Abstract
Guidelines recommend targeted antifungal prophylaxis for liver transplant recipients based on tiers of risk, rather than universal prophylaxis. The feasibility and efficacy of tiered, targeted prophylaxis is not well-established. We performed a retrospective study of liver transplant recipients who received targeted prophylaxis (n=145; voriconazole (54%), fluconazole (8%), no antifungal (38%))vs. universal voriconazole prophylaxis (n=237). Median durations of targeted and universal prophylaxis were 11 and 6 days, respectively (p<0.0001). The incidence of invasive fungal infections (IFIs)in targeted and universal groups was 6.9% and 4.2% (p= 0.34). Overall, intra-abdominal candidiasis (73%) was the most common IFI. Post-transplant bile leaks (p=0.001) and living donor transplants (p=0.04) were independent risk factors for IFI. IFIs occurred in 6% of high-risk transplants who received prophylaxis and 4% of low-risk transplants who did not receive prophylaxis (p=1.0).Mortality rates (100 days) were 10% (targeted) and 7% (universal) (p=0.26); attributable mortality due to IFI was 10%. Compliance with prophylaxis recommendations was 97%. Prophylaxis was discontinued for toxicity in 2% of patients. Targeted antifungal prophylaxis in liver transplant recipients was feasible and safe, effectively prevented IFIs, and reduced the number of patients exposed to antifungals. Bile leaks and living donor transplants should be considered high-risk indications for prophylaxis.
Introduction
Prior to the 2000s, invasive fungal infections (IFIs) were diagnosed in up to 40% of liver transplant recipients and associated with mortality rates ranging from 25 – 70%(1).IFIs were caused predominantly by Candida species (spp.) (60%- 80%). Infections due to Aspergillus spp. (1%– 8%), other moulds, and Cryptococcus spp. were less common(2).Most IFIs occurred within the first month after transplant(1, 3), and were associated with surgical factors, including the complexity of the transplant(1). The epidemiology of IFIs has changed in the recent era of liver transplantation, as surgical techniques and immunosuppressive and antifungal prophylaxis strategies have evolved(4). Over the past 10 years, the rate of IFIs has decreased to <10%(5–8),and risk factors such as retransplant, post-transplant renal failure requiring renal replacement therapy, and reoperation have been consistently identified(2).While the predominant pathogens remain consistent with older cohorts, the majority of infections due to Aspergillus spp. now occur ≥ 90 days post-transplant(3). More recent data have established the safety of withholding antifungal prophylaxis in low-risk patients.(4)
Taken together, guidelines from the American Society of Transplantation (AST) and Infectious Diseases Society of America (IDSA) currently recommend a three-tiered approach to antifungal prophylaxis following liver transplantation: 1) no prophylaxis for low-risk patients; 2) prophylaxis targeted against Candida spp. for patients with complicated operations, choledochojejunostomy anastomosis, or peri-operative Candida colonization; or 3) prophylaxis targeted against Candida and Aspergillus spp. for patients with risk factors of retransplantation, renal replacement therapy post-transplant, and reoperation(9–11).At present, there is a lack of data establishing the feasibility and efficacy of the AST/IDSA recommendations. Moreover, the appropriate antifungal agent, dose, and duration have not been determined(9–11).
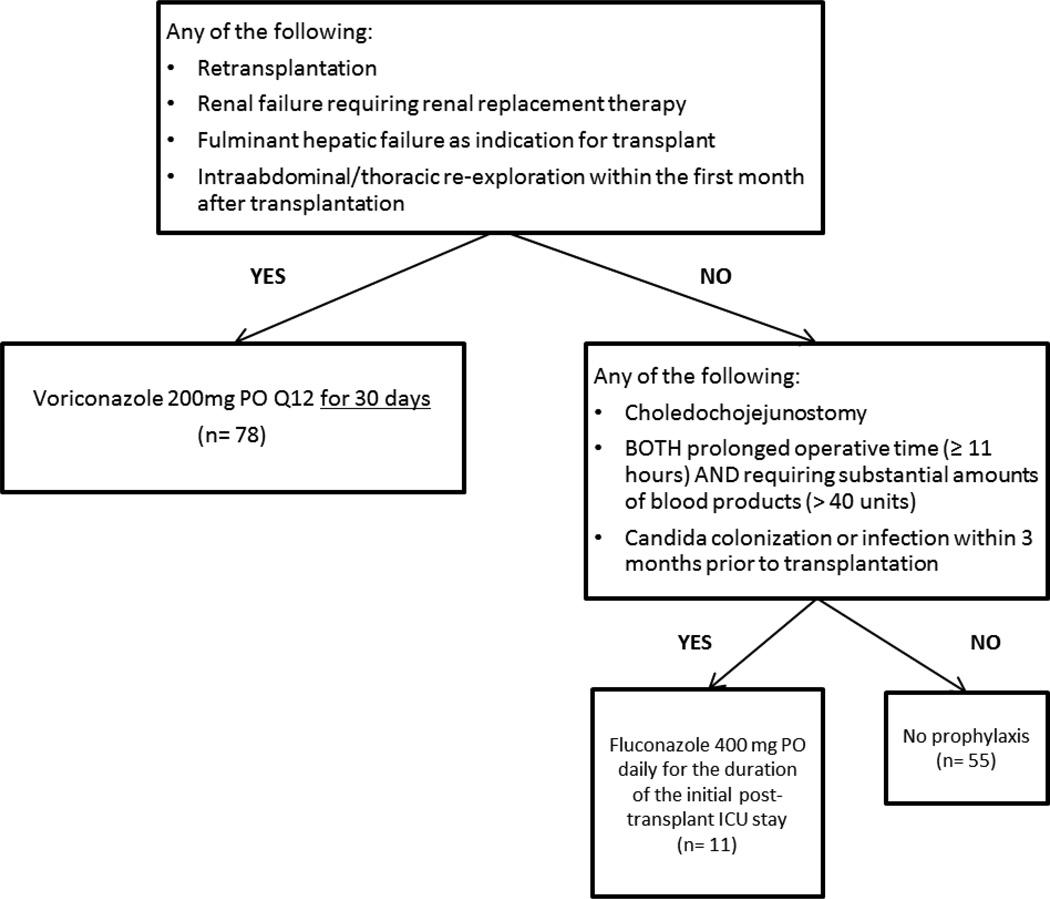
During a period of construction at our institution in mid-2007, several cases of invasive mould infections were diagnosed among organ transplant recipients residing in the transplant intensive care unit (ICU). In response to these developments, our program instituted a policy of universal antifungal prophylaxis with voriconazole for newly transplanted liver recipients during their stays in the transplant ICU. In 11/1/2010, were vised our approach from universal voriconazoleprophyl axis to a targeted algorithm that was adapted from AST/IDSA guidelines (Figure 1)(9, 12, 13). In the algorithm, patients were assigned to receive no antifungal prophylaxis, fluconazole, or voriconazole. In this study, we analyzed the comparative efficacy of targeted versus universal prophylaxis, and assessed the safety and efficacy of voriconazole, an agent that has not been investigated as a prophylactic agent in liver transplant recipients.
Figure 1.
Algorithm for targeted antifungal prophylaxis
Methods
Study design and patients
We performed a retrospective study of consecutive adult patients undergoing liver transplantation at the University of Pittsburgh Medical Center (UPMC) between November 1, 2008 and December 1, 2012 to compare IFI rates in patients receiving either targeted or universal antifungal prophylaxis. Patients were excluded if they under went multi visceral transplantation or dual transplant with lung(s) or heart, had an IFI at the time of transplant, died on the day of transplant, or if the transplant was performed at an outside institution. The University of Pittsburgh Institutional Review Board approved the study.
Antifungal prophylaxis
Patients were divided into two groups, based on antifungal prophylaxis regimen. The universal prophylaxis group consisted of patients transplanted between 11/1/2008 and 10/31/2010, during which the standard practice was voriconazole 200 mg oral twice daily for all patients. Voriconazole was started within one day of transplant and continued for the duration of the immediate post-transplant ICU stay. In November 2010, we instituted our tiered, targeted approach to antifungal prophylaxis (Figure 1). The targeted prophylaxis group consisted of patients transplanted between 11/1/2010 and 12/1/2012.
Definitions
IFIs were defined according to EORTC/MSG criteria(14); superficial fungal infections, mucocutaneous candidiasis and Candida colonization were not included. Death was attributed to IFI if there was ongoing positive fungal culture or infectious process at the time of death. Compliance with prophylaxis algorithms was defined by the use of the recommended agent. As there was a 3-month roll-out for the change in antifungal prophylaxis strategy, compliance in the targeted prophylaxis group was assessed after this period. The primary outcome of this study was the development of IFI within 100 days of transplantation. The secondary outcomes were attributable mortality for IFI, all-cause mortality at 100 days, compliance with antifungal prophylaxis algorithms, and toxicity of voriconazole.
Immunosuppressive regimen and prophylaxis
The standard immunosuppression during the study period consisted of methylprednisolone at the time of transplant followed post-operatively by tacrolimus, mycophenolate, and prednisone. For patients with underlying renal insufficiency (SCr> 1.5 mg/dL)(15), an early calcineurin-sparing regimen consisting of basiliximab induction followed by steroid taper and mycophenolate was used. Peri-operative antibacterial prophylaxis consisted of ampicillin-sulbactam for 72 hours; for patients allergic to β-lactam agents, aztreonam and vancomycin were recommended. Beginning in May 2011, patients known to be colonized with vancomycin-resistant Enterococcus received tigecycline for prophylaxis. Throughout the study period, trimethoprim/sulfamethoxazole was given for one year post-transplant. Prior to November 2011, pre-emptive valganciclovir was administered to all liver transplant recipients with CMV viremia, regardless of donor and recipient serology status; all other patients received acyclovir prophylaxis for the first month post-transplant. After November 2011, CMV seronegative recipients of livers from seropositive donors received universal prophylaxis with valganciclovir for 6 months; other patients received pre-emptive valganciclovir.
Statistical analysis
Instat Software (Graphpad Software Inc., San Diego, CA) was used. Comparisons of dichotomous and continuous variables were performed using Fisher's exact test and Mann-Whitney U test, respectively. P-values <0.05 were considered significant. To identify risk factors for IFIs, variables suggested by univariate analysis (p≤ 0.05) were entered into a multivariate logistic regression model; the type of antifungal prophylaxis (targeted vs. universal) was included in the model to address its impact on IFI. Stata/SE 12.1 was used to compute p-values, odds ratios and 95% confidence intervals. Kaplan–Meier curves were used to calculate event-free survival; curves were compared by log-rank test. Significance was defined as p-value 𢉤 0.05 (two-tailed).
Results
Patient characteristics
Three hundred ninety-eight transplants were performed at UPMC during the study period. Sixteen transplants were excluded (7 multivisceral transplants, 3 cotransplants with lung or heart, 5 deaths on day of transplant, and 1 IFI at time of transplant), leaving 382 transplants in 367 unique patients available for analysis (15 patients were re-transplanted in the study period).For purposes of our review, each transplant was considered to represent a patient at-risk for IFI. Two-hundred thirty-seven and 145 transplants were performed in the universal and targeted prophylaxis time periods, respectively. Among the universal prophylaxis group, 99.6% (236/237) of patients received voriconazole prophylaxis. Among the targeted prophylaxis group, 38% (55/145) of patients received no antifungal prophylaxis, and 54% (78/145), 8% (11/145) and <1% (1/145) of patients received voriconazole, fluconazole and itraconazole, respectively; the patient who received itraconazole had a remote history of pulmonary histoplasmosis. Patient demographics, baseline characteristics, and risk factors for IFIs are noted in Table 1. Patients in the targeted prophylaxis group were more likely to receive a kidney-liver dual transplant (p=0.003), undergo an operation exceeding 11 hours(without need for substantial transfused blood products) (p=0.02), or have a bile leak post-transplant (p=0.04).
Table 1.
Patient demographics, baseline characteristics and risk factors for invasive fungal infections.
| TOTAL (N=382) |
Universal Prophylaxis (N=237) |
Targeted Prophylaxis (N=145) |
P-value | |
|---|---|---|---|---|
| Age, years (mean ± standard deviation) | 55.7 ± 10.7 | 55.8 ± 11.0 | 55.4 ± 10.2 | 0.73 |
| Male | 65% (248/382) | 64% (152/237) | 66% (96/145) | 0.74 |
| Caucasian | 94% (359/382) |
95% (224/237) | 93% (135/145) |
0.66 |
| Indication for transplanta | ||||
| Fulminant hepatic failure | 2% (9/382) | 3% (6/237) | 2% (3/145) | 1.0 |
| Cryptogenic cirrhosis | 7% (26/382) | 8% (18/237) | 6% (8/145) | 0.53 |
| Nonalcoholic steatohepatitis | 10% (37/382) | 10% (24/237) | 9% (13/145) | 0.86 |
| Hepatocellular carcinoma | 14% (52/382) | 12% (28/237) | 17% (24/145) | 0.22 |
| Hepatitis C virus infection | 17% (66/382) | 19% (45/237) | 14% (21/145) | 0.27 |
| Alcoholic liver disease | 21% (80/382) | 22% (52/237) | 19% (28/145) | 0.60 |
| Others | 30% (114/382) |
27% (64/237) | 34% (50/145) | 0.13 |
| Re-transplant | 8% (29/382) | 6% (15/237) | 10% (14/145) | 0.24 |
| Re-transplant within 30 days | 1% (3/382) | 1% (2/237) | 1% (1/145) | 1.0 |
| HIV | 1% (5/382) | 2% (5/237) | 0% (0/145) | 0.16 |
| MELD (mean ± standard deviation) | 26.4 ± 8.2 | 25.8 ± 8.1 | 27.4 ± 8.4 | 0.07 |
| End-stage renal disease prior to transplant | 16% (62/382) | 16% (37/237) | 17% (25/145) | 0.67 |
| Candida colonization within 4 weeks prior to transplant | 7% (26/382) | 7% (17/237) | 6% (9/145) | 0.84 |
| Kidney/liver dual transplant | 4% (14/382) | 1% (3/237) | 8% (11/145) | 0.003 |
| Living donor | 13% (49/382) | 12% (28/237) | 14% (21/145) | 0.53 |
| Choledochojejunostomyanastomosis | 10% (40/382) | 10% (23/237) | 12% (17/145) | 0.61 |
| Operation length > 11 hours | 16% (63/382) | 13% (31/237) | 22% (32/145) | 0.02 |
| Intra-operative use of >40 units blood products | 11% (42/382) | 12% (28/237) | 10% (14/145) | 0.61 |
| Operation length >11 hours AND intra-operative use of >40 Units blood products | 4% (17/382) | 4% (10/237) | 5% (7/145) | 0.80 |
| Bile leak within 30 days post- transplant | 6% (22/382) | 4% (9/237) | 9% (13/145) | 0.04 |
| Reoperation within 30 days post transplant | 25% (96/382) | 22% (53/237) | 30% (43/145) | 0.12 |
| Renal replacement therapy within 30 days post-transplant | 27% (104/382) |
27% (63/237) | 28% (41/145) | 0.72 |
The targeted prophylaxis total adds up to 147 transplants because 2 patients with fulminant hepatic failure also had other indications for transplant
HIV = human immunodeficiency virus; MELD= Model for End-Stage Liver Disease
Invasive Fungal Infections
Twenty transplant recipients developed twenty-two IFIs (2 patients had two infections), for an overall rate of 5.2% (20/382). Twenty-three pathogens were recovered from the 20 patients. Ten patients in both the targeted and universal prophylaxis groups were diagnosed with an IFI (6.9% (10/145) vs.4.2% (10/237), respectively; p= 0.34).Details of IFIs are presented in Table 2. All patients developed their first episode of IFI within 30 days of transplant. Forty percent (8/20) of patients had breakthrough IFIs while receiving universal or targeted prophylaxis (n=4 each). Thirty-five percent (7/20) of patients developed IFI after prophylaxis had been discontinued (median of 17 days after the antifungal prophylaxis was stopped) and 25% (5/20) developed IFI in the absence of prophylaxis. Seventy-eight percent (18/23) of pathogens were Candida spp.; the predominant spp. were C. glabrata (39%, 9/23) and C. albicans(22%, 5/23). There were two infections due to Cryptococcus neoformans, and one infection each due to Saccharomyces cerevisiae, Aspergillus fumigatus and Rhizomucor. Eight-five percent (17/20) of patients developed peritonitis or another intra-abdominal infection. In each instance, intra-abdominal infection was caused by a Candida spp. or S. cerevisiae; 19% (3/16) of patients with intra-abdominal candidiasis had candidemia due to the same spp. C. neoformans and A. fumigatus caused pulmonary infections. Rhizomucor caused disseminated infection in an HIV/HCV co-infected patient. All patients with IFI were given algorithm-compliant prophylaxis, but prophylaxis was discontinued early in two patients. The reasons for discontinuation were voriconazole toxicity and an inability to continue voriconazole due to a lack of insurance coverage. Fifty percent (5/10) of patients with IFI in the targeted group did not receive antifungal prophylaxis, in keeping with the recommendations of the algorithm.
Table 2.
Invasive fungal infection case descriptions.
| Age (years) |
MELD score |
IFI risk factors | Living Donor |
Bile leak |
Prophylaxis | Days of Prophylaxis prior to IFI |
Days From Transplant to IFI |
Fungal organisms |
Types of infection | Treatment | Outcomea |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Universal Antifungal Prophylaxis | |||||||||||
| 50 | 33 | RRT post-transplant | - | - | Vori | 22 | 22 |
Saccharomyces cerevisiae |
Peritonitis, infected Hematoma (proven) |
FLU | Alive |
| 55 | 34 | None | Y | Y | Vori | 5 | 12 | C. tropicalis | Bile leak peritonitis (proven) |
FLU | Alive |
| 51 | 46 | HIV, Candida colonized, RRT post-transplant | - | - | Vori | 8 | 8 | Rhizomucor | Disseminated infection (proven at autopsy) |
None | Died |
| 64b | 29 | Choledochojejunostomy, RRT post-transplant |
- | - | Vori | 4 | 26 62 |
C. glabrata | Peritonitis (proven) Fungemia, catheter- associated (proven) |
VORI CAS |
Alive |
| 69 | 24 | None | - | - | Vori | 6 | 25 | C. glabrata | Intra-abdominal abscess (proven) |
CAS | Alive |
| 71 | 25 | None | - | - | Vori | 5 | 14 | C. parapsilosis | Peritonitis (proven) | VORI | Alive |
| 59 | 14 | None | Y | - | Vori | 5 | 22 | C. glabrata | Intra-abdominal abscess (proven) |
CAS | Alive |
| 23 | 22 | Fulminant hepatic failure, choledochojejunostomy, RRT post-transplant |
- | - | Vori | 4 | 4 | C. glabrata | Peritonitis (proven) | CAS | Alive |
| 40 | 29 | Choledochojejunostomy | - | - | Vori | 3 | 3 | C. glabrata | Peritonitis, infected Hematoma (proven) |
CAS | Alive |
| 71 | 25 | None | - | - | Vori | 3 | 8 |
Cryptococcus neoformans |
Pulmonary infection (proven) |
VORI | Alive |
| Targeted Antifungal Prophylaxis | |||||||||||
| 58 | 25 | None | Y | Y | None | 0 | 6 |
C. albicans and C. tropicalis |
Bile leak peritonitis (proven) |
CAS | Died |
| 57 | 28 | Reoperation | - | Y | Vori | 10 | 29 42 |
C. glabrata Aspergillusfumigatus |
Intra-abdominal abscess (proven) Pulmonary Infection (probable) |
VORI CAS |
Alive |
| 66 | 25 | Reoperation | - | Y | Vori | 8 | 8 | C. albicans | Bile leak peritonitis (proven) |
CAS | Alive |
| 58 | 18 | Reoperation | Y | Y | Vori | 10 | 10 | C. albicans | Bile leak peritonitis (proven) |
VORI | Alive |
| 54 | 48 | RRT post-transplant | - | - | None | 0 | 10 | C. glabrata | Fungemia and peritonitis (proven) |
CAS VORI |
Died |
| 57 | 13 | Reoperation | Y | Y | Vori | 7 | 7 | C. parapsilosis | Bile leak peritonitis (proven) |
CAS | Alive |
| 58 | 25 | None | - | - | None | 0 | 6 | C. albicans | Peritonitis (proven) | VORI | Alive |
| 67 | 19 | None | Y | - | None | 0 | 7 | C. albicans | Peritonitis (proven) | CAS | Alive |
| 59 | 25 | None | - | - | None | 0 | 18 |
Cryptococcus neoformans |
Pulmonary infection (proven) |
VORI AmB |
Alive |
| 57 | 15 | Re-transplant, choledochojejunostomy, reoperation |
Y | - | Vori | 16 | 16 | C. glabrata | Intra-abdominal abscess, fungemia (proven) |
VORI | Alive |
Mortality at 100 days
Note: 1) Ten patients had candiduria (5 each in the universal and targeted prophylaxis groups). All episodes were asymptomatic and associated with <25,000 colony forming units of Candida spp/mL. Most of these patients were evaluated by the Transplant Infectious Diseases service, and none was treated. Therefore, the cases were considered colonization and not included as IFIs. . 2) Among patients with IFI, only 1 patient had CMV viremia (asymptomatic) preceding IFI (3 days before IFI). The remaining patients developed viremia at a median time of 26 days after IFI (and 36.5 days after transplant).
AmB = Liposomal amphotericin B; CAS = Caspofungin; FLU = Fluconazole; HIV = human immunodeficiency virus; MELD= Model for End-Stage Liver Disease; RRT = renal replacement therapy; VORI = Voriconazole
Risk factors for IFIs are presented in Table 3. Prophylaxis group (targeted versus universal) designation was not associated with IFI on univariate analysis, nor was MELD score (p=0.78). Of the 46 patients in the targeted prophylaxis group with a MELD > 30, 59% (27/46) received prophylaxis and 41% (19/46) did not; the only patient who developed an IFI (IFI rate of 2%, 1/46)did not receive prophylaxis prior to the IFI. From the entire cohort, 12% (47/382) of patients developed acute cellular rejection (ACR) requiring pulsed corticosteroid therapy. There was no association between IFI and ACR; 8% (4/47) and 5% (16/335) of patients with ACR and no ACR developed IFI, respectively; p=0.29. Development of a bile leak within 30 days of transplant and living donor transplant were the only risk factors for IFI by univariate analysis (p=0.0004 and 0.008, respectively), and the only independent predictors of IFI by multivariate analysis (p=0.001 and 0.04, respectively; odds ratios = 7.13 and 2.96, respectively). Overall, 27% (6/22) of patients with post-transplant bile leak and 14% (7/49) of liver donor transplant recipients developed an IFI. Fifty-seven percent (4/7) of living donor recipients who developed IFIs had a bile leak.
Table 3.
Risk factors for invasive fungal infections.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Factors | IFI (n=20) | No IFI (n= 362) | P-value Odds ratio (95% Confidence interval) |
P-value Odds ratio (95% confidence interval) |
| Age (years), median | 58.1 | 56.6 | 0.51 | |
| MELD, median (mean) | 25 (26.1) | 25 (26.4) | 0.78 | |
| Re-transplant | 5% (1/20) | 8% (28/362) | 1.0 0.63 (0.08–4.87) |
|
| Candida colonization before or at time of transplant | 5% (1/20) | 7% (25/362) | 1.0 0.71 (0.09–5.52) |
|
| Reoperationa | 25% (5/20) | 22% (81/362) | 0.78 1.16 (0.41–3.28) |
|
| Renal failure prior to transplant | 15% (3/20) | 16% (59/362) | 1.0 0.91 (0.26–3.19) |
|
| Renal replacement therapy post- transplanta | 25% (5/20) | 27% (96/362) | 1.0 0.92 (0.33–2.61) |
|
| Fulminant hepatic failure | 5% (1/20) | 2% (8/362) | 0.39 2.33 (0.28–19.60) |
|
| Living donor | 35% (7/20) | 12% (42/362) | 0.008 4.10 (1.55–10.86) |
0.04 2.96 (1.05–8.40) |
| Choledochojejunostomy | 20% (4/20) | 10% (36/362) | 0.15 2.26 (0.72–7.14) |
|
| Operation length >11 hours | 25% (5/20) | 16% (58/362) | 0.35 1.75 (0.61–5.00) |
|
| Intra-operative use of >40 units blood products | 0% (0/20) | 12% (42/362) | 0.15 0.18 (0.01–3.10) |
|
| Operation length >11 hours AND intra-operative use of >40 Units blood products | 0% (0/20) | 5% (17/362) | 1.0 0.48 (0.03–8.30) |
|
| Targeted antifungal prophylaxis | 50% (10/20) | 37% (135/362) | 0.34 1.68 (0.68–4.14) |
|
| Bile leaks within 30 days post-transplant | 30% (6/20) | 4% (16/362) | 0.0004 9.27 (3.15–27.29) |
0.001 7.13 (2.31–22.04) |
Note: Bile leak within 30 days post-transplant and living donor remained independent risk factors for IFI when the types of antifungal prophylaxis was forced in the multivariate analysis model.
Total numbers of patients who required reoperation or renal replacement therapy post-transplant are lower than in Table 1 because only factors antecedent to the IFI are included
MELD= Model for End-Stage Liver Disease
The IFI rate in high-risk patients (defined as possessing a risk factor in Figure 1, living donor, or bile leak) who received prophylaxis was 6% (12/202) compared to 4% (2/46) in low-risk patients (defined as the absence of risk factors) not receiving prophylaxis (p=1.0).
Mortality
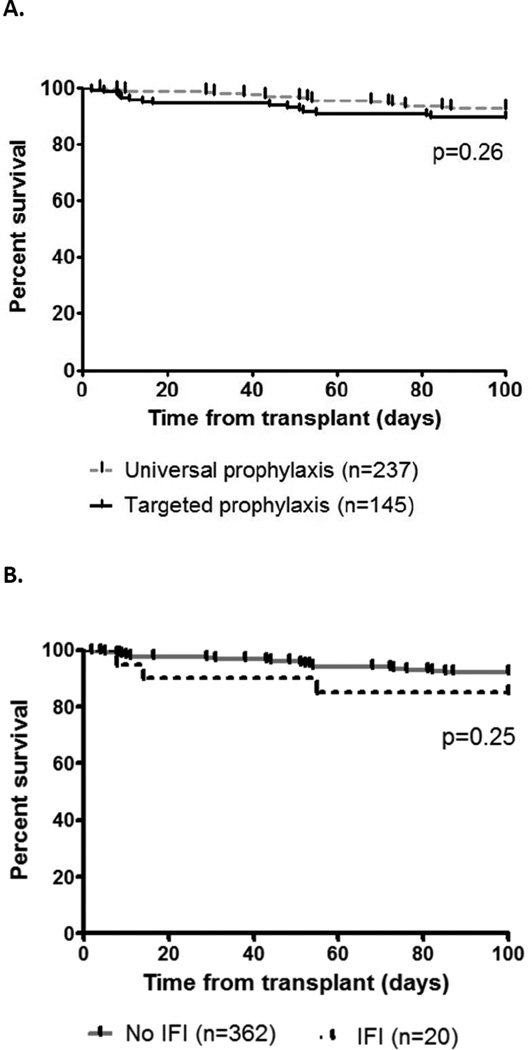
The overall mortality at 100 days and 1 year was 8% (32/382) and 16% (62/382), respectively. The 100 day mortality rate was 10% (15/145) in the targeted prophylaxis group and 7% (17/237) in the universal prophylaxis group (p=0.26, log-rank test; Figure 2A). The 100-day mortality rate among patients with IFI was 15% (3/20) compared to 8% (29/362) among patients without IFIs (p=0.25, log-rank test; Figure 2B).The attributable mortality rate for IFIs was 10% (2/20). The HIV/HCV coinfected patient who died due to disseminated Rhizomucor infection was in the universal prophylaxis group; the diagnosis was established at autopsy. A patient in the targeted prophylaxis group died due to persistent C. glabrata intra-abdominal infection.
Figure 2.
Survival among liver transplant recipients, stratified by type of antifungal prophylaxis (Fig. 2A) and presence or absence of IFIs (Fig 2B)
Compliance with prophylaxis algorithms
Overall compliance with the recommendations for antifungal prophylaxis was 97% (341/353). In the universal prophylaxis group, the median duration of prophylaxis in patients who would have been defined as high-risk was 9 days, with a 5% (5/107) IFI rate; corresponding data for low-risk patients were 4 days and 4% (5/130), respectively. After excluding the 20 patients with IFI, the duration of prophylaxis among patients who received an antifungal in the targeted group was significantly longer than in the universal group (median: 11 vs.6 days, IQR5–25 and 3–10, respectively; p<0.0001).No IFIs occurred during the 3-month roll-out period or in patients receiving non-compliant prophylaxis.
Toxicity
Antifungal prophylaxis was discontinued early due to concerns of toxicity in 2% (7/327) of patients; all received voriconazole prophylaxis. The reasons for early discontinuation were hepatotoxicity (3 patients), mental status changes (2), diarrhea (1), and supratherapeutictacrolimus levels due to a voriconazole interaction (1). Only the supratherapeutictacrolimus level was definitively ascribed to voriconazole.
Discussion
This large study of antifungal prophylaxis in liver transplant recipients has two particularly important findings. First, targeted antifungal prophylaxis was as effective as universal prophylaxis. Comparable out comes were achieved in the targeted prophylaxis group, even though 38% of patients did not receive an antifungal agent, and significantly higher percentages of patients had more complicated surgeries (prolonged lengths of transplant and kidney/liver dual transplants) and post-transplant complications (bile leaks). Our results are consistent with, and expand upon, previous findings. The observation that IFIs in low-risk patients were sufficiently uncommon as to not require prophylaxis (Supplemental Table 1)(4, 8, 16) supports AST and IDSA recommendations for targeted prophylaxis in liver transplantation(9–11). Specific definitions of “high-risk” liver transplant recipients differ dramatically across studies; nevertheless, our data clearly verify that antifungal prophylaxis in this population is effective (Supplemental Table 2)(5–8, 17–19). In particular, our IFI rate of 6% among high-risk patients compares favorably to rates of 15–35% in studies of high-risk patients not receiving prophylaxis(6, 20). Most importantly, the low attributable mortality in our study and others (Supplemental Table 2) suggests that prophylaxis attenuates the severity of IFIs that do develop(21, 22).
A second important finding is that bile leaks within the first 30 days post-transplant and living donor liver transplants were newly-identified, independent risk factors for IFI. Bile leaks have not commonly been assessed in studies of IFIs following liver transplantation. Biliary candidiasis and peritonitis are potentially problematic in liver transplant recipients since Candida has an affinity for growth in the biliary tract. Indeed, in one study, Candida was recovered in 44% of bile samples collected from patients undergoing endoscopic retrograde cholangiopancreatography (ERCP)(23).Bile extracts significantly decrease the susceptibility of C. albicans to various antifungals in vitro (24). In mouse models, C. albicans mutant strains that are attenuated for gastrointestinal colonization are more susceptible to bile salts than wild-type strains(25).Moreover, the gall bladder and biliary tree is a reservoir for the persistence of C. albicans within mice following antifungal therapy (24). Living donor liver transplants are highly technical procedures that are uncommonly performed in the United States(26); data from the Organ Procurement and Transplantation Network note that only 3.6% (252/7025) of liver transplants in 2013 were from living donors (http://optn.transplant.hrsa.gov/latestData/rptData.asp). Compared to deceased donor transplants, a higher percentage of our living donor transplants underwent operations lasting >11 hours (51% versus 11%, p<0.0001), and recipients were at higher risk for bile leak (16% versus 4%, p=0.003). These findings are concordant with results from a retrospective cohort study of 9 institutions in the United States(26).It is likely that the risk of IFI is increased in living donor liver recipients by both the complexity of the surgery and disruptions to the biliary tract. Taken together, our data suggest that antifungal prophylaxis should be instituted immediately following living donor transplant and when a bile leak is suspected or diagnosed. In fact, incorporating living donor transplant into our algorithm may result in a number of patients receiving antifungal treatment prior to the development of a bile leak.
Along related lines, previously identified risk factors for IFIs such as renal failure requiring dialysis, Candida colonization, fulminant hepatic failure, choledochojejunostomy, prolonged operation, receipt of numerous blood products, and reoperations were not identified as significant in this study. The data offer further support for the effectiveness of antifungal prophylaxis in these high-risk settings. In contrast to two recent studies(27, 28), we did not identify pre-transplant model for end stage liver disease (MELD) score to be associated with IFIs. In fact, the IFI rate in patients with MELD score ≥ 30 was only 3.4% and did not significantly differ between targeted and universal groups. Therefore, prophylaxis was effective even among patients in whom fulminant liver failure was the indication for transplant.
To the best of our knowledge, our study is the first to report the efficacy and tolerability of voriconazole as prophylaxis in liver transplant recipients. Among patients at high risk, the rate of IFIs was 6%, which is consistent with recent studies of other antifungal agents (Supplemental Table 2). Voriconazole is less expensive than echinocandins and lipid amphotericin B formulations and offers the advantage of oral administration. It is less nephrotoxic than amphotericin B products and provides broader-spectrum activity than fluconazole (covering moulds) or echinocandins (covering Cryptococcus neoformans and certain non-Aspergillus moulds)(29). There are obvious concerns with the use of voriconazole in liver transplant patients, including hepatotoxicity(30), significant interactions with calcineurin inhibitors(31), and highly variable pharmacokinetics(30).Voriconazole was discontinued in only 3 patients due to suspected hepatotoxicity, and in a single patient due to an interaction with tacrolimus. However, more prolonged use of voriconazole than in our cohort may result in higher toxicity rates(32, 33). Our study does not suggest that voriconazole is superior to any other prophylactic agent, as the number of patients receiving other antifungals was too limited to draw any conclusions. Furthermore, the literature (Supplemental Table 2) suggests that other agents may achieve similar prophylactic efficacy.
The pathogens causing IFI in our study were consistent with previous reports(2, 5–8, 18, 19).Yeasts (Candida spp., Cryptococcus neoformans and Saccharomyces cerevisiae) accounted for 91% (21/23) of disease-causing isolates. The remaining 9% (2/23) of pathogens were moulds (Aspergillus fumigatus and Rhizomucor). Our findings that C. glabrata and C. albicans were the predominant fungi, and that intra-abdominal candidiasis was the most common type of IFI, were in keeping with other studies of liver transplant recipients(Supplemental Table 2).The 19% (3/16) rate of candidemia among patients with intra-abdominal candidiasis was also in agreement with earlier studies(34, 35). Like Candida, S. cerevisiae(baker's yeast) caused an intra-abdominal infection. S. cerevisiae has been reported to cause invasive infections in immunocompromised patients, but infections in liver transplant recipients are exceedingly rare(36, 37).
C. neoformans caused pulmonary infections in 2 patients who were at low-risk for IFIs. Indeed, C. neoformans is recognized to cause disease among low-risk patients(4, 8).Cirrhosis, a common indication for liver transplantation, has been shown to predispose to cryptococcosis even in the absence of further immunosuppression(38).In addition, 52% of solid organ transplant recipients who develop cryptococcosis have serologic evidence of cryptococcal infection pre-transplant; seropositive patients develop disease earlier after transplant than patients without pre-transplant Cryptococcus antibodies(39).Liver transplant recipients appear particularly predisposed to infection within the first 30 days post-transplant (40). The two cryptococcal infections in our study were diagnosed 8 and 18 days post-transplant, suggesting that they may have been acquired pre-transplant but not eradicated due to impaired complement activity or other immune defects associated with cirrhosis(38, 40).It is plausible, then, that cryptococcosis developed rapidly following transplant with the administration of immunosuppressive agents. Donor-derived infection is possible, but less likely, since early-onset cryptococcosis has been predominantly reported to involve the allograft or surgical site(40). The identification of risk factors for cryptococcosis in our population would facilitate the development of prophylactic strategies. Pulmonary infections are the most common manifestation of aspergillosis. Although the majority of aspergillosis now occurs ≥ 90 days post-liver transplant, our case highlights that early-onset infections remain important(3). Finally, two cases of zygomycosis in liver transplant patients were reported previously from our center, corresponding to an incidence (in all solid organ transplant recipients) of 2 infections per 1000(41). The HIV/HCV patient in the present study who developed disseminated Rhizomucor disease died within 8 days.
Our data may inform recommendations for the duration of antifungal prophylaxis. The efficacy of short-course antifungal therapy in our universal prophylaxis group suggests that a duration of 30 days is unnecessary for a large proportion of patients. Neither the AST/IDSA guidelines nor the available literature (Supplemental Table 2) provide consistent recommendations for duration (9–11).Some studies examined defined, albeit widely variable, durations (range of 5 days to 10 weeks), while others recommended continuing prophylaxis until risk factors were no longer present or the patient was discharged from the hospital (Supplemental Table 2). We currently advocate an individualized approach, with antifungal regimens discontinued upon discharge from the ICU (as a marker of medical stability) and resolution of ongoing surgical complications.
There are several limitations to our study, in addition to those raised above. First, this was a retrospective review and data were limited to existing medical records. Our single-center experience should be cautiously extrapolated to other programs. Of particular note, our center has extensive experience with using voriconazole in lung and liver transplant recipients(42), and is comfortable managing voriconazole interactions with calcineurin inhibitors. Second, we did not routinely perform voriconazole therapeutic drug monitoring (TDM) during prophylaxis. A study of lung transplant recipients who received voriconazole prophylaxis at our center demonstrated a correlation between trough concentrations ≤ 1.5 µg/mL and IFI/fungal colonization (42). The comparable efficacy of voriconazole in our experience and other antifungal agents in previous studies of liver transplant recipients suggests TDM might not be necessary. Nevertheless, the issue warrants future study. Third, we did not routinely test infecting strains for antifungal susceptibility. The role of fluconazole or voriconazole prophylaxis in selecting for resistant pathogens like Rhizomucor or potentially resistant pathogens like C. glabrata remains unproven. Nevertheless, programs should be aware that widespread use of these agents may have an impact on institutional ecology. In this regard, the reduction in numbers of patients exposed to antifungal agents by targeted prophylaxis may be beneficial.
In conclusion, targeted antifungal prophylaxis in liver transplant recipients was feasible and safe, effectively prevented IFIs, and associated with attributable mortality similar to overall mortality among patients without IFI. The incorporation of bile leaks within 30 days post-transplant and living donor transplants into a prophylaxis algorithm as high-risk indications may further reduce the rate of IFIs. Future studies should validate tiered approaches to targeted prophylaxis, define optimal antifungal agents and durations of administration, and establish cost-effectiveness. Ideally, these issues should be addressed in a carefully-designed multi-center study.
Supplementary Material
Acknowledgments
MHN and CJC have received investigator-initiated research funding from Pfizer, Merck, and Astellas. FPS has received investigator-initiated research funding from Pfizer and Merck. RKS is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number KL2TR000146, and has received investigator-initiated funding from Merck and Astellas. All other authors report no disclosures. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- ABLC
amphotericin B lipid complex
- AmB
liposomal amphotericin B
- AmBd
amphotericin B deoxycholate
- AST
American Society of Transplantation
- ATB
antibiotics
- CAS
caspofungin
- CMV
cytomegalovirus
- CrCL
creatinine clearance
- FLU
fluconazole
- H
hours
- HIV
human immunodeficiency virus
- ICU
intensive care unit
- IDSA
Infectious Diseases Society of America
- IFI
invasive fungal infection
- ITR
itraconazole
- IV
intravenous
- LAmB
lipid amphotericin B preparations
- LT
liver transplant
- MELD
model for end stage liver disease
- PRBC
packed red blood cells
- PO
by mouth
- Q
every
- RRT
renal replacement therapy
- SCr
serum creatinine
- SOLN
solution
- TDM
therapeutic drug monitoring
- UNOS
United Network Organ Sharing
- UPMC
University of Pittsburgh Medical Center
- VORI
Voriconazole
Footnotes
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation.
Description of Supporting Information
Additional Supporting Information may be found in the online version of this article.
Table S1. Studies defining low-risk populations and rate of IFIs in the absence of prophylaxis
Table S2. Studies defining high-risk populations and rate of IFIs with prophylaxis
References
- 1.Husain S, Tollemar J, Dominguez EA, Baumgarten K, Humar A, Paterson DL, et al. Changes in the spectrum and risk factors for invasive candidiasis in liver transplant recipients: prospective, multicenter, case-controlled study. Transplantation. 2003;75(12):2023–2029. doi: 10.1097/01.TP.0000065178.93741.72. [DOI] [PubMed] [Google Scholar]
- 2.Eschenauer GA, Lam SW, Carver PL. Antifungal prophylaxis in liver transplant recipients. Liver Transpl. 2009;15(8):842–858. doi: 10.1002/lt.21826. [DOI] [PubMed] [Google Scholar]
- 3.Singh N, Avery RK, Munoz P, Pruett TL, Alexander B, Jacobs R, et al. Trends in risk profiles for and mortality associated with invasive aspergillosis among liver transplant recipients. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2003;36(1):46–52. doi: 10.1086/345441. [DOI] [PubMed] [Google Scholar]
- 4.Pappas PG, Andes D, Schuster M, Hadley S, Rabkin J, Merion RM, et al. Invasive fungal infections in low-risk liver transplant recipients: a multi-center prospective observational study. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6(2):386–391. doi: 10.1111/j.1600-6143.2005.01176.x. [DOI] [PubMed] [Google Scholar]
- 5.Shah T, Lai WK, Gow P, Leeming J, Mutimer D. Low-dose amphotericin for prevention of serious fungal infection following liver transplantation. Transplant infectious disease : an official journal of the Transplantation Society. 2005;7(3–4):126–132. doi: 10.1111/j.1399-3062.2005.00108.x. [DOI] [PubMed] [Google Scholar]
- 6.Reed A, Herndon JB, Ersoz N, Fujikawa T, Schain D, Lipori P, et al. Effect of prophylaxis on fungal infection and costs for high-risk liver transplant recipients. Liver Transpl. 2007;13(12):1743–1750. doi: 10.1002/lt.21331. [DOI] [PubMed] [Google Scholar]
- 7.Fortun J, Martin-Davila P, Montejo M, Munoz P, Cisneros JM, Ramos A, et al. Prophylaxis with caspofungin for invasive fungal infections in high-risk liver transplant recipients. Transplantation. 2009;87(3):424–435. doi: 10.1097/TP.0b013e3181932e76. [DOI] [PubMed] [Google Scholar]
- 8.Sun HY, Cacciarelli TV, Singh N. Micafungin versus amphotericin B lipid complex for the prevention of invasive fungal infections in high-risk liver transplant recipients. Transplantation. 2013;96(6):573–578. doi: 10.1097/TP.0b013e31829d674f. [DOI] [PubMed] [Google Scholar]
- 9.Pappas PG, Kauffman CA, Andes D, Benjamin DK, Jr, Calandra TF, Edwards JE, Jr, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009;48(5):503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silveira FP, Kusne S, Practice ASTIDCo. Candida infections in solid organ transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13(Suppl 4):220–227. doi: 10.1111/ajt.12114. [DOI] [PubMed] [Google Scholar]
- 11.Singh N, Husain S, Practice ASTIDCo. Aspergillosis in solid organ transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13(Suppl 4):228–241. doi: 10.1111/ajt.12115. [DOI] [PubMed] [Google Scholar]
- 12.Pappas PG, Silveira FP. Candida in solid organ transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9(Suppl 4):S173–S179. doi: 10.1111/j.1600-6143.2009.02909.x. [DOI] [PubMed] [Google Scholar]
- 13.Singh N, Husain S. Invasive aspergillosis in solid organ transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9(Suppl 4):S180–S191. doi: 10.1111/j.1600-6143.2009.02910.x. [DOI] [PubMed] [Google Scholar]
- 14.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008;46(12):1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verna EC, Farrand ED, Elnaggar AS, Pichardo EM, Balducci A, Emond JC, et al. Basiliximab induction and delayed calcineurin inhibitor initiation in liver transplant recipients with renal insufficiency. Transplantation. 2011;91(11):1254–1260. doi: 10.1097/TP.0b013e318218f0f5. [DOI] [PubMed] [Google Scholar]
- 16.San-Juan R, Aguado JM, Lumbreras C, Fortun J, Len O, Munoz P, et al. Universal prophylaxis with fluconazole for the prevention of early invasive fungal infection in low-risk liver transplant recipients. Transplantation. 2011;92(3):346–350. doi: 10.1097/TP.0b013e3182247bb4. [DOI] [PubMed] [Google Scholar]
- 17.Winston DJ, Busuttil RW. Randomized controlled trial of oral itraconazole solution versus intravenous/oral fluconazole for prevention of fungal infections in liver transplant recipients. Transplantation. 2002;74(5):688–695. doi: 10.1097/00007890-200209150-00017. [DOI] [PubMed] [Google Scholar]
- 18.Fortun J, Martin-Davila P, Moreno S, Barcena R, de Vicente E, Honrubia A, et al. Prevention of invasive fungal infections in liver transplant recipients: the role of prophylaxis with lipid formulations of amphotericin B in high-risk patients. The Journal of antimicrobial chemotherapy. 2003;52(5):813–819. doi: 10.1093/jac/dkg450. [DOI] [PubMed] [Google Scholar]
- 19.Hadley S, Huckabee C, Pappas PG, Daly J, Rabkin J, Kauffman CA, et al. Outcomes of antifungal prophylaxis in high-risk liver transplant recipients. Transplant infectious disease : an official journal of the Transplantation Society. 2009;11(1):40–48. doi: 10.1111/j.1399-3062.2008.00361.x. [DOI] [PubMed] [Google Scholar]
- 20.Singh N, Paterson DL, Gayowski T, Wagener MM, Marino IR. Preemptive prophylaxis with a lipid preparation of amphotericin B for invasive fungal infections in liver transplant recipients requiring renal replacement therapy. Transplantation. 2001;71(7):910–913. doi: 10.1097/00007890-200104150-00016. [DOI] [PubMed] [Google Scholar]
- 21.Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrobial agents and chemotherapy. 2005;49(9):3640–3645. doi: 10.1128/AAC.49.9.3640-3645.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, et al. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2006;43(1):25–31. doi: 10.1086/504810. [DOI] [PubMed] [Google Scholar]
- 23.Lenz P, Conrad B, Kucharzik T, Hilker E, Fegeler W, Ullerich H, et al. Prevalence, associations, and trends of biliary-tract candidiasis: a prospective observational study. Gastrointestinal endoscopy. 2009;70(3):480–487. doi: 10.1016/j.gie.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 24.Jacobsen ID, Luttich A, Kurzai O, Hube B, Brock M. In vivo imaging of disseminated murine Candida albicans infection reveals unexpected host sites of fungal persistence during antifungal therapy. The Journal of antimicrobial chemotherapy. 2014 doi: 10.1093/jac/dku198. [DOI] [PubMed] [Google Scholar]
- 25.Prieto D, Roman E, Correia I, Pla J. The HOG pathway is critical for the colonization of the mouse gastrointestinal tract by Candida albicans. PloS one. 2014;9(1):e87128. doi: 10.1371/journal.pone.0087128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freise CE, Gillespie BW, Koffron AJ, Lok AS, Pruett TL, Emond JC, et al. Recipient morbidity after living and deceased donor liver transplantation: findings from the A2ALL Retrospective Cohort Study. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008;8(12):2569–2579. doi: 10.1111/j.1600-6143.2008.02440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saliba F, Delvart V, Ichai P, Kassis N, Botterel F, Mihaila L, et al. Fungal infections after liver transplantation: outcomes and risk factors revisited in the MELD era. Clin Transplant. 2013;27(4):E454–E461. doi: 10.1111/ctr.12129. [DOI] [PubMed] [Google Scholar]
- 28.Lichtenstern C, Hochreiter M, Zehnter VD, Brenner T, Hofer S, Mieth M, et al. Pretransplant model for end stage liver disease score predicts posttransplant incidence of fungal infections after liver transplantation. Mycoses. 2013;56(3):350–357. doi: 10.1111/myc.12041. [DOI] [PubMed] [Google Scholar]
- 29.Dodds-Ashley ESLR, Lewis JS, Martin C, Andes D. Pharmacology of Systemic Antifungal Agents. Clinical Infectious Diseases. 2006;43:S28–S39. [Google Scholar]
- 30.Johnson HJ, Han K, Capitano B, Blisard D, Husain S, Linden PK, et al. Voriconazole pharmacokinetics in liver transplant recipients. Antimicrobial agents and chemotherapy. 2010;54(2):852–859. doi: 10.1128/AAC.00429-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saad AH, DePestel DD, Carver PL. Factors influencing the magnitude and clinical significance of drug interactions between azole antifungals and select immunosuppressants. Pharmacotherapy. 2006;26(12):1730–1744. doi: 10.1592/phco.26.12.1730. [DOI] [PubMed] [Google Scholar]
- 32.Husain S, Paterson DL, Studer S, Pilewski J, Crespo M, Zaldonis D, et al. Voriconazole prophylaxis in lung transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6(12):3008–3016. doi: 10.1111/j.1600-6143.2006.01548.x. [DOI] [PubMed] [Google Scholar]
- 33.Vadnerkar A, Nguyen MH, Mitsani D, Crespo M, Pilewski J, Toyoda Y, et al. Voriconazole exposure and geographic location are independent risk factors for squamous cell carcinoma of the skin among lung transplant recipients. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2010;29(11):1240–1244. doi: 10.1016/j.healun.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 34.Tissot F, Lamoth F, Hauser PM, Orasch C, Fluckiger U, Siegemund M, et al. beta-glucan antigenemia anticipates diagnosis of blood culture-negative intra abdominal candidiasis. American journal of respiratory and critical care medicine. 2013;188(9):1100–1109. doi: 10.1164/rccm.201211-2069OC. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen MH, Wissel MC, Shields RK, Salomoni MA, Hao B, Press EG, et al. Performance of Candida real-time polymerase chain reaction, beta-D-glucan assay, and blood cultures in the diagnosis of invasive candidiasis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;54(9):1240–1248. doi: 10.1093/cid/cis200. [DOI] [PubMed] [Google Scholar]
- 36.Alexander J, Limaye AP, Ko CW, Bronner MP, Kowdley KV. Association of hepatic iron overload with invasive fungal infection in liver transplant recipients. Liver Transpl. 2006;12(12):1799–1804. doi: 10.1002/lt.20827. [DOI] [PubMed] [Google Scholar]
- 37.Enache-Angoulvant A, Hennequin C. Invasive Saccharomyces infection: a comprehensive review. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2005;41(11):1559–1568. doi: 10.1086/497832. [DOI] [PubMed] [Google Scholar]
- 38.Singh N, Husain S, De Vera M, Gayowski T, Cacciarelli TV. Cryptococcus neoformans Infection in Patients With Cirrhosis, Including Liver Transplant Candidates. Medicine (Baltimore) 2004;83(3):188–192. doi: 10.1097/01.md.0000126760.45299.69. [DOI] [PubMed] [Google Scholar]
- 39.Saha DC, Goldman DL, Shao X, Casadevall A, Husain S, Limaye AP, et al. Serologic evidence for reactivation of cryptococcosis in solid-organ transplant recipients. Clinical and vaccine immunology : CVI. 2007;14(12):1550–1554. doi: 10.1128/CVI.00242-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun HY, Alexander BD, Lortholary O, Dromer F, Forrest GN, Lyon GM, et al. Unrecognized pretransplant and donor-derived cryptococcal disease in organ transplant recipients. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;51(9):1062–1069. doi: 10.1086/656584. [DOI] [PubMed] [Google Scholar]
- 41.Almyroudis NG, Sutton DA, Linden P, Rinaldi MG, Fung J, Kusne S. Zygomycosis in solid organ transplant recipients in a tertiary transplant center and review of the literature. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6(10):2365–2374. doi: 10.1111/j.1600-6143.2006.01496.x. [DOI] [PubMed] [Google Scholar]
- 42.Mitsani D, Nguyen MH, Shields RK, Toyoda Y, Kwak EJ, Silveira FP, et al. Prospective, observational study of voriconazole therapeutic drug monitoring among lung transplant recipients receiving prophylaxis: factors impacting levels of and associations between serum troughs, efficacy, and toxicity. Antimicrobial agents and chemotherapy. 2012;56(5):2371–2377. doi: 10.1128/AAC.05219-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.