Abstract
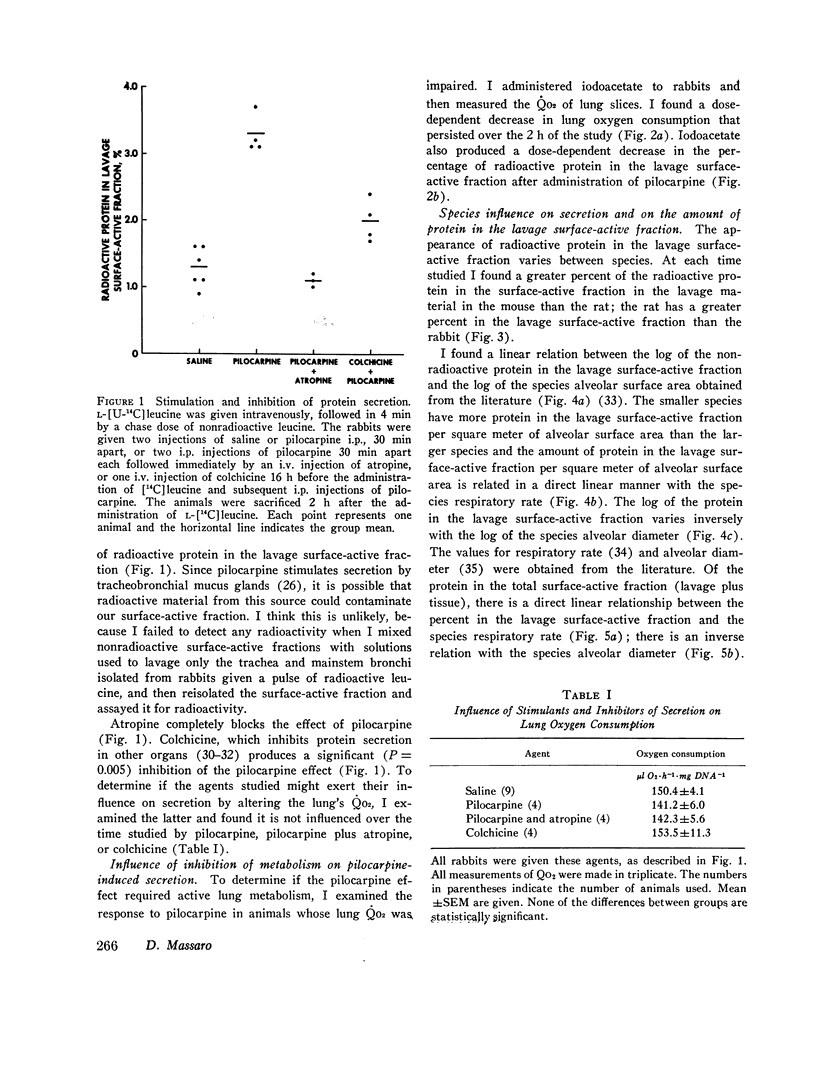
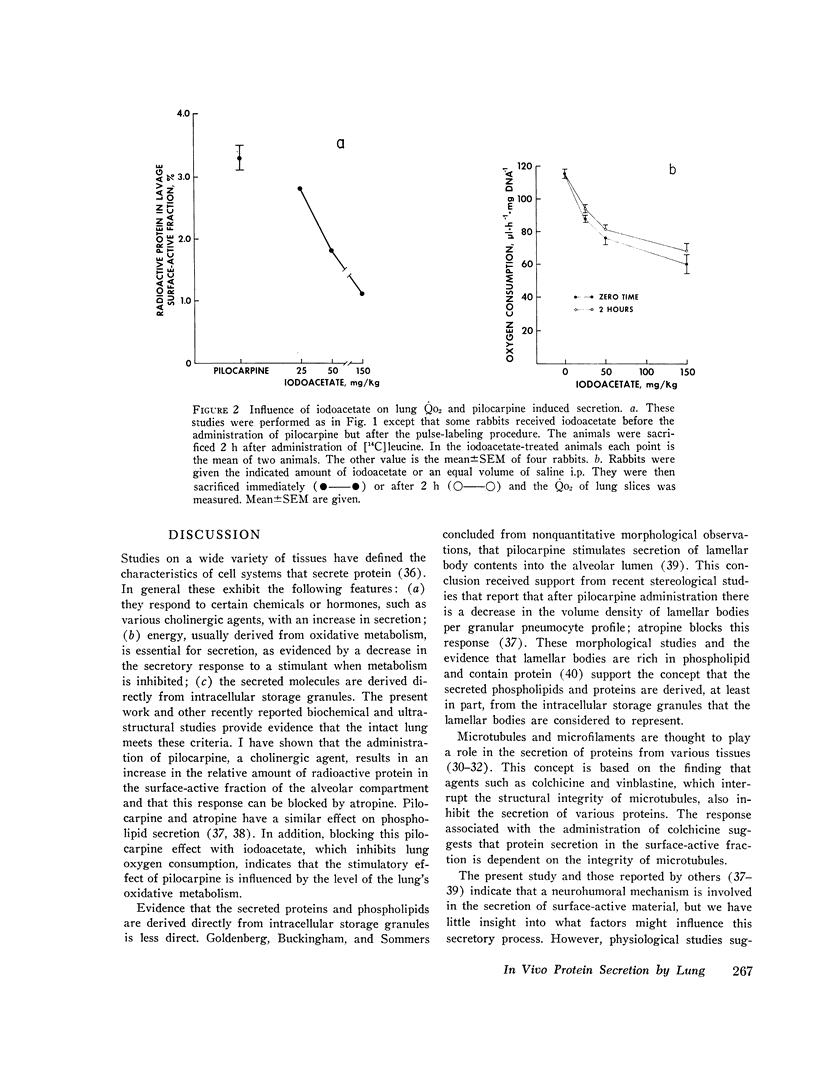
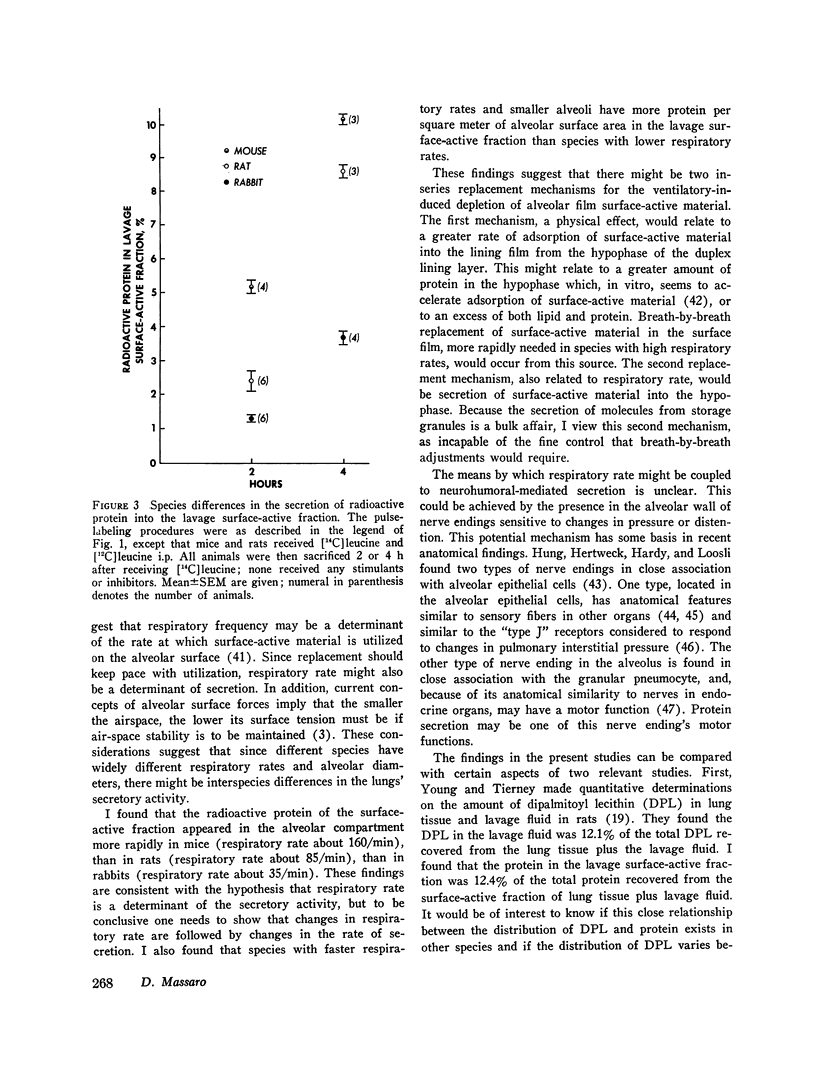
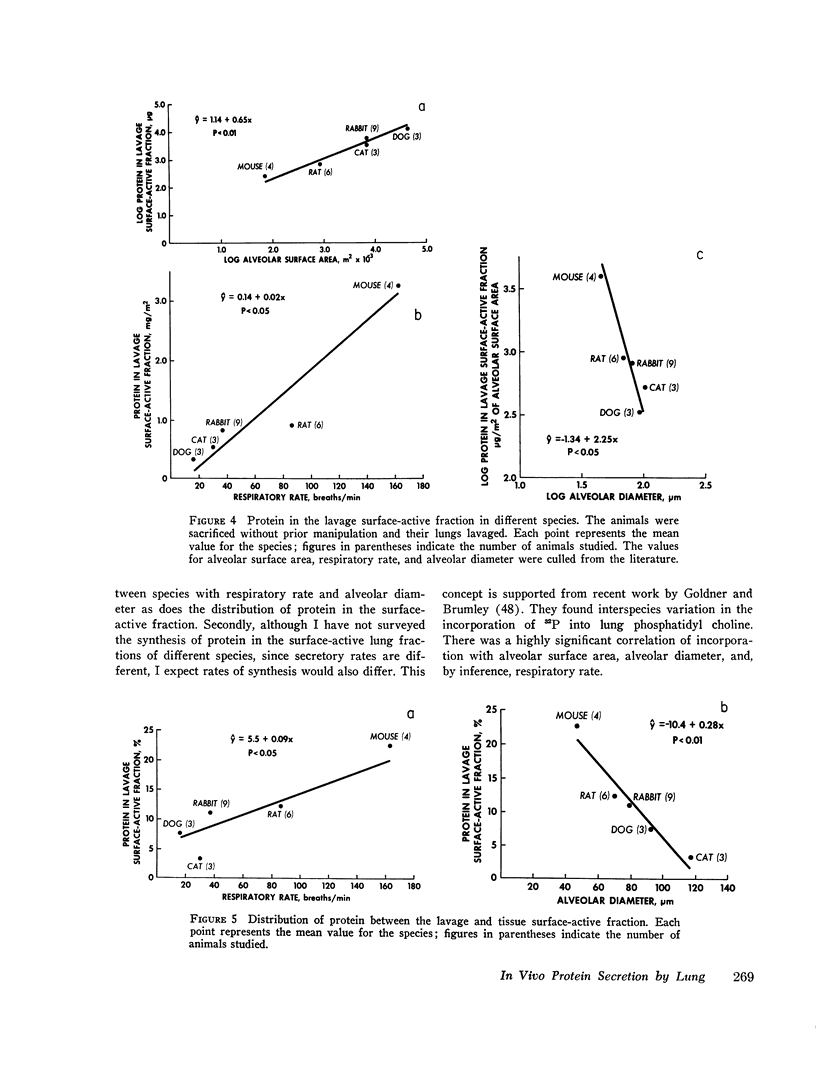
The present study is an attempt to determine (a) if the lung actively secretes protein into the surface-active fraction of lung lavage returns; (b) if there are interspecies differences in this secretory activity; and (c) if the amount of nonradioactive protein in the lavage surface-active fraction shows interspecies variation. I found that pilocarpine stimulates the release of radioactive protein into the lavage surface-active fraction of rabbits and that this pilocarpine effect is completely blocked by atropine. Inhibition of lung oxygen consumption by iodoacetate is associaged with a dose-dependent inhibition of the pilocarpine-induced secretion. Microtubules may be involved in this secretory process because colchicine inhibits the pilocarpine effect. Of the radioactive protein in the total surface-active fraction (tissue plus lavage returns), a greater percent appears in the lavage surface-active fraction at 2 and 4 h, after a pulsed injection [U-14C] leucine, in the mouse than in the rat, which in turn has a greater amount than the rabbit. There is also a difference in the amount of nonradioactive protein per square meter of alveolar surface area in the lavage surface-active fraction of different species: mouse greater than rabbit greater than cat greater than dog. The amount of nonradioactive protein per square meter of alveolar surface area in the lavage surface-active fraction is directly proportional to the species respiratory rate; the log of the nonradioactive protein in the lavage surface-active fraction is inversely proportional to the log of the species alveolar diameter. I conclude that the lung actively secretes protein into the lavage surface-active fraction, that this secretion is under neurohumoral regulation, and that respiratory rate and alveolar size may influence this secretory activity and the amount of protein in this surface-active fraction.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altenähr E. Electron microscopical evidence for innervation of chief cells in human parathyroid gland. Experientia. 1971 Sep 15;27(9):1077–1077. doi: 10.1007/BF02138889. [DOI] [PubMed] [Google Scholar]
- Buckingham S., Heinemann H. O., Sommers S. C., McNary W. F. Phospholipid synthesis in the large pulmonary alveolar cell. Its relation to lung surfactants. Am J Pathol. 1966 Jun;48(6):1027–1041. [PMC free article] [PubMed] [Google Scholar]
- Butcher F. R., Goldman R. H. Effect of cytochalasin B and colchicine on the stimulation of -amylase release from rat parotid tissue slices. Biochem Biophys Res Commun. 1972 Jul 11;48(1):23–29. doi: 10.1016/0006-291x(72)90338-5. [DOI] [PubMed] [Google Scholar]
- Chevalier G., Collet A. J. In vivo incorporation of choline- 3 H, leucine- 3 H and galactose- 3 H in alveolar type II pneumocytes in relation to surfactant synthesis. A quantitative radoautographic study in mouse by electron microscopy. Anat Rec. 1972 Nov;174(3):289–310. doi: 10.1002/ar.1091740303. [DOI] [PubMed] [Google Scholar]
- Chida N., Adams F. H. Incorporation of acetate into fatty acids and lecithin by lung slices from fetal and newborn lambs. J Lipid Res. 1967 Jul;8(4):335–341. [PubMed] [Google Scholar]
- Dickie K. J., Massaro G. D., Marshall V., Massaro D. Amino acid incorporation into protein of a surface-active lung fraction. J Appl Physiol. 1973 May;34(5):606–614. doi: 10.1152/jappl.1973.34.5.606. [DOI] [PubMed] [Google Scholar]
- Dickie K., Massaro D. Protein transport by lung. Proc Soc Exp Biol Med. 1974 Jan;145(1):154–156. doi: 10.3181/00379727-145-37767. [DOI] [PubMed] [Google Scholar]
- FELTS J. M. CARBOHYDRATE AND LIPID METABOLISM OF LUNG TISSUE IN VITRO. Med Thorac. 1965;22:89–99. doi: 10.1159/000386167. [DOI] [PubMed] [Google Scholar]
- Fujiwara T. Biochemical aspects of pulmonary alveolar surface lining layer. Acta Pathol Jpn. 1972 Nov;22(4):805–810. doi: 10.1111/j.1440-1827.1972.tb00762.x. [DOI] [PubMed] [Google Scholar]
- Gacad G., Massaro D. Hyperoxia: influence on lung mechanics and protein synthesis. J Clin Invest. 1973 Mar;52(3):559–565. doi: 10.1172/JCI107216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil J., Reiss O. K. Isolation and characterization of lamellar bodies and tubular myelin from rat lung homogenates. J Cell Biol. 1973 Jul;58(1):152–171. doi: 10.1083/jcb.58.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg V. E., Buckingham S., Sommers S. C. Pilocarpine stimulation of granular pneumocyte secretion. Lab Invest. 1969 Feb;20(2):147–158. [PubMed] [Google Scholar]
- Goldner R. D., Brumley G. W. Comparative incorporation of P32 into lung phosphatidyl choline in mammals with different metabolic and pulmonary morphologic characteristics. Proc Soc Exp Biol Med. 1974 Apr;145(4):1343–1347. doi: 10.3181/00379727-145-38010. [DOI] [PubMed] [Google Scholar]
- Hung K. S., Hertweck M. S., Hardy J. D., Loosli C. G. Innervation of pulmonary alveoli of the mouse lung: an electron microscopic study. Am J Anat. 1972 Dec;135(4):477–495. doi: 10.1002/aja.1001350404. [DOI] [PubMed] [Google Scholar]
- Iwayama T., Furness J. B., Burnstock G. Dual adrenergic and cholinergic innervation of the cerebral arteries of the rat. An ultrastructural study. Circ Res. 1970 May;26(5):635–646. doi: 10.1161/01.res.26.5.635. [DOI] [PubMed] [Google Scholar]
- King R. J., Clements J. A. Surface active materials from dog lung. II. Composition and physiological correlations. Am J Physiol. 1972 Sep;223(3):715–726. doi: 10.1152/ajplegacy.1972.223.3.715. [DOI] [PubMed] [Google Scholar]
- Klein R. M., Margolis S. Purification of pulmonary surfactant by ultracentirfugation. J Appl Physiol. 1968 Dec;25(6):654–658. doi: 10.1152/jappl.1968.25.6.654. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Le Marchand Y., Singh A., Assimacopoulos-Jeannet F., Orci L., Rouiller C., Jeanrenaud B. A role for the microtubular system in the release of very low density lipoproteins by perfused mouse livers. J Biol Chem. 1973 Oct 10;248(19):6862–6870. [PubMed] [Google Scholar]
- Massaro D. Alveolar cells: incorporation of carbohydrate into protein and evidence for intracellular protein transport. J Clin Invest. 1968 Feb;47(2):366–374. doi: 10.1172/JCI105733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaro D., Weiss H., Simon M. R. Protein synthesis and secretion by lung. Am Rev Respir Dis. 1970 Feb;101(2):198–206. doi: 10.1164/arrd.1970.101.2.198. [DOI] [PubMed] [Google Scholar]
- Massaro D., Weiss H., White G. Protein synthesis by lung following pulmonary artery ligation. J Appl Physiol. 1971 Jul;31(1):8–14. doi: 10.1152/jappl.1971.31.1.8. [DOI] [PubMed] [Google Scholar]
- Massaro G. D., Massaro D. Granular pneumocytes. Electron microscopic radioautographic evidence of intracellular protein transport. Am Rev Respir Dis. 1972 Jun;105(6):927–931. doi: 10.1164/arrd.1972.105.6.927. [DOI] [PubMed] [Google Scholar]
- McClenahan J. B., Urtnowski A. Effect of ventilation on surfactant, and its turnover rate. J Appl Physiol. 1967 Aug;23(2):215–220. doi: 10.1152/jappl.1967.23.2.215. [DOI] [PubMed] [Google Scholar]
- NASR K., HEINEMANN H. O. LIPID SYNTHESIS BY RABBIT LUNG TISSUE IN VITRO. Am J Physiol. 1965 Jan;208:118–121. doi: 10.1152/ajplegacy.1965.208.1.118. [DOI] [PubMed] [Google Scholar]
- Naimark A. Cellular dynamics and lipid metabolism in the lung. Fed Proc. 1973 Sep;32(9):1967–1971. [PubMed] [Google Scholar]
- Naimark A., Klass D. The incorporation of palmitate-1-14C by rat lung in vitro. Can J Physiol Pharmacol. 1967 Jul;45(4):597–607. doi: 10.1139/y67-072. [DOI] [PubMed] [Google Scholar]
- PATTLE R. E. SURFACE LINING OF LUNG ALVEOLI. Physiol Rev. 1965 Jan;45:48–79. doi: 10.1152/physrev.1965.45.1.48. [DOI] [PubMed] [Google Scholar]
- POPJAK G., BEECKMANS M. L. Extrahepatic lipid synthesis. Biochem J. 1950 Aug;47(2):233–238. doi: 10.1042/bj0470233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlowski R., Frosolono M. F., Charms B. L., Przybylski R. Intra- and extracellular compartmentalization of the surface-active fraction in dog lung. J Lipid Res. 1971 Sep;12(5):538–544. [PubMed] [Google Scholar]
- Salisbury-Murphy S., Rubinstein D., Beck J. C. Lipid metabolism in lung slices. Am J Physiol. 1966 Oct;211(4):988–992. doi: 10.1152/ajplegacy.1966.211.4.988. [DOI] [PubMed] [Google Scholar]
- Scalzi H. A., Price H. M. The arrangement and sensory innervation of the intrafusal fibers in the feline muscle spindle. J Ultrastruct Res. 1971 Aug;36(3):375–390. doi: 10.1016/s0022-5320(71)80111-9. [DOI] [PubMed] [Google Scholar]
- Scholz R. W., Woodward B. M., Rhoades R. A. Utilization in vitro and in vivo of glucose and glycerol by rat lung. Am J Physiol. 1972 Oct;223(4):991–996. doi: 10.1152/ajplegacy.1972.223.4.991. [DOI] [PubMed] [Google Scholar]
- TENNEY S. M., REMMERS J. E. Comparative quantitative morphology of the mammalian lung: diffusing area. Nature. 1963 Jan 5;197:54–56. doi: 10.1038/197054a0. [DOI] [PubMed] [Google Scholar]
- Thomas T., Jr, Rhoades R. A. Incorporation of palmitate-1-14C into lung tissue and "alveolar" lecithin. Am J Physiol. 1970 Dec;219(6):1535–1538. doi: 10.1152/ajplegacy.1970.219.6.1535. [DOI] [PubMed] [Google Scholar]
- Williams J. A., Wolff J. Colchicine-binding protein and the secretion of thyroid hormone. J Cell Biol. 1972 Jul;54(1):157–165. doi: 10.1083/jcb.54.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager H., Jr, Massaro D. Glucose metabolism and glycoprotein synthesis by lung slices. J Appl Physiol. 1972 Apr;32(4):477–482. doi: 10.1152/jappl.1972.32.4.477. [DOI] [PubMed] [Google Scholar]
- Young S. L., Tierney D. F. Dipalmitoyl lecithin secretion and metabolism by the rat lung. Am J Physiol. 1972 Jun;222(6):1539–1544. doi: 10.1152/ajplegacy.1972.222.6.1539. [DOI] [PubMed] [Google Scholar]



