Abstract
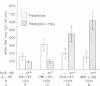
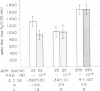
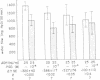
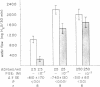
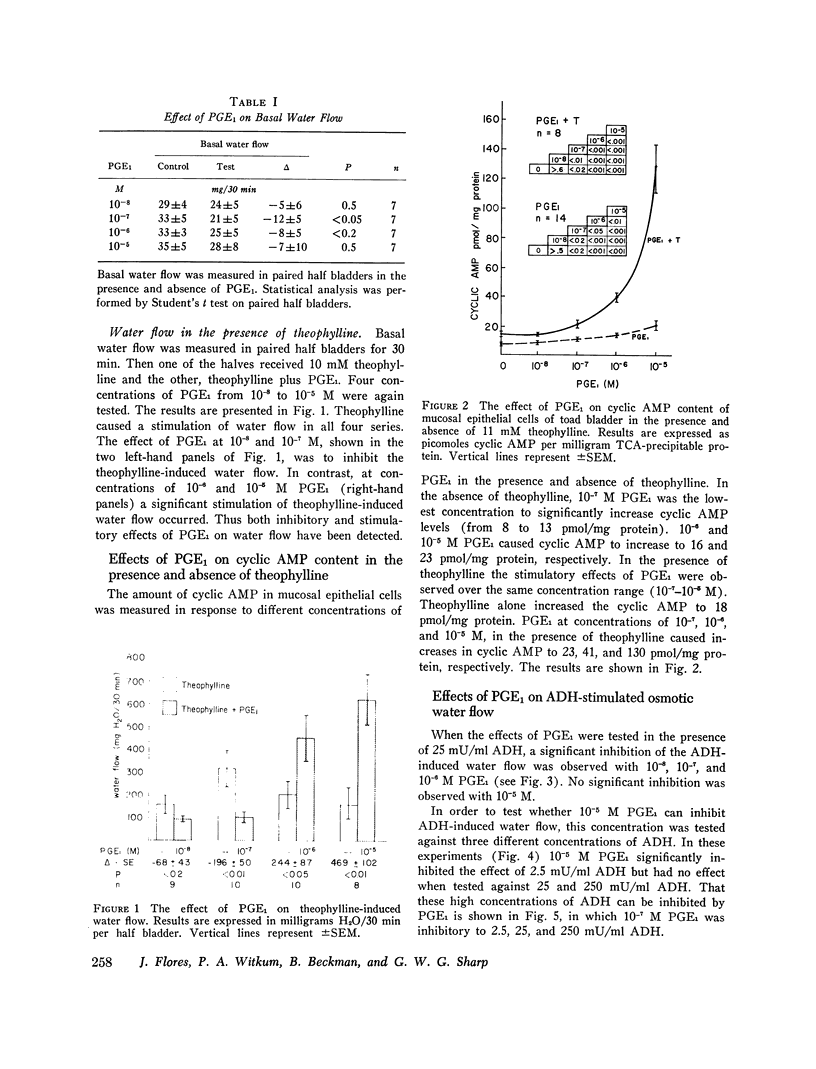
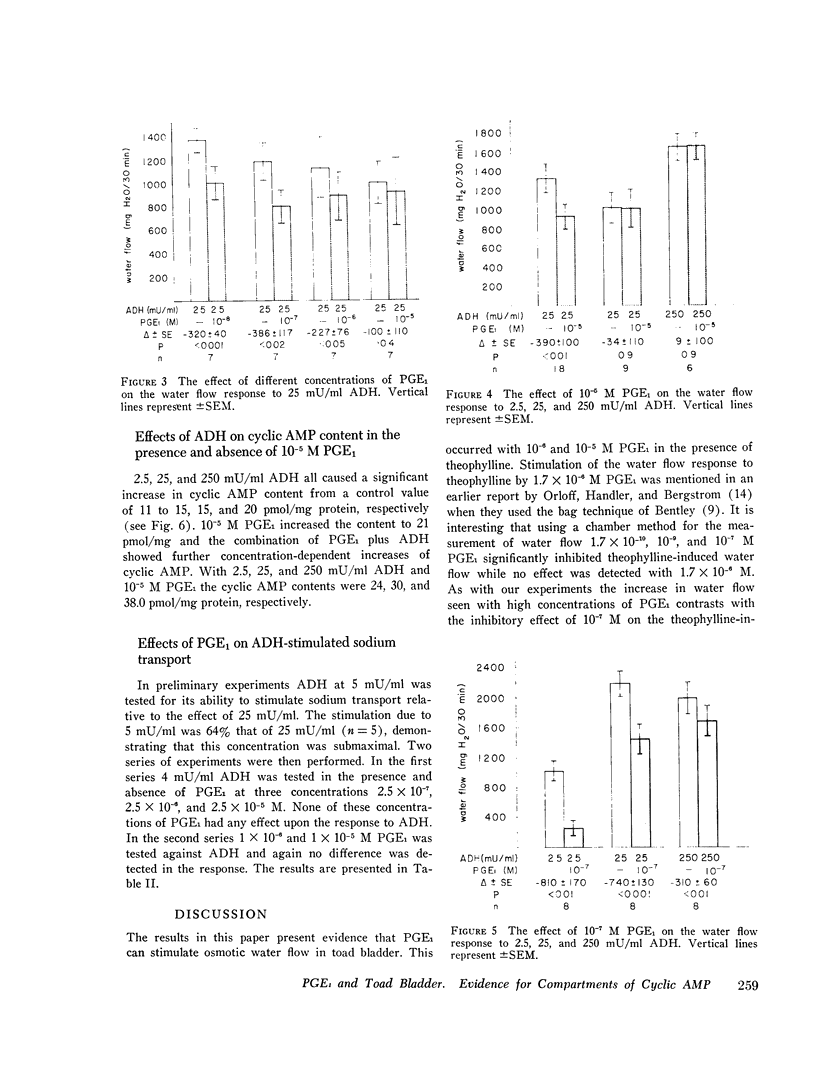
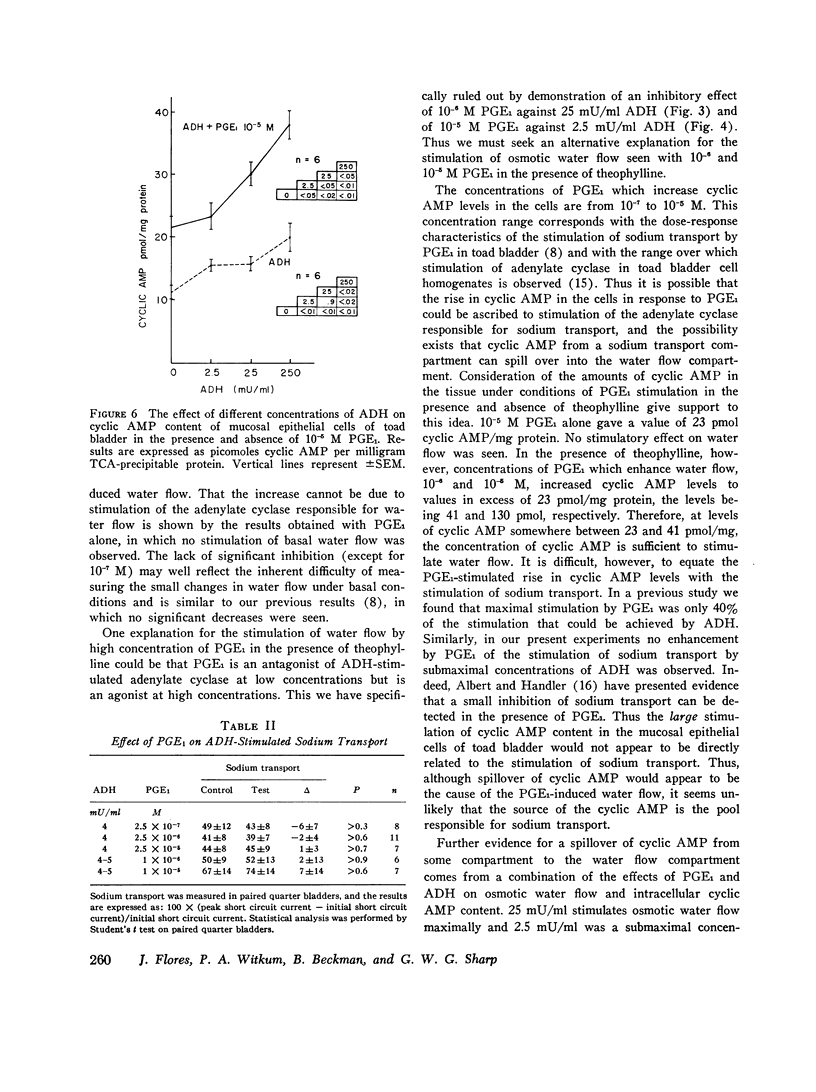
The effect of prostaglandin E1 (PGE1) on osmotic water flow across toad bladder and cyclic AMP content of the mucosal epithelial cells has been determined under basal conditions and in the presence of either theophylline or antidiuretic hormone (ADH); Under basal conditions and with PGE1 concentrations from 10(-8) to 10(-5) M no evidence of stimulation of water flow was observed, and with 10(-7) M PGE1 a significant inhibition was foundmcyclic AMP content under control conditions was 8 pmol/mg protein. It was 9 at 10(-8) M PGE1, 13 at 10(-7) M, 16 at 10(-6) M, and 23 at 10(-5) M. In the presence of theophylline, 10(-8) and 10(-7) M PGE1 inhibited the theophylline-induced water flow as expected. In contrast, 10(-6) and 10(-5) M PGE1 enhanced the rate of water flow. Theophylline increased cyclic AMP content from 8 to 18 pmol/mg protein. PGE1 in the presence of theophylline caused marked increases in cyclic AMP content; The content was 23 at 10(-7) M, 41 at 10(-6) M, and 130 at 10(-5) M; Thus PGE1 stimulates theophylline-induced water flow at cyclic AMP concentrations somewhere between 23 and 41 pmol/mg. Further evidence along these lines was obtained from experiments in which the effects of PGE1 on ADH-induced water flow were studied. Inhibitory effects of PGE1 were not observed at concentrations of PGE1 which raised the level of intracellular cyclic AMP to 30 pmol/mg protein or higher. These results were obtained despite the fact that all four concentrations of PGE1 tested were found capable of inhibiting ADH-induced water flow under appropriate conditions or, in other words, were inhibiting the adenylate cyclase controlling water flow, Thus the increase in cyclic AMP content in response to PGE1 is not derived from this enzyme. Thus the stimulation of water flow by PGE1 in the presence of theophylline is thought to be caused by cyclic AMP spilling over from one compartment to the water flow compartment. No evidence was obtained to directly suggest spillover into the sodium transport compartment. Furthermore evidence is discussed to suggest that most of the cyclic AMP generated in the tissue does not originate from the enzyme controlling sodium transport. As cyclic AMP-stimulated water flow and sodium transport are thought to occur in one cell type, the granular cells, distinct pools of cyclic AMP are thought to be present in one and the same cell type. Thus one pool controls water flow and one controls sodium transport. With high concentrations of PGE1 in the presence of theophylline or high concentrations of ADH, the adenylate cyclase responsible for water flow is inhibited; However, PGE1 can stimulate a tissue adenylate cyclase to sufficiently high levels that cyclic AMP spills over into the "water flow compartment" and thus stimulates water flow.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert W. C., Handler J. S. Effect of PGE1, indomethacin, and polyphloretin phosphate on toad bladder response to ADH. Am J Physiol. 1974 Jun;226(6):1382–1386. doi: 10.1152/ajplegacy.1974.226.6.1382. [DOI] [PubMed] [Google Scholar]
- Argy W. P., Jr, Handler J. S., Orloff J. Ca++ and Mg++ effects on toad bladder response to cyclic AMP, theophylline, and ADH analogues. Am J Physiol. 1967 Sep;213(3):803–808. doi: 10.1152/ajplegacy.1967.213.3.803. [DOI] [PubMed] [Google Scholar]
- BENTLEY P. J. The effects of neurohypophysial extracts on the water transfer across the wall of the isolated urinary bladder of the toad Bufo marinus. J Endocrinol. 1958 Sep;17(3):201–209. doi: 10.1677/joe.0.0170201. [DOI] [PubMed] [Google Scholar]
- Brown B. L., Albano J. D., Ekins R. P., Sgherzi A. M. A simple and sensitive saturation assay method for the measurement of adenosine 3':5'-cyclic monophosphate. Biochem J. 1971 Feb;121(3):561–562. doi: 10.1042/bj1210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOI J. K. The fine structure of the urinary bladder of the toad, Bufo marinus. J Cell Biol. 1963 Jan;16:53–72. doi: 10.1083/jcb.16.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civan M. M., Frazier H. S. The site of the stimulatory action of vasopressin on sodium transport in toad bladder. J Gen Physiol. 1968 May;51(5):589–605. doi: 10.1085/jgp.51.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis W. L., Goodman D. B., Martin J. H., Matthews J. L., Rasmussen H. Vasopressin-induced changes in the toad urinary bladder epithelial surface. J Cell Biol. 1974 May;61(2):544–547. doi: 10.1083/jcb.61.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBona D. R., Civan M. M., Leaf A. The anatomic site of the transepithelial permeability barriers of toad bladder. J Cell Biol. 1969 Jan;40(1):1–7. doi: 10.1083/jcb.40.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handler J. S., Butcher R. W., Sutherland E. W., Orloff J. The effect of vasopressin and of theophylline on the concentration of adenosine 3',5'-phosphate in the urinary bladder of the toad. J Biol Chem. 1965 Nov;240(11):4524–4526. [PubMed] [Google Scholar]
- Hynie S., Sharp G. W. Adenyl cyclase in the toad bladder. Biochim Biophys Acta. 1971 Jan 26;230(1):40–51. doi: 10.1016/0304-4165(71)90052-3. [DOI] [PubMed] [Google Scholar]
- KELLER A. R. A HISTOCHEMICAL STUDY OF THE TOAD URINARY BLADDER. Anat Rec. 1963 Nov;147:367–377. doi: 10.1002/ar.1091470308. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leaf A. Transepithelial transport and its hormonal control in toad bladder. Ergeb Physiol. 1965;56:216–263. [PubMed] [Google Scholar]
- Lipson L. C., Sharp G. W. Effect of prostaglandin E1 on sodium transport and osmotic water flow in the toad bladder. Am J Physiol. 1971 Apr;220(4):1046–1052. doi: 10.1152/ajplegacy.1971.220.4.1046. [DOI] [PubMed] [Google Scholar]
- Lipson L., Hynie S., Sharp G. Effect of prostaglandin E-1 on osmotic water flow and sodium transport in the toad bladder. Ann N Y Acad Sci. 1971 Apr 30;180:260–277. doi: 10.1111/j.1749-6632.1971.tb53196.x. [DOI] [PubMed] [Google Scholar]
- ORLOFF J., HANDLER J. S., BERGSTROM S. EFFECT OF PROSTAGLANDIN (PGE-1) ON THE PERMEABILITY RESPONSE OF TOAD BLADDER TO VASOPRESSIN, THEOPHYLLINE AND ADENOSINE 3',5'-MONOPHOSPHATE. Nature. 1965 Jan 23;205:397–398. doi: 10.1038/205397a0. [DOI] [PubMed] [Google Scholar]
- ORLOFF J., HANDLER J. S. The similarity of effects of vasopressin, adenosine-3',5'-phosphate (cyclic AMP) and theophylline on the toad bladder. J Clin Invest. 1962 Apr;41:702–709. doi: 10.1172/JCI104528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orloff J., Handler J. The role of adenosine 3',5'-phosphate in the action of antidiuretic hormone. Am J Med. 1967 May;42(5):757–768. doi: 10.1016/0002-9343(67)90093-9. [DOI] [PubMed] [Google Scholar]
- PEACHEY L. D., RASMUSSEN H. Structure of the toad's urinary bladder as related to its physiology. J Biophys Biochem Cytol. 1961 Aug;10:529–553. doi: 10.1083/jcb.10.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSEN M. J., EDELMAN I. S. CALCIUM INHIBITION OF THE ACTION OF VASOPRESSIN ON THE URINARY BLADDER OF THE TOAD. J Clin Invest. 1964 Apr;43:583–594. doi: 10.1172/JCI104943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARP G. W., LEAF A. BIOLOGICAL ACTION OF ALDOSTERONE IN VITRO. Nature. 1964 Jun 20;202:1185–1188. doi: 10.1038/2021185a0. [DOI] [PubMed] [Google Scholar]
- USSING H. H., ZERAHN K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand. 1951 Aug 25;23(2-3):110–127. doi: 10.1111/j.1748-1716.1951.tb00800.x. [DOI] [PubMed] [Google Scholar]