Abstract
Objective
Elevated intracranial pressure (ICP) is one of the proposed mechanisms leading to poor outcomes in patients with intraventricular hemorrhage (IVH). We sought to characterize the occurrence and significance of intracranial hypertension in severe IVH requiring extraventricular drainage (EVD).
Design
Prospective analysis from two randomized multicenter clinical trials.
Setting
Intensive care units of 23 academic hospitals.
Patients
One hundred patients with obstructive IVH, and intracerebral hemorrhage (ICH) volume < 30cc requiring emergency EVD from two randomized multicenter studies comparing intraventricular recombinant tissue plasminogen activator (rt-PA) (n=78) to placebo (n=22).
Interventions
ICP was recorded every 4 hours in all patients and before and after a 1 hr EVD closure period post-injection. ICP readings were analyzed at pre-defined thresholds and compared between treatment groups, pre- and post-injection of study agent, and pre- and post-opening of 3rd and 4th ventricles on CT. Impact on 30 day outcomes was assessed.
Measurements and Main Results
Initial ICP ranged from −2 to 60 mm Hg (median, interquartile range; 11,10). Of 2576 ICP readings, 91.5% (2359) were ≤ 20 mm Hg, 1.6% were >30, 0.5% were >40, and 0.2% were > 50 mm Hg. In a multivariate analysis threshold events > 20 and > 30 mm Hg were more frequent in placebo vs. rt-PA treated groups (p=0.03 and p=0.08, respectively). ICP elevation > 20 mm Hg occurred during a required 1 hr EVD closure interval in 207/868 (23.8%) injections of study agent although early re-opening of the EVD only occurred in 7.9%. After radiographic opening of the lower ventricular system, ICP events > 20 mmHg remained significantly associated with initial IVH volume (p=0.002), and EVD placement ipsilateral to the largest IVH volume (p=0.001), but not with thrombolytic treatment (p=0.05) or ICH volume (p=0.14). VP shunts were required in 13.6% of Pcb and 6.4% of rt-PA treated patients (p=0.37). Percentage of ICP readings per patient > 30 mmHg, and initial ICH and IVH volumes were independent predictors of 30 day mortality after adjustment for other outcome predictors (p=0.003; p=0.03; p<0.001, respectively). Independent predictors of poor modified Rankin Score (mRS) at 30 days were % of ICP events > 30 mmHg/patient (p=0.01) (but not > 20 mmHg), both ICH and IVH volume and pulse pressure.
Conclusions
ICP is not frequently elevated during monitoring and drainage with an EVD in patients with severe IVH although ICP > 30 mm Hg predicts higher short-term mortality. Thrombolytic therapy may reduce the frequency of high ICP events. ICP elevation appears to be significantly correlated with EVD placement in the ventricle with greatest clot volume.
Keywords: Intracranial pressure, intraventricular hemorrhage, hydrocephalus, thrombolysis, intracerebral hemorrhage
Introduction
The relationship between the occurrence of intraventricular hemorrhage (IVH) in patients with intracerebral hemorrhage (ICH) and the likelihood of death or poor neurologic outcome has been consistently demonstrated in a growing number of prospective studies and clinical trials including the International Surgical Trial in Intracerebral Hemorrhage (STICH) study and the recombinant activated factor VII for acute intracerebral hemorrhage (NovoSeven) trials (1-9).
Massive IVH leads to acute hydrocephalus which compresses the rostral brainstem and thalamus causing injury to the reticular activating system and may decrease cerebral perfusion pressure contributing to ischemic injury (10-11). Elevated intracranial pressure (ICP) is thought to be fundamental to these injury mechanisms. A prospective investigation of ICP in 11 patients with IVH who had ICP recorded every 4-6 hours while an external ventricular drain (EVD) was in place demonstrated that both initial and subsequent ICP readings were not commonly elevated > 20 mm Hg (14% of readings) despite acute obstructive hydrocephalus (12). To better understand the occurrence of elevated ICP in IVH and its role in neurological deterioration, we performed a more detailed analysis of ICP using data from the CLEAR IVH trial, a large placebo-controlled multisite trial to evaluate low-dose intraventricular rt-PA for the treatment of ICH with IVH. This represents the largest cohort to date of prospectively collected severe IVH patients managed with aggressive neurointensive care. The goal of this study was to investigate frequency of, and factors associated with ICP elevation during EVD use, tolerance of EVD closure for intraventricular study agent administration, and impact of ICP on early mortality and neurologic outcomes.
Materials and Methods
Patients
One hundred adult patients with obstructive hydrocephalus secondary to IVH, and ICH volume < 30cc (by ABC/2 method) (13) requiring EVD (per the treating physician), from two multicenter trials (the Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage (CLEAR IVH) Trials) were prospectively studied. The CLEAR IVH Trial study procedures have been published previously (14,15). All eligible patients with an EVD inserted within the initial 24 hours of the diagnostic CT scan were approached for randomization. Patients were enrolled within 48 hours after the diagnostic head CT. Exclusion criteria included: posterior fossa hematoma; intraparenchymal hemorrhage volume > 30 cc; suspected intracerebral aneurysm or arteriovenous malformation (excluded by appropriate diagnostic studies); any severe complicating illness; active internal bleeding, current use of heparin, coagulopathy with prothrombin time (PT) or partial thromboplastin time (PTT) outside of normal range, platelet count < 75 IU/mm3; pregnancy; and age < 18 years.
Randomization and masking
The CLEAR IVH trials comprised a Safety Trial, (n = 48) and a Dose Finding Trial, (n = 52). In the Safety trial, patients were randomly assigned (1:1) to intraventricular administration of placebo (Pcb) (n=22) or recombinant tissue plasminogen activator (rt-PA) (n=26) at a dose of 3.0 mg q12h. In the Dose Finding study, patients were either randomized 1:1 (n=16) to receive intraventricular rt-PA at doses 0.3 or 1.0 mg q12h (CLEAR A) or to receive 1.0 mg q12h or q8h (n=36) (CLEAR B) (Fig. 1). Patients in this study include all enrolled patients in the CLEAR IVH trials.
Figure 1.
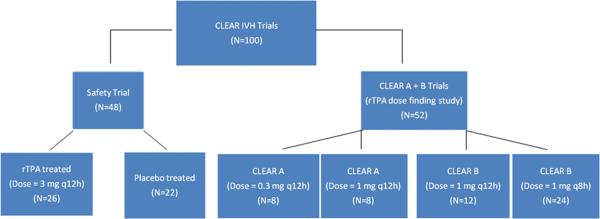
Study design of the CLEAR IVH trials showing randomized groups from 3 trials.
Procedures
Treatments for ICP management such as hyperventilation and osmotic therapy were administered per the treating physician for sustained (> 5 minutes) ICP greater than 20 mm Hg. EVDs were placed into the frontal horn of the lateral ventricle and tunneled under the scalp by neurosurgical staff. In the absence of accepted practice guidelines regarding catheter location, the choice of catheter placement ipsilateral or contralateral to dominant lateral ventricular IVH was left up to each site's neurosurgical team. Please refer to appendix for other procedural details.
ICP and CPP management were based on the Brain Trauma Foundation Guidelines and were performed continuously per each ICU's protocol (16,17). ICP management was protocolized by the study only for the hour after dosing when the EVD was closed. The EVD was to be reopened within the initial hour only if needed to control medically intractable ICP elevation (ICP > 20 mm Hg refractory to hyperventilation and mannitol administration). The daily CSF volume drained was recorded. The decision for ventriculoperitoneal (VP) shunt placement was at the discretion of the treating neurosurgeon.
ICP and CPP determination
ICP was recorded at the end of every 4 hour interval in all patients and every 1 hr in 36 patients. ICP was also recorded before and after a 1 hr EVD closure period post-injection. For each recorded ICP measurement, the EVD was closed for 10 minutes and the ICP was recorded at the end of this time period.
CPP was recorded with each ICP measurement. Low CPP events were defined at thresholds, CPP < 60 or CPP < 70 mm Hg. CPP < 60 mm Hg and ICP > 20 mm Hg were considered threshold levels for treatment, based on Brain Trauma Foundation Guidelines (16,17).
IVH and ICH volume determination
Daily head CT scans were obtained during study agent administration per protocol, and additional CT studies were performed for acute neurological deterioration per the treating physician. The first CT scan performed at least 6 hours after EVD placement that demonstrated a stable IVH and ICH volume and either no or stable catheter tract hemorrhage was designated the “stability CT”. The volumes of intraventricular and intraparenchymal hematomas were measured independently by a blinded investigator using standard computerized volumetric analysis (Alice 5.1, Perceptive Informatics, Boston MA). This represents a more rigorous method to recalculate volumes for analysis compared to the ABC/2 method used for ICH volume determination for patient enrollment. Clearance of blood from the 3rd and 4th ventricles (ability to visualize a clear pathway of hypodense CSF through the lower ventricular system) was similarly assessed. This protocol was approved by the Internal Review Boards of all enrolling institutions.
Statistical analysis
Demographic variables, stability IVH and ICH volumes, total duration of ICP monitoring, and differences in median ICP before and after injection of study agent as well as before and after blood clearance from the third and fourth ventricles were compared between rt-PA and Pcb-treated patients. Frequency of high ICP threshold events (ICP above 20, 30, 40, or 50 mm Hg) by treatment group, and by frequency of ICP data recordings (q1 hr vs. q4h) was also compared. Wilcoxon rank-sum test was used to compare variables with non-normal distributions. Student's t-test was used for continuous variables with normal distributions and Chi-squared or Fisher's exact test was used for analysis of categorical data as appropriate. Univariate and multivariate analysis of factors associated with ICP and CPP levels and thresholds were performed with linear and logistic regression models, with clustering by patient to adjust for multiple readings per patient. The following outcome variables were considered in the analysis: (i) the number of ICP readings per patient > 20 and > 30 mmHg over the entire monitoring period, during EVD closure for study agent administration, and after the third and fourth ventricles had opened on CT; (ii) early opening of the EVD prior to the one hour recommended closure time; (iii) ventriculoperitoneal (VP) shunt placement; (iv) average daily CSF volume drained from the EVD; (v) the proportion of CPP readings < 60 and < 70 mmHg; and (vi) 30 day outcomes (mortality and modified Rankin Score (mRS) ).
Independent predictors considered for ICP outcomes were treatment group, stability ICH and IVH volumes, catheter location, presenting Glasgow Coma Scale (GCS), ICP immediately before EVD closure, ICP > 30 mmHg during EVD closure, and proportion of ICP events > 20 mmHg not associated with EVD closure. Independent predictors considered for VP shunt placement were proportion of ICP readings > 20 and > 30 mmHg, IVC duration, time from EVD placement to opening of 3rd and 4th ventricles, IVH volume, presenting GCS and treatment. Independent predictors considered for average daily CSF volume drained from the EVD were treatment group, proportion ICP readings > 30 mmHg, IVC duration, IVH volume and ICH volume.
Independent predictors considered for the proportion of CPP readings < 60 and < 70 mmHg were proportion of ICP readings > 20 mmHg, ICH volume, and IVH volume.
For outcome evaluation, the mRS at 30 days was dichotomized as good outcome (mRS 0-4) and poor outcome (mRS 5-6). Covariates considered in the final outcome models were number of ICP events per patient > 30 mm Hg and variables known to be associated with outcome after ICH if they were significantly associated with 30 day mortality or poor mRS, respectively on univariate analysis (P<0.1). These included: presentation GCS and pulse pressure (PP), age, baseline ICH and IVH volume, and treatment with any dose of intraventricular rt-PA. Statistical significance was assigned for P≤0.05. Data are presented as median ± interquartile range (iqr) or mean ± SD, unless otherwise indicated.
Results
Baseline clinical characteristics
One hundred patients were enrolled with age range 28 to 75 years (mean 55 ± 10 years) (Table 1). Treatment groups did not differ with respect to age, race, stroke risk factors or baseline clinical and radiologic parameters. There were significantly more males in the rt-PA treated group (68% M vs. 32% M in Pcb group; p=0.002).
Table 1.
Demographic and presenting characteristics
| Characteristic, amean (SD); bmedian [iqr] | rt-treated (N=78) | Placebo treated (N=22) | Total (N=100) | P-value |
|---|---|---|---|---|
| Age, ya | 54.9 (11.0) | 56.1 (7.6) | 55 (10.0) | 0.65 |
| Gender Male/Female (n) | 53/25 | 7/15 | 60/40 | 0.002 |
| Stability IVH Volume, ccb | 33.3 [47.2] | 37.6 [46.1] | 35 [47] | 0.36 |
| Stability ICH Volume, ccb | 5.6 [12.4] | 10.6 [20.9] | 6 [15] | 0.37 |
| Ethnicity (%) | ||||
| Caucasion | 19 (24) | 7 (32) | 26 (26) | 0.18 |
| African American | 42 (54) | 10 (45) | 52 (52) | |
| Hispanic | 4 (5) | 4 (18) | 8 (8) | |
| Asian or Pacific Islander | 8 (10) | 1 (5) | 9 (9) | |
| Other/Unknown | 5 (7) | 0 (0) | 5 (5) | |
| Hypertension (%) | 69 (89) | 19 (86) | 88 (88) | 0.47 |
| Diabetes Mellitus (%) | 17 (22) | 3 (14) | 20 (20) | 0.40 |
| Migraine (%) | 1 (1) | 1(5) | 2 (2) | 0.33 |
| History of Seizure (%) | 7 (9) | 0 (0) | 7 (7) | 0.14 |
| Hx of Alcohol Abuse (%) | 13 (17) | 6 (27) | 19 (19) | 0.26 |
| Hx of tobacco Use (%) | 20 (26) | 2 (9) | 22 (22) | 0.10 |
| Hx of Cocaine Abuse (%) | 12 (15) | 2 (9) | 14 (14) | 0.45 |
| Presenting Parameter | ||||
| Systolic Blood Pressurea | 194 (40) | 189 (34) | 193 (39) | 0.62 |
| Diastolic Blood Pressurea | 108 (25) | 101 (27) | 107 (25) | 0.26 |
| Mean Arterial Pressurea | 137 (28) | 130 (28) | 135 (28) | 0.37 |
| Pulse Pressurea | 86 (26) | 88 (21) | 86 (25) | 0.71 |
| Opening ICPb | 11[9] | 13 [11] | 11 [10] | 0.83 |
| CPPb | 84 [18] | 87 [24] | 85 [24] | 0.90 |
| GCSb | 8 [8] | 7 [4] | 8 [7] | 0.20 |
| NIHSSb | 22 [16] | 22 [18] | 22 [17] | 0.75 |
| IVH Graeb Scoreb* | 7[3] | 8 [4] | 7 [3] | 0.45 |
| Clot Location (%) | ||||
| Caudate | 6 (8) | 3 (14) | 9 (9) | 0.39 |
| Thalamus | 35 (45) | 8 (36) | 43 (43) | |
| Putamen | 7 (9) | 3 (14) | 10 (10) | |
| Globus Pallidus | 8 (10) | 0 (0) | 8 (8) | |
| Lobar | 7 (9) | 1 (4) | 8 (8) | |
| Probable Primary IVH | 15 (19) | 7 (32) | 22 (22) | |
Abbreviations: SD: standard deviation; IQR: interquartile range; rt-PA: recombinant tissue plasminogen activator; Hx: history of; ICP: intracranial pressure; CPP: cerebral perfusion pressure; GCS: Glasgow Coma Scale; NIHSS: NIH Stroke Scale; IVH: intraventricular hemorrhage
Graeb score [2].
All patients had EVDs inserted within 24 hours of the diagnostic CT scan (median 0.2 days; min-max 0.01-1.1 days). With reference to the majority of lateral ventricular blood, the initial EVD was inserted in the ipsilateral lateral ventricle in 22 patients and the contralateral lateral ventricle in 71. Patients with ipsilateral or contralateral EVD placement did not differ with regard to treatment group, stability ICH and IVH volume, average daily CSF volume and all demographic and presenting characteristics. Seven patients had two simultaneous catheters (one on each side). These patients had significantly larger IVH volume (median 104.8 vs. 34.7cc; p=0.004) and non-significantly lower presentation GCS (median 5 vs. 8; p=0.36) compared to patients with single EVDs only. More than 1 EVD was placed in 47 patients (27 had 2 EVDs; 11 had 3; 7 had 4 and 2 had 5). The EVD became obstructed more often (requiring replacement) in Pcb vs. rt-PA treated patients (40.9% vs. 11.8%; p=0.002). VP shunts were required in 14% of Pcb and 6% of rt-PA treated patients (p=0.27).
Stability IVH and ICH volumes were not different between treatment groups. Stability IVH volume was positively correlated with time to clearance of blood from the 3rd, but not the 4th ventricle (Spearman's rho = 0.22; p=0.03; rho = 0.09, p=0.42 respectively).
ICP monitoring results
Initial ICP reading
Opening pressure at EVD placement ranged from −2 to 60 mm Hg (median, iqr; 11, 10). Fourteen patients (16.1%) had initial ICP above 20 mm Hg. Opening pressure was not associated with stability IVH volume, or with occurrence of ICP elevation > 20 or 30 mmHg at other times during the patient's ICP monitoring course. Opening pressure was marginally associated with stability ICH volume (spearman's rho=0.21; p=0.05).
ICP readings during EVD monitoring period
The median duration of EVD (and ICP monitoring) was 8.6 days (iqr 7.3) and was shorter in the rt-PA treated group (8.4), but was not statistically different from Pcb (11.0) (Table 2).
Table 2.
Comparison of EVD related variables between treatment groups
| Factor: | rt-PA Treated Group (N=78) | Placebo Treated Group (N=22) | p-value |
|---|---|---|---|
| Median duration of EVD, days [interquartile range] | 8.4 [5.6] | 11.0 [10.6] | 0.41 |
| Number who failed to clear visible blood from 3rd and 4th ventricles on CT | 3rd only – 5 4th only – 4 Both - 3 |
3rd only – 4 4th only – 3 Both – 3 |
0.12 0.09 |
| Median day of visible blood clearance from 3rd ventricle* | 2.3 [1.9] | 7.0 [5.0] | <0.001 |
| Median day of visible blood clearance from 4th ventricle* | 1.9 [1.3] | 5.3 [5.3] | 0.001 |
| Difference in median ICP before and after blood clearance from both 3rd and 4th ventricle [ICP (after clearance) – ICP (before clearance)] (mmHg) | −1.0 [5.5] | 1.3 [6.0] | 0.11 |
| Median change in ICP from pre-injection to 1 hour post-injection of study agent (mmHg) | 4 [8] | 4 [8] | 0.54 |
| EVD obstruction requiring replacement | 9 (11.5%) | 9 (40.9%) | 0.002 |
| Bacterial ventriculitis | 3 (3.9%) | 2 (9.1%) | 0.32 |
| Symptomatic rebleeding | 9 (11.5%) | 1 (4.5%) | 0.33 |
From time of EVD placement; excludes patients with absent blood in 3rd (1 patient) or 4th (6 patients) ventricle on stability CT respectively.
Abbreviations: EVD: external ventricular drain; rt-PA: recombinant tissue plasminogen activator; CT: computed tomography; ICP: intracranial pressure
Of 2576 ICP readings (taken every 4 hrs), 91.5% (2359) were ≤ 20 mm Hg, 8.5% were > 20 (in n=49 patients), 1.6% were >30 (n=15), 0.5% were > 40 (n=5) and 0.2% were > 50 mm Hg (n=4). Groups are not mutually exclusive. In a multivariate analysis, the proportion of threshold ICP events > 20 mm Hg and > 30 mm Hg per patient were significantly more frequent in the Pcb treated group (ICP > 20 mm Hg only) (p=0.03 and p=0.08, respectively) (Fig. 2), in patients with initial EVD placement ipsilateral to the side of greatest IVH clot volume (vs. contralateral) (p=0.01 and p<0.001, respectively) (Fig. 3), with larger stability IVH volume (p<0.001 and p<0.001, respectively), but not with stability ICH volume (p=0.37 and p=0.26, respectively), and in patients with a higher presentation Glasgow Coma Scale (GCS) score (p=0.007 and p=0.01, respectively).
Figure 2.

Bar graph comparing percentage of ICP threshold events > 20, 30, 40 and 50 mm Hg in rt-PA and placebo treated patients. Percent of ICP events > 20 mm Hg was significantly greater in placebo treated patients (*). ICP threshold events are additive from left to right.
Figure 3.

Bar graph comparing percentage of ICP threshold events > 20, 30, 40 and 50 mm Hg in patients with EVDs placed ispi- and contralateral to side of greatest initial volume of IVH. Percent of ICP events > 20 and > 30 mm Hg was significantly greater in patients with ipsilateral placed EVDs (*). ICP threshold events are additive from left to right.
Fifty-one patients, 43 (55%) rt-PA and 8 (36%) Pcb-treated, had no ICP readings > 20 mm Hg for the duration of their EVD. Table 3 shows the characteristics of patients with and without ICP readings > 20 mmHg. Other demographic and presenting characteristics (not shown) were not different between these 2 groups with the exception that presenting mean arterial pressure was higher in the group with any ICP reading > 20 mm Hg (127 (32) vs. 113 (29) with no ICP reading > 20 mm Hg; p=0.03). Clot location was not associated with any occurrence of elevated ICP. All 7 patients treated with simultaneous initial placement of dual EVDs had at least one ICP reading > 20 mmHg, although only one of these had ICP > 30 mmHg.
Table 3.
Characteristics of patients with and without any ICP readings > 20 mm Hg for duration of ICP monitoring
| Characteristic | No ICP reading > 20 mm Hg (N=51) | Any ICP reading > 20 mmHg (N=49) | P=Value |
|---|---|---|---|
| Presenting GCSa | 7 [8] | 8 [9] | 0.79 |
| Stability ICH Volumea | 5.9 [12.4] | 6.0 [12.7] | 0.98 |
| Stability IVH Volumea | 31.0 [31.8] | 49.1 [38.8] | 0.002 |
| Stability IVH Graeba,b | 7 [3] | 8[2] | 0.22 |
| ICH Location: (%) | 0.31 | ||
| Thalamic | 23 (45) | 20 (41) | |
| Caudate | 3 (6) | 6 (12) | |
| Globus Pallidus | 6 (12) | 2 (4) | |
| Putamen | 7 (14) | 3 (6) | |
| Lobar | 3 (6) | 5 (10) | |
| Primary IVH | 9 (18) | 13 (26) | |
| rt-PA treated (%) | 43 (84) | 35 (71) | 0.12 |
| Placebo treated (%) | 8 (16) | 14 (29) |
median [iqr:interquartile range]
Graeb score [2]
Abbreviations: ICP: intracranial pressure; GCS: Glasgow Coma Scale; ICH: intracerebral hemorrhage; IVH: intraventricular hemorrhage; rt-PA: recombinant tissue plasminogen activator
Comparison of 2676 q1h vs. 894 q4h ICP readings in a subset of 36 patients showed no significant difference in proportion of readings above ICP threshold events (p=0.97).
ICP monitoring during EVD closure for study agent administration
The median change in ICP per patient during 1 h EVD closure period post-injection of study agent was an increase of 4 mm Hg (iqr 8) and was not different between treatment groups (p=0.54). Of 868 intraventricular injections of study agent, ICP elevations > 20 and > 30 mm Hg occurred during EVD closure in 23.8% (n=59 patients; 207 injections) and 6.2% (n=26; 54 injections) of injections respectively. ICP elevation > 30 mmHg by the end of the closure period was independently associated with higher pre-EVD closure ICP (p<0.001) and with higher initial GRAEB score (p=0.04), but not with treatment group (p=0.59), stability IVH volume (p=0.15), stability ICH volume (p=0.57), or side of EVD placement relative to greatest IVH clot volume (p=0.13). The number of ICP elevations > 20 mmHg per patient during EVD-closure for the first 10 doses of study agent was significantly associated with having any ICP readings >20 mmHg during non-EVD closure times (p=0.003). Early opening of the EVD before 90% of the recommended 1 h closure time had elapsed occurred in 69 (7.9%) injections (n=35 patients) and was independently associated with ICP > 30 mm Hg during closure (p=0.003, adjusted for number of injections/patient) and with rt-PA treatment (p=0.004), but not with the immediate pre-injection ICP (p=0.61), nor with stability IVH volume (p=0.12) or ICH volume (p=0.40).
ICP monitoring before and after clearance of lower ventricular system
Clearance of blood from both the 3rd and 4th ventricles, as visualized on CT scan occurred at a median of 2.5 days (2.0) in the rt-PA group compared to 6.4 days (4.6) in the Pcb group (p<0.001). The proportion of ICP readings > 20 mmHg after clearance was significantly greater in patients with ipsilateral placement of EVD (p=0.001), larger stability IVH volume (p=0.002), longer time to opening of 3rd ventricle (p=0.001), and was marginally greater in rt-PA treated patients (p=0.05), but was not associated with stability ICH volume (p=0.14). Median ICP was unchanged pre vs. post clearance (p=0.09). Three surviving patients in each group (3.8% - rtPA; 13.6% - Pcb) failed to radiographically clear both the 3rd and 4th ventricles.
CSF drainage and VP shunt insertion
Median Daily CSF drainage was 109cc (153) in the Pcb group compared with 53 cc (138) in the rt-PA treated group. Average daily CSF volume drained/patient was significantly higher with longer EVD duration (p<0.001), and larger stability ICH volume (p=0.02), but was not associated with stability IVH volume (p=0.41), treatment (p=0.54), or number of high ICP events > 20 mm Hg (p=0.87) or > 30 mm hg (p=0.47). We did not adjust the model for daily IVH volume because not all patients had daily CT scans beyond the first 2 days of admission.
In the multivariate analysis of factors associated with VP shunt insertion, there was a significant association with proportion of ICP readings > 20 mmHg (p=0.03), but only marginal for ICP > 30 mmHg (p=0.05). VP shunt insertion was also significantly associated with Pcb treatment (p=0.04), lower presentation GCS score (p=0.03), longer time to opening of the 3rd ventricle (p=0.01), longer EVD duration (p=0.004), but not with stability IVH volume (p=0.40) or GRAEB score (p=0.24). The number of VP shunts placed was not significantly different between treatment groups (6.4%-rtPA vs. 13.6%-Pcb; p=0.37).
Cerebral perfusion pressure monitoring
Baseline CPP ranged from 52 to 147 mm Hg (median 83.5, iqr 21.3). Over the entire ICP monitoring period low CPP events < 60 but not < 70 mm Hg per patient were less frequent in rtPA vs. Pcb treated patients (p=0.02 and p=0.25 respectively). CPP events < 60 mm Hg were associated with elevated ICP (> 20 mm Hg) in 7/25 (28%) of readings (p=0.04); CPP events < 70 were associated with elevated ICP in 25/102 (24.5%) of readings (p=0.01).
30 Day neurologic outcomes
Median 30-day modified Rankin Score (mRS) was 5 in both treatment groups (p=0.23). Mortality was 19 (19.1%) at 30 days. In the multivariate analysis (adjusting for known predictors of ICH outcome), mortality was significantly associated with the percentage of high ICP events per patient > 30 mm Hg (p=0.003) and with a trend for % ICP readings > 20 mmHg (p=0.08). Mortality was also associated with both IVH and ICH stability volume (Table 4). Poor mRS at 30 days was independently associated with % of ICP events > 30 mmHg/patient (p=0.01) (but not > 20 mmHg), both ICH and IVH volume and pulse pressure. Rt-PA treatment was not associated with 30 day outcomes.
Table 4.
Multivariate logistic regression: risk of 30 day mortality and 30 day poor outcome (mRS 5 or 6) compared with 30 day good outcome (mRS 0-4) by known determinants of ICH and IVH outcome
| Characteristics | 30 day Mortality OR | 30 day Mortality 95% CI | 30 day Mortality P value | 30 day Poor Outcome OR | 30 day Poor Outcome 95% CI | 30 day Poor Outcome P-value |
|---|---|---|---|---|---|---|
| % ICP readings > 30 mm Hg† | 1.15 | 1.05-1.26 | 0.003 | 1.11 | 1.02-1.20 | 0.01 |
| IVH volume* | 1.04 | 1.02-1.06 | <0.001 | 1.05 | 1.03-1.08 | <0.001 |
| ICH volume* | 1.08 | 1.01-1.15 | 0.03 | 1.16 | 1.05-1.29 | 0.003 |
| Presentation GCS | 0.85 | 0.66-1.10 | 0.23 | 0.89 | 0.75-1.06 | 0.19 |
| Presentation PP | 1.01 | 0.99-1.03 | 0.45 | 1.03 | 1.00-1.05 | 0.04 |
| Age | 1.05 | 0.97-1.13 | 0.19 | 1.04 | 0.98-1.10 | 0.20 |
| Rt-PA treated | 1.99 | 0.55-7.21 | 0.29 | 0.60 | 0.13-2.86 | 0.52 |
per patient
on stability CT
Abbreviations: mRS: modified Rankin Scale; OR: odds ratio; CI: confidence interval; ICP: intracranial pressure; IVH: intraventricular hemorrhage; ICH: intracerebral hemorrhage; GCS: Glasgow Coma Scale: PP: pulse pressure: rt-PA: recombinant tissue plasminogen activator
Discussion
During monitoring, significant ICP elevation occurs in 8.5% of readings after initiation of external CSF drainage for spontaneous IVH with associated hydrocephalus. The corollary is that CSF drainage effectively controls ICP over 90% of the time. However, ICP elevation > 30 mm Hg, when it occurs, is an independent predictor of short-term mortality suggesting that EVDs play both a therapeutic and diagnostic role in this disease.
This study raises several important issues. Firstly although ICP elevation was not a frequent event in most patients, the duration of high ICP (especially ICP > 30 mm Hg) appears to be important. The use of EVDs should not be considered only for ICP relief, but equally important for CSF drainage or clearance of blood to prevent occurrence of ICP elevation. This is supported by the finding that ICP is dependent on ventricular clot volume.
Impact of intraventricular thrombolysis on ICP
The hypothesis of the CLEAR IVH studies on which this data is based is that intraventricular clot volume reduction assisted by intraventricular rtPA will improve long-term neurologic outcomes. The finding that the proportion of high ICP events (> 20 mm Hg) was significantly less frequent in the rtPA treated group (and borderline significant for > 30 mm Hg) (vs. Pcb treated group) suggests that more effective clot removal lowers the risk of ICP elevation. There was also a non-significantly higher percentage of rt-PA treated patients who had no ICP elevation > 20 mm Hg (vs. any ICP elevation). We previously reported a small study of ICP in urokinase vs. placebo treated patients with IVH in which EVDs were equally effective in controlling ICP in both treatment groups during the first 5 days of monitoring (12). The current larger study appears consistent with the explanation that thrombolysis improves ICP control. Compared with placebo, rt-PA treated patients in the current study had less common EVD obstruction requiring replacement, shorter duration of EVD, faster clearance of the lower ventricular system, less frequent VP shunt placement and fewer low CPP readings. All of these treatment effects may be associated with decreased occurrence of intracranial hypertension.
Thrombolytic therapy did not have an impact on ICP control after radiographic clearance of the third and fourth ventricles while ICP was monitored although tPA treatment had stopped by this time. High ICP after apparent establishment of clearance of the lower CSF pathways may occur due to non-obstructive (communicating) hydrocephalus, lack of clearance of blood from lateral ventricles (obstruction of the Foramina of Munro), and challenging the need for EVD by raising the “pop-off” for CSF drainage. The definition of ventricle clearance used was dependent on visualization of hyperdense blood products in the 3rd and 4th ventricles which may become less reliable with time-related changes in the appearance of blood on CT.
EVD closure-related ICP elevation occurred after nearly a quarter of injections indicating that many patients are highly dependent on CSF drainage to control ICP. However, these events were apparently readily controlled with medical management (or tolerated by the treating physician) such that only a small percentage of injections (7.9%) required early re-opening of the EVD. Patients who experienced elevated ICP during EVD closure for study agent administration were also more likely to have high ICP events during CSF drainage. This group may benefit from alternative strategies for delivery of intraventricular thrombolysis. It is conventional to perform equivolumetric exchange of CSF for thrombolytic agent and to close the EVD for some period of time after drug administration to ensure adequate pharmacologic activity. In patients who are not expected to tolerate EVD closure (due to preexisting ICP elevation), techniques such as pre-emptive ICP management with sedatives to decrease cerebral metabolism (propofol or barbiturates), and use of dual catheters to facilitate CSF removal may provide superior ICP control to that achieved in this study.
Compartmentalization of ICP
The finding that initial EVD placement ipsilateral to the side of greatest IVH clot burden was significantly associated with high ICP (> 20 and > 30 mmHg) compared to patients with contralateral EVD placement suggests that ICP may be lateralized in this disease. There are several possible explanations. There may be an ICP gradient within the ventricular system with ICP highest on the side of the greatest clot volume; patients who had ipsilateral drains may have been sicker, had local mass effect or a trapped lateral ventricle at the time of EVD placement; or EVDs placed in the more open ventricle allowed better ICP control due to increased CSF drainage. The concept of ICP compartmentalization has been described in stroke. In patients with bilateral epidural sensors, ICP is higher ipsilateral to the stroke with a difference up to 15 mm Hg (18).
Compartmentalization of ICP within the ventricle has not been described but has clinical implications because EVDs are often placed contralaterally when IVH produces casting of the most involved ventricle. If ICP is lateralized, monitoring ICP from the less involved ventricle may fail to demonstrate treatable ICP elevation that occurs on the side of greater clot. Our study results cannot support specific recommendations as to the most appropriate side of EVD placement because EVD location was chosen at the discretion of the inserting proceduralist and we did not record simultaneous ICP readings in the few patients with dual EVDs.
Mortality and neurologic outcome
It is often assumed that a major contributing factor to poor outcomes in patients with ICH and IVH is hydrocephalus and accompanying elevated ICP which injures the reticular activating system and compromises cerebral perfusion. This study found that the percentage of high ICP (> 30 mm Hg) recordings per patient (not in association with EVD clamping for study agent administration) was an independent predictor of 30 day mortality after adjusting for ICH and IVH volumes, age and presentation GCS and PP. This is consistent with the traumatic brain injury literature on the significance of ICP elevation (≥ 20 mm Hg) in the first 48 hours after head injury as a poor prognostic indicator especially if ICP is refractory to treatment (19,20). We also found a significant association between baseline ICH and especially IVH volumes and 30 day outcomes in this cohort. These findings support previously established associations between larger IVH volume and greater degree of ventricular dilatation on early CT scans with worse outcomes (21-23). Since ICP was aggressively managed in this protocol, it is not clear whether outcomes could be improved with more intensive treatment of ICP elevations or if the observed intracranial hypertension is a treatment-refractory marker of disease severity.
Our finding that admission pulse pressure was an independent predictor of poor outcome (but not mortality at 30 days) is consistent with the literature (24,25). This finding was independent of proportion of ICP readings above 30 mm Hg. Other possible mechanisms for this association are that patients with higher blood pressure had increased perihematomal edema or hematoma expansion which was not captured as higher ICP, or had ischemic strokes which we did not evaluate. Early recurrence of acute ischemic stroke has been associated with both high systolic and diastolic blood pressure (24,26). It may also be that initial higher blood pressures were a physiologic response to very high ICP which was not recorded prior to EVD placement and resulted in worse outcomes.
Previous observations in patients with ICH and IVH suggest that EVD placement alone is not sufficient to result in improved outcomes, and that morbidity associated with hydrocephalus may precede EVD placement. Adams and Diringer reported EVD placement was associated with “adequate” control of ICP in 20/22 patients with supratentorial ICH and hydrocephalus, although neither ventricular size nor level of consciousness improved in the short-term (27). Diringer et al. reported that hydrocephalus was associated with higher intubation rates and mortality in 81 patients with supratentorial ICH although outcomes in patients treated with EVD were not different from those not treated with EVDs (23). Coplin et al (28) reported that ICP at insertion of EVD does not appear to have any particular prognostic significance either as an indicator of future high ICP events or as a prognostic indicator of outcome. While our results do not refute any of these prior findings, this is the first study to show with serial ICP recordings that high ICP (>30 mm Hg) is an independent predictor of short-term survival and therefore aggressive ICP management may be a sufficient rationale for EVD placement independent of the decision to use thrombolytics.
Study limitations
The sample size, duration of monitoring and frequency/intensity of monitoring could have limited our ability to observe ICP events and define relationships. That being said, the frequency of high ICP events is plausible and consistent with the frequency of ICP elevation in the previous study using urokinase thrombolysis (12). Although ICP was aggressively managed in this study, we cannot exclude the possibility of missed events; for example, it is possible that ICP was not managed at standard thresholds in patients who were expected to do poorly. Additionally, in only considering 30 day outcomes, we were not able to determine whether ICP elevation impacts long-term neurologic function. As in our previous study, generalizability is limited to patients with small ICH (< 30 cc) and large IVH requiring EVDs. This may also explain why ICH volume and clot location were not associated with ICP. There were relatively few lobar hemorrhages (n=8) which are often larger and may contribute more to ICP elevation. Variability in clot localization and size were constrained by study inclusion criteria thus limiting the influence of these otherwise important factors on outcome and mortality. Our study showed that median ICP and proportion of high ICP events above threshold were not different for q1h compared to q4h readings. However, because ICP was not collected continuously, transient ICP elevations may have been missed. This should not imply that therapeutic response to high ICP events only occurred every 4 hours as all centers recorded ICP continuously with more frequent internal monitoring than required by the study protocol.
Conclusions
In summary, EVD placement with CSF drainage controlled ICP over 90% of the time and thrombolytic therapy appears to contribute to ICP reduction in the acute phase of IVH. Refractory intracranial hypertension is an independent risk factor for mortality. This study also supports that administration of thrombolytic agents can be performed without additional ICP management in the majority of patients, but that close ICP monitoring during EVD closure is essential in order to treat ICP elevations which in most cases respond quickly to hyperventilation and/or mannitol without the need for EVD opening.
Our study suggests there could be further benefit in preventing episodes of ICP elevation > 30 mm Hg during the acute phase of ICH with IVH. Assuming ICP management was optimized in these patients, further strategies to treat intractable ICP elevation should be investigated as to whether they may improve mortality or whether this reflects a more severe injury with permanent structural damage. A better understanding of the pathophysiology of intracranial hypertension in this disease will likely come from studies using cerebral microdialysis and brain tissue oxygen probes to elucidate the association of disturbances in cerebral metabolism with ICP elevation and also the relationship with long-term radiographic markers of cognitive function such as cerebral atrophy and ventricular size. ICP should be monitored early in all IVH patients during drainage and during administration of intraventricular drugs so that elevated ICP can be treated rapidly and EVDs can be appropriately managed.
Supplementary Material
Acknowledgments
Financial support:
This work was supported in part by the Eleanor Naylor Dana Fellowship (DFH and WCZ), American Academy of Neurology (WCZ), NIH/NINDS 1RO1 NS 24282-08 (DFH), Grant FD-R-001693-01-1 from the Food and Drug Administration Orphan Products Development Agency (DFH), and the France Merrick Foundation Grant (DFH). Genentech assisted by donating drug.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Weerd AW. The prognosis of intraventricular hemorrhage. J Neurol. 1979;222:46–51. doi: 10.1007/BF00313266. [DOI] [PubMed] [Google Scholar]
- 2.Graeb DA, Robertson WD, Lapointe JS, Nugent RA, Harrison PB. Computed tomographic diagnosis of intraventricular hemorrhage. Etiology and prognosis. Radiology. 1982;143:91–6. doi: 10.1148/radiology.143.1.6977795. [DOI] [PubMed] [Google Scholar]
- 3.Todo T, Usui M, Takakura K. Treatment of severe intraventricular hemorrhage by intraventricular infusion of urokinase. J Neurosurg. 1991;74:81–6. doi: 10.3171/jns.1991.74.1.0081. [DOI] [PubMed] [Google Scholar]
- 4.Bhattathiri PS, Gregson B, Prasad KS, Mendelow AD. STICH Investigators. Intraventricular hemorrhage and hydrocephalus after spontaneous intracerebral hemorrhage: results from the STICH trial. Acta Neurochir Suppl. 2006;96:65–8. doi: 10.1007/3-211-30714-1_16. [DOI] [PubMed] [Google Scholar]
- 5.Steiner T, Diringer MN, Schneider D, Mayer SA, Begtrup K, Broderick J, Skolnick BE, Davis SM. Dynamics of intraventricular hemorrhage in patients with spontaneous intracerebral hemorrhage: risk factors, clinical impact, and effect of hemostatic therapy with recombinant activated factor VII. Neurosurgery. 2006 Oct;59(4):767–73. doi: 10.1227/01.NEU.0000232837.34992.32. [DOI] [PubMed] [Google Scholar]
- 6.Tuhrim S, Dambrosia JM, Price TR, Mohr JP, Wolf PA, Heyman A, Kase CS. Prediction of intracerebral hemorrhage survival. Ann Neurol. 1988;24:258–263. doi: 10.1002/ana.410240213. [DOI] [PubMed] [Google Scholar]
- 7.Tuhrim S, Dambrosia JM, Price TR. Intracerebral hemorrhage: External validation and extension of a model for prediction of 30-day survival. Ann Neurol. 1991;29:658–663. doi: 10.1002/ana.410290614. [DOI] [PubMed] [Google Scholar]
- 8.Hemphill JC, 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–897. doi: 10.1161/01.str.32.4.891. [DOI] [PubMed] [Google Scholar]
- 9.Hallevi H, Albright KC, Aronowski J, Barreto AD, Martin-Schild S, Khaja AM, Gonzales NR, Illoh K, Noser EA, Grotta JC. Intraventricular hemorrhage: anatomic relationships and clinical implications. Neurology. 2008;70:848–852. doi: 10.1212/01.wnl.0000304930.47751.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naff NJ, Carhuapoma JR, Williams MA, Bhardwaj A, Ulatowski JA, Bederson J, Bullock R, Schmutzhard E, Pfausler B, Keyl PM, Tuhrim S, Hanley DF. Treatment of intraventricular hemorrhage with urokinase : effects on 30- Day survival. Stroke. 2000;31:841–7. doi: 10.1161/01.str.31.4.841. [DOI] [PubMed] [Google Scholar]
- 11.Steinke W, Sacco RL, Mohr JP, Foulkes MA, Tatemichi TK, Wolf PA, Price TR, Hier DB. Thalamic stroke. Presentation and prognosis of infarcts and hemorrhages. Arch Neurol. 1992;49:703–10. doi: 10.1001/archneur.1992.00530310045011. [DOI] [PubMed] [Google Scholar]
- 12.Ziai WC, Torbey MT, Naff NJ, Williams MA, Bullock R, Marmarou A, Tuhrim S, Schmutzhard E, Pfausler B, Hanley DF. Frequency of sustained intracranial pressure elevation during treatment of severe intraventricular hemorrhage. Cerebrovasc Dis. 2009;27:403–410. doi: 10.1159/000209241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–1305. doi: 10.1161/01.str.27.8.1304. [DOI] [PubMed] [Google Scholar]
- 14.Naff N, Williams M, Keyl PM, Tuhrim S, Bullock RM, Mayer S, et al. Low-dose rt-PA enhances clot resolution in brain hemorrhage: The intraventricular hemorrhage thrombolysis trial. Stroke. 2011 doi: 10.1161/STROKEAHA.110.610949. In-press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan T, Awad I, Keyl P, Lane K, Hanley D, editors. Preliminary report of the clot lysis evaluating accelerated resolution of intraventricular hemorrhage (CLEAR-IVH) clinical trial. Acta Neurochir Suppl. 2008;105:217–20. doi: 10.1007/978-3-211-09469-3_41. [DOI] [PubMed] [Google Scholar]
- 16.The Brain Trauma Foundation The American Association of Neurological Surgeons. The Joint Section on Neurotrauma and Critical Care. Intracranial pressure treatment threshold. J Neurotrauma. 2000;17(6-7):493–5. doi: 10.1089/neu.2000.17.493. [DOI] [PubMed] [Google Scholar]
- 17.The Brain Trauma Foundation The American Association of Neurological Surgeons. The Joint Section on Neurotrauma and Critical Care. Guidelines for cerebral perfusion pressure. J Neurotrauma. 2000;17(6-7):507–11. doi: 10.1089/neu.2000.17.507. [DOI] [PubMed] [Google Scholar]
- 18.Schwab S, Aschoff A, Spranger M, Albert F, Hacke W. The value Neurology in acute hemispheric stroke. Neurology. Aug. 1996;47(2):393–8. doi: 10.1212/wnl.47.2.393. [DOI] [PubMed] [Google Scholar]
- 19.Alberico AM, Ward JD, Choi SC, Marmarou A, Young HF. Outcome after severe head injury. Relationship to mass lesions, diffuse injury, and ICP course in pediatric and adult patients. J Neurosurg. 1987;67:648–56. doi: 10.3171/jns.1987.67.5.0648. [DOI] [PubMed] [Google Scholar]
- 20.Miller JD, Becker DP, Ward JD, Sullivan HG, Adams WE, Rosner MJ. Significance of intracranial hypertension in severe head injury. J Neurosurg. 1977;47:503–16. doi: 10.3171/jns.1977.47.4.0503. [DOI] [PubMed] [Google Scholar]
- 21.Mayfrank L, Lippitz B, Groth M, Bertalanffy H, Gilsbach JM. Effect of recombinant tissue plasminogen activator on clot lysis and ventricular dilatation in the treatment of severe intraventricular haemorrhage. Acta Neurochir. 1993;122:32–8. doi: 10.1007/BF01446983. [DOI] [PubMed] [Google Scholar]
- 22.Tuhrim S, Horowitz DR, Sacher M, Godbold JH. Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage. Crit Care Med. 1999;27:617–21. doi: 10.1097/00003246-199903000-00045. [DOI] [PubMed] [Google Scholar]
- 23.Diringer MN, Edwards DF, Zazulia AR. Hydrocephalus: A Previously Unrecognized Predictor of Poor Outcome From Supratentorial Intracerebral Hemorrhage. Stroke. 1998;29:1352–1357. doi: 10.1161/01.str.29.7.1352. [DOI] [PubMed] [Google Scholar]
- 24.Willmot M, Leonardi-Bee J, Bath PM. High blood pressure in acute stroke and subsequent outcome: a systematic review. Hypertension. 2004;43:18–24. doi: 10.1161/01.HYP.0000105052.65787.35. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Reilly KH, Tong W, et al. Blood pressure and clinical outcome among patients with acute stroke in Inner Mongolia, China. J Hypertens. 2008;26:1446–1452. doi: 10.1097/HJH.0b013e328300a24a. [DOI] [PubMed] [Google Scholar]
- 26.Leonardi-Bee J, Bath PMW, Phillips SJ, Sandercock PAG, for the IST Collaborative Group Blood pressure and clinical outcomes in the International Stroke Trial. Stroke. 2002;33:1315–1320. doi: 10.1161/01.str.0000014509.11540.66. [DOI] [PubMed] [Google Scholar]
- 27.Adams RE, Diringer MN. Response to external ventricular drainage in spontaneous intracerebral hemorrhage with hydrocephalus. Neurology. 1998;50:519–23. doi: 10.1212/wnl.50.2.519. [DOI] [PubMed] [Google Scholar]
- 28.Coplin WM, Vinas FC, Agris JM, Buciuc R, Michael DB, Diaz FG, Muizelaar JP. A cohort study of the safety and feasibility of intraventricular urokinase for nonaneurysmal spontaneous intraventricular hemorrhage. Stroke. 1998;29:1573–9. doi: 10.1161/01.str.29.8.1573. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


