Abstract
The current therapeutic options for acute decompensated heart failure are limited to afterload reducers and positive inotropes. The latter increases myocardial contractility through changes in myocyte calcium (Ca2+) handling (mostly through stimulation of the β-adrenergic pathways [β-ADR]) and is associated with paradoxical effects of arrhythmias, cell death, and subsequently increased mortality. We have previously demonstrated that probenecid can increase cytosolic Ca2+ levels in the cardiomyocyte resulting in an improved inotropic response in vitro and in vivo without activating the β-ADR system. We hypothesize that, in contrast to other commonly used inotropes, probenecid functions through a system separate from that of β-ADR and hence will increase contractility and improve function without damaging the heart. Furthermore, our goal was to evaluate the effect of probenecid on cell death in vitro and its use in vivo as a positive inotrope in a mouse model of ischemic cardiomyopathy. Herein, we demonstrate that probenecid induced an influx of Ca2+ similar to isoproterenol, but does not induce cell death in vitro. Through a series of in vivo experiments we also demonstrate that probenecid can be used at various time points and with various methods of administration in vivo in mice with myocardial ischemia, resulting in improved contractility and no significant difference in infarct size. In conclusion, we provide novel data that probenecid, through its activity on cellular Ca2+ levels, induces an inotropic effect without causing or exacerbating injury. This discovery may be translatable if this mechanism is preserved in man.
Keywords: echocardiography, inotrope, Ca2+ influx, ischemia/reperfusion, infarct
Introduction
The current management of acute decompensated heart failure is based on afterload reduction and increasing myocardial contractility.1 The therapies for the former are well established and include direct and indirect vasodilators; unfortunately, the therapeutic options available for the latter are limited to digoxin and sympathomimetics (ie, dopamine, dobutamine, and milrinone).2 The sympathomimetic drugs share the common end point of increasing cyclic adenosine monophosphate (cAMP) production in the cardiomyocyte (either directly through β-adrenergic [β-ADR] stimulation or indirectly via phosphodiesterase inhibition [PDI]) and have repeatedly been shown to be associated with poor outcomes.3,4 Several mechanisms have been shown to be related to the deleterious outcomes associated with these medications including arrhythmias,5 stimulation of apoptotic signaling,6 and increased myocardial energetic demand.7
We have recently published that probenecid has positive inotropic properties which were previously not recognized.8 Our previous experiments demonstrated that treatment with probenecid in isolated myocytes resulted in an increase in fractional shortening, while in Langendorff perfused hearts and in healthy wild-type mice in vivo, the result was an increase in contractility. However, these experiments did not use probenecid as a potential therapy for heart failure or investigate its use in ischemically damaged animals. This critically important step is needed as probenecid is an Food and Drug Administration (FDA)-approved drug used for the treatment of gout for decades9 and as adjuvant therapy for use with certain antibiotics10 and antivirals.11 It also has been used safely for over 50 years with a very limited adverse effect profile.12 Several years ago, Bang et al13 found that it is also a potent agonist of the transient receptor potential vanilloid 2 (TRPV2) channel. More recently, we have shown that TRPV2 is expressed in the murine heart and, when activated with probenecid, increases cytosolic calcium (Ca2+) concentration which results in a positive inotropic response both in vitro and in vivo.8
Our previous work further demonstrated that probenecid does not have significant malignant electrophysiologic properties (ie, no arrhythmias were noted) unlike other adrenergic drugs. Furthermore, as other positive inotropes are known to be cell injurious, we examined the potential cytotoxic/apoptotic properties of probenecid for its use as a positive inotrope in a mouse model of ischemic heart disease. Hence, we hypothesized that probenecid functions through a separate mechanism than conventionally used inotropes and that it will increase contractility and improve heart function after ischemia without decreasing cell survival or increasing apoptosis. We tested this in vitro using the HL-1 cardiac cell line in addition to a well-established and clinically relevant mouse model of ischemia/reperfusion (I/R)14 and monitored cardiac function via echocardiography.8
Methods
Imaging and Measurement of Ca2+ Fluorescence
Briefly, the HL-1 cells were seeded in 96-well plates at a density of 1 × 104 cells/well and allowed to grow overnight in Claycomb media supplemented with 10% fetal bovine serum, 100 U/mL penicillin/streptomycin, and 2 mmol/L L-glutamine. Dissociated HL-1 cells were plated on glass coverslips (GG-25-polylysine #1, Neuvitro, California) placed inside each well of 6-well plates (BD Falcon; BD Falcon; Fisher Scientific, Pittsburgh, Pennsylvania). Acquisition of images was done at 40 to 65 Hz using LSM 510 Meta system equipped with the inverted Axiovert 200 M BP (Carl Zeiss Microscopy, LLC, Thornwood, New York) and LSM5 Software. Image acquisition was done with Plan-Apochromat 40×/water with frame size of 512 (X) and 256 (Y). To optimize the speed of acquisition, the acquisition area was limited to a few cells. The cells were loaded with the Ca2+ indicator dye FLUO4-AM (Molecular Probes, Life Technologies, Carlsbad, California) diluted in Tyrode solution (Sigma-Aldrich, St Louis, Missouri) to a final concentration of 1 µmol/L for 20minutes at 37°C and then washed twice for 5minutes in Tyrode solution. Coverslips with adherent HL-1 cells preloaded with dye were placed in the recording chamber that was mounted on an inverted microscope and subsequently bathed in Tyrode solution before perfusion of probenecid. After focusing and taking baseline images, the cells were perfused with probenecid (10−8-10−4 mol/L in different experiments) at a rate of 0.5 mL/15 s. For these experiments, image exposure time was 5 milliseconds with a camera on-chip multiplication gain of 500 to 600, and there were 700 cycles for each run of the different concentrations. The 488-nm line of a multiline argon laser excited FLUO-4 and an electronic shutter-controlled cell exposure to the laser. Offline image processing was performed with LSM5 software and statistical analyses on the data were done using MS Excel (Microsoft, Redmond, Washington) and SAS 9.2 (SAS Institute, Cary, North Carolina).
Average fluorescence values for before perfusion (F0) and after perfusion (F) were determined manually after acquisition of images from the saved LSM files. Circular areas within the cytosol of each cell of the saved images over the entire duration of respective experiments were used to determine changes in fluorescence before and after the perfusion.
Normalized fluorescence, determined as a quotient of F/F0, was used as a variable to compare the relative changes in fluorescence at different incremental concentrations during statistical analyses. Maximal likelihood estimates of the dependent variable (log [F/F0]) were determined as a function of concentration of the probenecid (log [probenecid], M) dose using “proc mixed modeling” with repeated measures in SAS. A dose–response relationship was graphed using linear regression plot of the model.
Cell Viability Assays
Cell viability was assessed using the ApoTox-Glo Triplex Assay (Promega, Madison, Wisconsin) as per the manufacturer’s instructions. The HL-1 cells were obtained as described above. In order to approximate the expected serum concentration of both probenecid (500 mg orally) and isoproterenol (10 mcg/min intravenously) at standard therapeutic doses, we treated the cells with a range of probenecid from 10−4 to 10−8 mol/L and of isoproterenol of 10−4 and 10−5 mol/L.
The cells were treated with indicated doses of probenecid or isoproterenol in supplemented growth media at 100 µL total volume and allowed to incubate for 4, 8, or 24 hours. The glycine phenylalanine amino-fluoro-coumarin (GF-AFC) viability reagent was then added to ApoTox-Glo Assay Buffer at a ratio of 10 µL GF-AFC to 2.0mL Assay buffer and 20 µL of this mixture was added to each well. The cells were incubated at 37°C for 30 minutes and fluorescence was measured at 400 nm (ex)/505 nm (em) and the data were normalized to untreated control cells.
Water soluble probenecid (Molecular Probes, Life Technologies, Grand Island, New York) was used for all of the experiments.
Animals
All animal procedures were performed with the approval of the Institutional Animal Care and Use Committee of the University of Cincinnati and in accordance with the Guide for the Care and Use of Laboratory Animals (NIH, revised 1996). All mice (C57BL6J, Jackson laboratories, Bar Harbor, Maine) were males at 12 to 16 weeks of age.
Detection of Cell Death
Hearts were obtained from mice which were treated with probenecid or sugar water (control) under various conditions. There were 4 groups of mice for these experiments, including control (n = 4), probenecid-treated water (n = 4), control after I/R injury (n = 4), and probenecid-treated water after I/R injury (n = 4). The hearts were removed, rinsed with phosphate-buffered saline (PBS), fixed with 3.7% buffered formaldehyde (after 30minutes the buffer was changed), and dehydrated (hearts were placed in 70%ethanol after 18–24 hours). The samples were taken to the Cincinnati Children’s Hospital Medical Center, Department of Pathology Research Core (Cincinnati, Ohio) where they were embedded in paraffin, sectioned into thicknesses of 6 µm, each 10 µm apart, and 2 heart sections were placed onto each slide. The slides were prepared using an in situ apoptosis detection kit (Cardio TACS; Trevigen, Gaithersburg, Maryland), which stains by terminal deoxynucleotide transferase-mediated deoxyuridine triphosphate nick end labeling, according to the manufacturer’s instructions. After staining, the slides were placed on amicroscope (Olympus1X71:Olympus, Melville, NewYork) and the number of apoptotic cells was counted by 2 separate and blinded readers in 5 fields of view for each heart section. The total number of cells was also determined for the same field, and the apoptotic cells were calculated to be a percentage of the total cells. These values were then averaged for each group, probenecid and control mice.
Ischemia/Reperfusion Methods
The I/R studies were performed as previously described.14,15 Briefly, mice were anesthetized with sodium pentobarbital and the heart was exposed through a left thoracotomy at the level of the fourth intercostal space. The mice were intubated and placed on a miniventilator (Harvard apparatus, Holliston, Massachusetts). A loop occluder was placed around the left anterior descending artery and tightened, occluding it for either 30 minutes (small infarct) or 45 minutes (larger infarct). After surgery, the thorocotomy was sutured in layers, and the animal was allowed to recover.
All mice had echocardiographic measurements as described after 24 hours post-IR to determine the severity of the injury in vivo.
Echocardiography Methods
All echocardiographic studies were performed as previously described.8 Briefly, mice were anesthetized with isoflurane and placed on the heated stage of the Vevo 2100. Parasternal long axis and short axis images were recorded and then analyzed on a separate work station with VevoStrain software (Vevo 2100, v1.1.1 B1455, Visualsonic, Toronto, Canada).
Ischemia/Reperfusion Followed by Probenecid Bolus
The I/R studies were performed as described above with 45 minutes of ischemia. Echocardiography was performed at 24 hours post-I/R and weekly thereafter. There were 4 groups of mice, saline and probenecid with subgroups of mice with a post-I/R ejection fraction (EF) of 50% to 60% (n = 5 for saline and n = 6 for probenecid) or those with a post I/R EF of 40% to 50% (n = 6 for saline and n = 6 for probenecid). The probenecid mice were treated with an intraperitoneal bolus of 100 µL (100 mg/kg) while the saline groups only received 100 µL of saline intraperitoneally. The mice were imaged at 5-minute intervals for a total of 30 minutes.
Ischemia/Reperfusion Followed by Oral Probenecid
The I/R studies were performed as described above with 45 minutes of ischemia and echocardiography was performed at 24 hours post-I/R and weekly thereafter for 4 weeks. After 24 hours, the mice were randomized to probenecid-treated water or untreated water cages. The probenecid was dissolved in water containing 5% sucrose at a concentration of 0.5 µg/mL. The water was changed twice a week and the volume was measured before and after the consumption to determine the approximate dose. The mice were housed 2 to a cage for more accurate assessment of the total dose administered.
Probenecid Bolus Followed by Ischemia/Reperfusion
Either saline or probenecid (100mg/kg) were administered intravenously 15 minutes prior to a 45-minute coronary occlusion (ie, at the time of maximal increase in contractility). At 24 hours post-I/R, echocardiography was performed and the hearts were subsequently removed for determination of infarct size via triphenyl tetrazolium chloride (TCC) staining and normalized to the area at risk region.14,16
Statistical Analysis
All data are expressed as means ± standard error of the mean. Results were analyzed with a paired and unpaired Student t test, 1-way analysis of variance (ANOVA), and 2-way ANOVA as needed. P values ≤.05 were considered significant. For all in vivo studies, power analysis was employed (α less than or equal to 0.05 and β greater than or equal to 0.80) to determine the group size necessary to determine whether significant differences exist between end point measures in control versus experimental groups as previously described.14
Results
Probenecid Enhances Ca2+ Flux Without Cytotoxic Effects
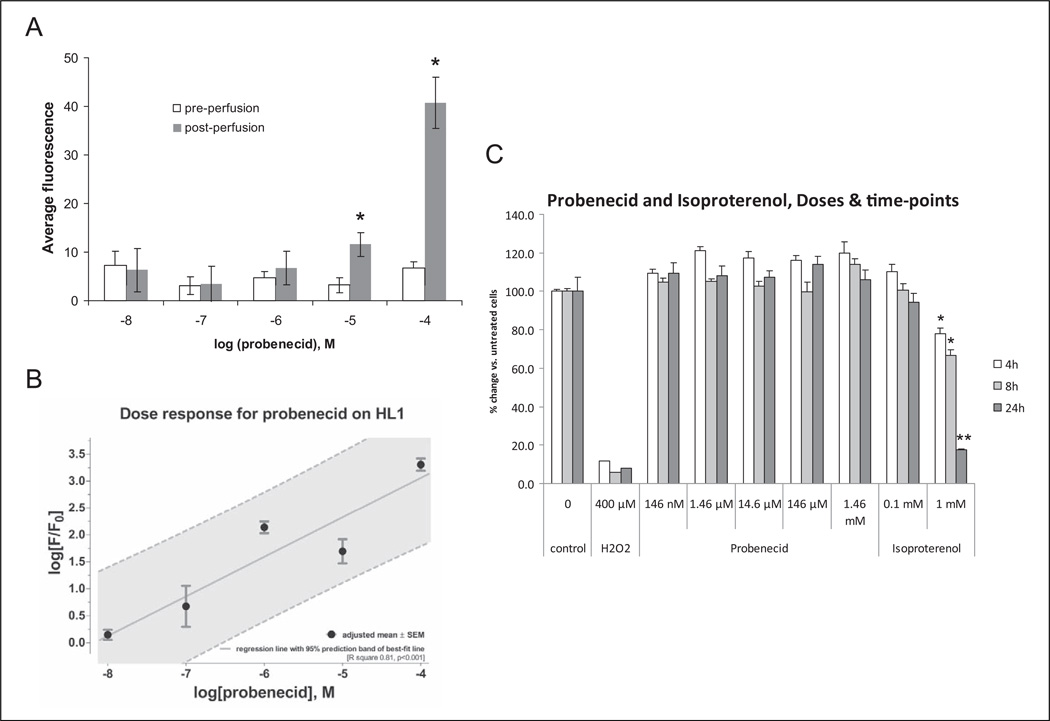
We previously demonstrated that treatment of isolated adult murine cardiomyocytes with probenecid resulted in increased cytosolic Ca2+ concentrations and increased contractility.8 For these experiments, we used HL-1 cells to assay the effect of probenecid. There were no significant differences in the mean measurement of the average cytosolic fluorescence before and after perfusion of probenecid 10−8 to 10−6 mol/L. For the remaining concentrations (10−5 and 10−4 mol/L), the mean fluorescence after the perfusion of probenecid was significantly higher than that before perfusion (P < .05, Figure 1A).
Figure 1.
Probenecid induces increased intracellular calcium concentrations without an increase in cell death in HL-1 cells in vitro. A, Average cystolic fluorescence before and after various doses of probenecid (*P < .01 vs preperfusion). B, Dose–response curve of normalized fluorescence for various doses of probenecid. C, HL-1 cells treated with increasing doses of probenecid (41.7 ng/mL to 417 µg/mL) did not show any detectable cell death for 4, 8, or 24 hours following treatment. In contrast, treatment with 1 mmol/L isoproterenol resulted in significant cell death at just 4 hours and was indistinguishable from hydrogen peroxide (H2O2)-positive controls by 24 hours after treatment. *P < .001 versus untreated controls. **P < .001 versus untreated controls and not significantly different from H2O2 controls.
A log dose–response curve for logarithm of normalized fluorescence (F/F0) with incremental concentrations (10−8-10−4 mol/L) of probenecid showed a linear increase in response with increasing concentrations of probenecid. The R2 value for best-fit regression line of the dose response was 0.81 (P < .001; Figure 1B). There were statistically significant differences in the response with the lowest probenecid concentration 10−8 mol/L versus other higher concentrations (P values ranging between .001 and .01 for these pairwise comparisons).
In addition, treatment of HL-1 cells with increasing doses of probenecid does not result in any detectable cytotoxicity at 4, 8, or 24 hours following treatment (Figure 1C). However, similar treatment with 1.0 mmol/L isoproterenol induced a significant amount of cell death after 4 and 8 hours of treatment (*P < .001) and substantial cell death at 24 hours (**P < .001) where less than 20% of the cells were still viable, which was indistinguishable from the hydrogen peroxide-positive control (Figure 1C).
Probenecid Does Not Cause Significant Myocyte Cell Death In Vivo
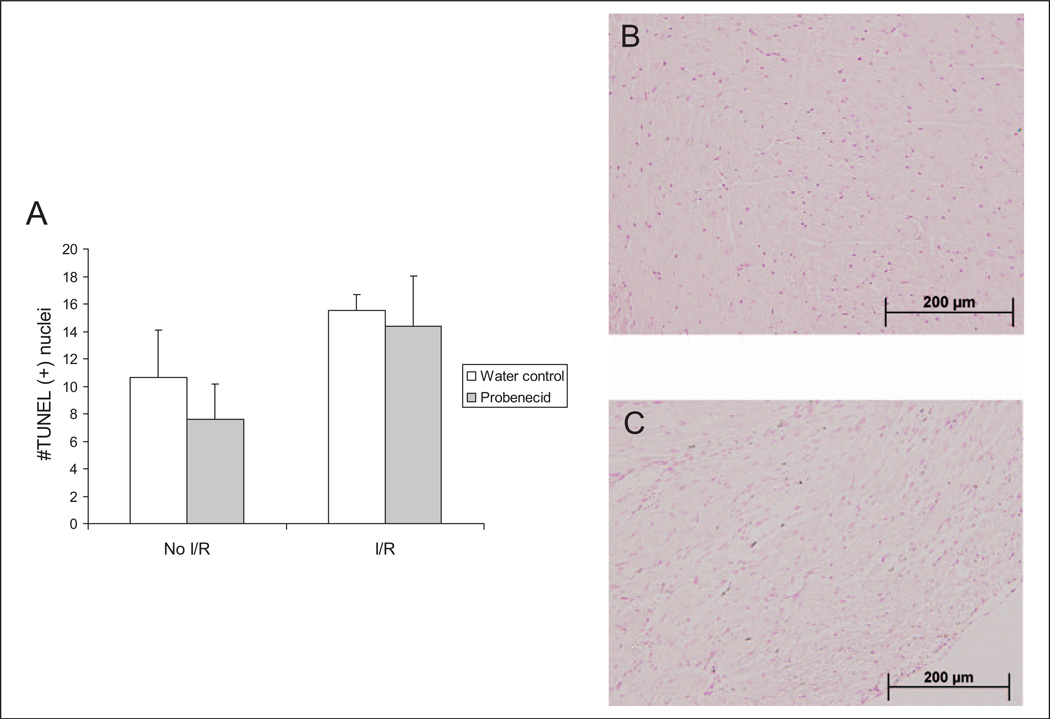
Oral probenecid therapy in healthy mice did not induce significant apoptosis after 2 weeks of treatment, where the average intake of probenecid was estimated to be 190.56 ± 6.33 mg/kg per d based on the water consumption of paired mice per cage. Furthermore, mice subjected to I/R and subsequently randomized to either probenecid-treated water or untreated water did not show significant differences in the amount of cell death between the probenecid treatment and untreated water (Figure 2A). Mice subjected to I/R injury did demonstrate higher levels of apoptosis (Figure 2B) in comparison to non-I/R mice (Figure 2C).
Figure 2.
Terminal deoxynucleotide transferase-mediated dUTP nick end labeling (TUNEL) was used to determine the number of positive apoptotic cells for mouse hearts with and without I/R treated with oral probenecid or water (A). Representative stained images for hearts treated with water without I/R (B) and after I/R (C). dUTP indicates deoxyuridine triphosphate; I/R, infusion/reperfusion.
Bolus Probenecid Improves Function Acutely in Ischemically Damaged Hearts
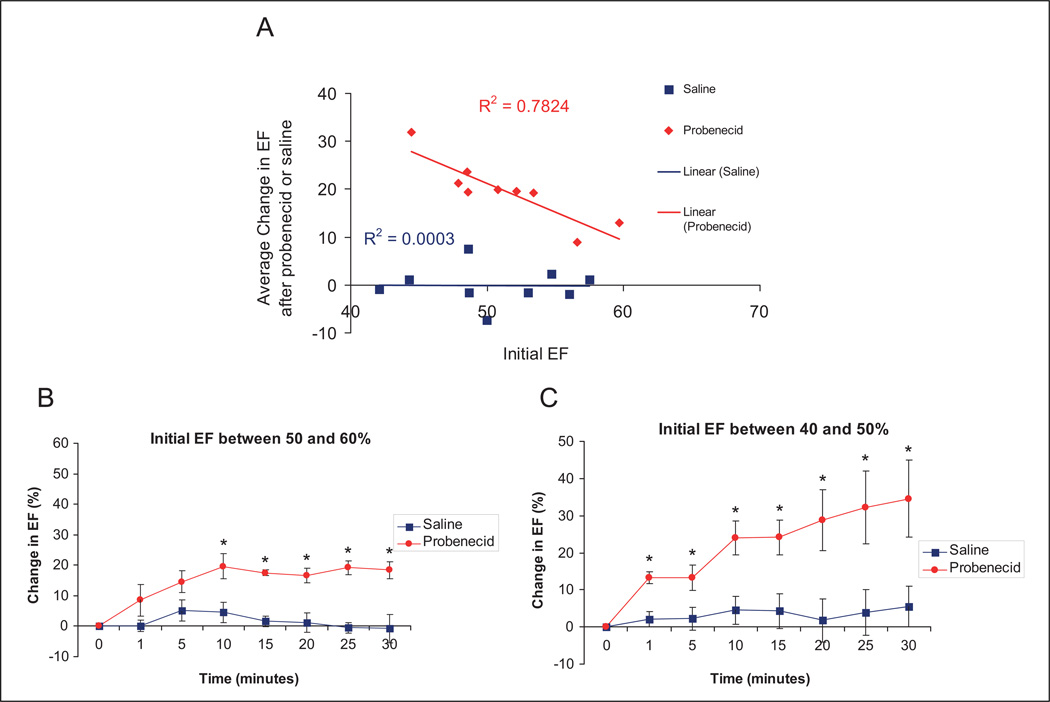
After I/R injury, probenecid administered intraperitoneally caused a statistically significant increase in EF in all treated mice. Interestingly, treatment with probenecid was able to more strongly enhance function in mice that display a lower initial EF, as indicated by the strong correlation between the probenecid-induced increase in EF and initial EF (Figure 3A). This effect occurred in a similar time frame as previously reported, with an initial rise observed at 1 and 5 minutes in both the groups. However, though the peak effect was found at 10 minutes (change in EF was 19.72% ± 4.10%) in mice with a higher initial EFs (50%–60%), probenecid continued to enhance contractility throughout the full 30-minute experiment in mice with lower initial EFs (40%–50%; Figure3B and 3C).
Figure 3.
Average change in EF after intraperitoneal administration of probenecid (100 mg/kg) or saline in mice after I/R injury. The correlation between initial EF (before injection) and the change in EF after probenecid administration (A), and the groups divided by initial EF with 50% to 60% (B) representing minimal damage, and 40% to 50% (C) representing more significant myocardial injury (*P < .05). EF indicates ejected fraction; I/R, infusion/reperfusion.
Oral Probenecid Improves Function After Ischemia and Reperfusion Injury
Twenty-four hours after I/R injury, there was no significant difference in EF or left ventricular diastolic volume (LV Vol;d) between the mice subsequently randomized to water control and probenecid treatment (EF: 43.44 ± 2.05 and 41.81 ± 3.40, respectively, P = .65; LV Vol;d: 66.51 ± 2.65 and 71.99 ± 5.44, respectively, P = .39).
Based on the amount of water consumed, we estimated that the probenecid dose for each control mouse (no I/R) was approximately 4.76 ± 0.16 mg/d, while each I/R probenecid mouse dose was 6.61 ± 0.72 mg/d, which is equivalent to 190.56 ± 6.33 and 264.35 ± 18.64 mg/kg/ d, respectively.
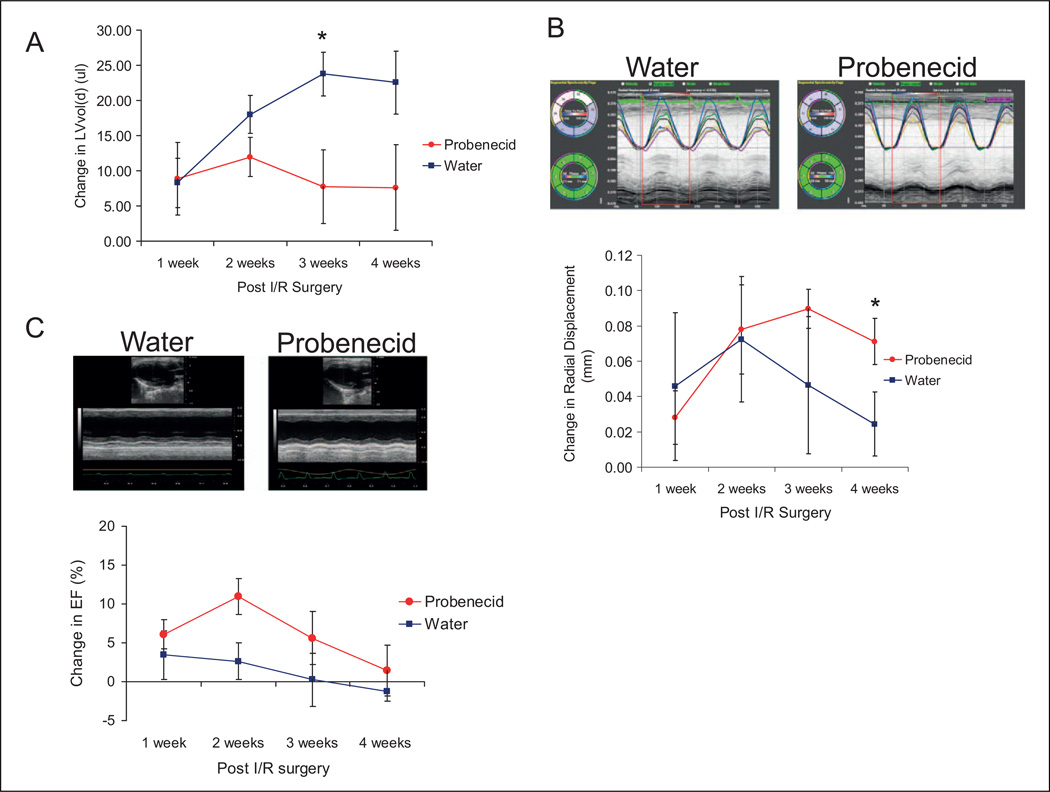
The LV cavity size was minimally increased after 1 week, as determined by the change in LV Vol;d but did not worsen over the course of the experiment in the probenecid group, though it increased further in the sham-treated mice (at 3 weeks, water 23.75 ± 3.07 and probenecid 7.72 ± 5.23, P < .05, Figure 4A). In addition, the water-treated mice had a decrease in radial displacement when compared to the probenecid-treated mice, indicating preserved myocardial function (at 4 weeks, water 0.018 ± 0.018 and probenecid 0.071 ± 0.013, P < .05, Figure 4B). Although the probenecid-treated mice demonstrated a trend toward a higher percentage of EF, there were no significant differences found between water-and probenecid-treated mice (Figure 4C).
Figure 4.
Echocardiogram of water- and probenecid-treated mice after infusion/reperfusion injury. Water-treated mice had an increased left ventricular volume during diastole (LV Vol;d) when compared to mice treated with probenecid (A; *P < .05). The probenecid mice had a higher radial displacement (*P < .05) compared to water-treated mice (B). There was a slight (but not statistically significant) increase in the change in ejected fraction (EF) from baseline with probenecid (C).
Probenecid Does Not Worsen Ischemia and Reperfusion Injury
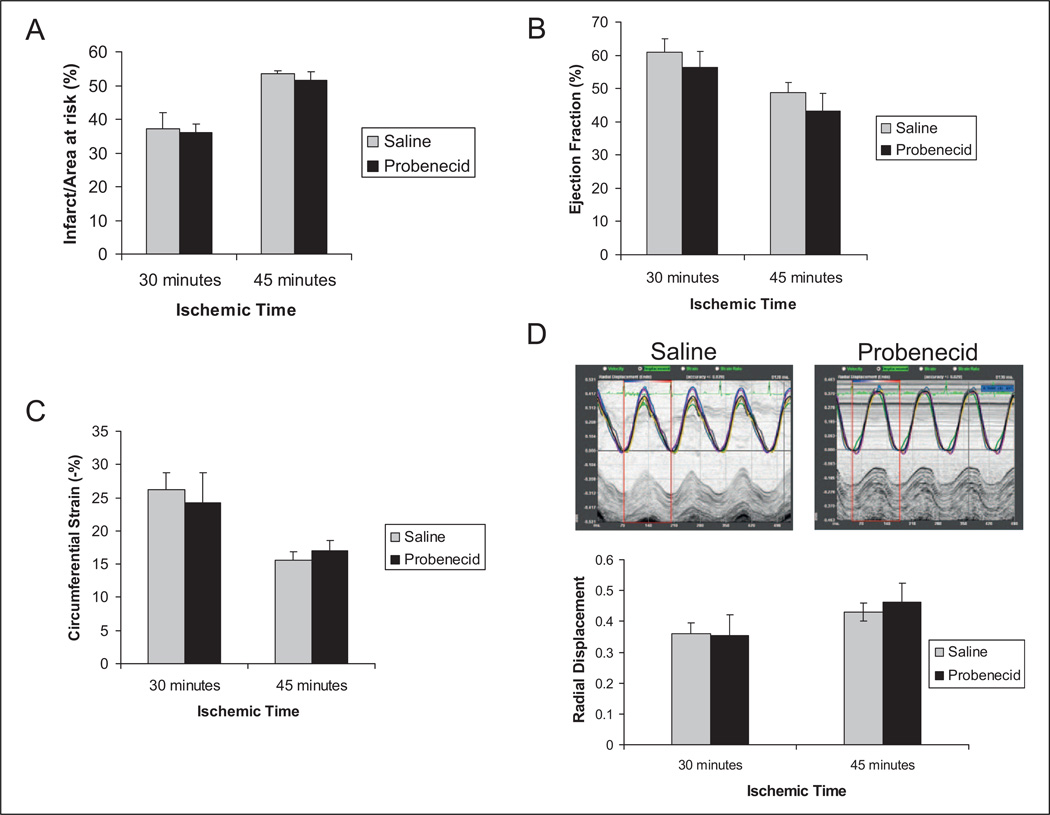
It is well established that adrenergic stimulation results not only in increased myocyte contractility but is also associated with cellular damage and death through several mechanisms including increased metabolic requirements and induction of apoptotic mechanisms.17,18 We sought to evaluate the effects of increased contractility observed with probenecid administration during an ischemic event. The infarct size as measured via histology using the TTC method for staining14,16 was not significantly different in mice pretreated with probenecid in comparison to saline for both the 30- and 45-minute ischemia groups after 24 hours of reperfusion (Figure 5A). Furthermore, the systolic function in these mice (as measured via EF and global circumferential strain) was not significantly different between the saline- and the probenecid-treated groups (Figure5B and 5C). Interestingly, when regional wall motion was determined using strain imaging measuring radial displacement, there was also a significant difference in the function of the anterior wall after 30 minutes when compared to 45 minutes of ischemia, but no difference between the treatment groups (30 minutes, saline 0.43 ± 0.02 mm vs probenecid 0.46 ± 0.06 mm and 45 minutes, saline 0.36 ± 0.03 mm. vs 0.35 ± 0.06 mm, P > .05 between both saline- and probenecid-treated groups; Figure 5D).
Figure 5.
Infusion/reperfusion (IR) with pretreatment with probenecid. The infarct size was higher for 45 minutes of ischemia compared to the 30-minute ischemia; however, there was no difference between control mice and mice pretreated with 200 mg/kg probenecid at either time point (A, P = .41 and .25, respectively). Furthermore, there was no difference between control and pretreated mice at either time point with regard to ejected fraction ([EF] B, P = .25 and .19, respectively), circumferential strain (C, P = .36 and .24, respectively), and radial displacement (D, P = .34 and .46, respectively).
Discussion
We have previously demonstrated that probenecid, an FDA-approved drug, has inotropic properties which went unrecognized for decades.8 Those experiments demonstrated that probenecid increased cytosolic Ca2+ levels resulting in increased contractility independent of β-ADR signaling. The lack of stimulation of the β-ADR pathway initiated the research presented herein, as ADR stimulation has been previously shown to increase myocardial metabolic demand,19 stimulate apoptotic pathways,20 and cause myocyte hypertrophy.17 This has been reflected in numerous clinical trials involving traditional β1-agonists (dobutamine, dopamine)21,22 as well as PDIs (ie, milrinone)23 which have found that even as symptoms and hemodynamic parameters improve, the short- and long-term mortality of the patients exposed to these drugs increases.
Although theoretically interesting, we were still unsure of the potential clinical implications of these findings, as a response in vitro and in healthy mice may not correlate with the effect after ischemic event. Hence, this work addresses 2 critical issues (1) the lack of cell death associated with the improved inotropic response and (2) the positive inotropic effect of probenecid in an ischemically damaged heart. Regarding the former, we calculated the expected serum concentration of both probenecid and isoproterenol under a standard therapeutic regimen and applied the dose to isolated cells and demonstrated a lack of toxicity for probenecid and confirmed the multiple studies that have previously proven cytotoxicity for isoproterenol.6,7
Regarding the latter, we initially used a clinically relevant model of ischemic heart disease in which ischemia is caused for a short period of time and subsequently the vessel is opened.15 This pathophysiology is not dissimilar to the common clinical course of an acute myocardial infarction in which revascularization is accomplished in a short period of time and the subsequent ischemic damage is minor.24 In our model, we were able to detect small decreases in systolic function due to the high-frequency probe used25 which permitted us to dichotomously divide the mice between mild damage (EF of 50%–60%) and moderate damage (EF of 40%–50%). Our finding that probenecid not only increases contractility as previously described but also that the increase is inversely correlated with postischemic EF (the moderately damaged group, EF of 40% to 50%, showed a larger increase in EF) is a crucial finding which lends credence to the translational potential of this drug for use in patients with moderate systolic dysfunction.
The mechanism of action of probenecid in the undamaged/nonischemic myocyte was reported by our group previously,8 and we are currently pursuing the effect of TRPV2 channels as modulators of contractility in response to stressors (such as ischemia). There is some evidence outside of the cardiovascular system which describes the TRPV2 channels as being mostly located in the intracellular compartment of uroepithelial cells,26 pancreatic cells,27 and neuroendocrine cells.28 In these studies, they have been found to be activated and/or to translocate to the cell membrane after stretch stimulation. Although this study did not seek to address the role of TRPV2 in this process, our results demonstrate that the effect of probenecid on the heart is enhanced in the ischemically damaged myocardium. This is consistent with previous studies demonstrating that TRPV2 translocation and activation are stress mediated (ie, via stretch or increased cAMP)29–32 and which may be a compensatory mechanism after injury.33
As a further evidence for the use of probenecid as a potential therapeutic agent in I/R, we also demonstrated that treatment with oral probenecid decreases deterioration of cardiac function (as evidenced by less ventricular dilation and improved systolic function) in comparison to sham-treated mice. The dose required (and self-administered via water) for the effect was about 200 mg/kg administered orally, clearly higher than the commonly used human dose of 500 mg orally twice daily (about 15 mg/kg for a 70 kg patient) and did not cause any noticeable adverse effects. Although this does not imply that such a high dose would be necessary in the treatment of patients with heart failure, it does suggest that very high doses do not appear to pose a substantial risk in an ischemically damaged mouse. Clearly, further studies with very careful dosing in other animal species would be required before we can propose the use of this drug in humans.
The second critical finding of this study was the lack of cellular damage that probenecid caused both in vivo and in vitro. This is a dramatic finding as the vast majority of the clinically available inotropes have been found to increase mortality3,4 and stimulate cardiotoxic pathways5,6 resulting in increased infarct size34 and apoptosis.17,18 However, even high-dose therapy did not result in significant cell death (compared to untreated water) in vivo after 2 weeks of therapy or in vitro at several different time points and at substantially higher concentrations than would be achieved when administered clinically. This finding, if confirmed in other models and species, may be potentially important, as only digoxin can be used as a positive inotrope over prolonged periods of time orally, though it requires tight monitoring as it has a very narrow therapeutic window. This is in stark contrast to probenecid that has a very positive safety profile over decades and as we have shown is safe in a murine model even when administered at very high doses.
Conclusion
In summary, we present the next step in a translational process that began with the interesting finding that probenecid is a positive inotropic drug. We present data that it improves function following I/R injury and does not induce an apoptotic response in vivo or in vitro. These findings, together with our previous work documenting a β-ADR-independent mechanism for probenecid and our ongoing work in larger animals, may have a future impact on how we treat heart failure in humans.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the NIH grants (HL007382, HL091478); University of Cincinnati CCTST grant (T1 UL1RR026314); and the Cardiovascular Center of Excellence at the University of Cincinnati, College of Medicine.
Footnotes
All work was conducted at the University of Cincinnati, Cincinnati, Ohio.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Gheorghiade M, Zannad F, Spoko G, et al. Acute heart failure syndromes. Current state and framework for future research. Circulation. 2005;112(25):3958–3968. doi: 10.1161/CIRCULATIONAHA.105.590091. [DOI] [PubMed] [Google Scholar]
- 2.Goldhaber JI, Hamilron MA. Role of inotropic agents in the treatment of heart failure. Circulation. 2010;121(14):1655–1660. doi: 10.1161/CIRCULATIONAHA.109.899294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abraham WT, Adams KF, Fonarow GS, et al. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE) J Am Coll Cardiol. 2005;46(1):57–64. doi: 10.1016/j.jacc.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 4.Felker GM, O’Connor CM. Inotropic therapy for heart failure: an evidence based approach. Am Heart J. 2001;142(3):393–401. doi: 10.1067/mhj.2001.117606. [DOI] [PubMed] [Google Scholar]
- 5.Burger AJ, Elkayam U, Naibaur MT, et al. Comparison of the occurrence of ventriculary arrhtyhmias in patients with acutely decompensated congestive heart failure receiving dobutamine versus nesiritide therapy. Am J Cardiol. 2001;88(1):35–39. doi: 10.1016/s0002-9149(01)01581-8. [DOI] [PubMed] [Google Scholar]
- 6.Singh K, Xiao L, Remondino A, Sawyer DB, Colucci WS. Adrenergic regulation of cardiac myocyte apoptosis. J Cell Physiol. 2001;189(3):257–265. doi: 10.1002/jcp.10024. [DOI] [PubMed] [Google Scholar]
- 7.Hasenfuss G, Holubarsch C, Blanchard EM, Mulieri LA, Alpert NR, Just HJ. Influence of isoproterenol on myocardial energetics. Experimental and clinical investigations. Basic Res Cardiol. 1989;84(suppl 1):147–155. doi: 10.1007/BF02650354. [DOI] [PubMed] [Google Scholar]
- 8.Koch SE, Gao X, Haar L, et al. Probenecid: novel use as a non-injurious positive inotrope acting via cardiac TRPV2 stimulation. J Mol Cell Cardiol. 2012;53(1):134–144. doi: 10.1016/j.yjmcc.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choudhury MR, Hassan MM, Hakim F, Haq SA. Review on treatment of gout & hyperuricemia. J Bangladesh Coll Phys Surg. 2011;29:85–95. [Google Scholar]
- 10.Robbins N, Koch SE, Tranter M, Rubinstein J. The history and future of probenecid. Cardiovasc Toxicol. 2012;12(1):1–9. doi: 10.1007/s12012-011-9145-8. [DOI] [PubMed] [Google Scholar]
- 11.Butler D. Wartime tactics doubles power of scarce bird-flu drug. Nature. 2005;438(7064):6. doi: 10.1038/438006a. [DOI] [PubMed] [Google Scholar]
- 12.Boger WP, Pitts FW, Gallagher ME. Benemid and carinamide: comparison of effect on para-amino-salicylic acid (PAS) plasma concentrations. J Lab Clin Med. 1950;36(2):276–282. [PubMed] [Google Scholar]
- 13.Bang S, Kim KY, Too S, Lee SH, Hwang SW. Transient receptor potential V2 expressed in sensory neurons is activated by probenecid. Neurosci Lett. 2007;425(2):120–125. doi: 10.1016/j.neulet.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 14.Ren X, Wang Y, Jones WK. TNF-α is required for late ischemic preconditioning but not for remote preconditioning of trauma. J Surg Res. 2004;121(1):120–129. doi: 10.1016/j.jss.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Guo Y, Wu WJ, Qiu Y, Tang XL, Yang Z, Bolli R. Demonstration of an early and a late phase of ischemic preconditioning in mice. Am J Physiol. 1998;275(4pt 2):H1375–H1387. doi: 10.1152/ajpheart.1998.275.4.H1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones WK, Fan GC, Liao S, et al. Peripheral nociception associated with surgical incision elicits remote nonischemic cardioprotection via neurogenic activation of protein kinase C signaling. Circulation. 2009;120(11 suppl):S1–S9. doi: 10.1161/CIRCULATIONAHA.108.843938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shizukuda Y, Buttrick PM, Geenen DL, Borczuk AC, Kitsis RN, Sonneblick EH. β-Adrenergic stimulation causes cardiocyte apoptosis: influence of tachycardia and hypertrophy. Am J Physiol. 1998;275(3 pt 2):H961–H968. doi: 10.1152/ajpheart.1998.275.3.H961. [DOI] [PubMed] [Google Scholar]
- 18.Saito S, Hiroi Y, Zou Y, et al. β-adrenergic pathway induces apoptosis through calcineurin activation in cardiac myocytes. J Biol Chem. 2000;275(44):34528–34533. doi: 10.1074/jbc.M002844200. [DOI] [PubMed] [Google Scholar]
- 19.Clark MG, Patten GS. Adrenergic regulation of glucose metabolism in rat heart. A calcium dependent mechanism mediated by both alpha and beta adrenergic receptors. J Biol Chem. 1984;25(24):15204–15211. [PubMed] [Google Scholar]
- 20.Hu A, Jiao X, Gao E, et al. Chronic β-adrenergic receptor stimulation induces cardiac apoptosis and aggravates myocardial ischemia/ reperfusion injury by provoking inducible nitric-oxide synthase-mediated nitrative stress. J Pharm Exp Ther. 2006;318(2):469–475. doi: 10.1124/jpet.106.102160. [DOI] [PubMed] [Google Scholar]
- 21.O’Connor CM, Gattis WA, Uretsky BF, et al. Continuos intravenous dobutamine is associated with an increased risk of death in patients with advanced heart failure: insights from the FLolan International Randomized Survival Trial (FIRST) Am Heart J. 1999;138(1):78–86. doi: 10.1016/s0002-8703(99)70250-4. [DOI] [PubMed] [Google Scholar]
- 22.Silver MA, Horton DP, Ghali JK, Elkayam U. Effect of nesiritide versus dobutamine on short-term outcomes in the treatment of patients with acutely decompensated heart failure. J Am Coll Cardiol. 2002;39(5):798–803. doi: 10.1016/s0735-1097(01)01818-6. [DOI] [PubMed] [Google Scholar]
- 23.Bayram M, De Luca L, Massie MB, Gheorghiade M. Reassessment of cobutamine, dopamine and milrinone in the management of acute heart failure syndromes. Am J Cardiol. 2005;96(6):47–58. doi: 10.1016/j.amjcard.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 24.Houser SR, Margulies KB, Murphy AM, et al. Animal models of heart failure: a scientific statement from the American Heart Association. Circ Res. 2012;111(1):131–150. doi: 10.1161/RES.0b013e3182582523. [DOI] [PubMed] [Google Scholar]
- 25.Foster FS, Mehi J, Lukacs M, et al. A new 15–50 MHz array-based micro-ultrasound scanner for preclinical imaging. Ultrasound Med Biol. 2009;35(10):1700–1708. doi: 10.1016/j.ultrasmedbio.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Everaerts W, Vriens J, Owsianik G, et al. Functional characterization of transient receptor potential channels in mouse urothelial cells. Am J Physiol Renal Physiol. 2010;298(3):F692–F701. doi: 10.1152/ajprenal.00599.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hisanaga E, Nagasawa M, Ueki K, Kulkarni RN, Mori M, Kojima I. Regulation of calcium-permeable TRPV2 channel by insulin in pancreatic β-Cells. Diabetes. 2009;58(1):174–184. doi: 10.2337/db08-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boels K, Glassmeier G, Herrmann D, et al. The neuropeptide head activator induces activation and translocation of the growth-factor-regulated Ca(2+)-permeable channel GRC. J Cell Sci. 2001;114(pt 20):3599–3606. doi: 10.1242/jcs.114.20.3599. [DOI] [PubMed] [Google Scholar]
- 29.Kanzaki M, Zhang YQ, Mashima H, Li L, Shibata H, Kojima I. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat Cell Biol. 1999;1(3):165–170. doi: 10.1038/11086. [DOI] [PubMed] [Google Scholar]
- 30.Rutter AR, Ma QP, Leveridge M, Bonnert TP. Heteromerization and colocalization of TrpV1 and TrpV2 in mammalian cell lines and rat dorsal root ganglia. Neuroreport. 2005;16(16):1735–1739. doi: 10.1097/01.wnr.0000185958.03841.0f. [DOI] [PubMed] [Google Scholar]
- 31.Penna A, Juvin V, Chemin J, Compan V, Monet M, Rassendren F-A. PI3-kinase promotes TRPV2 activity independently of channel translocation to the plasma membrane. Cell Calcium. 2006;39(6):495–507. doi: 10.1016/j.ceca.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 32.Nagasawa M, Nakagawa Y, Tanaka S, Kojima I. Chemotactic Peptide fMetLeuPhe Induces Translocation of the TRPV2 Channel in Macrophages. J Cell Physiol. 2007;210(3):692–702. doi: 10.1002/jcp.20883. [DOI] [PubMed] [Google Scholar]
- 33.Frederick J, Buck ME, Matson DJ, Contright DN. Increased TRPA1, TRPM8 and TRPV2 expression in dorsal root ganglia by nerve injury. Biochem Biophys Res Commun. 2007;358(4):1058–1064. doi: 10.1016/j.bbrc.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 34.Maroko PR, Kjekshus JK, Sobel BE, et al. Factors influencing infarct size following experimental coronary artery occlusions. Circulation. 1971;43(1):67–82. doi: 10.1161/01.cir.43.1.67. [DOI] [PubMed] [Google Scholar]