Abstract
The development and maintenance of cocaine addiction depend heavily on learned reward-environment associations that can induce drug-seeking behavior and relapse. Understanding the mechanisms underlying these cue-induced conditioned responses is important for relapse prevention. To test whether intracellular responses measured after cocaine conditioned place preference (CPP) expression are context-dependent, we re-exposed cocaine-treated rats (drug-free) to an environment previously paired with cocaine or saline, 24 h after the CPP test. After 8 days of cocaine CPP training with one of two cocaine doses (5 mg/kg or 20 mg/kg, i.p.), CPP was expressed only after conditioning with the higher cocaine dose. In CPP expressing rats, locomotor responses after re-exposure to the cocaine-chamber were greater than in rats re-exposed to the saline-paired chamber. Nucleus Accumbens (NAc) phosphorylated ERK (pERK) levels were increased after re-exposure to the cocaine-paired, but not the saline-paired chamber, regardless of whether or not CPP behavior was expressed. Caudate Putamen (CPu) pERK and FosB protein levels increased after re-exposure to the cocaine chamber only after conditioning with the higher cocaine dose. Conversely, the higher cocaine dose, independent of environment, resulted in increased NAc FosB, ΔFosB and phosphorylated CREB (pCREB) protein levels compared to those conditioned with 5 mg/kg cocaine (non-CPP-expressing). Our results suggest that NAc ERK phosphorylation may be involved with retrieving the contextual information of a cocaine-association, without necessarily motivating the expression of CPP behavior. Additionally, we show distinct patterns of intracellular responses in the NAc and CPu indicating a region-specific role for pERK/pCREB/FosB intracellular signaling in the retrieval of cocaine-context associations.
Keywords: cocaine, conditioned place preference (CPP), ERK, CREB, FosB/ΔFosB, striatum
INTRODUCTION
Although cocaine addiction is a major clinical problem, an understanding of the intracellular underpinnings that drive the high rate of relapse observed in abstinent cocaine users is lacking (McHugh et al., 2013). Relapse is particularly common when preceded by drug-associated stimuli; stimuli (including but not limited to, paraphernalia, persons and/or environments) associated with the rewarding effects produced by drugs of abuse (Nestler, 2002; Hyman, 2005). Drug reward increases the motivational valence of stimuli in the environment that through Pavlovian learning mechanisms, become conditioned stimuli that directly motivate behavior in the absence of the original unconditioned stimulus (Cardinal and Everitt, 2004; Kelley, 2004). To date, we seem to have a clear understanding of the neural circuitry involved with the development of these learned environment associations. However, elucidating the underlying molecular mechanisms is necessary for the development and advancement of relapse prevention and treatment methods (Milton, 2012).
The striatum, made up of the Nucleus Accumbens (NAc) and Caudate Putamen (CPu), is a key brain region important for the regulation of reward, reward-learning and habitual responses (Wickens et al., 2007). Cocaine produces neuroplastic changes in intracellular signaling in these regions known to underlie long-term memory processes (Madsen et al., 2012). The NAc and CPu undergo changes in several of the same intracellular signaling proteins after acute and repeated cocaine exposure. For example, acute cocaine treatment increases Fos protein expression, ERK (extracellular regulated kinase) and CREB (cAMP response element binding protein) phosphorylation (Jenab et al., 2005; Sun et al., 2007) and chronic cocaine induces persistent increases in ΔFosB protein levels in both striatal regions (McClung and Nestler, 2003). ERK phosphorylation (pERK) has been extensively implicated in cocaine reward and plasticity (Lu et al., 2006) and is required for associative learning (Atkins et al., 1998). CPP rodent models indirectly measure drug-reward and allow for exploration of changes in signal transduction pathways that occur after exposure to an environment paired with cocaine (Taylor et al., 2008). Studies investigating ERK’s role in cocaine CPP suggest that ERK phosphorylation is necessary for both the development of a cocaine-context association (CPP acquisition) and for subsequent memory retrieval (CPP expression) (Valjent et al., 2000; Miller and Marshall, 2005; Ferguson et al., 2006). Specifically, recruitment of the ERK pathway during acute cocaine exposure is thought to promote neural plasticity while cocaine is present allowing for the encoding and consolidation of drug-related memories (Girault et al., 2007).
It is clear that striatal pERK plays a role in the acquisition and expression of CPP behaviors. However, a complete understanding of the relationship between changes in intracellular signaling pathways activated during the formation of cocaine-reward associations and those activated upon retrieval of the association is lacking. Additionally, the extent to which these changes are context-induced rather than a result of cocaine administration is unclear. To test whether intracellular responses measured after cocaine-CPP expression are conditioned responses to the cocaine-associated context, rather than to cocaine administration itself or withdrawal, we re-exposed cocaine-treated rats to an environment previously paired with cocaine or saline, drug-free after 8 days of cocaine-CPP training. Rats were conditioned with one of two doses of cocaine (5 or 20 mg/kg) previously shown to induce CPP (Russo et al., 2003) and reintroduced to either the cocaine-paired or saline-paired environment 24 h after CPP testing. We hypothesized that after CPP acquisition, rats expressing cocaine-CPP and reintroduced to the cocaine-paired environment would have increased pERK, CREB, and FosB protein levels compared to rats re-exposed to the context previously paired with saline.
EXPERIMENTAL PROCEDURES
Animals
Individually housed eight-week-old male Fischer rats (Charles River, Kingston, NY, USA) with free access to food and water were kept on a 12-h light/dark cycle. Animal care was in adherence with the guide for the care and use of laboratory animals and approval was obtained by the Institutional Animal Care and Use committee at Hunter College.
CPP apparatus
A three chamber place preference apparatus (Med Associates, Georgia, VT, USA), was used as previously described (Russo et al., 2003; Nygard et al., 2013). Briefly, a rectangular neutral chamber with gray walls and a smooth PVC floor separated two square conditioning chambers with distinct visual and tactile cues. The apparatus was equipped with a computerized photobeam system and used MED-PC software to record the time spent in each chamber, locomotor responses, entrances into each chamber, and explorations.
CPP procedure
During the preconditioning test, guillotine doors were open and rats were placed into the neutral middle chamber and allowed to freely explore all three chambers for 20 min. Rats were then randomly assigned to one of two groups to be conditioned with 5 mg/kg or 20 mg/kg cocaine. Conditioning consisted of alternating saline/cocaine treatments over the next 8 days. On days 1, 3, 5 and 7 of conditioning, rats were administered an intra-peritoneal (i.p.) injection of saline and immediately confined to one of the conditioning chambers for 30 min. On days 2, 4, 6, and 8, rats were treated with cocaine and immediately confined to the opposite chamber for 30 min. Conditioning was counterbalanced so that half of the animals received black side cocaine pairings and the other half received white side cocaine pairings. CPP testing was conducted with rats in a drug-free state the day after the last conditioning session and followed the same procedure as the preconditioning test (24 h after the last cocaine treatment rats were given free access to all three chambers for 20 min). All rats in this experiment were conditioned with one of the two cocaine doses. The unbiased design of our CPP protocol makes the comparison of time spent in the cocaine-paired chamber to time spent in the saline-paired chamber during the CPP test sufficient to establish CPP behavior, (Cunningham et al., 2003).
The day after the CPP test, (48 h after the last cocaine treatment), each group of rats was split into two subgroups matched for CPP scores (four groups total) for confinement to either the cocaine-paired or saline-paired chamber for 30 min without cocaine treatment (n = 4–5 per group). Subgroups were as follows: 5 mg/kg cocaine-paired: conditioned with 5 mg/kg cocaine and re-exposed to the cocaine-chamber; 5 mg/kg saline-paired: conditioned with 5 mg/kg cocaine and re-exposed to the saline-paired chamber; 20 mg/kg cocaine-paired: conditioned with 20 mg/kg cocaine and re-exposed to the cocaine-chamber; 20 mg/kg saline-paired: conditioned with 20 mg/kg cocaine and re-exposed to the saline-paired chamber. After 30-min re-exposure to one of the conditioning chambers (drug-free), rats were briefly (30 s or less) exposed to CO2 and rapidly decapitated. We chose 30 min as the time point for protein measurement because in addition to being the same duration as a conditioning session, our lab has shown that 30 min is ideal to observe levels of pERK, phosphorylated CREB (pCREB), and FosB in the same animals.
Protein preparation
Following decapitation brains were quickly removed, flash frozen in 2-methylbutane (−40 °C) and stored at −80 °C for 7 days until tissue was dissected. Tissue punches of NAc and CPu were dissected on a cold glass plate and homogenized with a Polytron handheld homogenizer (Kinematica, Luzern, Switzerland) in lysis buffer (50 mM Tris–HCl, 150 mM NaCl, 2 mM EDTA, 10% Glycerol, 1% Triton X-100, 1% sodium deoxycholic acid) containing a phosphatase inhibitor cocktail. Homogenates were incubated for 30 min and then centrifuged (15 min, 13,000 rpm, 4 °C). Supernatants were collected and stored at −80 °C until used for Western blot analysis.
Drugs and antibodies
Cocaine hydrochloride was purchased from Sigma Chemical Co. (St. Louis, MO, USA). Primary antibodies for pERK (9101), ERK (9102), CREB (9197), and FosB (5G4) were purchased from Cell Signaling Technologies (Beverly, MA, USA). pCREB primary antibody (06-519) was purchased from Millipore (Billercia, MA, USA) and α-tubulin primary antibody (sc-8035) was purchased from Santa Cruz Technologies (Santa Cruz, CA, USA). Horseradish peroxidase-conjugated anti-rabbit (NA-934) and anti-mouse (NA-931) immunoglobulin G (IgG) were obtained from Amersham Pharmacia (Piscataway, NJ, USA).
Protein measurement and Western blot analysis
Total protein content was determined using a Bradford kit from Bio-Rad laboratories (Hercules, CA, USA). Protein extracts (30–50 µg) were boiled for 5 min in Lammeli buffer with 1% Beta-mercaptoethanol, followed by electrophoresis onto 10–12% Tris–HCl SDS–PAGE gels and transferred onto PVDF membranes. Membranes were first blocked at room temperature with a solution of 5% nonfat dry milk in Tris-buffered saline with Tween-20 (TBST; pH=7.4) for 1 h. After three washes with TBST, membranes were incubated overnight at 4 °C with the primary antibody for pERK, pCREB or FosB (1:3000). Membranes were then washed with TBST three more times and incubated for 1 h at room temperature with the appropriate secondary antibody (1:1000). After three more washes with TBST, a chemiluminescence kit (Clarity ECL, BioRad) was used and membranes were exposed to X-ray film to detect antibody binding. All membranes were re-probed with the antibody for α-tubulin (1:3000) and their respective total protein (phosphorylated proteins) to normalize protein levels. Films were scanned and analyzed using ImageJ (NIH) to quantify the intensity of the protein bands.
Data analysis
CPP scores are defined as the time spent in the cocaine-paired chamber minus the time spent in the saline-paired chamber during testing. Time spent, entrances, and explorations of the saline and cocaine-paired chamber during the CPP test were analyzed with paired samples t-tests for each cocaine dose. Comparisons of behavioral and Western blot data made between the four subgroups were done with 2 × 2 (Conditioning-chamber × Dose) ANOVAs. Significant main effects were followed by independent samples t-tests with Bonferonni corrections and significant interactions were followed by pairwise comparisons of simple main effects. Main effects of the “Conditioning-chamber” variable refer to differences between rats re-exposed to the saline-paired and cocaine-paired chamber the day after the CPP test, regardless of cocaine-conditioning dose. Main effects of the “Dose” variable refer to differences between rats conditioned with 5 and 20 mg/kg, regardless of chamber re-exposure subgroup.
Locomotor responses were measured at two time points: (1) during the CPP test and (2) during re-exposure to the cocaine-paired or saline-paired chamber 24 h after the CPP test. Pearson correlation analysis was used to assess the relationship between CPP scores and locomotor responses recorded during both time points. Western blot data were converted to a ratio of phosphorylated protein levels to total protein levels, which were normalized to α-tubulin as a loading control, using arbitrary densitometric units. Statistical significance was determined at the p < 0.05 level for all comparisons.
RESULTS
Cocaine effects on CPP behaviors are dose dependent
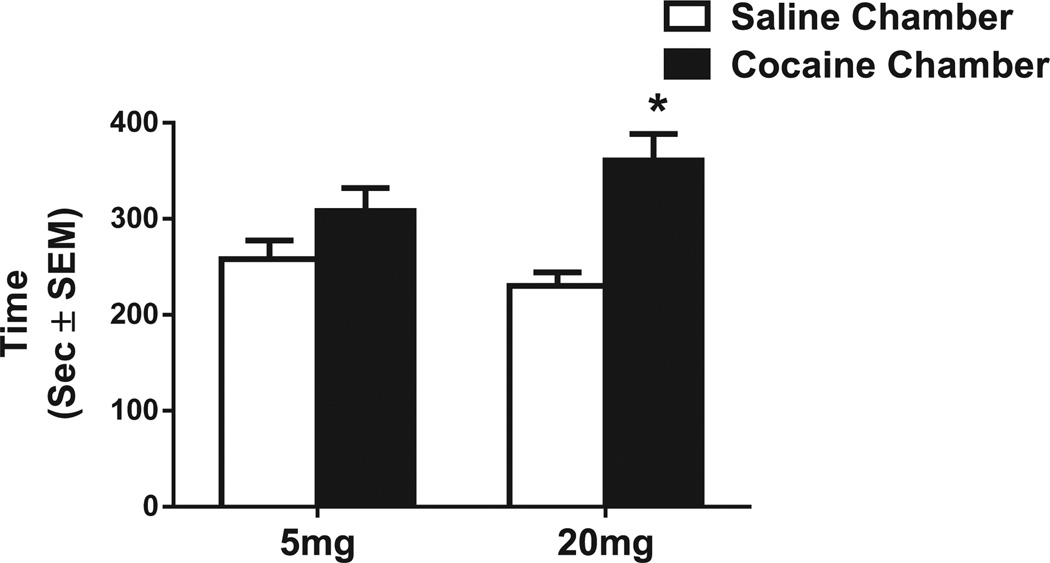
Unexpectedly, CPP was not expressed in rats conditioned with 5 mg/kg cocaine—no differences were seen between time spent in the cocaine-paired chamber and time spent in the saline-paired chamber [t(8) = 1.77, p = 0.20; Fig. 1]. Conversely, rats conditioned with 20 mg/kg cocaine spent significantly more time in the cocaine-paired than in the saline-paired chamber during the CPP test [t(8) = 2.72, p < 0.01; Fig. 1]. During the CPP test, no significant differences were found in total locomotor responses explorations or entrances into the cocaine-paired or saline paired-chamber after conditioning with either dose (Table 1).
Fig. 1.
Average time spent in seconds (± SEM) in the saline-paired chamber and cocaine-paired chamber during the CPP test after conditioning with 5 or 20 mg/kg cocaine. *Significant difference at the p < 0.05 level (n = 9 animals per group).
Table 1.
Cocaine effects on entrances and explorations during CPP test
| Dose (mg/kg) | Explorations | Entrances | Total locomotor counts | ||
|---|---|---|---|---|---|
| Saline-paired | Cocaine-paired | Saline-paired | Cocaine-paired | ||
| 5 | 69.30 ± 20.26 | 64.40 ± 17.20 | 89.20 ± 6.80 | 104.03 ± 15.50 | 1728 ± 79.92 |
| 20 | 52.60 ± 15.21 | 59.33 ± 7.01 | 75.22 ± 7.92 | 77.44 ± 13.50 | 1760 ± 51.21 |
Data are shown as mean (± SEM) number of entrances and explorations while in the saline-paired and cocaine-paired chambers during CPP testing (n = 9 animals per group).
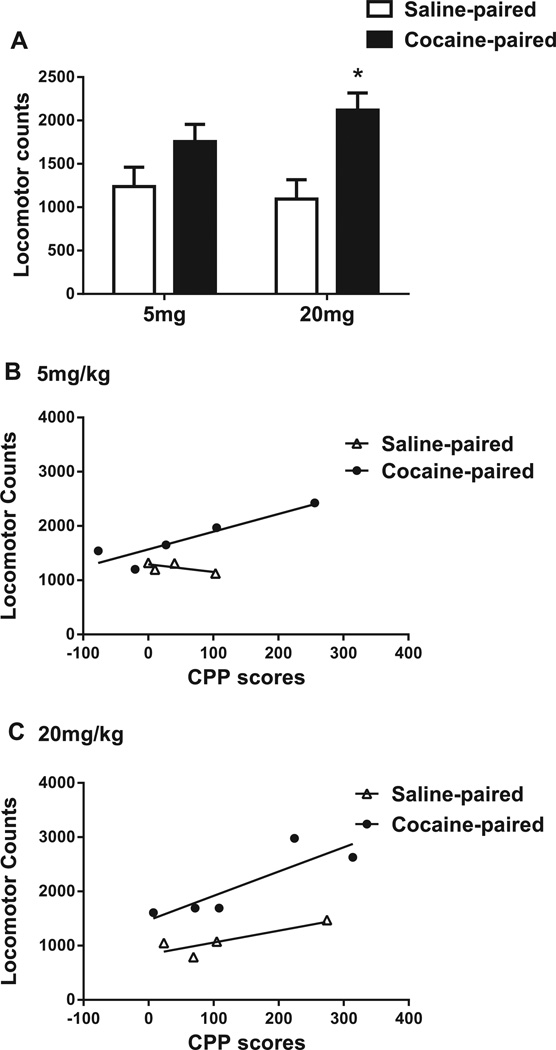
A significant main effect of Conditioning-chamber indicated that rats re-exposed to the chamber previously paired with cocaine were more active than those re-exposed to the chamber previously paired with saline [F(1,14) = 13.52, p < 0.01]; locomotor activity of 20 mg/kg cocaine-paired rats was significantly higher than 20 mg/kg saline-paired rats [p < 0.05; Fig. 2A]. Regardless of cocaine conditioning dose, locomotor responses in rats re-exposed to the cocaine-paired chamber were significantly correlated to CPP scores [5 mg/kg: r = 0.9, p < 0.05; 20 mg/kg: r = 0.8, p = 0.05; Fig. 2B and C, respectively].
Fig. 2.
Locomotor responses during re-exposure to the cocaine-paired or saline-paired chamber 24 h after the CPP test after conditioning with 5 or 20 mg/kg cocaine. (A) Total locomotor counts (mean ± SEM) in rats re-exposed to the saline-paired (white bars) or cocaine-paired (black bars) chamber. *Significant difference at the p < 0.05 level (n = 4–5 animals per group). (B, C) Correlations between CPP scores and locomotor counts during saline-chamber (triangles) or cocaine-chamber (circles) re-exposure after conditioning with (B) 5 mg/kg or (C) 20 mg/kg.
NAc pERK, pCREB and FosB/ΔFosB protein levels
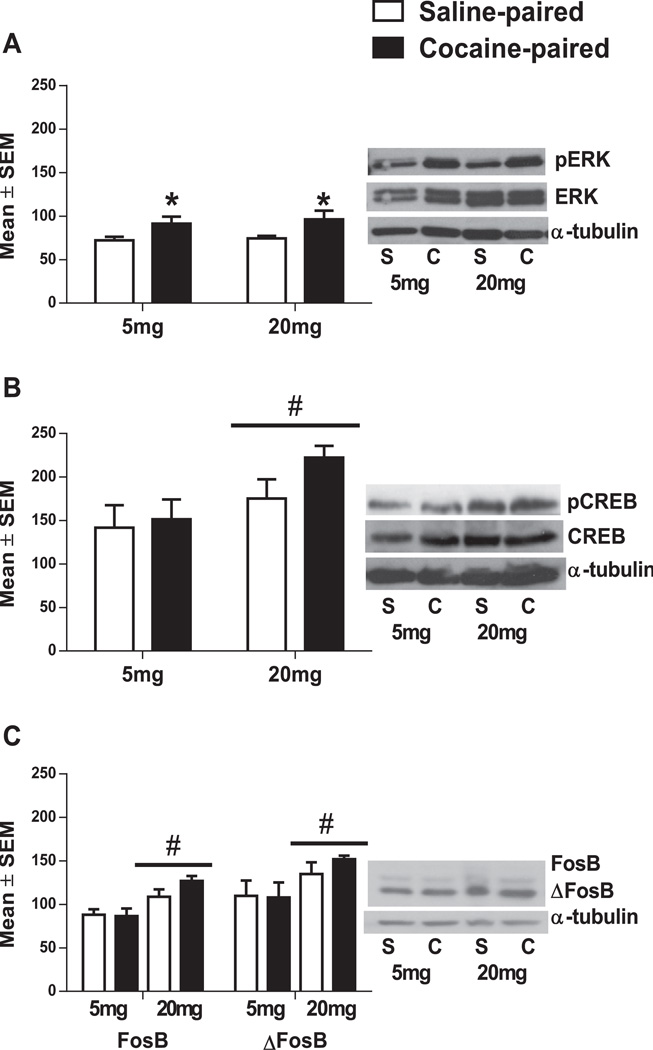
No differences were seen in total ERK or CREB protein levels in the NAc or CPu (Table 2). A significant main effect of Conditioning-chamber on NAc pERK levels was found [F(1,14) = 9.72, p < 0.05; Fig. 3A]. NAc pERK levels increased after re-exposure to the cocaine-paired chamber regardless of cocaine conditioning dose [p < 0.05 for all comparisons; Fig. 3A]. Conversely, we found significant main effects of Dose on NAc pCREB, FosB, and ΔFosB protein levels [pCREB: F(1,14) = 7.33, p < 0.05: Fig. 3B; FosB: F(1,12) = 15.24, p < 0.01: Fig. 3C; ΔFosB: F(1,12) = 5.98, p < 0.05; Fig. 3C]. NAc pCREB, FosB, and ΔFosB levels were higher after conditioning with 20 mg/kg cocaine compared to 5 mg/kg cocaine regardless of re-exposure to the cocaine-paired or saline-paired chamber [p < 0.05 for all comparisons; Fig. 3B and C].
Table 2.
Total ERK and CREB protein levels after CPP expression
| Region | 5 mg/kg | 20 mg/kg | ||
|---|---|---|---|---|
| Saline-paired | Cocaine-paired | Saline-paired | Cocaine-paired | |
| ERK | ||||
| NAc | 89.92 ± 6.10 | 80.54 ± 8.30 | 88.24 ± 7.90 | 96.12 ± 7.63 |
| CPu | 103.90 ± 6.40 | 102.14 ± 4.40 | 102.01 ± 2.72 | 90.30 ± 8.60 |
| CREB | ||||
| NAc | 218.30 ± 7.10 | 221.71 ± 5.53 | 224.30 ± 4.63 | 225.10 ± 4.30 |
| CPu | 204.70 ± 14.10 | 204.43 ± 4.00 | 199.02 ± 2.90 | 197.50 ± 9.10 |
Data are shown as mean arbitrary densitometric units (± SEM) normalized to α-tubulin (n = 4–5 animals per group). No significant differences were observed based on treatment.
Fig. 3.
NAc (A) pERK1/2, (B) pCREB and (C) FosB/ΔFosB protein levels after re-exposure to the cocaine paired or saline paired chamber after conditioning with 5 mg/kg (left) or 20 mg/kg (right) cocaine (24 h after initial CPP test). Phosphorylated protein levels are expressed as a ratio to total protein levels (normalized to α tubulin). *Significant difference from rats re-exposed to the saline-paired chamber of the same dose at the p < 0.05 levels. #Significant difference between rats trained with 5 and 20 mg/kg cocaine (n = 4– 5 animals per group).
CPu pERK, pCREB and FosB/ΔFosB protein levels
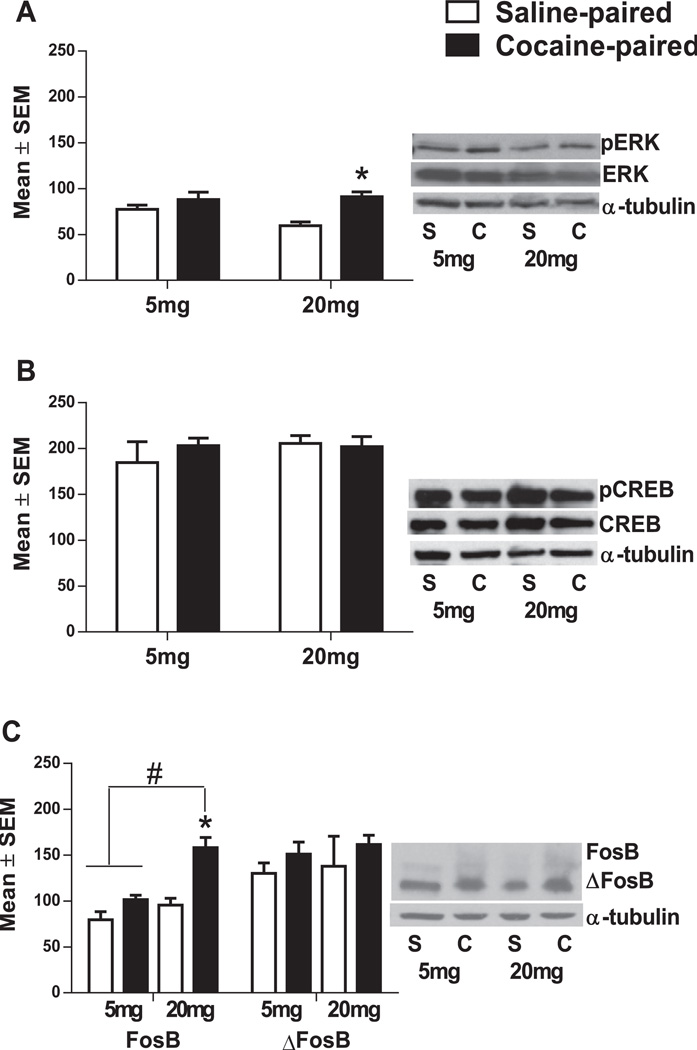
A main effect of Conditioning chamber showed CPu pERK was increased in rats re-exposed to the cocaine-paired chamber only after conditioning with 20 mg/kg cocaine [F(1,14)=11.30, p < 0.01; Fig. 4A]. CPu pCREB levels did not change based on cocaine Dose or Conditioning chamber re-exposure (Fig. 4B). A significant dose by Conditioning-chamber interaction effect on CPu FosB levels was also observed [F(1,12) = 5.10, p < 0.05]. CPu FosB levels in rats re-exposed to the cocaine-paired chamber after conditioning with 20 mg/kg were increased compared to 20 mg/kg saline-paired rats and rats conditioned with 5 mg/kg cocaine and re-exposed to the saline-paired chamber or the cocaine-paired chamber [F(1,12) = 14.82, p < 0.01; Fig. 4C].
Fig. 4.
CPu (A) pERK1/2, (B) pCREB and (C) FosB/ΔFosB protein levels after re-exposure to the cocaine-paired or saline-paired chamber after conditioning with 5 mg/kg (left) or 20 mg/kg (right) cocaine (24 h after initial CPP test). Phosphorylated protein levels are expressed as a ratio to total protein levels (normalized to α-tubulin). *Significant difference from rats re-exposed to the saline-paired chamber of the same dose at the p < 0.05 level. #Significant difference between rats trained with 5 and 20 mg/kg cocaine (n = 4–5 animals per group).
DISCUSSION
The aim of the present study was to determine the extent to which increases in phosphorylated protein levels previously observed after cocaine-CPP expression (Nygard et al., 2013), are context-induced. Consistent with our hypothesis, drug-free re-exposure to the environment paired with cocaine during CPP training context dependently increased NAc pERK levels when compared to animals re-exposed to the control environment. Because all rats were treated with cocaine and re-exposure to the saline conditioning chamber did not increase pERK levels, the increase cannot be attributed to cocaine treatment history or withdrawal. We also saw increased CPu pERK and FosB levels after CPP expression and re-exposure to the cocaine-paired environment. Rats conditioned with the higher cocaine dose (CPP expressing) and re-exposed to the cocaine-chamber had higher CPu pERK and FosB protein levels compared to those re-exposed to the saline-chamber and rats that did not express CPP behavior (rats conditioned with 5 mg/kg cocaine). The different patterns of intracellular responses we observed after exposure to a cocaine-paired environment are in agreement with previous studies suggesting that these striatal regions may be associated with different aspects of a drug-environment association. Specifically, our results suggest that the CPu and NAc may have differential roles in the expression of cocaine-CPP behavior and may regulate dissociable aspects of the learned association. The similar pattern of context-induced NAc pERK with or without the expression of CPP behavior suggests that pERK may not be sufficient to regulate the motivational properties that control the behavioral expression of CPP.
Unexpectedly, we observed differences in CPP behavior after training with two doses of cocaine previously shown to be effective in inducing CPP (Russo et al., 2003). We only saw CPP behavior after conditioning with the higher cocaine dose (20 mg/kg cocaine, but not 5 mg/kg cocaine). Rats trained with 20 mg/kg cocaine and re-introduced to the cocaine-chamber the day after the CPP test also displayed more locomotor responses than 20 mg/kg conditioned rats re-introduced to the saline- paired chamber. However, we saw some evidence of conditioned locomotion after conditioning with both doses. CPP scores were positively correlated to locomotor counts during forced re-exposure to the cocaine-paired context regardless of conditioning cocaine dose. Although rats trained with the lower cocaine dose (5 mg/kg) did not develop a preference for the cocaine-paired chamber, the positive correlation between CPP scores and locomotor counts during re-exposure to the cocaine-paired chamber, may be an indication of conditioned locomotion. Therefore, an association may have been made between the effects of cocaine and the context in which it was administered in response to both doses, but the lower dose may have lacked the rewarding properties to motivate CPP behavior during the CPP test. The extent to which the changes in protein levels reported here are involved with conditioned locomotor responses is still unclear. However, ERK phosphorylation has been implicated in the development of conditioned locomotor behavior in a cocaine-paired context (Valjent et al., 2006). Increased CPu pERK and FosB levels may also be related to the increased locomotor counts in rats re-exposed to the cocaine-chamber and may play a role in the expression of a conditioned locomotor response. Therefore, the CPu may be more important to CPP expression and the motivational aspects of the cocaine-contextual association; probably those driving the motivation to engage in motor behaviors (Wickens et al., 2007; Everitt and Robbins, 2013). Our observation of context-induced increases in CPu pERK and FosB only after CPP expression, may also suggest a potential role for these proteins in contributing to extinction learning that occurs after repeated exposure to a previously drug-paired chamber without drug (Auber et al., 2013). Although this postulate requires further testing, regardless of the precise memory component involved, it is clear that the protein level increases we observed were context-induced.
Recent research has shown that the motivational and associative components of cocaine-environment associations may be dissociable and are mediated by different brain regions (Theberge et al., 2010; Ding et al., 2013; Wells et al., 2013). NAc pERK may be more involved with the associative component due to the increase after cocaine-context re-exposure regardless of CPP expression. Wells et al. (2013) recently found that NAc ERK inhibition during reconsolidation of an instrumental cocaine-context association had no effect on subsequent cocaine-seeking behavior. Based on our results, the lack of behavioral impairment makes sense because NAc ERK phosphorylation was increased even when cocaine-seeking behaviors were not exhibited. According to this hypothesis, inhibiting ERK during reconsolidation (exposure to the cocaine-context) would not alter subsequent cocaine seeking because the motivational components of the association could remain intact. The increase in NAc pERK observed in the present study is contradictory to the results of Tropea et al. (2008) in which an increase in NAc pERK was not seen after cocaine-paired environment re-exposure. The differences in results can potentially be explained through the differences in cocaine dose, time points examined and method of CPP testing. However, the role of NAc pERK has been extensively studied and our results are consistent with most previous research. Marin et al. (2009) found different patterns of context-specific cocaine-induced NAc ERK and CREB phosphorylation after a cocaine-challenge. In the present study, rats were drug-free indicating that changes in protein levels reported were not due to the presence of cocaine. Rather, the increases in protein levels after re-exposure to the cocaine-chamber (increased NAc pERK and CPu FosB and pERK) were most likely context-induced and may be necessary for the retrieval of a cocaine-associated memory.
Future research should examine connections between these brain regions and others known to be involved with associative learning, attention, and emotional processing. For example, a role of the amygdala in the attention processes necessary for motivational associative learning has recently been shown in humans (Li et al., 2011) and rodents (Wells et al., 2013). NAc pCREB, FosB/ΔFosB dose dependently increased, regardless of re-exposure context. In addition to temporal differences in protein expression in the NAc and CPu, these proteins may be regulating different aspects of a cocaine-contextual memory. It is necessary to explore the precise mechanisms regulating these differences. Future studies need to be done in order to clarify the increased NAc pERK we observed after cocaine-CPP training and re-exposure to the cocaine-paired environment (but not the control environment) regardless of whether or not CPP behavior was expressed. However, given the role of ERK phosphorylation in associative learning (Atkins et al., 1998), our data are in agreement with previous research implicating NAc pERK as a necessary component to form the association, but may not be sufficient on its own to motivate drug-seeking behavior when in contact with the drug-associated context. Our observation that NAc pCREB and ΔFosB protein levels dose dependently increased further implicates NAc ΔFosB accumulation in cocaine-reward and motivation (Lobo et al., 2013). These dose-dependent effects may represent downstream regulators of drug-reward processing, and thus may work in combination with NAc pERK to establish the motivation necessary for the expression of drug-seeking behaviors such as CPP.
CONCLUSIONS
The present study provides evidence for the role of ERK and CREB intracellular signaling pathways in mediating the neuronal plasticity involved with cocaine-context associations. Our data indicate a region-specific role for pERK/pCREB/FosB intracellular signaling in the acquisition and subsequent expression/retrieval of cocaine-context associations. Overall, the changes we observed in CPu and NAc pERK suggest a multifunctional role for pERK in the retrieval of cocaine-context associations. Disrupting reconsolidation of the motivational component may be helpful for the development of new treatments (Tronson and Taylor, 2013). Therefore, these results will aid in the advancement of the general knowledge about the molecular formation and retrieval of cocaine-associated memories that can be used in the future when designing treatments for cocaine addiction to assist in both the cessation of addiction and prevention of relapse.
Acknowledgements
This work was supported by DA12136, MD007599, and PSC-CUNY to S.J. and V.Q.J.
Abbreviations
- CPP
conditioned place preference
- CPu
Caudate Putamen
- CREB
cAMP response element binding protein
- ERK
extracellular regulated kinase
- NAc
Nucleus Accumbens
- pCREB
phosphorylated CREB
- pERK
phosphorylated ERK
Footnotes
AUTHOR CONTRIBUTIONS
S.K.N., V.Q.J., and S.J. designed experiments. S.K.N. performed experiments, statistical analysis, and wrote the manuscript. A.K. helped with behavioral testing, sacrificing and tissue preparation. B.B. ran and analyzed western blots. All authors helped edit the manuscript and have approved the final article for submission.
Contributor Information
S. K. Nygard, Email: stephanienygard@gmail.com.
A. Klambatsen, Email: anthonyklambatsen@yahoo.com.
B. Balouch, Email: bbalouch@gmail.com.
V. Quinones-Jenab, Email: vaquinon@hunter.cuny.edu.
S. Jenab, Email: sjenab@hunter.cuny.edu.
REFERENCES
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt DJ. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- Auber A, Tedesco V, Jones CE, Monfils MH, Chiamulera C. Post-retrieval extinction as reconsolidation interference: methodological issues or boundary conditions? Psychopharmacology. 2013;226:631–647. doi: 10.1007/s00213-013-3004-1. http://dx.doi.org/10.1007/s00213-013-3004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Everitt BJ. Neural and psychological mechanisms underlying appetitive learning: links to drug addiction. Curr Opin Neurobiol. 2004;14:156–162. doi: 10.1016/j.conb.2004.03.004. http://dx.doi.org/10.1016/j.conb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Ferree NK, Howard MA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology (Berl) 2003;170:409–422. doi: 10.1007/s00213-003-1559-y. [DOI] [PubMed] [Google Scholar]
- Ding ZB, Wu P, Luo YX, Shi HS, Shen HW, Wang SJ, Lu L. Region-specific role of Rac in nucleus accumbens core and basolateral amygdala in consolidation and reconsolidation of cocaine-associated cue memory in rats. Psychopharmacology. 2013;228:427–437. doi: 10.1007/s00213-013-3050-8. http://dx.doi.org/10.1007/s00213-013-3050-8. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci Biobehav Rev. 2013;37:1946–1954. doi: 10.1016/j.neubiorev.2013.02.010. http://dx.doi.org/10.1016/j.neubiorev.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Fasano S, Yang P, Brambilla R, Robinson TE. Knockout of ERK1 enhances cocaine-evoked immediate early gene expression and behavioral plasticity. Neuropsychopharmacology. 2006;31:2660–2668. doi: 10.1038/sj.npp.1301014. [DOI] [PubMed] [Google Scholar]
- Girault JA, Valjent E, Caboche J, Herve D. ERK2: a Logical AND gate critical for drug-induced plasticity? Curr Opin Pharmacol. 2007;7:77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Addiction: a disease of learning and memory. AM J Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Jenab S, Festa ED, Nazarian A, Wu HBK, Sun WL, Hazim R, et al. Cocaine induction of ERK proteins in dorsal striatum of Fischer rats. Mol Brain Res. 2005;142:134–138. doi: 10.1016/j.molbrainres.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Li J, Schiller D, Schoenbaum G, Phelps EA, Daw ND. Differential roles of human striatum and amygdala in associative learning. Nat Neurosci. 2011;14:1250–1252. doi: 10.1038/nn.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Zaman S, Damez-Werno D, Koo JW, Bagot R, DiNieri J, Nugent A, Finkel E, Chaudhury D, Chandra R, Riberio E, Rabkin J, Mouzon E, Cachope R, Cheer J, Han M-H, Dietz DM, Self D, Hurd Y, Vialou V, Nestler EJ. DFosB induction in striatal medium spiny neuron subtypes in response to chronic pharmacological, emotional, and optogenetic stimuli. J Neurosci. 2013;33:18381–18395. doi: 10.1523/JNEUROSCI.1875-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Madsen HB, Brown RM, Lawrence AJ. Neuroplasticity in addiction: cellular and transcriptional perspectives. Front Mol Neurosci. 2012;5:99. doi: 10.3389/fnmol.2012.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin MT, Berkow A, Golden SA, Koya E, Planeta CS, Hope BT. Context-specific modulation of cocaine-induced locomotor sensitization and ERK and CREB phosphorylation in the rat nucleus accumbens. Eur J Neurosci. 2009;30:1931–1940. doi: 10.1111/j.1460-9568.2009.06982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ. Regulation of gene expression and cocaine reward by CREB and DeltaFosB. Nat Neurosci. 2003;6:1208–1215. doi: 10.1038/nn1143. http://dx.doi.org/10.1038/nn1143. [DOI] [PubMed] [Google Scholar]
- McHugh MJ, Demers CH, Braud J, Briggs R, Adinoff B, Stein EA. Striatal-insula circuits in cocaine addiction: implications for impulsivity and relapse risk. Am J Drug Alcohol Abuse. 2013;39:424–432. doi: 10.3109/00952990.2013.847446. [DOI] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Milton AL. Drink, drugs and disruption: memory manipulation for the treatment of addiction. Curr Opin Neurobiol. 2012;23:706–712. doi: 10.1016/j.conb.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Common molecular and cellular substrates of addiction and memory. Neurobiol Learn Mem. 2002;78:637–647. doi: 10.1006/nlme.2002.4084. [DOI] [PubMed] [Google Scholar]
- Nygard SK, Klambetsen A, Hazim R, Eltareb MH, Blank JC, Chang AJ, Quinones-Jenab V, Jenab S. Sexually dimorphic intracellular responses after cocaine-induced conditioned place preference expression. Brain Res. 2013;1520:121–133. doi: 10.1016/j.brainres.2013.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Jenab S, Fabian SJ, Festa ED, Kemen LM, Quinones-Jenab V. Sex differences in the conditioned rewarding effects of cocaine. Brain Res. 2003;970:214–220. doi: 10.1016/s0006-8993(03)02346-1. [DOI] [PubMed] [Google Scholar]
- Sun WL, Zhou L, Hazim R, Quinones-Jenab V, Jenab S. Effects of acute cocaine on ERK and DARP-32 phosphorylation pathways in the caudate-putamen of Fischer rats. Brain Res. 2007;1178:12–19. doi: 10.1016/j.brainres.2007.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Olausson P, Quinn JJ, Torregrossa MM. Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology. 2008;56:186–195. doi: 10.1016/j.neuropharm.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theberge FRM, Milton AL, Belin D, Lee JLC, Everitt BJ. The basolateral amygdala and nucleus accumbens core mediate dissociable aspects of drug memory reconsolidation. Learn Mem. 2010;17:444–453. doi: 10.1101/lm.1757410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR. Addiction: a drug-induced disorder of memory reconsolidation. Curr Opin Neurobiol. 2013;23:573–580. doi: 10.1016/j.conb.2013.01.022. http://dx.doi.org/10.1016/j.conb.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropea TF, Kosofsky BE, Rajadhyaksha AM. Enhanced CREB and DARP-32 phosphorylation in the nucleus accumbens and CREB, ERK, and GluR1 phosphorylation in the dorsal hippocampus is associated with cocaine-conditioned place preference behavior. J Neurochem. 2008;106:1780–1790. doi: 10.1111/j.1471-4159.2008.05518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Trzaskos JM, Girault JA, Herve D. Role of the ERK pathway in psychostimulant-induced locomotor sensitization. BMC Neurosci. 2006;7:1–11. doi: 10.1186/1471-2202-7-20. http://dx.doi.org/10.1186/1471-2202/7/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AM, Arguello AA, Xie X, Blanton MA, Lassete HC, Reittinger AM, Fuchs RA. Extracellular signal-regulated kinase in the basolateral amygdala, but not the nucleus accumbens core, is critical for context-response-cocaine memory reconsolidation in rats. Neuropsychopharmacology. 2013;38:753–762. doi: 10.1038/npp.2012.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens JR, Budd CS, Hyland BI, Arbuthnott GW. Striatal contributions to reward and decision making: making sense of regional variations in a reiterated processing matrix. Ann N Y Acad Sci. 2007;1104:192–212. doi: 10.1196/annals.1390.016. http://dx.doi.org/10.1196/annals.1390.016. [DOI] [PubMed] [Google Scholar]