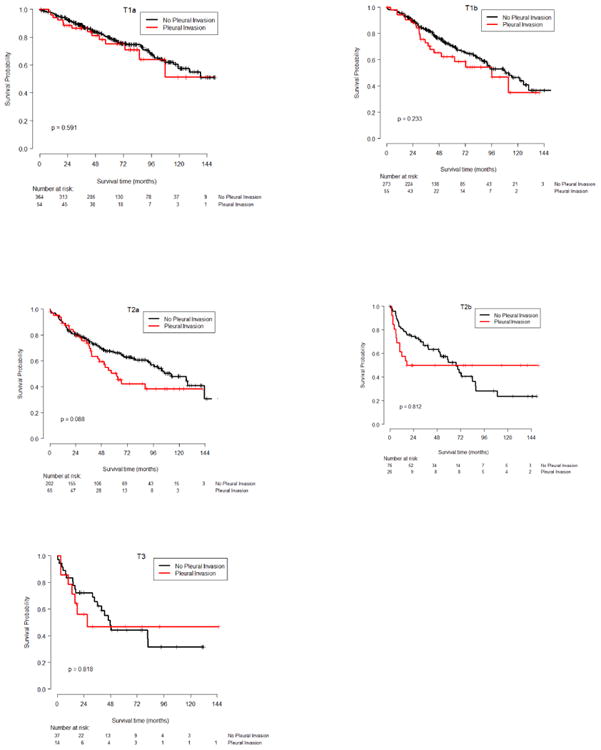
Figure 2. Kaplan-Meier survival plots for overall survival for each T group.
Results
Patient Characteristics
There were 1166 patients who underwent lobectomy for pathologic N0M0 NSCLC from January 1998 to December 2009. Two hundred fourteen (18%) were found to have VPI. Patient demographics and preoperative factors are listed in Table 1. VPI was found to be significantly associated with both tumor size and histology, with patients having adenocarcinoma or other non-squamous histology more likely to have pleural invasion (Table 2). Patients with tumors larger than T1a were more likely to have VPI. The mean tumor size was 3.6cm in patients with VPI and 3.0cm in patients without VPI (p<0.0001).
Table 1. Patient Demographics.
| Factor | Frequency (percent) |
|---|---|
| Gender | |
| M | 564 (48.4%) |
| F | 602 (51.6%) |
|
| |
| Recurrence | |
| No | 997 (85.5%) |
| Yes | 169 (14.5%) |
|
| |
| Histology | |
| Adenocarcinoma | 645 (55.3%) |
| Squamous cell carcinoma | 281 (24.1%) |
| Other Non-small cell | 240 (20.6%) |
|
| |
| Zubrod Performance status | |
| Normal activity | 627 (53.8%) |
| Symptoms, but nearly fully ambulatory | 524 (44.9%) |
| Some bed time, but needs to be in bed < 50% of normal day | 15 (1.3%) |
|
| |
| Pleural Invasion | |
| No | 952 (81.6%) |
| Yes | 214 (18.4%) |
|
| |
| Tumor Size (pathologic) | |
| T1a | 418 (35.8%) |
| T1b | 328 (28.1%) |
| T2a | 267 (22.9%) |
| T2b | 102 (8.7%) |
| T3 | 51 (4.4%) |
Table 2. Tumor size, histology, and performance status, dichotomized by pleural invasion.
| Covariate | Levels | No Pleural Invasion N (column %) |
Pleural Invasion N (column %) |
Total | Fisher's exact test |
|---|---|---|---|---|---|
| Tumor size | T1a | 364(38.2%) | 54(25.2%) | 418(35.8%) | <0.001 |
| T1b | 273(28.7%) | 55(25.7%) | 328(28.1%) | ||
| T2a | 202(21.2%) | 65(30.4%) | 267(22.9%) | ||
| T2b | 76(8%) | 26(12.1%) | 102(8.7%) | ||
| T3 | 37(3.9%) | 14(6.5%) | 51(4.4%) | ||
| Histology | Adenocarcinoma | 498(52.3%) | 147(68.7%) | 645(55.3%) | <0.001 |
| Squamous cell carcinoma | 239(25.1%) | 42(19.6%) | 281(24.1%) | ||
| Other Non-small cell | 215(22.6%) | 25(11.7%) | 240(20.6%) | ||
| Zubrod | Normal | 522(54.8%) | 105(49.1%) | 627(53.8%) | 0.121 |
| some symptoms | 420(44.1%) | 104(48.6%) | 524(44.9%) | ||
| < 50% of time in bed | 10(1.1%) | 5(2.3%) | 15(1.3%) |
Regression Analyses of Survival and Disease –Free Survival
Median follow-up was 59 months (Min 5 days, Max 156 months). The fitted multivariable regression model for OS showed that worse survival was significantly associated with older age, VPI, larger tumor size, poor performance status (PS), and female gender (Table 3). The fitted model for DFS (Table 4) showed that shorter DFS was significantly associated with larger tumor size, poor PS, and female gender, but that VPI was not significant (p = 0.181). Simple two-subgroup comparisons to assess the effects of VPI versus no VPI on OS and DFS, while ignoring all other covariates, showed significant harmful VPI effects for both outcomes. Median OS was 89.6 months for patients with VPI versus 127.1 months for patients without VPI, p=0.007, while median DFS was 62.9 months in patients with VPI and 107.76 in patients without VPI, p=0.018. Since the VPI effect on DFS in the multivariate model was not significant, we wondered whether this large disagreement between the one-variable and multivariable effects of VPI on DFS might be due to a possible interaction between VPI and tumor size. Such an interactive effect is strongly suggested graphically by the Kaplan-Meier plots in Figure 1, and also numerically by the Kaplan-Meier-based estimates of 48-month DFS and OS given in Table 5. To assess this, we fit extended versions of the two regression models summarized in Tables 3 and 4 that included four additional interactive effects of VPI and tumor size, for DFS and for OS. The estimated VPI-tumor size interactive effects on DFS, summarized in Table 6, show much larger estimates for the VPI-T2b and VPI-T3 interactions, with the latter statistically significant in that the upper 95% confidence limit is below 0. For DFS, these analyses implicate VPI as being harmful for patients with tumor size T2b, very harmful for patients with tumor size T3, but not harmful for patients with tumor sizes T2a or smaller.
Table 3. Multivariable exponential regression model for overall survival.
| estimate | sd | z | HR | 95% CI Lower | 95% CI Upper | p value | |
|---|---|---|---|---|---|---|---|
| (Intercept) | 7.82 | 0.38 | 20.6 | ||||
| Age (each 5 yrs) | -0.173 | 0.0264 | -6.57 | 1.19 | 1.13 | 1.25 | <0.001 |
| Pleural Invasion (yes.vs.no) | -0.271 | 0.124 | -2.19 | 1.31 | 1.03 | 1.67 | 0.029 |
| Tumor Size | |||||||
| T1a (baseline group) | - | - | - | 1.0 | - | - | |
| T1b | -0.298 | 0.137 | -2.17 | 1.35 | 1.03 | 1.76 | 0.030 |
| T2a | -0.481 | 0.138 | -3.48 | 1.62 | 1.23 | 2.12 | <0.001 |
| T2b | -0.768 | 0.177 | -4.34 | 2.16 | 1.52 | 3.05 | <0.001 |
| T3 | -0.892 | 0.221 | -4.03 | 2.44 | 1.58 | 3.77 | <0.001 |
| Performance status: some symptoms vs. normal | -0.272 | 0.103 | -2.64 | 1.31 | 1.07 | 1.61 | 0.008 |
| Performance status: < 50% of time in bed vs. normal | -1.09 | 0.305 | -3.56 | 2.97 | 1.63 | 5.4 | <0.001 |
| Gender F vs. M | 0.325 | 0.104 | 3.14 | 0.722 | 0.59 | 0.885 | <0.001 |
| Histology: Squamous vs. Adenocarcinomas | -0.203 | 0.121 | -1.68 | 1.22 | 0.967 | 1.55 | 0.093 |
| Histology: Other non-small cell vs. Adenocarcinomas | -0.0722 | 0.134 | -0.537 | 1.07 | 0.826 | 1.4 | 0.592 |
Table 4. Multivariable exponential regression model for disease free survival.
| estimate | sd | z | HR | 95% CI Lower | 95% CI Upper | p value | |
|---|---|---|---|---|---|---|---|
| (Intercept) | 6.011 | 0.54 | 11.134 | ||||
| Age (each 5 years) | -0.048 | 0.038 | -1.278 | 1.05 | 0.974 | 1.131 | 0.201 |
| Pleural Invasion (yes.vs.no) | -0.249 | 0.186 | -1.337 | 1.282 | 0.891 | 1.846 | 0.181 |
| Tumor Size | |||||||
| T1a (baseline group) | - | - | - | 1.0 | - | - | |
| T1b | -0.224 | 0.205 | -1.093 | 1.251 | 0.837 | 1.869 | 0.274 |
| T2a | -0.82 | 0.194 | -4.222 | 2.271 | 1.552 | 3.324 | <0.001 |
| T2b | -0.599 | 0.285 | -2.103 | 1.821 | 1.042 | 3.184 | 0.035 |
| T3 | -1.217 | 0.343 | -3.549 | 3.376 | 1.724 | 6.611 | <0.001 |
| Performance status: some symptoms vs. normal | -0.061 | 0.155 | -0.395 | 1.063 | 0.785 | 1.441 | 0.692 |
| Performance status: < 50% of time in bed vs. normal | -1.521 | 0.36 | -4.224 | 4.577 | 2.26 | 9.271 | <0.001 |
| Gender F vs. M | 0.313 | 0.154 | 2.036 | 0.731 | 0.541 | 0.988 | 0.042 |
| Histology: Squamous vs. Adenocarcinomas | 0.009 | 0.188 | 0.047 | 0.991 | 0.686 | 1.432 | 0.963 |
| Histology: Other non-small cell vs. Adenocarcinomas | 0.092 | 0.203 | 0.452 | 0.912 | 0.612 | 1.359 | 0.651 |
Table 5.
Estimate of disease free survival rate and overall survival rate at 48 month with 95% CI by T-stage and pleural invasion status based on Kaplan-Meier estimates.
| 48 month disease free survival rate 95% CI | 48 month survival rate 95% CI | |||
|---|---|---|---|---|
| No PI | With PI | No PI | With PI | |
| T1a | 0.795 (0.734,0.861) | 0.708 (0.526,0.952) | 0.840 (0.795,0.877) | 0.814 (0.669,0.900) |
| T1b | 0.752 (0.681,0.831) | 0.713 (0.516,0.986) | 0.766 (0.706,0.815) | 0.654 (0.495,0.774) |
| T2a | 0.591 (0.495,0.707) | 0.599 (0.431,0.833) | 0.698 (0.626,0.759) | 0.595 (0.453,0.712) |
| T2b | 0.759 (0.635,0.907) | 0.642 (0.446,0.924) | 0.633 (0.508,0.735) | 0.500 (0.299,0.672) |
| T3 | 0.708 (0.507,0.988) | 0.225 (0.047,1.000) | 0.479 (0.297,0.641) | 0.469 (0.192,0.707) |
Table 6.
Estimates of visceral pleural invasion (VPI)_effects within T-stage subgroups based on extended exponential regression models including VPI-tumor size interaction terms.
| Estimate of effect (95%CI) | ||
|---|---|---|
| Tumor Size | DFS | OS |
| T1a | -0.063 (-0.914, 0.788) | -0.115 (-0.678, 0.448) |
| T1b | -0.187(-0.992, 0.618) | -0.269(-0.743, 0.205) |
| T2a | -0.016(-0.633, 0.601) | -0.375(-0.790, 0.040) |
| T2b | -0.379(-1.327, 0.569) | -0.007(-0.635,0.621) |
| T3 | -1.753(-2.940,-0.566) | -0.074(-0.935,0.787) |
We did not get the same large and significant effects for the VPI-T2b and VPI-T3 interactions for OS as we obtained for DFS. One can observe from the KM plots of OS (Figure 2) that the general pattern is actually the same for OS, but the magnitudes of the VPI-T2b and VPI-T3 interactions effects are smaller than for DFS, probably due to limited sub-sample sizes. In other words, for OS the effects of VPI were not significant.
Patterns of recurrence
Sixteen percent of patients without VPI had a recurrence compared to 20% in the VPI group (p=0.58). The pattern of tumor recurrence for patients with VPI was similar to the one of patients without VPI with no difference in the rate of local, regional and distal recurrences.
Comments
VPI remains a controversial and important clinical factor for thoracic surgeons, medical oncologists, and radiation oncologists treating patients with NSCLC. Here we have presented our experience with VPI in the context of N0M0 NSCLC with the aim to better characterize the impact of VPI and the T factor of TNM staging systems on patient outcomes. Our data demonstrate a survival advantage for patients with tumors ≤ 2cm with or without VPI, suggesting that these patients should not be upstaged to T2 tumors. The key finding is that VPI and tumor size appear to have interactive effects on DFS, with VPI significantly harmful only for the larger tumor sizes T2a and T3. In an effort to achieve a homogenous cohort, we chose to exclude patients who had resections other than lobectomy and thus eliminate confounders for OS and DFS related to incomplete resection or larger tumors that required larger resection. Given the limitations of our database, we were unable to identify patients who were treated with adjuvant therapy, but this should only have impacted the patients with larger tumor sizes. Very few series have excluded patients with nodal metastases as we chose to do, in order to closely examine the relationship between tumor size and VPI, but this limited the size of our cohort. In addition, routine elastic staining is not performed at our institution and pathologic evaluation of VPI is assessed only by hematoxylin and eosin (H&E) staining analysis, and not using the Hammar classification system.
The majority of data examining the impact of VPI on survival come from Asian series, where elastic staining is routine, which makes direct comparison between our data and these series difficult. Shimizu et al. examined 1074 pathologic specimens of T1/2 NSCLC and found a survival advantage for patients with tumors ≤ 3cm without VPI over tumors with VPI [8]. However, they did not segregate the patients with tumors ≤ 2cm. Yoshida et al. analyzed nearly 10,000 patients and separated the groups by tumor size using the AJCC 7th edition staging system and included patients with nodal metastases [9]. They suggested that T status in tumors of < 7cm with VPI, should be upgraded to the next T level.
Very few series have looked specifically at the relationship between VPI and tumor size in early-stage NSCLC. Results from the prospective multicenter ACOSOG trial Z0030 suggest that there is a significant link between tumor size and VPI for tumors >3cm [11]. Larger tumors, whether associated with VPI or not, are more prone to nodal and distant metastases and have a worse prognosis. Intuitively, one would imagine that patients whose prognosis is altered by the presence of VPI should have a pattern of recurrence that is different than patients with no VPI such as a higher rate of pleural metastatic disease but we did not observe such association.
Yoshida and al have also suggested an increased rate of nodal disease associated with VPI [9]. They reported in their series that half of the patients with VPI who had nodal disease had a T3 tumor or a tumor larger than 7cm making it impossible to differentiate whether nodal disease is a factor of size or pleural invasion.
Finally, the paper may give a mixed message as to the effect of VPI on OS. In the fitted multivariable exponential regression model summarized in Table 3, the effect of pleural invasion is significant (p=0.029). However looking at the 95% CI data in Table 6 the effect of VPI disappeared. The likely explanation is that the effects being estimated in Table 6 are tumor size-VPI interactive parameters. Hence, they are a second order effects. In contrast the significant VPI effect in Table 3 is a main effect, not an interaction. The effect on nodal disease, recurrence and survival from VPI may just be a reflection of its association with larger tumor size and not an independent negative effect by the VPI itself.
Conclusion
VPI and tumor size have different, but closely related effects on OS and DFS in NSLC. For DFS, VPI is harmful for patients with T2b, very harmful for patients with T3, and not harmful for patients with smaller tumors. This VPI-T interaction could not be observed for OS. Consequently, VPI should not be used to modify tumor size categories. In tumors of equal or less than 5cm, using VPI as a poor risk factor is not warranted.
Footnotes
Presented at the STS Annual meeting, Los Angeles, January 2013.
The authors have no disclosures to report.
References
- 1.American Cancer Society. Cancer Facts and Figures 2012. Available at www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-031941.pdf.
- 2.Butnor KJ, Travis WD. Recent advances in our understanding of lung cancer visceral pleural invasion and other forms of minimal invasion: implications for the next TNM classification. Eur J Cardiothorac Surg. 2012;0:1–3. doi: 10.1093/ejcts/ezs429. [DOI] [PubMed] [Google Scholar]
- 3.Rami-Porta R, Ball D, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the T descriptors in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol. 2007;2(7):593–602. doi: 10.1097/JTO.0b013e31807a2f81. [DOI] [PubMed] [Google Scholar]
- 4.Groome PA, Bolejack V, Crowley JJ, et al. The IASLC Lung Cancer Staging Project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2(8):694–705. doi: 10.1097/JTO.0b013e31812d05d5. [DOI] [PubMed] [Google Scholar]
- 5.Travis WD, Brambilla E, Rami-Porta R, et al. Visceral pleural invasion: pathologic criteria and use of elastic stains: proposal for the 7th edition of the TNM classification for lung cancer. J Thorac Oncol. 2008;3(12):1384–90. doi: 10.1097/JTO.0b013e31818e0d9f. [DOI] [PubMed] [Google Scholar]
- 6.Hammar S. Common Tumors. In: H S, Dail DH, editors. Pulmonary Pathology. Springer-Verlag; New York: 1994. p. 1138. [Google Scholar]
- 7.Hammar S. Common Tumors. In: H S, Dail DH, editors. Pulmonary Pathology. Springer-Verlag; New York: 1988. pp. 727–845. [Google Scholar]
- 8.Shimizu K, Yoshida J, Nagai K, et al. Visceral pleural invasion is an invasive and aggressive indicator of non-small cell lung cancer. J Thorac Cardiovasc Surg. 2005;130(1):160–5. doi: 10.1016/j.jtcvs.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida J, Nagai K, Asamura H, et al. Visceral pleura invasion impact on non-small cell lung cancer patient survival: its implications for the forthcoming TNM staging based on a large-scale nation-wide database. J Thorac Oncol. 2009;4(8):959–63. doi: 10.1097/JTO.0b013e3181a85d5e. [DOI] [PubMed] [Google Scholar]
- 10.Chang YL, Lin MW, Shih JY, et al. The significance of visceral pleural surface invasion in 321 cases of non-small cell lung cancers with pleural retraction. Ann Surg Oncol. 2012;19(9):3057–64. doi: 10.1245/s10434-012-2354-y. [DOI] [PubMed] [Google Scholar]
- 11.Fibla JJ, Cassivi SD, Brunelli A, et al. Re-evaluation of the prognostic value of visceral pleura invasion in Stage IB non-small cell lung cancer using the prospective multicenter ACOSOG Z0030 trial data set. Lung Cancer. 2012;78(3):259–62. doi: 10.1016/j.lungcan.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher R. On the interpretation of X2 from contingency tables, and the calculation of P. Journal of the Royal Statistical Society. 1922;85:87–94. [Google Scholar]
- 13.Randles R, Wolfe D. Introduction to the Theory of Nonparametric Statistics. John Wiley; 1979. [Google Scholar]
- 14.Kaplan EL, Meier P. Nonparametric estimator from incomplete observations. J American Statistical Association. 1958;53:457–81. [Google Scholar]
- 15.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemotherapy Reports. 1966;60:163–170. [PubMed] [Google Scholar]
- 16.Grambasch P, Therneau TT. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1997;81:515–526. [Google Scholar]
- 17.Schwarz G. Estimating the dimension of a model. Annals of Statistics. 1978;6(2):461–64. [Google Scholar]
- 18.The Cran Project. Available at: http://cran.r-project.org/web/packages/survival/index.html.


