Abstract
Psychosis has been recognized as a common feature in neurodegenerative diseases and a core feature of dementia that worsens most clinical courses. It includes hallucinations, delusions including paranoia, aggressive behaviour, apathy and other psychotic phenomena that occur in a wide range of degenerative disorders including Alzheimer’s disease, synucleinopathies (Parkinson’s disease, dementia with Lewy bodies), Huntington’s disease, frontotemporal degenerations, motoneuron and prion diseases. Many of these psychiatric manifestations may be early expressions of cognitive impairment, but often there is a dissociation between psychotic/behavioural symptoms and the rather linear decline in cognitive function, suggesting independent pathophysiological mechanisms. Strictly neuropathological explanations are likely to be insufficient to explain them, and a large group of heterogeneous factors (environmental, neurochemical changes, genetic factors, etc.) may influence their pathogenesis. Clinico-pathological evaluation of behavioural and psychotic symptoms (PS) in the setting of neurodegenerative and dementing disorders presents a significant challenge for modern neurosciences. Recognition and understanding of these manifestations may lead to the development of more effective preventive and therapeutic options that can serve to delay long-term progression of these devastating disorders and improve the patients’ quality of life. A better understanding of the pathophysiology and distinctive pathological features underlying the development of PS in neurodegenerative diseases may provide important insights into psychotic processes in general.
Keywords: neurodegenerative disorders, dementia, psychotic and behavioural symptoms, Alzheimer’s disease, Lewy body disease, frontotemporal dementia, neuroimaging, neuropathology
Introduction
Dementia is an acquired disorder characterized by progressive loss of memory and other cognitive functions that are severe enough to compromise an individual’s daily activities [1]. Until recently clinico-pathological correlation studies focused on cognitive functions and neglected psychiatric symptoms [2]. In Alzheimer’s disease (AD), the progression of cognitive impairment relates to a defined topographical pattern of neurodegenerative changes that first affect the medial aspect of the temporal lobes, i.e. entorhinal cortex and hippocampus, with a gradual extension to isocortical association areas [3, 4]. This hierarchical pathology translates into a chronology of symptoms which first give rise to memory loss then followed by other cognitive dysfunction [5, 6]. It is now recognized that psychotic symptoms (PS) in neurodegenerative diseases arise from specific dysfunctions of brain systems that may or may not be associated with cognitive impairment [7-9].
Psychiatric symptoms in dementing disorders include hallucinations, delusions, restlessness, aggressive behaviour, an argumentative nature and personality changes such as apathy. They show expression profiles that vary according to the underlying condition and thus represent a heterogeneous array of manifestations that punctuate individual differences among affected patients. Their presence in some types of dementia is so pervasive that they are part of consensus criteria for clinical diagnosis, e.g. visual hallucinations (VHs) and sleep disturbances as common features for dementia with Lewy bodies (DLB) [10, 11], behavioural, impulsive and activity disturbances being frequent in frontotemporal dementia (FTD) [12, 13], anxieties and phobias in AD [14] and affective disturbances in vascular dementia [15]. In the absence of severe behavioural symptoms, executive impairment is more characteristic of AD than FTD [16]. Neurobiological studies have reinforced the diagnostic validity of psychosis in AD [5, 7, 8, 14]. The occurrence of VHs predicts postmortem Lewy pathology with 93% accuracy, indicating their high specificity for Lewy body (LB) disorders [17]. Thus, psychiatric symptoms may offer worthwhile clues for the diagnosis of these disorders [18].
In 1996 the term ‘behavioural and psychological symptoms in dementia’ (BPSD) was introduced [19], and panels of experts have developed specific diagnostic instruments for various types of BPSD in AD and other disorders [20, 21], which will increase our knowledge by correlating them with neuroimaging, neurochemical and neuropathological changes. Recent studies have provided models for BPSD clusters which include psychotic, affective and behavioural elements [22]. The significance of these studies is demonstrated by the fact that some of these symptoms may respond to the same drugs [23]. Although AD patients display a high variability in the evolutionary course of BPSD, groups of patients with similar frequency and severity of these symptoms may be identified [22]. Untreated psychiatric manifestations in patients with dementia have a tremendous impact on the patient, family and caregiver. They lead to greater rates of institutionalization, worse outcome and increased mortality [24-28]. Therefore, psychotic conditions are an important source of comorbidity in patients with dementia.
The goal of this article is to review the current state of neuroimaging and neuropathological correlates to BPSD, in particular PSs in the most frequent neurodegenerative diseases. The cerebral correlates of effective and behavioural disorders in dementia will be reviewed elsewhere [29]. The multiplicity of causes, influence of premorbid personality, the overlap of various lesions and sparse literature limits this review to clinico-pathological correlates for PSs. One should bear in mind that clinico-pathological studies often suffer from limitations and conclusions should therefore be considered with caution.
Old age and Alzheimer’s disease
Prevalence estimates (Table 1)
Table 1.
Epidemiology of psychotic symptoms in Alzheimer’s and Lewy body diseases
| % | Author | |
|---|---|---|
| Dementia—prevalence | ||
| Delusions | 60 | [30] |
| Agitation | 42 | |
| Aggression | 33 | |
| Hallucination | 20 | |
| AD—prevalence | ||
| Initial presentation | 64 | [31] |
| Total | 88–90 | [32, 33] |
| Average | 28–38 | [34] |
| Total | 50–80 | [35] |
| Delusions | 36 | [27] |
| Hallucinations | 18 | |
| Delusions + hallucinations | 13 | |
| —” —, persistent | 40–50 | [36] |
| PD—prevalence | ||
| Hallucinations | 42 | [37] |
| Delusions | 21 | |
| Minor symptoms | 45 | |
| VHs lifetime prevalence | ∼50 | [38] |
| PD—overall incidence | 30.8 (range 22–65) | [39] |
| PD—VHs prevalence | 15.8–50 | [40] |
| PDD—overall incidence | 45–68 | [41, 42] |
| Hallucinations | 45–79 | [43] |
| Delusions | 25–30 | [41, 42, 44] |
| DLB—prevalence | ||
| Hallucinations | 13–92 | [45, 46] |
| Hallucinations mean | 63–78 | |
| Misidentification | 56 | |
| Paranoid delusions | 25–28.6 | [47, 48] |
| Paranoid delusions | 49 | [49] |
PD: Parkinson disease; PDD: Parkinson disease dementia; DLB: dementia with Lewy bodies; VHs: visual hallucinations.
Psychiatric symptoms in old age are frequent and have been observed in 43% of non-demented and 58% of demented persons [50]. Others reported a higher prevalence of PSs in elderly people with dementia than in those without, suggesting that dementia with psychosis is not a reflection of cognitive impairment associated with aging but rather a distinct syndrome that warrants specific treatment [51]. In fact, psychosis was the presenting symptom followed by rapid memory loss in the original case described by Alzheimer [52]. A study of psychiatric symptoms in AD found a prevalence rate of 64% at initial evaluation [31], which may precede diagnosis by up to 3 years [53]. The prevalence of psychosis in AD patients increases from the earliest stages of cognitive impairment to reach a maximum value by the middle stages of illness [24]. Sleep disorders and hallucinations, on the other hand, tend to occur in later stages [54]. Among 226 demented patients (174 with postmortem examination), 27% had transient agitation and delirant confusion, 18.9% predominant confusion and 9.2% paranoid symptoms [55]. Conservative estimates of the prevalence of psychosis in AD have ranged from 10% to 73%, with an average range of 28% to 38% [34], an overall mean of 24% in clinic populations and 7–20% in community and clinical trial populations, depending on definition. Others have claimed 88% to over 90% [32, 56]. In longitudinal studies, PSs have been reported to emerge in approximately half of the patients with clinically diagnosed or histologically confirmed AD, with frequencies of hallucinations between 15% and 30% [57] and delusional misidentifications between 20% and 30% [27, 58].
In nursing home patients two thirds have persistent symptoms over 12 weeks. Among AD outpatients hallucinations and delusions may persist in 40–50% over periods of 3 months up to 1 year [36]. Analysis of frontal lobe features in AD showed associations not only with more pronounced cognitive deficits but also with increased severity of PSs [59]. Others reported frequencies of delusions in AD (range 16–70%, median 36.5%), hallucinations (range 4–76%, median 23%) [60] and of VHs, ranging from 25% to 83% [58, 61, 62]. A review evaluating 55 studies with 9749 patients showed a median prevalence of AD + psychosis (AD + P) of 41% (range 12.2–74.1%), of delusions of 36% (range 9.3–63%) and other PSs, e.g. misidentification delusions of 25% (range 3.6–38.9%), hallucinations (median 18%, range 4–41%), with VHs (median 18.7%) being more frequent than auditory hallucinations (median 9.2.%), both hallucinations and delusions (median 13%, range 7.8–20%) [27]. In a population-based sample of 85-year-old demented individuals, the prevalence of PSs was 36% among AD patients compared to 54% in vascular dementia cases (P < 0.04); the proportion with PSs increased with increasing dementia severity in AD [63]. In a sample of 95 year olds in Goteborg, Sweden, 1 year prevalence of any PS in AD was 7.4% [64]. In a study of 56 autopsy-confirmed AD cases, 23% had hallucinations (18% visual, 13% auditory), 16% paranoid delusions and 24% delusional misidentifications, but there was no relationship between the presence of psychosis and differences of cognitive performance or the state of illness [65]. However, recent studies supported the relationship between greater cognitive impairment and AD + P [66, 67].
According to the International Psychogeriatric Association [30], the most common psychiatric manifestations in dementia, without specifying the underlying aetiology, appear to be delusions (60%), affective symptoms (40%), anxiety (35%), verbal outbursts (33%) and hallucinations (20%). Apathy and agitation (41% and 42%) were most common in severe dementia; others reported apathy was the most common psychiatric symptom with almost 65% in AD [25, 68]. Variability within prevalence estimates for any given neuropsychiatric symptom depends on the type of populations (outpatients versus nursing home patients, cross-sectional versus longitudinal), the diagnostic criteria used and the severity/stage of dementia.
Clinical manifestations
Psychosis occurs in a subset of AD patients (AD + P) during progression of the disease in whom it is associated with a more aggressive cognitive deterioration and worse outcome [84-86].
Clinical studies have identified AD + P as a distinct subtype of AD [14, 79]. There is growing evidence that AD + P aggregates within families and may result, in part, from effects of genetic variations and risk factors [21, 35, 87, 88], and studies revealed that the heritability of psychosis in late-onset AD doubled from 30% to 60% by including only patients with multiple and/or recurrent PSs [89].
Although no definite association with the apo-lipoprotein E (ApoE) 4 allele has been found [79, 90], some studies have suggested relations to putative schizophrenia risk genes, e.g. neuroregulin-1 [91], but in recent times no association between neuroregulin-1, involved in neurodevelopment and in schizophrenia risk [91], and PSs in AD has been found [92]. Several studies showed linkage of chromosomes 2p, 15 and 7, and 8p, respectively, with AD + P [88, 89, 91], whereas one linkage analysis implicated the region of chromosome 14 near the locus of presenilin-1 in AD patients without hallucinations [93]. Previous findings indicating relations to serotonin receptors/transporters and dopamine-3 receptor were not reproduced [68, 94-96], whereas serotonin system genes [97], dopaminergic receptors [98, 99], genetic variations of catechol-O-methyltransferase [100] and of neurotrophins, such as brain-derived neurotrophic factor [35] may increase the risk of psychosis in AD. Recent studies found no association of AD + P with neurodegenerative pathway genes (β-secretase 1, microtubule-associated protein τ, amyloid precursor protein, sortilin-related receptor, etc.) [101]. Although it is evident that AD + P is a distinct subtype with a frequent genetic base, the inconsistent findings of genetic association studies indicate that many challenges still face elucidation of genetics behind this syndrome [102].
Neuroimaging
PS are produced by distributed neuronal dysfunction. Neuroimaging investigations found interesting changes in AD + P patients. Single photon emission tomography (SPECT) studies showed a pattern of cerebral blood flow (CBF) deficits significantly different from that in non-psychotic patients. The latter had hypoperfusion of the left frontal lobe, those with hallucinations in the parietal lobe [103]. Others reported significant correlation between psychotic factor and metabolism in the frontal lobe, and between agitation/inhibition score and metabolism in the frontal and temporal lobes [104]. AD patients with visuospatial deficits revealed severe glucose deficits in parietal and occipital visual cortical areas [105], AD + P cases significantly lower regional perfusion in bilateral dorsolateral frontal, left anterior cingulate and left ventral striatal regions along with the left pulvinar and dorsolateral parietal cortex in comparison to a non-psychotic group [106].
Perfusion positron emission tomography (PET) studies in female AD + P subjects showed lower perfusion in right infero-lateral frontal cortex and inferior temporal regions compared to females without such symptoms, whereas in male AD + P patients, perfusion was higher in the right striatum compared to those without such symptoms, indicating gender differences in regional perfusion [107]. Aggressive AD patients compared with non-aggressive ones displayed significantly more severe hypoperfusion of the right middle temporal region, suggesting it as an important neuronal correlate of aggression [108]. Comparison of an AD group with delusions with a non-delusional one in the first group found significant flow reductions in the prefrontal, anterior cingulate, inferior and middle temporal, and parietal cortices of the right hemisphere [109], whereas an AD group with autobiographic delusions had significant hypoperfusion in the right frontal lobe including Brodman area 9 and 10, suggesting that a focal functional damage was liable for the content-specific delusions [110, 111].
Structural imaging revealed increase in frontal and temporal asymmetry in AD + P [112]. Volumetric magnetic resonance imaging (MRI) studies showed an association between VHs and decreased occipital-to-whole brain ratio [113]. Voxel-based morphometry from T(1) weighted MRI revealed association of delusions with decreased grey matter (GM) density in the left frontal lobe, in the right frontoparietal cortex and left claustrum; apathy with GM density loss in the anterior cingulate and frontal lobe bilaterally, the head of left caudate nucleus and bilateral putamen, whereas agitation was associated with decreased GM values in the left insula and anterior cingulate bilaterally. These data indicated that psychiatric symptoms in AD seem to associate with neurodegeneration affecting specific neural networks [114].
In amnestic mild cognitive impairment patients, delusions were associated with GM volume reduction in the right hippocampus; disinhibition was strongly associated with GM volume in bilateral cingulate and right middle frontal gyrus [115]. The authors suggested that PSs represent clinical features of neurodegenerative changes in AD. MRI showed white matter lesions (WML) in the bilateral frontal or parieto-occipital region and left basal ganglia significantly correlating with the score of Psychotic Symptoms Subscale of Behavioural Rating Scale for Dementia. However, WMLs were only associated with delusional misinterpretation but not with hallucinations and paranoid delusions. These findings suggested that white matter changes in AD patients, especially located in frontal and parieto-occipital regions may contribute to the development of a specific type of PSs, namely delusional misidentification, whereas other neuropsychiatric symptoms seemed not to be related with WMLs [116]. The ratio of MRI measured occipital volumes to whole brain volumes in AD patients with VHs was significantly smaller than in those without them, suggesting that VHs in AD may be associated with atrophy of the occipital lobe [113]. A small MRI study of five AD patients each with and without VHs showed a higher occipital periventricular hyperintensity score in those with VHs, whereas the occipital deep white matter hyperintensity score was zero in both groups, implying that structural lesions in the geniculocalcarine region and preserved subcortical connections with visual association areas are involved in the genesis of VHs in AD [117]. Older magnetic resonance spectroscopy studies showed significant elevation of glycerophosphoethanolamine and reduction of N-acetyl-L-aspartate in prefrontal, superior temporal and inferior parietal cortex of postmortem brains of AD + P patients, indicating impairment of neocortical neuronal and synaptic integrity as the structural substrate of AD + P [118].
Neuropathology
Early studies on neuropathological and neurochemical correlates of psychosis in 48% of autopsy-confirmed cases of AD showed significantly increased densities of senile plaques and neurofibrillary tangles in the prosubiculum and middle frontal cortex, respectively, with trends towards increased densities of these lesions in other cortical areas (entorhinal and superior temporal cortex). These findings were consistent with the increased rate of cognitive decline that accompanies behavioural and psychotic disorders. Psychosis was also associated with relative preservation of norepinephrine (NE) in substantia nigra, with trends in this direction for several other subcortical regions (thalamus, amygdala and caudate nucleus), and significant reduction of serotonin in prosubiculum, and trends towards reduced levels of serotonin and 5-hydroxyindole acetic acid in other regions. The NE metabolite 3-methoxy-4-hydroxyphenylglycol showed mild but insignificant increase in cerebral cortex and caudate nucleus. In contrast, psychosis was not associated with significant changes in dopamine, its metabolites homovanillic acid and 3,4-dihydroxy phenylacetic acid, or choline acetyltransferase activities in any of the brain regions studied. The profile of neuropathological and neurochemical changes in AD + P was distinct from that reported for major depression in primary dementia, and no effects of neuroleptic treatment on biogenic amine concentrations were detected [119] (Fig. 1).
Fig 1.
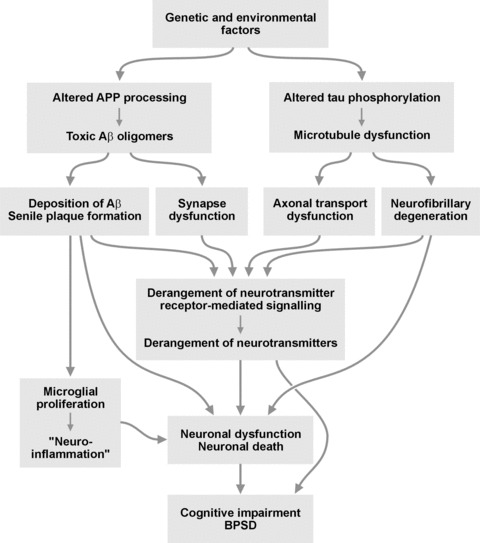
scheme of the interplay of neurochemical and neuropathological factors in the pathogenesis of AD and its neuropsychiatric disorders.
Other studies suggested that imbalance of different neurotransmission systems and involvement of specific brain regions are responsible for BPSD (parahippocampal cortex, locus ceruleus and dorsal raphe [120]). Although cholinergic cell loss is an important attribute of the pathological process underlying BPSD, including psychosis, aggression and depression, an early feature in AD pathology is degeneration of the locus ceruleus which serves as the main source of NE supply of cortical and subcortical areas [121].
Other studies have described a relationship between prominent hallucinations and the presence of LBs in cortex and brainstem [122-124], or between delusions and basal ganglia mineralization [69, 70, 125].
In a prospective clinico-pathological study of 56 patients with autopsy-confirmed AD (23% with hallucinations, 16% with paranoid delusions and 25% with delusional misidentification), the subgroup with auditory hallucinations and paranoid delusions showed less severe neuron loss in the parahippocampal gyrus and non-significantly lower neuron numbers in the serotonergic dorsal raphe nucleus than patients without those symptoms, but no differences in the locus ceruleus (these were associated with depression). Delusional misidentifications were seen in patients with lower neuron counts in the hippocampal area cornu ammonis (CA)-1. Apart from a higher parahippocampal tangle count in AD patients with delusions, no significant differences of plaque and tangle counts between AD patients with and without psychotic phenomena, and no significant differences in substantia nigra, frontal and parietal lobes in AD + P patients were found [65]. These authors found no evidence for a relationship between LBs and PSs, whereas basal ganglia mineralization was observed in a subset of five patients with a high rate of psychotic disturbances [7]. In a family with idiopathic basal ganglia calcifications, clinically presenting parkinsonism, cognitive deterioration and schizophrenia-like psychoses, (18)FDG uptake was significantly reduced in striatal or cortical areas, including the precuneus and posterior cingulate gyrus [126]. The reduction of pyramidal cell neurons in hippocampal area CA-1 of AD patients with delusional misidentifications appeared in good agreement with their previously suggested association with a lesion in the mediobasal temporal lobe [127]. A recent semi-quantitative study of neuropathological changes in postmortem hippocampus revealed that an increased tangle load, but no other hippocampal morphologic variables, were associated with increased severity of aggressive behaviours and presence of chronic aggression, suggesting a pathogenic link between tangle load and aggressive behaviour in the hippocampus of dementia patients [128]. Another recent study in AD patients with a history of major depression showed higher tangle and plaque counts in hippocampus and more rapid cognitive decline than in similar demented patients without depression [129]. Apathy in AD is related to disruption of transmission of decision value signals from orbitofrontal cortex to nucleus accumbens and midbrain dopaminergic ascending pathways and of frontostriatal circuits [130].
Lewy body diseases
Clinical features and prevalence (see Table 1)
Psychotic symptoms are frequent and disabling in patients with LB diseases-DLB and Parkinson’s disease (PD) [41]. They share a similar profile in both disorders, including complex hallucinations (i.e. false sensory perception) and delusions (i.e. fixed false beliefs) of a paranoid type that are less frequent [131]. In PD, hallucinations were already observed in the prelevodopa era, and for early authors, such as Gowers and Charcot, hallucinations that occurred in the course of PD either accompanied the final phase of the disease or reflected comorbidities, and disease duration is one of the risk factors for their development [132-134]. International consensus criteria have been proposed [37], as well as a critical review of the available rating scales [134]. Psychiatric symptoms in early untreated PD included 17% apathy, whereas 30% had at least one such symptom [41]. The average overall incidence of PSs is 30.8%, ranging from 22% to 65% [39]. Reported prevalence of hallucinations and psychosis in PD is up to 48% and 80%, respectively. PSs generally develop 10 or more years after the onset of PD [37, 132]. Their prevalence in PD with dementia (PDD) is markedly higher than in non-demented PD patients [44, 135], ranging from 45% to 68% [41, 42], while chronic disorientation frequently occurs in PDD patients. Assessment of 116 PD patients for the presence of PSs using the recent new criteria for psychosis associated with PD [37] revealed hallucinations in 42% (visual 16%, non-visual 35%), delusions in 21% and minor symptoms in 45%. The prevalence of psychosis associated with PD was 43% when the usual definitions were applied, and 60% when the new National Institute of Mental Health/National Institute of Neurological Disorders criteria were used [136]. Most frequent are VHs, that occur in about 25–44% of PD patients, auditory hallucinations in up to 20% [38, 137], whereas tactile [138], gustatory [137, 139], auditory [140] and olfactory [139, 141] hallucinations are less frequent. VHs are generally complex, well formed and kinematic [137, 142, 143]; preserved or disturbed insight on the nature of the hallucinations is a major prognostic factor, although eventually all hallucinations will present with reduced insight [144]. VHs in PD are facilitated by impaired colour and contrast discrimination, and may be associated with visual cognitive impairment attributable to PDD and DLB pathology [145]. Impaired visual processing precedes image recognition in PD patients with VHs [146]. Other phenomena, e.g. sense of presence and VHs affect 17–72% and delusions about 5%.
Delusions are less common than hallucinations, and typically co-occur with hallucinations [43]. Most frequent forms are ‘phantom boarder’ phenomenon (the belief that a stranger is living in the patient’s home), feeling of presence and delusions of infidelity. Imposter phenomenon is rare but can occur. Prevalence rates for delusions are reported to be 17% in PD patients overall and 25–30% in PDD patients [41, 42]. Studies investigating the prevalence of hallucinations and delusions in PD patients yielded a large difference range from 3% to 60% and for the incidence of hallucinations from 16% to 37% [147]. Prevalence rates for delusions were 17% in PD patients overall and 25–30% in PDD patients [42]. In a community based study that used the neuropsychiatric inventory (NPI) to identify neuropsychiatric symptoms in 100 PD patients with (43%) and without (57%) dementia, five NPI clusters were identified. The clusters with the highest representation of PDD patients were a group characterized primarily by hallucinations (79% PDD) and a group with high scores on several NPI items (57% PDD) [44].
Psychotic symptoms are frequent during night and associated with sleep disturbances, vivid dreams and sleep-related phenomena may lead to hallucinations [144]. They are often related to autonomic impairment or mechanisms underlying motor fluctuations [148] and other non-psychotic psychiatric disturbances, especially affective symptoms, are frequent comorbidities [149]. Olfactory impairment early in the disease course may be a useful marker for the risk of psychotic complications in PD. Lifetime prevalence of VHs ranges between 50 and 74% [17]. They persist and worsen, their prevalence increases with time [38]. A total of 30–40% of PD patients show fluctuating PSs [150], but, once present, they tend to persist and progress. Psychosis is a key feature to nursing home placement [151], and PSs are more common in patients living in nursing homes compared with home dwelling patients. They correlate with stage of the disease and cognitive impairment, but not with age or duration of disease [41, 152]. Longitudinal studies show a prevalence of hallucinations of 16–17% in population-based surveys and 30–40% in hospital-based series [153]. In a 10 year prospective longitudinal study of 89 patients, hallucination prevalence and severity increased significantly with time. They were highly associated with concurrent vivid dreams/nightmares [154].
Dementia is the most robust risk factor for the onset of hallucinations in PD, and hallucinations again are a possible risk factor for dementia [39, 155]. PD patients with hallucinations but without dementia have affection of executive functions [156].
In a 12 year population-based study of 230 PD patients, 60% developed hallucinations or delusions. Their incidence rate was 79.7/1000 patient/years, increasing with higher age at disease onset [157]. In a longitudinal study of PD patients, 95% had hallucinations and 60% had paranoia; psychosis was persistent in 69% and dementia was diagnosed in 68%. Persistent psychosis was associated with younger age at onset of PD and longer disease duration; paranoia with more frequent nursing home placement. A total of 28% of nursing home patients died within 2 years, paranoia being associated with a poorer prognosis [158]. A recent population-based long-term study demonstrated that, in addition to age at onset, chronological age, motor severity, dementia, PSs independently predict increased mortality in PD. Early prevention of motor progression and development of both dementia and psychosis may be the most prominent strategies to increase life expectancy in PD [159].
In DLB—both ‘common’ and ‘mixed’ forms (DLB + AD, LB variant of DLB/LBV/AD)—PSs are extremely common, predominantly VHs, misidentifications, delusions and other syndromes [160]. Although delusions and misinterpretation syndromes have a similar phenomenology in both DLB and PDD patients, they may be more prevalent in DLB, possibly due to morphological and neurochemical differences between both disorders [160]. Hallucinations are the most frequent PSs, with a prevalence range between 13% [46] and 92% [58] and means of 63–78%, followed by misidentifications [56%] and paranoid delusions (25–28.6%) [47, 48], whereas another group reported PS in 50% of the DLB patients, showing no association with the degree of motor disability [161]. VHs are generally present early in the course of illness with characteristics as described in the original report [10]. They are significantly more frequent in common DLB than in LBV/AD [162-164]. VHs are the presenting feature in 33–65% of DLB versus 8–15% in AD [45]. Unlike AD, 18% of common DLB and 14% of LBV/AD present with VHs prior to the dementia onset. Recurrent, complex VHs are one of most useful signposts to a clinical diagnosis of DLB and, together with visuospatial/constructional dysfunctions are a validated diagnostic criterion for DLB [165], and differentiate it in the earliest states from AD [164, 166]. VHs tend to persist; e.g. hallucinations were stable in a group over 20 weeks [165], and in a cohort over 52 weeks [167]. They are similar to those in PD in that they are vivid, complex, colourful, three dimensional and generally mute images of animals, less frequent of other objects [42, 165, 168]. Patients with DLB have more frequent VHs than those with AD [169], showing more prominent visuospatial dysfunction compared to those without VHs [170, 171].
In DLB, most frequent are delusional misidentifications, followed by paranoid beliefs, Capgras syndrome (belief that an acquaintance has been replaced by an identical looking impostor), phantom syndrome and reduplication of people and places [42, 162]. A meta-analysis calculated the mean prevalence of delusions in DLB at 49% compared to 31% in AD [49]. The persistence of delusions is similar in DLB and AD [167]. VHs and delusions are usually independent of each other, and the relationship between hallucinations/delusions and cognition is obscure [45]. In the population-based Vantaa 85+ study, among 109 patients with autopsy-proven LBD, 48% were reported to have VHs associated with dementia [172].
Neuroimaging
(18)-F-FDG PET studies in PD patients with compared to those without VHs revealed reduction in the regional cerebral glucose consumption in the occipito-temporo-parietal regions, the ventral and dorsal visual streams (P < 0.05). Sparing the occipital pole indicated functional disorders of visual association areas in higher-order visual processing [173]. These data extended previous and partial contradictory SPECT and PET studies showing decreased rCBF in left temporal cortex in PD with VHs [173], greater relative glucose metabolism rates in the frontal areas, in particular the left superior frontal gyrus and in posterior cortical areas [175] or hypoperfusion in the right fusiform gyrus and hyperperfusion in the right superior and middle temporal gyri in non-psychotic PD patients with VHs [176]. Most other studies in PD and DLB reported stronger functional involvement of posterior brain regions than in AD, including the primary visual cortex and the visual pathways [34], whereas reductions in all cortical areas were reported in PDD [177].
Tc-Hexamethylpropyleneamine Oxime SPECT studies in DLB patients with hallucinations and fluctuations in consciousness showed a significant correlation between increased perfusion in midline posterior cingulate and decrease in hallucination severity, and between increased fluctuations of consciousness and increased thalamic and decreased inferior occipital perfusion [178]. There was reduced occipital uptake in areas identified as primary and secondary visual cortex [61, 179-183]. Hallucinators showed greater right posterior and parietal glucose uptake than non-hallucinators [184]. 18-F-FDG PET studies in DLB patients with VHs revealed hypometabolic regions at the right occipito-temporal junction and in the right middle frontal gyrus, suggesting hypometabolism in visual association areas rather than in the primary visual cortex [48], whereas delusions were associated with hypometabolism of the right prefrontal cortex. Although there was a significant correlation between the frequency and severity of delusions and other clinical variables such as VHs, the Mini Mental State Examination (MMSE) score and the regional glucose uptake of the right prefrontal cortex, only the regional metabolic alteration was a significant predictor of delusion symptomatology in this DLB group [48]. Functional MRI during visual stimulation showed hypometabolism in occipital and parietal cortex and hyperactivity in prefrontal regions [185]. SPECT studies in DLB patients revealed that factor 1 symptoms (Capgras syndrome, etc.) were significantly related to hypoperfusion in the left hippocampus, insula, ventral striatum and bilateral inferior gyri; factor 3 symptoms (VHs) were related to hypoperfusion in the left ventral occipital gyrus and bilateral parietal areas, whereas delusions were associated with hypoperfusion in the right rostral medial frontal cortex, left medial superior frontal gyrus and bilateral dorsolateral frontal cortices. These data suggest that VHs are related to dysfunction of the parietal and occipital association cortices, misidentifications to dysfunction of the limbic-paralimbic structures and delusions to dysfunctions of the frontal cortex [186]. Despite the relative heterogeneity of previous reports [178, 184], which can partly be explained by differences in patient characteristics and imaging modalities, more recent studies imply that dysfunction of extrastriate downstream visual association areas, rather than the primary visual cortex, are involved in the occurrence of VHs [48, 186]. They consistently indicate an alteration of the ventral visual pathways along with disordered cortical inhibition (Fig. 2).
Fig 2.
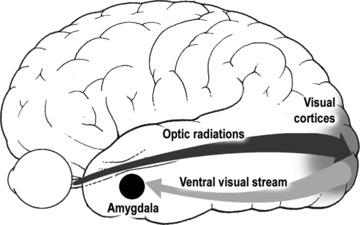
Diagram of the major visual pathways implicated pathologically in VH in PD.
Volumetric studies in DLB showed no occipital volume differences between hallucinators and non-hallucinators [187], whereas voxel-based morphometry in subjects with VHs revealed greater GM loss than non-hallucinators, specifically in the right inferior frontal gyrus (BA 45) in DLB patients, and in the orbitofrontal lobe (BA 10) in PDD patients. Decreased volume in association visual areas, namely left precuneus and inferior frontal lobe, correlated with VHs in DLB but not in PDD patients. In conclusion, DLB and PDD patients with VHs had more frontal GM atrophy than non-hallucinators, the impairment being greater in the DLB group [188]. Other MRI studies showed that PD + VH patients had significant hippocampal cell loss compared to controls [188]; they frequently develop dementia and show a widespread atrophy, involving limbic, paralimbic and neocortical structures [190].
Changes in DLB associated with psychosis include lowered choline acetyltransferase activity and increased muscarinic receptor binding (types 2 and 4) [191].
Neuropathology
Some important associations between histopathology and PSs have been described in PD and DLB. Psychopathology including VHs and dementia corresponds to LB distribution in the limbic system [192, 193], the latter being significantly associated with α-synuclein (αSyn) deposition in anterior cingulate gyrus, entorhinal cortex, amygdaloid complex and nucleus basalis of Meynert as well as τ in the CA-2 sector of hippocampus. On the other hand, αSyn burden in the amygdala is strongly related to the presence of VHs, but only in those PD cases with concomitant dementia [194]. VHs in DLB relate to amygdala and parahippocampal LB density and, when present early in the course of disease, to parahippocampal and inferior temporal cortical LB density [61, 195]. Cases with DLB had higher LB densities in the inferior temporal cortex than cases with PDD. Patients with VHs had nearly double the density of LBs in the basolateral nucleus compared to non-hallucinators, however, without differences in neuronal loss, which suggests neuronal dysfunction rather than amygdala’s ability to integrate coordinated behavioural responses between the two visual systems [61].
Alzheimer-related pathologies, including plaques and tangles, and ApoE status, do not appear related to psychosis in PD [196, 197]. Although there was no association across groups between any neuropathological variable and the presence or absence of fluctuating cognition, there was a striking association between the distribution of temporal lobe LBs and well-formed VHs. These data confirmed previous ones showing a strong correlation between cortical LBs with early onset VHs, their persistence and severity [124, 198], whereas others found no significant differences in LB density in any brain region among patients with and without VHs and delusions [199]. A recent study of 129 cases of pathologically proven PD showed that patients with VHs had significantly higher cortical LB scores (7.7) than those without VHs (6.6; P = 0.02) [200]. Cases with well-formed VHs had high LB densities in amygdala and parahippocampus, with early hallucinations relating to higher densities in parahippocampal and inferior temporal cortices. These data suggest that the distribution of LBs is more related to the presence and duration of VHs in LB diseases than to the presence and severity or duration of dementia [61]. Other studies showed an association between neuronal loss and the severity of αSyn deposition in the intralaminar nuclei of the thalamus and VHs, fluctuations in consciousness and other symptoms in PD [201]; these changes correlated with disease duration [202]. In a series of 112 autopsy-confirmed cases of DLB, the main neuropathological correlate of persistent VHs was the presence of less severe tangle pathology, but there was no significant association between tangle pathology and persistent delusions, whereas LB staging was associated with persistent VHs and delusions. All baseline psychiatric features were significantly more frequent in DLB than in AD, as were persistent VHs, but DLB patients with severe tangle pathology (LBV/AD) had a clinical symptom profile more similar to that of AD patients and were less likely to have neocortical LBs. Thus, unlike AD, DLB cases showed a significant inverse association between tangle burden and psychosis [11].
Several clinico-pathological studies have addressed the morphologic features underlying VHs in dementia. They have consistently reported a higher frequency and earlier onset of VHs in subjects with Lewy-related pathology, i.e. subjects with DLB or PDD [124, 167, 202], than in demented subjects with AD. Most prospective studies have reported VH frequencies greater than 50% in subjects with confirmed Lewy-related pathology [58, 204]. Some have suggested that concomitant AD pathology obscures the clinical presentations of VHs in subjects with Lewy pathology [10, 205-207], whereas others suggested that VH frequencies are especially associated with the density of LBs in the medial temporal lobe [208].
In a community-based clinico-pathological study of 148 demented subjects, those with VHs (18%) had significantly more frequent Lewy pathology than those without VHs (78 versus 45%). A higher frequency of VHs was observed in subjects with neocortical LBs than in subjects with limbic-, amygdala- or brainstem-predominant LB pathology. Although AD with concomitant Lewy pathology was the most common neuropathological subtype in the VH+ group (59%), the frequency of subjects with AD did not differ significantly between those with and without VHs (74% versus 62%) [207].
In the population-based Vantaa 85+ study of DLB in individuals at least 85 years of age, VHs, reported in 48% of the patients, were associated with Braak stage independent of the presence of dementia (P = 0.041), but no association was seen with the type of Lewy pathology (P = 0.78). The probability for VHs was more than three times higher in subjects with neuritic Braak stages III–VI compared to those with Braak stages 0–II [172]. These findings differ from others which reported that subjects with extensive neurofibrillary pathology show fewer clinical features of DLB, like VHs [11, 208].
Huntington’s disease (HD)
Psychosis is more common in HD than in the general population, occurring in up to 15% of HD patients [210-212]. Paranoia rather than schizophrenia-like symptoms are the important and underestimated manifestations. PSs include paranoid delusions in up to 11%, although auditory hallucinations and VHs are rare, occurring in about 2% [212, 214]. HD patients may have a familial predisposition to develop psychosis and it has been suggested that other genetic factors may influence susceptibility to a particular phenotype precipitated by cytokine-adenine-guanine (CAG) expansion in the HD gene [212]. Gene carriers and non-carriers do not differ in terms of the lifetime frequency of psychiatric disorders or subclinical symptoms, but gene carriers had a significantly higher rate of depressive symptoms [215, 216], whereas symptomatic mutation carriers showed an increased prevalence of non-affective psychosis [216]. Specific psychiatric symptoms (e.g. paranoid ideation and psychoticisms) differentiate non-mutation carriers from individuals in the early preclinical stage of HD who are either symptom free or have minor non-specific motor anomalies [216]. Subclinical psychiatric symptoms are present in about one third of preclinical HD patients, often occurring more than 10 years before HD diagnosis, thus probably being the earliest markers of the disease [218]. Psychiatric symptoms generally do not correlate with cognitive decline, abnormal movements, or CAG repeat length [219].
Most of PSs in HD are believed to arise from subcortical neuropathological changes [220]. Although we are not aware of specific studies of neuropathology in HD cases with psychosis, PSs in early stages of the disease may be related to dysfunction of vulnerable striosomal spiny neurons in the neostriatum and centre median of thalamus [221], involving the basal ganglia-thalamocortical circuit, but the models of striatal connectivity and pathology are insufficient to explain such features in early HD [222].
Frontotemporal dementia (frontotemporal lobe degeneration [FLTD])
Although most patients with FTD present with neuropsychiatric symptoms, the frequency of PSs is unclear. Among 86 patients who met consensus criteria for FTD, only two (2.3%) had delusions, one of whom had paranoid ideation, whereas no FTD patient had hallucinations; this was significantly less than in AD patients [223]. These and other case reports [224, 225], and an evidence-based review of the psychopathology of FTD showed rare occurrence of delusions and hallucinations [226], possibly due to limited temporo-limbic pathology in this disorder. There are rare clinical reports about FTD patients presenting as acute later onset schizophrenia [227-229]. Among 61 autopsy cases of FTD (43 with behavioural [bvFTD] and 18 with FTD + amyotrophic lateral sclerosis [FTD/ALS]), there was a significant association of the presence of delusions with FTD/ALS (50%), and their occurrence in bvFTD may early indicate combination with ALS features. The median survival from symptom onset was significantly shorter for the FTD/ALS group than for bvFTD cases (mean 2.4 versus 6.6 years) [230]. Apathy and disinhibition in FTLD was associated with lesions in the anterior cingulate and dorsolateral prefrontal cortex [13].
In a small number of FTD cases presenting with schizophrenia-like psychosis years prior to the dementia diagnosis, neuropathology was consistent with TDP-43 and ubiquitin+ FTD [231-233]. Recent studies identified a novel group of FTD patients with clinical features that overlap with DLB presenting, among others, with fluctuating cognition and hallucinations; SPECT showed frontal lobe hypoperfusion. Pathology was consistent with FTLD with ubiquitin-only immunoreactive changes, type 1 [234], which represent a significant, clinically heterogeneous subtype of FTLD [235]. Another subtype clinically characterized by early onset and progressive and behavioural and personality changes, morphologically being τ- and TDP-43–, with fused in sarcoma pathology, has been classified as ‘atypical’ FTLD [236, 237].
PSs, occasionally mimicking schizophrenia, have been reported in rare clinical cases of ALS, but no neuropathological findings were available [238, 239].
Prion diseases
Sporadic Creutzfeld–Jakob disease (sCJD) is characterized by prominent neurological symptoms and less common psychiatric disorders, whereas the variant form (vCJD) is described as primary psychiatric in presentation and course [240, 241]. A retrospective review of 126 sCJD patients at the Mayo Clinic revealed that 80% of the cases demonstrated psychiatric symptoms within the first 100 days of illness, with 26% occurring at presentation. The most common symptoms included sleep disturbances, PSs and depression. Psychiatric manifestations as an early and prominent feature of sCJD often occurred prior to formal diagnosis [241, 242]. In one patient, anxiety and irritability also occurred early, followed by transitory hallucinatory psychosis [243], whereas others presented as depressive disorder with psychotic features [244]. Psychiatric symptoms as a consistent early clinical feature in vCJD, in addition to depression, include transient delusions and auditory hallucinations or VHs [245], and are difficult to distinguish from common psychiatric disorders [246]. CJD affects multiple brain areas, which causes multifocal deficits involving movement, cognition and psychiatric status. Modern neuroimaging helps to track the progression of the disease course and provides insight into clinico-anatomical correlations [247], but relevant neuropathological data about PSs in prion diseases, to the best of our knowledge, are not available so far.
Conclusions and future prospects
The aetiology of PSs in neurodegenerative disorders is complex and likely includes a variety of interactions between genetic and personality factors, medication exposure, morphological, neurochemical, hitherto unknown molecular changes and comorbid conditions, particularly cognitive impairment, visual disturbances and others. There has been an ongoing debate as to whether BPSD are non-specific aspects of neurodegeneration or distinct phenotypes. Because several of them may precede cognitive impairment by several years and can be considered as prodromal symptoms to dementia [68], the question arises which neuropathological changes may be important as risk factors for PSs in neurodegenerative disorders. The separation of BPSD and cognition into relative distinct domains suggests that different neuroanatomical systems may be involved in the genesis of these symptoms [248], but recent imaging studies have suggested that behavioural symptoms in AD are directly associated with neurodegeneration [115]. Because the onset of psychiatric symptoms varies for each individual manifestation and may appear at any stage of illness, temporal correlation is difficult to assess as all these processes and their psychiatric manifestations tend to have insidious onsets that preclude accurate staging and a fluctuating course, causing difficulties in their relation to anatomical and molecular biological changes. PSs are frequently present in patients with executive deficits, suggesting an association with frontal lobe dysfunctions [2, 59], related to interruption of corticosubcortical circuits involving the basal ganglia and frontal lobes [249]. They are associated with lesions in meso/paralimbic, prefrontal and temporal cortical association areas [250], suggesting that regional rather than diffuse brain pathology may be a primary response (Fig. 3).
Fig 3.
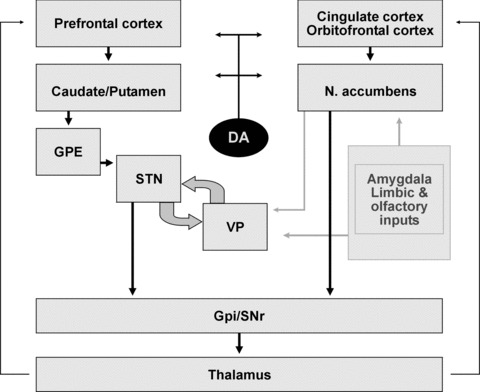
Circuiting interconnections between psychotic and cognitive functions. GPE: external globus pallidus; STN: subthalamic nucleus; VP: ventral pallidum; GPi-SNpr: internal globus pallidus and substantia nigra pars reticulata; DA: dopamine. Modified from Barone P, Santangelo G. Interaction between affect and executive functions in Parkinson’s disease. In: Emre M, editor. Cognitive Impairment and Dementia in Parkinson’s Disease. Oxford, UK: Oxford University Press; 2010. pp. 65–73.
There is increasing information about the neurobiological substrate of VHs and other PSs in PD and DLB [251]. These symptoms have been commonly viewed as an adverse effect of anti-Parkinson treatment [252, 253]; but more recent studies indicated that VHs do not directly relate to medication doses, and factors intrinsic to the disease process may also cause PSs [254-256]. Other studies linked VHs to cholinergic deficits with up-regulation of post-synaptic muscarinic acetylcholine (ACh) receptors [122], delusions correlating with post-synaptic up-regulation of nicotinic receptors [62], and hallucinations with decreased binding to nicotinic receptors (α7 nAChR) in the temporal cortex [257]. Serotonergic/dopaminergic imbalance has also been implicated in the development of psychosis in PD [123, 253, 258]. Moreover, altered visual information processing in PD and DLB with predominant visual cognitive impairments and alterations of the extrastriatal visual pathways were discussed [259]. Increase of blood flow in the ventral occipital regions and improvement of VHs in DLB after donepezil administration suggested that hypofunction in the visual association areas caused by a lack of cholinergic inputs from the forebrain or brainstem may be a key for the genesis of VHs [260]. Others suggested alterations of brainstem sleep-wake and dream regulation, and impaired attentional focus [261]. Genetic variation analysis in PD patients with and without VHs showed they were neither associated with the genetic pattern seen in patients with AD nor with an ApoE genotype [262].
Dopamine has been linked to DLB + psychosis (Fig. 3). Neuronal loss in the substantia nigra, albeit not as severe as that in PD, occurs in DLB [123, 205]. Dopamine uptake in the basal ganglia is more uniformly but less decreased compared with PD; dopamin receptor D-2 binding is decreased in DLB and increased in PD [263]. Although in psychotic AD patients, receptor D-3 density is increased, in DLB cases D-1 are increased and D-2 and D-3 receptor densities are decreased [264]. These data suggest that dopaminergic compounds may indirectly influence VHs, increasing the dopaminergic/cholinergic imbalance [45]. NE may also contribute to DLB psychopathology, because NE is depleted in the putamen [265], and the neuronal loss in the locus ceruleus is more severe compared with AD [122, 123, 205].
Although previous studies showed that VHs in PD are not influenced by polymorphisms of serotonin 5-HT2A receptor and transporter genes [266], recent pilot studies provided in vivo evidence suggesting a role for serotonin 2A receptors in mediating VHs via the ventral visual pathway [267]. DLB showed a significantly higher frontal 5-HTA1 receptor binding compared with PDD, suggesting distinct neurochemical features for the two disorders [252].
Some of the correlations between PSs and neuropathology may be the result of comorbid conditions, e.g. cerebrovascular or Lewy pathology which, however, frequently are missed clinically and could not be identified without neuropathological examination [268-277]. Some authors have avoided reporting BPSD by dementia subtypes because of the high degree of mixed pathology [269, 278].
It would be difficult to evaluate how neuropathology alone counts for the heterogeneity of psychiatric manifestations in neurodegenerative disorders, because current opinion suggests a complex network of genetic, morphological, neurochemical, neuropsychological and hitherto poorly understood molecular factors to be responsive for PSs. The burden of different lesions, although frequently overlapping, is considered to be independent of each other, and is consistent with an additive or synergistic effect, but this effect could hardly be measured so far [15, 273, 275, 276]. It should be emphasized that patients with PSs differ clinically and neuropathologically from those without psychiatric symptoms. Despite the increased focus on psychiatric symptoms associated with neurodegenerative diseases and dementia in neurobiological research, many challenges remain. Clinical phenotypes are imperfect and at times overlap increasing the difficulty of finding significant biological correlates, particularly in small samples. Although functional imaging, genetic and clinico-pathological studies hold immense promise, many studies will require replication and validation in larger samples. Understanding of these manifestations may lead to the development of more effective preventive and therapeutic options that may delay long-term progression of these devastating disorders and improve the patients’ quality of life [279]. It is important to recognize these subtypes and to better understand the pathophysiological cascade underlying the development of psychiatric syndromes in neurodegenerative and dementing diseases, which also may provide important insights into psychotic processes in general [280].
Conflict of interest
The author confirms that there are no conflicts of interest.
References
- 1. Wikipedia. Dementia. http://enwikipediaorg/wiki/Dementia 2010; accessed January 17, 2011.
- 2.Piccininni M, Di Carlo A, Baldereschi M, et al. Behavioral and psychological symptoms in Alzheimer’s disease: frequency and relationship with duration and severity of the disease. Dement Geriatr Cogn Disord. 2005;19:276–81. doi: 10.1159/000084552. [DOI] [PubMed] [Google Scholar]
- 3.Braak H, Alafuzoff I, Arzberger T, et al. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 5.Lopez OL, Becker JT, Sweet RA, et al. Psychiatric symptoms vary with the severity of dementia in probable Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 2003;15:346–53. doi: 10.1176/jnp.15.3.346. [DOI] [PubMed] [Google Scholar]
- 6.Nelson PT, Braak H, Markesbery WR. Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J Neuropathol Exp Neurol. 2009;68:1–14. doi: 10.1097/NEN.0b013e3181919a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forstl H, Besthorn C, Geiger-Kabisch C, et al. Psychotic features and the course of Alzheimer’s disease: relationship to cognitive, electroencephalographic and computerized tomography findings. Acta Psychiatr Scand. 1993;87:395–9. doi: 10.1111/j.1600-0447.1993.tb03394.x. [DOI] [PubMed] [Google Scholar]
- 8.Reisberg B, Auer SR, Monteiro IM. Behavioral pathology in Alzheimer’s disease (BEHAVE-AD) rating scale. Int Psychogeriatr. 1996;8:301–8. doi: 10.1097/00019442-199911001-00147. discussion 51–4. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez M, Gobartt AL, Balana M. Behavioural symptoms in patients with Alzheimer’s disease and their association with cognitive impairment. BMC Neurol. 2010;10:87. doi: 10.1186/1471-2377-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–24. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 11.Ballard CG, Jacoby R, Del Ser T, et al. Neuropathological substrates of psychiatric symptoms in prospectively studied patients with autopsy-confirmed dementia with Lewy bodies. Am J Psychiatry. 2004;161:843–9. doi: 10.1176/appi.ajp.161.5.843. [DOI] [PubMed] [Google Scholar]
- 12.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 13.Massimo L, Powers C, Moore P, et al. Neuroanatomy of apathy and disinhibition in frontotemporal lobar degeneration. Dement Geriatr Cogn Disord. 2009;27:96–104. doi: 10.1159/000194658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeste DV, Finkel SI. Psychosis of Alzheimer’s disease and related dementias. Diagnostic criteria for a distinct syndrome. Am J Geriatr Psychiatry. 2000;8:29–34. doi: 10.1097/00019442-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Chiu MJ, Chen TF, Yip PK, et al. Behavioral and psychologic symptoms in different types of dementia. J Formos Med Assoc. 2006;105:556–62. doi: 10.1016/S0929-6646(09)60150-9. [DOI] [PubMed] [Google Scholar]
- 16.Woodward M, Brodaty H, Boundy K, et al. Does executive impairment define a frontal variant of Alzheimer’s disease? Int Psychogeriatr. 2010;22:1280–90. doi: 10.1017/S1041610210001596. [DOI] [PubMed] [Google Scholar]
- 17.Williams DR, Lees AJ. Visual hallucinations in the diagnosis of idiopathic Parkinson’s disease: a retrospective autopsy study. Lancet Neurol. 2005;4:605–10. doi: 10.1016/S1474-4422(05)70146-0. [DOI] [PubMed] [Google Scholar]
- 18.Bozeat S, Gregory CA, Ralph MA, et al. Which neuropsychiatric and behavioural features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer’s disease? J Neurol Neurosurg Psychiatry. 2000;69:178–86. doi: 10.1136/jnnp.69.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finkel SI, Costa e Silva J, Cohen G, et al. Behavioral and psychological signs and symptoms of dementia: a consensus statement on current knowledge and implications for research and treatment. Int Psychogeriatr. 1996;8:497–500. doi: 10.1017/s1041610297003943. [DOI] [PubMed] [Google Scholar]
- 20.Robert PH, Verhey FR, Byrne EJ, et al. Grouping for behavioral and psychological symptoms in dementia: clinical and biological aspects. Consensus paper of the European Alzheimer disease consortium. Eur Psychiatry. 2005;20:490–6. doi: 10.1016/j.eurpsy.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 21.Meeks TW, Ropacki SA, Jeste DV. The neurobiology of neuropsychiatric syndromes in dementia. Curr Opin Psychiatry. 2006;19:581–6. doi: 10.1097/01.yco.0000245746.45384.0e. [DOI] [PubMed] [Google Scholar]
- 22.Garre-Olmo J, Lopez-Pousa S, Vilalta-Franch J, et al. Grouping and trajectories of the neuropsychiatric symptoms in patients with Alzheimer’s disease, part I: symptom clusters. J Alzheimers Dis. 2010;22:1157–67. doi: 10.3233/JAD-2010-101212. [DOI] [PubMed] [Google Scholar]
- 23.Aalten P, de Vugt ME, Lousberg R, et al. Behavioral problems in dementia: a factor analysis of the neuropsychiatric inventory. Dement Geriatr Cogn Disord. 2003;15:99–105. doi: 10.1159/000067972. [DOI] [PubMed] [Google Scholar]
- 24.Drevets WC, Rubin EH. Psychotic symptoms and the longitudinal course of senile dementia of the Alzheimer type. Biol Psychiatry. 1989;25:39–48. doi: 10.1016/0006-3223(89)90145-5. [DOI] [PubMed] [Google Scholar]
- 25.Aalten P, Verhey FR, Boziki M, et al. Neuropsychiatric syndromes in dementia. Results from the European Alzheimer Disease Consortium: part I. Dement Geriatr Cogn Disord. 2007;24:457–63. doi: 10.1159/000110738. [DOI] [PubMed] [Google Scholar]
- 26.Cummings JL, Diaz C, Levy M, et al. Neuropsychiatric syndromes in neurodegenerative disease: frequency and significance. Semin Clin Neuropsychiatry. 1996;1:241–7. doi: 10.1053/SCNP00100241. [DOI] [PubMed] [Google Scholar]
- 27.Ropacki SA, Jeste DV. Epidemiology of and risk factors for psychosis of Alzheimer’s disease: a review of 55 studies published from 1990 to 2003. Am J Psychiatry. 2005;162:2022–30. doi: 10.1176/appi.ajp.162.11.2022. [DOI] [PubMed] [Google Scholar]
- 28.Wilson RS, Krueger KR, Kamenetsky JM, et al. Hallucinations and mortality in Alzheimer disease. Am J Geriatr Psychiatry. 2005;13:984–90. doi: 10.1176/appi.ajgp.13.11.984. [DOI] [PubMed] [Google Scholar]
- 29.Casanova MF, Starkstein SE, Jellinger KA. Clinicopathological correlates of behavioral and psychological symptoms of dementia. Acta Neuropathol. 2011 doi: 10.1007/s00401-011-0821-3. ; doi: 10.1007/s00401-011-0821-3. [DOI] [PubMed] [Google Scholar]
- 30. International Psychogeriatric Association. BPSD: introduction to behavioral and psychological symptoms of dementia. http://ipa-onlineorg/ipaonlinev3/ipaprograms/taskforces/bpsd/introasp. 2010; accessed February 24, 2011.
- 31.Devanand DP, Jacobs DM, Tang MX, et al. The course of psychopathologic features in mild to moderate Alzheimer disease. Arch Gen Psychiatry. 1997;54:257–63. doi: 10.1001/archpsyc.1997.01830150083012. [DOI] [PubMed] [Google Scholar]
- 32.Brodaty H, Draper B, Saab D, et al. Psychosis, depression and behavioural disturbances in Sydney nursing home residents: prevalence and predictors. Int J Geriatr Psychiatry. 2001;16:504–12. doi: 10.1002/gps.382. [DOI] [PubMed] [Google Scholar]
- 33.Mega MS, Cummings JL, Fiorello T, et al. The spectrum of behavioral changes in Alzheimer’s disease. Neurology. 1996;46:130–5. doi: 10.1212/wnl.46.1.130. [DOI] [PubMed] [Google Scholar]
- 34.Matsui H, Nishinaka K, Oda M, et al. Hypoperfusion of the visual pathway in parkinsonian patients with visual hallucinations. Mov Disord. 2006;21:2140–4. doi: 10.1002/mds.21140. [DOI] [PubMed] [Google Scholar]
- 35.Borroni B, Costanzi C, Padovani A. Genetic susceptibility to behavioural and psychological symptoms in Alzheimer disease. Curr Alzheimer Res. 2010;7:158–64. doi: 10.2174/156720510790691173. [DOI] [PubMed] [Google Scholar]
- 36.Schneider LS, Dagerman KS. Psychosis of Alzheimer’s disease: clinical characteristics and history. J Psychiatr Res. 2004;38:105–11. doi: 10.1016/s0022-3956(03)00092-x. [DOI] [PubMed] [Google Scholar]
- 37.Ravina B, Marder K, Fernandez HH, et al. Diagnostic criteria for psychosis in Parkinson’s disease: report of an NINDS, NIMH work group. Mov Disord. 2007;22:1061–8. doi: 10.1002/mds.21382. [DOI] [PubMed] [Google Scholar]
- 38.Fenelon G, Alves G. Epidemiology of psychosis in Parkinson’s disease. J Neurol Sci. 2010;289:12–7. doi: 10.1016/j.jns.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 39.Molho ES, Factor SA. Psychosis. In: Pfeiffer RF, Bodis-Wollner I, editors. Current clinical neurology: Parkinson’s disease and nonmotor dysfunction. Totowa, NJ: Humana Press; 2005: 49–74.
- 40.Williams DR, Poewe W. Lesions associated with visual hallucinations and psychoses. In: Halliday G, Barker RA, Rowe DB, editors. Non-dopamine lesions in Parkinson’s disease. Oxford, New York: Oxford University Press; 2011: 242–60.
- 41.Aarsland D, Marsh L, Schrag A. Neuropsychiatric symptoms in Parkinson’s disease. Mov Disord. 2009;24:2175–86. doi: 10.1002/mds.22589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aarsland D, Ballard C, Larsen JP, et al. A comparative study of psychiatric symptoms in dementia with Lewy bodies and Parkinson’s disease with and without dementia. Int J Geriatr Psychiatry. 2001;16:528–36. doi: 10.1002/gps.389. [DOI] [PubMed] [Google Scholar]
- 43.Weintraub D, Mamikonyan E. Neuropsychiatric symptoms in Parkinson’s disease dementia. In: Emre M, editor. Cognitive impairment and dementia in Parkinson’s disease. Oxford, UK: Oxford University Press; 2010: 45–64.
- 44.Bronnick K, Aarsland D, Larsen JP. Neuropsychiatric disturbances in Parkinson’s disease clusters in five groups with different prevalence of dementia. Acta Psychiatr Scand. 2005;112:201–7. doi: 10.1111/j.1600-0447.2005.00562.x. [DOI] [PubMed] [Google Scholar]
- 45.Apostolova LG, Cummings JL. Neuropsychiatric features of dementia with Lewy bodies. In: O’Brien J, McKeith I, Ames D, Chiu E, editors. Dementia with Lewy bodies. London: Taylor & Francis; 2006: 73–94.
- 46.Byrne EJ, Lennox G, Lowe J, et al. Diffuse Lewy body disease: clinical features in 15 cases. J Neurol Neurosurg Psychiatry. 1989;52:709–17. doi: 10.1136/jnnp.52.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagahama Y, Okina T, Suzuki N, et al. Classification of psychotic symptoms in dementia with Lewy bodies. Am J Geriatr Psychiatry. 2007;15:961–7. doi: 10.1097/JGP.0b013e3180cc1fdf. [DOI] [PubMed] [Google Scholar]
- 48.Perneczky R, Drzezga A, Boecker H, et al. Cerebral metabolic dysfunction in patients with dementia with Lewy bodies and visual hallucinations. Dement Geriatr Cogn Disord. 2008;25:531–8. doi: 10.1159/000132084. [DOI] [PubMed] [Google Scholar]
- 49.Simard M, van Reekum R, Cohen T. A review of the cognitive and behavioral symptoms in dementia with Lewy bodies. J Neuropsychiatry Clin Neurosci. 2000;12:425–50. doi: 10.1176/jnp.12.4.425. [DOI] [PubMed] [Google Scholar]
- 50.Okura T, Plassman BL, Steffens DC, et al. Prevalence of neuropsychiatric symptoms and their association with functional limitations in older adults in the United States: the aging, demographics, and memory study. J Am Geriatr Soc. 2010;58:330–7. doi: 10.1111/j.1532-5415.2009.02680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lyketsos CG, Steinberg M, Tschanz JT, et al. Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging. Am J Psychiatry. 2000;157:708–14. doi: 10.1176/appi.ajp.157.5.708. [DOI] [PubMed] [Google Scholar]
- 52.Alzheimer A. Ueber eine eigenartige Erkrankung der Hirnrinde (About a peculiar disease of the cerebral cortex) Allgem Z Psychiatr. 1907;64:146–8. [Google Scholar]
- 53.Jost BC, Grossberg GT. The evolution of psychiatric symptoms in Alzheimer’s disease: a natural history study. J Am Geriatr Soc. 1996;44:1078–81. doi: 10.1111/j.1532-5415.1996.tb02942.x. [DOI] [PubMed] [Google Scholar]
- 54.Michel BF, Luciani V, Geda YE, et al. [In Alzheimer’s disease, the clinical expression of behavioral and psychological signs and symptoms is early and specific of neuropathological stages] Encephale. 2010;36:314–25. doi: 10.1016/j.encep.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 55.Gülden W. Über anatomische Befunde und klinische Verlaufsformen bei senilen Psychosen (About anatomical findings and clinical types of senile psychoses). Univ. Hamburg Medical School: Thesis, 1946.
- 56.Schreinzer D, Ballaban T, Brannath W, et al. Components of behavioral pathology in dementia. Int J Geriatr Psychiatry. 2005;20:137–45. doi: 10.1002/gps.1263. [DOI] [PubMed] [Google Scholar]
- 57.Rosen J, Zubenko GS. Emergence of psychosis and depression in the longitudinal evaluation of Alzheimer’s disease. Biol Psychiatry. 1991;29:224–32. doi: 10.1016/0006-3223(91)91284-x. [DOI] [PubMed] [Google Scholar]
- 58.Ballard C, Holmes C, McKeith I, et al. Psychiatric morbidity in dementia with Lewy bodies: a prospective clinical and neuropathological comparative study with Alzheimer’s disease. Am J Psychiatry. 1999;156:1039–45. doi: 10.1176/ajp.156.7.1039. [DOI] [PubMed] [Google Scholar]
- 59.Engelborghs S, Maertens K, Marien P, et al. Behavioural and neuropsychological correlates of frontal lobe features in dementia. Psychol Med. 2006;36:1173–82. doi: 10.1017/S003329170600777X. [DOI] [PubMed] [Google Scholar]
- 60.Bassiony MM, Lyketsos CG. Delusions and hallucinations in Alzheimer’s disease: review of the brain decade. Psychosomatics. 2003;44:388–401. doi: 10.1176/appi.psy.44.5.388. [DOI] [PubMed] [Google Scholar]
- 61.Harding AJ, Broe GA, Halliday GM. Visual hallucinations in Lewy body disease relate to Lewy bodies in the temporal lobe. Brain. 2002;125:391–403. doi: 10.1093/brain/awf033. [DOI] [PubMed] [Google Scholar]
- 62.Ballard C, Piggott M, Johnson M, et al. Delusions associated with elevated muscarinic binding in dementia with Lewy bodies. Ann Neurol. 2000;48:868–76. [PubMed] [Google Scholar]
- 63.Ostling S, Gustafson D, Blennow K, et al. Psychotic symptoms in a population-based sample of 85-year-old individuals with dementia. J Geriatr Psychiatry Neurol. 2010 doi: 10.1177/0891988710373596. [DOI] [PubMed] [Google Scholar]
- 64.Ostling S, Borjesson-Hanson A, Skoog I. Psychotic symptoms and paranoid ideation in a population-based sample of 95-year-olds. Am J Geriatr Psychiatry. 2007;15:999–1004. doi: 10.1097/JGP.0b013e31814622b9. [DOI] [PubMed] [Google Scholar]
- 65.Forstl H, Burns A, Levy R, et al. Neuropathological correlates of psychotic phenomena in confirmed Alzheimer’s disease. Br J Psychiatry. 1994;165:53–9. doi: 10.1192/bjp.165.1.53. [DOI] [PubMed] [Google Scholar]
- 66.Wilkosz PA, Miyahara S, Lopez OL, et al. Prediction of psychosis onset in Alzheimer disease: the role of cognitive impairment, depressive symptoms, and further evidence for psychosis subtypes. Am J Geriatr Psychiatry. 2006;14:352–60. doi: 10.1097/01.JGP.0000192500.25940.1b. [DOI] [PubMed] [Google Scholar]
- 67.Weamer EA, Emanuel JE, Varon D, et al. The relationship of excess cognitive impairment in MCI and early Alzheimer’s disease to the subsequent emergence of psychosis. Int Psychogeriatr. 2009;21:78–85. doi: 10.1017/S1041610208007734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Assal F, Cummings JL. Neuropsychiatric symptoms in the dementias. Curr Opin Neurol. 2002;15:445–50. doi: 10.1097/00019052-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 69.Burns A, Jacoby R, Levy R. Psychiatric phenomena in Alzheimer’s disease. IV: disorders of behaviour. Br J Psychiatry. 1990;157:86–94. doi: 10.1192/bjp.157.1.86. [DOI] [PubMed] [Google Scholar]
- 70.Burns A, Jacoby R, Levy R. Psychiatric phenomena in Alzheimer’s disease. III: disorders of mood. Br J Psychiatry. 1990;157:81–6. doi: 10.1192/bjp.157.1.81. [DOI] [PubMed] [Google Scholar]
- 71.Tariot PN, Mack JL, Patterson MB, et al. The Behavior Rating Scale for Dementia of the Consortium to Establish a Registry for Alzheimer’s Disease. The Behavioral Pathology Committee of the Consortium to Establish a Registry for Alzheimer’s Disease. Am J Psychiatry. 1995;152:1349–57. doi: 10.1176/ajp.152.9.1349. [DOI] [PubMed] [Google Scholar]
- 72.Rubin EH, Drevets WC, Burke WJ. The nature of psychotic symptoms in senile dementia of the Alzheimer type. J Geriatr Psychiatry Neurol. 1988;1:16–20. doi: 10.1177/089198878800100104. [DOI] [PubMed] [Google Scholar]
- 73.Ballard CG, Bannister CL, Patel A, et al. Classification of psychotic symptoms in dementia sufferers. Acta Psychiatr Scand. 1995;92:63–8. doi: 10.1111/j.1600-0447.1995.tb09544.x. [DOI] [PubMed] [Google Scholar]
- 74.Hwang JP, Tsai SJ, Yang CH, et al. Persecutory delusions in dementia. J Clin Psychiatry. 1999;60:550–3. doi: 10.4088/jcp.v60n0808. [DOI] [PubMed] [Google Scholar]
- 75.Perez-Madrinan G, Cook SE, Saxton JA, et al. Alzheimer disease with psychosis: excess cognitive impairment is restricted to the misidentification subtype. Am J Geriatr Psychiatry. 2004;12:449–56. doi: 10.1176/appi.ajgp.12.5.449. [DOI] [PubMed] [Google Scholar]
- 76.Capitani E, Francescani A, Spinnler H. Are hallucinations and extrapyramidal signs associated with a steeper cognitive decline in degenerative dementia patients? Neurol Sci. 2007;28:245–50. doi: 10.1007/s10072-007-0830-0. [DOI] [PubMed] [Google Scholar]
- 77.Scarmeas N, Brandt J, Albert M, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. 2005;62:1601–8. doi: 10.1001/archneur.62.10.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stern Y, Mayeux R, Sano M, et al. Predictors of disease course in patients with probable Alzheimer’s disease. Neurology. 1987;37:1649–53. doi: 10.1212/wnl.37.10.1649. [DOI] [PubMed] [Google Scholar]
- 79.Sweet RA, Nimgaonkar VL, Devlin B, et al. Psychotic symptoms in Alzheimer disease: evidence for a distinct phenotype. Mol Psychiatry. 2003;8:383–92. doi: 10.1038/sj.mp.4001262. [DOI] [PubMed] [Google Scholar]
- 80.Reisberg B, Borenstein J, Salob SP, et al. Behavioral symptoms in Alzheimer’s disease: phenomenology and treatment. J Clin Psychiatry. 1987;48:9–15. [PubMed] [Google Scholar]
- 81.Ballard CG, Chithiramohan RN, Bannister C, et al. Paranoid features in the elderly with dementia. Int J Geriatr Psychiatry. 1991;6:155–7. [Google Scholar]
- 82.Chen JY, Stern Y, Sano M, et al. Cumulative risks of developing extrapyramidal signs, psychosis, or myoclonus in the course of Alzheimer’s disease. Arch Neurol. 1991;48:1141–3. doi: 10.1001/archneur.1991.00530230049020. [DOI] [PubMed] [Google Scholar]
- 83.Gilley DW, Whalen ME, Wilson RS, et al. Hallucinations and associated factors in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 1991;3:371–6. doi: 10.1176/jnp.3.4.371. [DOI] [PubMed] [Google Scholar]
- 84.Deutsch LH, Bylsma FW, Rovner BW, et al. Psychosis and physical aggression in probable Alzheimer’s disease. Am J Psychiatry. 1991;148:1159–63. doi: 10.1176/ajp.148.9.1159. [DOI] [PubMed] [Google Scholar]
- 85.Lopez OL, Becker JT, Brenner RP, et al. Alzheimer’s disease with delusions and hallucinations: neuropsychological and electroencephalographic correlates. Neurology. 1991;41:906–12. doi: 10.1212/wnl.41.6.906. [DOI] [PubMed] [Google Scholar]
- 86.Gilley DW, Wilson RS, Beckett LA, et al. Psychotic symptoms and physically aggressive behavior in Alzheimer’s disease. J Am Geriatr Soc. 1997;45:1074–9. doi: 10.1111/j.1532-5415.1997.tb05969.x. [DOI] [PubMed] [Google Scholar]
- 87.Sweet RA, Nimgaonkar VL, Devlin B, et al. Increased familial risk of the psychotic phenotype of Alzheimer disease. Neurology. 2002;58:907–11. doi: 10.1212/wnl.58.6.907. [DOI] [PubMed] [Google Scholar]
- 88.Hollingworth P, Hamshere ML, Holmans PA, et al. Increased familial risk and genomewide significant linkage for Alzheimer’s disease with psychosis. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:841–8. doi: 10.1002/ajmg.b.30515. [DOI] [PubMed] [Google Scholar]
- 89.Bacanu SA, Devlin B, Chowdari KV, et al. Heritability of psychosis in Alzheimer disease. Am J Geriatr Psychiatry. 2005;13:624–7. doi: 10.1176/appi.ajgp.13.7.624. [DOI] [PubMed] [Google Scholar]
- 90.Del Prete M, Spaccavento S, Craca A, et al. Neuropsychiatric symptoms and the APOE genotype in Alzheimer’s disease. Neurol Sci. 2009;30:367–73. doi: 10.1007/s10072-009-0116-9. [DOI] [PubMed] [Google Scholar]
- 91.Go RC, Perry RT, Wiener H, et al. Neuregulin-1 polymorphism in late onset Alzheimer’s disease families with psychoses. Am J Med Genet B Neuropsychiatr Genet. 2005;139B:28–32. doi: 10.1002/ajmg.b.30219. [DOI] [PubMed] [Google Scholar]
- 92.Middle F, Pritchard AL, Handoko H, et al. No association between neuregulin 1 and psychotic symptoms in Alzheimer’s disease patients. J Alzheimers Dis. 2010;20:561–7. doi: 10.3233/JAD-2010-1405. [DOI] [PubMed] [Google Scholar]
- 93.Avramopoulos D, Fallin MD, Bassett SS. Linkage to chromosome 14q in Alzheimer’s disease (AD) patients without psychotic symptoms. Am J Med Genet B Neuropsychiatr Genet. 2005;132B:9–13. doi: 10.1002/ajmg.b.30074. [DOI] [PubMed] [Google Scholar]
- 94.Pritchard AL, Pritchard CW, Bentham P, et al. Role of serotonin transporter polymorphisms in the behavioural and psychological symptoms in probable Alzheimer disease patients. Dement Geriatr Cogn Disord. 2007;24:201–6. doi: 10.1159/000107081. [DOI] [PubMed] [Google Scholar]
- 95.Assal F, Alarcon M, Solomon EC, et al. Association of the serotonin transporter and receptor gene polymorphisms in neuropsychiatric symptoms in Alzheimer disease. Arch Neurol. 2004;61:1249–53. doi: 10.1001/archneur.61.8.1249. [DOI] [PubMed] [Google Scholar]
- 96.Craig D, Donnelly C, Hart D, et al. Analysis of the 5HT-2A T102C receptor polymorphism and psychotic symptoms in Alzheimer’s disease. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:126–8. doi: 10.1002/ajmg.b.30409. [DOI] [PubMed] [Google Scholar]
- 97.Ramanathan S, Glatt SJ. Serotonergic system genes in psychosis of Alzheimer dementia: meta-analysis. Am J Geriatr Psychiatry. 2009;17:839–46. doi: 10.1097/JGP.0b013e3181ab8c3f. [DOI] [PubMed] [Google Scholar]
- 98.Pritchard AL, Ratcliffe L, Sorour E, et al. Investigation of dopamine receptors in susceptibility to behavioural and psychological symptoms in Alzheimer’s disease. Int J Geriatr Psychiatry. 2009;24:1020–5. doi: 10.1002/gps.2214. [DOI] [PubMed] [Google Scholar]
- 99.Sweet RA, Nimgaonkar VL, Kamboh MI, et al. Dopamine receptor genetic variation, psychosis, and aggression in Alzheimer disease. Arch Neurol. 1998;55:1335–40. doi: 10.1001/archneur.55.10.1335. [DOI] [PubMed] [Google Scholar]
- 100.Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry. 2006;60:141–51. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 101.Demichele-Sweet MA, Klei L, Devlin B, et al. No association of psychosis in Alzheimer disease with neurodegenerative pathway genes. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.10.003. ; online 2010–11-17: doi:10.1016/j.neurobiolaging.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.DeMichele-Sweet MA, Sweet RA. Genetics of psychosis in Alzheimer’s disease: a review. J Alzheimers Dis. 2010;19:761–80. doi: 10.3233/JAD-2010-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kotrla KJ, Chacko RC, Harper RG, et al. SPECT findings on psychosis in Alzheimer’s disease. Am J Psychiatry. 1995;152:1470–5. doi: 10.1176/ajp.152.10.1470. [DOI] [PubMed] [Google Scholar]
- 104.Sultzer DL, Mahler ME, Mandelkern MA, et al. The relationship between psychiatric symptoms and regional cortical metabolism in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 1995;7:476–84. doi: 10.1176/jnp.7.4.476. [DOI] [PubMed] [Google Scholar]
- 105.Pietrini P, Furey ML, Graff-Radford N, et al. Preferential metabolic involvement of visual cortical areas in a subtype of Alzheimer’s disease: clinical implications. Am J Psychiatry. 1996;153:1261–8. doi: 10.1176/ajp.153.10.1261. [DOI] [PubMed] [Google Scholar]
- 106.Mega MS, Lee L, Dinov ID, et al. Cerebral correlates of psychotic symptoms in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2000;69:167–71. doi: 10.1136/jnnp.69.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Moran EK, Becker JA, Satlin A, et al. Psychosis of Alzheimer’s disease: gender differences in regional perfusion. Neurobiol Aging. 2008;29:1218–25. doi: 10.1016/j.neurobiolaging.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 108.Lanctot KL, Herrmann N, Nadkarni NK, et al. Medial temporal hypoperfusion and aggression in Alzheimer disease. Arch Neurol. 2004;61:1731–7. doi: 10.1001/archneur.61.11.1731. [DOI] [PubMed] [Google Scholar]
- 109.Nakano S, Yamashita F, Matsuda H, et al. Relationship between delusions and regional cerebral blood flow in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;21:16–21. doi: 10.1159/000089215. [DOI] [PubMed] [Google Scholar]
- 110.Staff RT, Venneri A, Gemmell HG, et al. HMPAO SPECT imaging of Alzheimer’s disease patients with similar content-specific autobiographic delusion: comparison using statistical parametric mapping. J Nucl Med. 2000;41:1451–5. [PubMed] [Google Scholar]
- 111.Sultzer DL, Brown CV, Mandelkern MA, et al. Delusional thoughts and regional frontal/temporal cortex metabolism in Alzheimer’s disease. Am J Psychiatry. 2003;160:341–9. doi: 10.1176/appi.ajp.160.2.341. [DOI] [PubMed] [Google Scholar]
- 112.Geroldi C, Bresciani L, Zanetti O, et al. Regional brain atrophy in patients with mild Alzheimer’s disease and delusions. Int Psychogeriatr. 2002;14:365–78. doi: 10.1017/s1041610202008566. [DOI] [PubMed] [Google Scholar]
- 113.Holroyd S, Shepherd ML, Downs JH., 3rd Occipital atrophy is associated with visual hallucinations in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 2000;12:25–8. doi: 10.1176/jnp.12.1.25. [DOI] [PubMed] [Google Scholar]
- 114.Bruen PD, McGeown WJ, Shanks MF, et al. Neuroanatomical correlates of neuropsychiatric symptoms in Alzheimer’s disease. Brain. 2008;131:2455–63. doi: 10.1093/brain/awn151. [DOI] [PubMed] [Google Scholar]
- 115.Serra L, Perri R, Cercignani M, et al. Are the behavioral symptoms of Alzheimer’s disease directly associated with neurodegeneration. J Alzheimers Dis. 2010;21:627–39. doi: 10.3233/JAD-2010-100048. [DOI] [PubMed] [Google Scholar]
- 116.Lee DY, Choo IH, Kim KW, et al. White matter changes associated with psychotic symptoms in Alzheimer’s disease patients. J Neuropsychiatry Clin Neurosci. 2006;18:191–8. doi: 10.1176/jnp.2006.18.2.191. [DOI] [PubMed] [Google Scholar]
- 117.Lin SH, Yu CY, Pai MC. The occipital white matter lesions in Alzheimer’s disease patients with visual hallucinations. Clin Imaging. 2006;30:388–93. doi: 10.1016/j.clinimag.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 118.Sweet RA, Panchalingam K, Pettegrew JW, et al. Psychosis in Alzheimer disease: postmortem magnetic resonance spectroscopy evidence of excess neuronal and membrane phospholipid pathology. Neurobiol Aging. 2002;23:547–53. doi: 10.1016/s0197-4580(02)00009-x. [DOI] [PubMed] [Google Scholar]
- 119.Zubenko GS, Moossy J, Martinez AJ, et al. Neuropathologic and neurochemical correlates of psychosis in primary dementia. Arch Neurol. 1991;48:619–24. doi: 10.1001/archneur.1991.00530180075020. [DOI] [PubMed] [Google Scholar]
- 120.Lanari A, Amenta F, Silvestrelli G, et al. Neurotransmitter deficits in behavioural and psychological symptoms of Alzheimer’s disease. Mech Ageing Dev. 2006;127:158–65. doi: 10.1016/j.mad.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 121.Weinshenker D. Functional consequences of locus coeruleus degeneration in Alzheimer’s disease. Curr Alzheimer Res. 2008;5:342–5. doi: 10.2174/156720508784533286. [DOI] [PubMed] [Google Scholar]
- 122.Perry EK, Kerwin J, Perry RH, et al. Visual hallucinations and the cholinergic system in dementia (letter) J Neurol Neurosurg Psychiatry doi: 10.1136/jnnp.53.1.88. . 1990; 53: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Perry EK, Marshall E, Kerwin J, et al. Evidence of a monoaminergic-cholinergic imbalance related to visual hallucinations in Lewy body dementia. J Neurochem. 1990;55:1454–6. doi: 10.1111/j.1471-4159.1990.tb03162.x. [DOI] [PubMed] [Google Scholar]
- 124.McShane R, Gedling K, Reading M, et al. Prospective study of relations between cortical Lewy bodies, poor eyesight, and hallucinations in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1995;59:185–8. doi: 10.1136/jnnp.59.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cummings JL. Organic delusions: phenomenology, anatomical correlations, and review. Br J Psychiatry. 1985;146:184–97. doi: 10.1192/bjp.146.2.184. [DOI] [PubMed] [Google Scholar]
- 126.Le Ber I, Marie RM, Chabot B, et al. Neuropsychological and 18FDG-PET studies in a family with idiopathic basal ganglia calcifications. J Neurol Sci. 2007;258:115–22. doi: 10.1016/j.jns.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 127.Staton RD, Brumback RA, Wilson H. Reduplicative paramnesia: a disconnection syndrome of memory. Cortex. 1982;18:23–35. doi: 10.1016/s0010-9452(82)80016-6. [DOI] [PubMed] [Google Scholar]
- 128.Lai MK, Chen CP, Hope T, et al. Hippocampal neurofibrillary tangle changes and aggressive behaviour in dementia. Neuroreport. 2010;21:1111–5. doi: 10.1097/WNR.0b013e3283407204. [DOI] [PubMed] [Google Scholar]
- 129.Rapp MA, Schnaider-Beeri M, Grossman HT, et al. Increased hippocampal plaques and tangles in patients with Alzheimer disease with a lifetime history of major depression. Arch Gen Psychiatry. 2006;63:161–7. doi: 10.1001/archpsyc.63.2.161. [DOI] [PubMed] [Google Scholar]
- 130.Guimaraes HC, Levy R, Teixeira AL, et al. Neurobiology of apathy in Alzheimer’s disease. Arq Neuropsiquiatr. 2008;66:436–43. doi: 10.1590/s0004-282x2008000300035. [DOI] [PubMed] [Google Scholar]
- 131.Fenelon G. Psychosis in Parkinson’s disease: phenomenology, frequency, risk factors, and current understanding of pathophysiologic mechanisms. CNS Spectr. 2008;13:18–25. doi: 10.1017/s1092852900017284. [DOI] [PubMed] [Google Scholar]
- 132.Eng ML, Welty TE. Management of hallucinations and psychosis in Parkinson's disease. Am J Geriatr Pharmacother. 2010;8:316–30. doi: 10.1016/j.amjopharm.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 133.Fenelon G, Goetz CG, Karenberg A. Hallucinations in Parkinson disease in the prelevodopa era. Neurology. 2006;66:93–8. doi: 10.1212/01.wnl.0000191325.31068.c4. [DOI] [PubMed] [Google Scholar]
- 134.Fernandez HH, Aarsland D, Fenelon G, et al. Scales to assess psychosis in Parkinson’s disease: critique and recommendations. Mov Disord. 2008;23:484–500. doi: 10.1002/mds.21875. [DOI] [PubMed] [Google Scholar]
- 135.Kulisevsky J, Pagonabarraga J, Pascual-Sedano B, et al. Prevalence and correlates of neuropsychiatric symptoms in Parkinson’s disease without dementia. Mov Disord. 2008;23:1889–96. doi: 10.1002/mds.22246. [DOI] [PubMed] [Google Scholar]
- 136.Fenelon G, Soulas T, Zenasni F, et al. The changing face of Parkinson’s disease-associated psychosis: a cross-sectional study based on the new NINDS-NIMH criteria. Mov Disord. 2010;25:755–9. doi: 10.1002/mds.22839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Holroyd S, Currie L, Wooten GF. Prospective study of hallucinations and delusions in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2001;70:734–8. doi: 10.1136/jnnp.70.6.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fenelon G, Thobois S, Bonnet AM, et al. Tactile hallucinations in Parkinson’s disease. J Neurol. 2002;249:1699–703. doi: 10.1007/s00415-002-0908-9. [DOI] [PubMed] [Google Scholar]
- 139.Goetz CG, Wuu J, Curgian L, et al. Age-related influences on the clinical characteristics of new-onset hallucinations in Parkinson’s disease patients. Mov Disord. 2006;21:267–70. doi: 10.1002/mds.20701. [DOI] [PubMed] [Google Scholar]
- 140.Inzelberg R, Kipervasser S, Korczyn AD. Auditory hallucinations in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1998;64:533–5. doi: 10.1136/jnnp.64.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tousi B, Frankel M. Olfactory and visual hallucinations in Parkinson’s disease. Parkinsonism Relat Disord. 2004;10:253–4. doi: 10.1016/j.parkreldis.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 142.Fenelon G, Mahieux F, Huon R, et al. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain. 2000;123:733–45. doi: 10.1093/brain/123.4.733. [DOI] [PubMed] [Google Scholar]
- 143.Mosimann UP, Rowan EN, Partington CE, et al. Characteristics of visual hallucinations in Parkinson disease dementia and dementia with Lewy bodies. Am J Geriatr Psychiatry. 2006;14:153–60. doi: 10.1097/01.JGP.0000192480.89813.80. [DOI] [PubMed] [Google Scholar]
- 144.Onofrj M, Thomas A, Bonanni L. New approaches to understanding hallucinations in Parkinson’s disease: phenomenology and possible origins. Expert Rev Neurother. 2007;7:1731–50. doi: 10.1586/14737175.7.12.1731. [DOI] [PubMed] [Google Scholar]
- 145.Kurita A, Murakami M, Takagi S, et al. Visual hallucinations and altered visual information processing in Parkinson disease and dementia with Lewy bodies. Mov Disord. 2010;25:167–71. doi: 10.1002/mds.22919. [DOI] [PubMed] [Google Scholar]
- 146.Meppelink AM, de Jong BM, Renken R, et al. Impaired visual processing preceding image recognition in Parkinson’s disease patients with visual hallucinations. Brain. 2009;132:2980–93. doi: 10.1093/brain/awp223. [DOI] [PubMed] [Google Scholar]
- 147.Aarsland D, Larsen JP. Mental and behavioral dysfunctions in movement disorders. Totowa, NJ: Humana Press Inc.; 2003. Diagnosis and treatment of hallucinations and delusions in Parkinson’s disease. In: Bédard M-A, Agid Y, Chouinard S, Fahn S, Korczyn AD, Lespérance P, editors. pp. 369–82. [Google Scholar]
- 148.Verbaan D, van Rooden SM, Visser M, et al. Psychotic and compulsive symptoms in Parkinson’s disease. Mov Disord. 2009;24:738–44. doi: 10.1002/mds.22453. [DOI] [PubMed] [Google Scholar]
- 149.Marsh L, Williams JR, Rocco M, et al. Psychiatric comorbidities in patients with Parkinson disease and psychosis. Neurology. 2004;63:293–300. doi: 10.1212/01.wnl.0000129843.15756.a3. [DOI] [PubMed] [Google Scholar]
- 150.Wolters E. PD-related psychosis: pathophysiology with therapeutical strategies. J Neural Transm Suppl. 2006;71:31–7. doi: 10.1007/978-3-211-33328-0_4. [DOI] [PubMed] [Google Scholar]
- 151.Lauterbach EC. The neuropsychiatry of Parkinson’s disease and related disorders. Psychiatr Clin North Am. 2004;27:801–25. doi: 10.1016/j.psc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 152.Aarsland D, Larsen JP, Lim NG, et al. Range of neuropsychiatric disturbances in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1999;67:492–6. doi: 10.1136/jnnp.67.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Williams-Gray CH, Foltynie T, Lewis SJ, et al. Cognitive deficits and psychosis in Parkinson’s disease: a review of pathophysiology and therapeutic options. CNS Drugs. 2006;20:477–505. doi: 10.2165/00023210-200620060-00004. [DOI] [PubMed] [Google Scholar]
- 154.Goetz CG, Ouyang B, Negron A, et al. Hallucinations and sleep disorders in PD: ten-year prospective longitudinal study. Neurology. 2010;75:1773–9. doi: 10.1212/WNL.0b013e3181fd6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sanchez-Ramos JR, Ortoll R, et al. Visual hallucinations associated with Parkinson disease. Arch Neurol. 1996;53:1265–8. doi: 10.1001/archneur.1996.00550120077019. [DOI] [PubMed] [Google Scholar]
- 156.Imamura K, Wada-Isoe K, Kitayama M, et al. Executive dysfunction in non-demented Parkinson’s disease patients with hallucinations. Acta Neurol Scand. 2008;117:255–9. doi: 10.1111/j.1600-0404.2007.00933.x. [DOI] [PubMed] [Google Scholar]
- 157.Forsaa EB, Larsen JP, Wentzel-Larsen T, et al. A 12-year population-based study of psychosis in Parkinson disease. Arch Neurol. 2010;67:996–1001. doi: 10.1001/archneurol.2010.166. [DOI] [PubMed] [Google Scholar]
- 158.Factor SA, Feustel PJ, Friedman JH, et al. Longitudinal outcome of Parkinson’s disease patients with psychosis. Neurology. 2003;60:1756–61. doi: 10.1212/01.wnl.0000068010.82167.cf. [DOI] [PubMed] [Google Scholar]
- 159.Forsaa EB, Larsen JP, Wentzel-Larsen T, et al. What predicts mortality in Parkinson disease?: a prospective population-based long-term study. Neurology. 2010;75:1270–6. doi: 10.1212/WNL.0b013e3181f61311. [DOI] [PubMed] [Google Scholar]
- 160.Ballard C, Aarsland D. Spectrum of Lewy body dementias: relationship of Parkinson’s disease dementia to dementia with Lewy bodies. In: Emre M, editor. Cognitive impairment and dementia in Parkinson’s disease. Oxford, UK: Oxford University Press; 2010: 199–214.
- 161.Borroni B, Agosti C, Padovani A. Behavioral and psychological symptoms in dementia with Lewy-bodies (DLB): frequency and relationship with disease severity and motor impairment. Arch Gerontol Geriatr. 2008;46:101–6. doi: 10.1016/j.archger.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 162.Klatka LA, Louis ED, Schiffer RB. Psychiatric features in diffuse Lewy body disease: a clinicopathologic study using Alzheimer’s disease and Parkinson’s disease comparison groups. Neurology. 1996;47:1148–52. doi: 10.1212/wnl.47.5.1148. [DOI] [PubMed] [Google Scholar]
- 163.Del Ser T, Hachinski V, Merskey H, et al. Clinical and pathologic features of two groups of patients with dementia with Lewy bodies: effect of coexisting Alzheimer-type lesion load. Alzheimer Dis Assoc Disord. 2001;15:31–44. doi: 10.1097/00002093-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 164.Ala TA, Yang KH, Sung JH, et al. Hallucinations and signs of parkinsonism help distinguish patients with dementia and cortical Lewy bodies from patients with Alzheimer’s disease at presentation: a clinicopathological study. J Neurol Neurosurg Psychiatry. 1997;62:16–21. doi: 10.1136/jnnp.62.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.McKeith IG, Ballard CG, Perry RH, et al. Prospective validation of consensus criteria for the diagnosis of dementia with Lewy bodies. Neurology. 2000;54:1050–8. doi: 10.1212/wnl.54.5.1050. [DOI] [PubMed] [Google Scholar]
- 166.Tiraboschi P, Salmon DP, Hansen LA, et al. What best differentiates Lewy body from Alzheimer’s disease in early-stage dementia? Brain. 2006;129:729–35. doi: 10.1093/brain/awh725. [DOI] [PubMed] [Google Scholar]
- 167.Ballard CG, O’Brien JT, Swann AG, et al. The natural history of psychosis and depression in dementia with Lewy bodies and Alzheimer’s disease: persistence and new cases over 1 year of follow-up. J Clin Psychiatry. 2001;62:46–9. doi: 10.4088/jcp.v62n0110. [DOI] [PubMed] [Google Scholar]
- 168.Ballard C, Lowery K, Harrison R, et al. Noncognitive symptoms in Lewy body dementia. In: Perry EK, McKeith IG, Perry EK, editors. Dementia with Lewy bodies. 1st ed. Cambridge, UK: Cambridge Univ. Press; 1996: 67–84.
- 169.Stavitsky K, Brickman AM, Scarmeas N, et al. The progression of cognition, psychiatric symptoms, and functional abilities in dementia with Lewy bodies and Alzheimer disease. Arch Neurol. 2006;63:1450–6. doi: 10.1001/archneur.63.10.1450. [DOI] [PubMed] [Google Scholar]
- 170.Mori E, Shimomura T, Fujimori M, et al. Visuoperceptual impairment in dementia with Lewy bodies. Arch Neurol. 2000;57:489–93. doi: 10.1001/archneur.57.4.489. [DOI] [PubMed] [Google Scholar]
- 171.Mosimann UP, Mather G, Wesnes KA, et al. Visual perception in Parkinson disease dementia and dementia with Lewy bodies. Neurology. 2004;63:2091–6. doi: 10.1212/01.wnl.0000145764.70698.4e. [DOI] [PubMed] [Google Scholar]
- 172.Oinas M, Polvikoski T, Sulkava R, et al. Neuropathologic findings of dementia with Lewy bodies (DLB) in a population-based Vantaa 85+ study. J Alzheimers Dis. 2009;18:677–89. doi: 10.3233/JAD-2009-1169. [DOI] [PubMed] [Google Scholar]
- 173.Boecker H, Ceballos-Baumann AO, Volk D, et al. Metabolic alterations in patients with Parkinson disease and visual hallucinations. Arch Neurol. 2007;64:984–8. doi: 10.1001/archneur.64.7.984. [DOI] [PubMed] [Google Scholar]
- 174.Okada K, Suyama N, Oguro H, et al. Medication-induced hallucination and cerebral blood flow in Parkinson’s disease. J Neurol. 1999;246:365–8. doi: 10.1007/s004150050364. [DOI] [PubMed] [Google Scholar]
- 175.Nagano-Saito A, Washimi Y, Arahata Y, et al. Visual hallucination in Parkinson’s disease with FDG PET. Mov Disord. 2004;19:801–6. doi: 10.1002/mds.20129. [DOI] [PubMed] [Google Scholar]
- 176.Oishi N, Udaka F, Kameyama M, et al. Regional cerebral blood flow in Parkinson disease with nonpsychotic visual hallucinations. Neurology. 2005;65:1708–15. doi: 10.1212/01.wnl.0000187116.13370.e0. [DOI] [PubMed] [Google Scholar]
- 177.Colloby S, O’Brien J. Functional imaging in Parkinson’s disease and dementia with Lewy bodies. J Geriatr Psychiatry Neurol. 2004;17:158–63. doi: 10.1177/0891988704267468. [DOI] [PubMed] [Google Scholar]
- 178.O’Brien JT, Firbank MJ, Mosimann UP, et al. Change in perfusion, hallucinations and fluctuations in consciousness in dementia with Lewy bodies. Psychiatry Res. 2005;139:79–88. doi: 10.1016/j.pscychresns.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 179.Lobotesis K, Fenwick JD, Phipps A, et al. Occipital hypoperfusion on SPECT in dementia with Lewy bodies but not AD. Neurology. 2001;56:643–9. doi: 10.1212/wnl.56.5.643. [DOI] [PubMed] [Google Scholar]
- 180.Colloby SJ, Fenwick JD, Williams ED, et al. A comparison of (99m)Tc-HMPAO SPET changes in dementia with Lewy bodies and Alzheimer’s disease using statistical parametric mapping. Eur J Nucl Med Mol Imaging. 2002;29:615–22. doi: 10.1007/s00259-002-0778-5. [DOI] [PubMed] [Google Scholar]
- 181.Donnemiller E, Heilmann J, Wenning GK, et al. Brain perfusion scintigraphy with 99mTc-HMPAO or 99mTc-ECD and 123I-beta-CIT single-photon emission tomography in dementia of the Alzheimer-type and diffuse Lewy body disease. Eur J Nucl Med. 1997;24:320–5. doi: 10.1007/BF01728771. [DOI] [PubMed] [Google Scholar]
- 182.Imamura T, Ishii K, Hirono N, et al. Occipital glucose metabolism in dementia with Lewy bodies with and without Parkinsonism: a study using positron emission tomography. Dement Geriatr Cogn Disord. 2001;12:194–7. doi: 10.1159/000051257. [DOI] [PubMed] [Google Scholar]
- 183.Pasquier J, Michel BF, Brenot-Rossi I, et al. Value of (99m)Tc-ECD SPET for the diagnosis of dementia with Lewy bodies. Eur J Nucl Med Mol Imaging. 2002;29:1342–8. doi: 10.1007/s00259-002-0919-x. [DOI] [PubMed] [Google Scholar]
- 184.Imamura T, Ishii K, Hirono N, et al. Visual hallucinations and regional cerebral metabolism in dementia with Lewy bodies (DLB) Neuroreport. 1999;10:1903–7. doi: 10.1097/00001756-199906230-00020. [DOI] [PubMed] [Google Scholar]
- 185.Stebbins GT, Goetz CG, Carrillo MC, et al. Altered cortical visual processing in PD with hallucinations: an fMRI study. Neurology. 2004;63:1409–16. doi: 10.1212/01.wnl.0000141853.27081.bd. [DOI] [PubMed] [Google Scholar]
- 186.Nagahama Y, Okina T, Suzuki N, et al. Neural correlates of psychotic symptoms in dementia with Lewy bodies. Brain. 2010;133:557–67. doi: 10.1093/brain/awp295. [DOI] [PubMed] [Google Scholar]
- 187.Middelkoop HA, van der Flier WM, Burton EJ, et al. Dementia with Lewy bodies and AD are not associated with occipital lobe atrophy on MRI. Neurology. 2001;57:2117–20. doi: 10.1212/wnl.57.11.2117. [DOI] [PubMed] [Google Scholar]
- 188.Sanchez-Castaneda C, Rene R, Ramirez-Ruiz B, et al. Frontal and associative visual areas related to visual hallucinations in dementia with Lewy bodies and Parkinson’s disease with dementia. Mov Disord. 2010;25:615–22. doi: 10.1002/mds.22873. [DOI] [PubMed] [Google Scholar]
- 189.Ibarretxe-Bilbao N, Ramirez-Ruiz B, Tolosa E, et al. Hippocampal head atrophy predominance in Parkinson’s disease with hallucinations and with dementia. J Neurol. 2008;255:1324–31. doi: 10.1007/s00415-008-0885-8. [DOI] [PubMed] [Google Scholar]
- 190.Ibarretxe-Bilbao N, Ramirez-Ruiz B, Junque C, et al. Differential progression of brain atrophy in Parkinson’s disease with and without visual hallucinations. J Neurol Neurosurg Psychiatry. 2010;81:650–7. doi: 10.1136/jnnp.2009.179655. [DOI] [PubMed] [Google Scholar]
- 191.Teaktong T, Piggott MA, McKeith IG, et al. Muscarinic M2 and M4 receptors in anterior cingulate cortex: relation to neuropsychiatric symptoms in dementia with Lewy bodies. Behav Brain Res. 2005;161:299–305. doi: 10.1016/j.bbr.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 192.Burkhardt CR, Filley CM, Kleinschmidt-DeMasters BK, et al. Diffuse Lewy body disease and progressive dementia. Neurology. 1988;38:1520–8. doi: 10.1212/wnl.38.10.1520. [DOI] [PubMed] [Google Scholar]
- 193.Gomez-Tortosa E, Ingraham AO, Irizarry MC, et al. Dementia with Lewy bodies. J Am Geriatr Soc. 1998;46:1449–58. doi: 10.1111/j.1532-5415.1998.tb06016.x. [DOI] [PubMed] [Google Scholar]
- 194.Kalaitzakis ME, Christian LM, Moran LB, et al. Dementia and visual hallucinations associated with limbic pathology in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:196–204. doi: 10.1016/j.parkreldis.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 195.Harding AJ, Halliday GM. Cortical Lewy body pathology in the diagnosis of dementia. Acta Neuropathol. 2001;102:355–63. doi: 10.1007/s004010100390. [DOI] [PubMed] [Google Scholar]
- 196.Papapetropoulos S, Lieberman A, Gonzalez J, et al. Can Alzheimer’s type pathology influence the clinical phenotype of Parkinson’s disease? Acta Neurol Scand. 2005;111:353–9. doi: 10.1111/j.1600-0404.2005.00411.x. [DOI] [PubMed] [Google Scholar]
- 197.Camicioli R, Rajput A, Rajput M, et al. Apolipoprotein E epsilon4 and catechol-O-methyltransferase alleles in autopsy-proven Parkinson’s disease: relationship to dementia and hallucinations. Mov Disord. 2005;20:989–94. doi: 10.1002/mds.20481. [DOI] [PubMed] [Google Scholar]
- 198.McShane R, Keene J, Gedling K, et al. Hallucinations, cortical Lewy body pathology, cognitive function and neuroleptics use in dementia. In: Perry EK, McKeith IG, Perry EK, editors. Dementia with Lewy bodies. 1st ed. Cambridge, UK: Cambridge Univ. Press; 1996: p. 85–98.
- 199.Gomez-Tortosa E, Newell K, Irizarry MC, et al. Clinical and quantitative pathologic correlates of dementia with Lewy bodies. Neurology. 1999;53:1284–91. doi: 10.1212/wnl.53.6.1284. [DOI] [PubMed] [Google Scholar]
- 200.Kempster PA, O’Sullivan SS, Holton JL, et al. Relationships between age and late progression of Parkinson’s disease: a clinico-pathological study. Brain. 2010;133:1755–62. doi: 10.1093/brain/awq059. [DOI] [PubMed] [Google Scholar]
- 201.Brooks D, Halliday GM. Intralaminar nuclei of the thalamus in Lewy body diseases. Brain Res Bull. 2009;78:97–104. doi: 10.1016/j.brainresbull.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 202.Halliday GM. Thalamic changes in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:S152–5. doi: 10.1016/S1353-8020(09)70804-1. [DOI] [PubMed] [Google Scholar]
- 203.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 204.Tsuang DW, Riekse RG, Purganan KM, et al. Lewy body pathology in late-onset familial Alzheimer’s disease: a clinicopathological case series. J Alzheimers Dis. 2006;9:235–42. doi: 10.3233/jad-2006-9302. [DOI] [PubMed] [Google Scholar]
- 205.Hansen L, Salmon D, Galasko D, et al. The Lewy body variant of Alzheimer’s disease: a clinical and pathologic entity. Neurology. 1990;40:1–8. doi: 10.1212/wnl.40.1.1. [DOI] [PubMed] [Google Scholar]
- 206.Hansen LA. The Lewy body variant of Alzheimer disease. J Neural Transm Suppl. 1997;51:83–93. doi: 10.1007/978-3-7091-6846-2_7. [DOI] [PubMed] [Google Scholar]
- 207.Galvin JE, Lee VM, Trojanowski JQ. Synucleinopathies: clinical and pathological implications. Arch Neurol. 2001;58:186–90. doi: 10.1001/archneur.58.2.186. [DOI] [PubMed] [Google Scholar]
- 208.Tsuang D, Larson EB, Bolen E, et al. Visual hallucinations in dementia: a prospective community-based study with autopsy. Am J Geriatr Psychiatry. 2009;17:317–23. doi: 10.1097/JGP.0b013e3181953b9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Merdes AR, Hansen LA, Jeste DV, et al. Influence of Alzheimer pathology on clinical diagnostic accuracy in dementia with Lewy bodies. Neurology. 2003;60:1586–90. doi: 10.1212/01.wnl.0000065889.42856.f2. [DOI] [PubMed] [Google Scholar]
- 210.Caine ED, Shoulson I. Psychiatric syndromes in Huntington’s disease. Am J Psychiatry. 1983;140:728–33. doi: 10.1176/ajp.140.6.728. [DOI] [PubMed] [Google Scholar]
- 211.Mendez MF. Huntington’s disease: update and review of neuropsychiatric aspects. Int J Psychiatry Med. 1994;24:189–208. doi: 10.2190/HU6W-3K7Q-NAEL-XU6K. [DOI] [PubMed] [Google Scholar]
- 212.Tsuang D, Almqvist EW, Lipe H, et al. Familial aggregation of psychotic symptoms in Huntington’s disease. Am J Psychiatry. 2000;157:1955–9. doi: 10.1176/appi.ajp.157.12.1955. [DOI] [PubMed] [Google Scholar]
- 213.Paulsen JS, Ready RE, Hamilton JM, et al. Neuropsychiatric aspects of Huntington’s disease. J Neurol Neurosurg Psychiatry. 2001;71:310–4. doi: 10.1136/jnnp.71.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Guttman M, Alpay M, Chouinard S, et al. Clinical management of psychosis and mood disorders in Huntington’s disease. In: BÈdard M-A, Agid Y, Chouinard S, Fahn S, Korczyn AD, editors. Mental and behavioral dysfunction in movement disorders. Totowa, NJ: Humana Press; 2003: 409–26.
- 215.Julien CL, Thompson JC, Wild S, et al. Psychiatric disorders in preclinical Huntington’s disease. J Neurol Neurosurg Psychiatry. 2007;78:939–43. doi: 10.1136/jnnp.2006.103309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Marshall J, White K, Weaver M, et al. Specific psychiatric manifestations among preclinical Huntington disease mutation carriers. Arch Neurol. 2007;64:116–21. doi: 10.1001/archneur.64.1.116. [DOI] [PubMed] [Google Scholar]
- 217.van Duijn E, Kingma EM, Timman R, et al. Cross-sectional study on prevalences of psychiatric disorders in mutation carriers of Huntington’s disease compared with mutation-negative first-degree relatives. J Clin Psychiatry. 2008;69:1804–10. doi: 10.4088/jcp.v69n1116. [DOI] [PubMed] [Google Scholar]
- 218.Duff K, Paulsen JS, Beglinger LJ, et al. Psychiatric symptoms in Huntington’s disease before diagnosis: the predict-HD study. Biol Psychiatry. 2007;62:1341–6. doi: 10.1016/j.biopsych.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 219.Zappacosta B, Monza D, Meoni C, et al. Psychiatric symptoms do not correlate with cognitive decline, motor symptoms, or CAG repeat length in Huntington’s disease. Arch Neurol. 1996;53:493–7. doi: 10.1001/archneur.1996.00550060035012. [DOI] [PubMed] [Google Scholar]
- 220.Rosenblatt A. Neuropsychiatry of Huntington’s disease. Dialogues Clin Neurosci. 2007;9:191–7. doi: 10.31887/DCNS.2007.9.2/arosenblatt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Benarroch EE. The midline and intralaminar thalamic nuclei: anatomic and functional specificity and implications in neurologic disease. Neurology. 2008;71:944–9. doi: 10.1212/01.wnl.0000326066.57313.13. [DOI] [PubMed] [Google Scholar]
- 222.Joel D. Open interconnected model of basal ganglia-thalamocortical circuitry and its relevance to the clinical syndrome of Huntington’s disease. Mov Disord. 2001;16:407–23. doi: 10.1002/mds.1096. [DOI] [PubMed] [Google Scholar]
- 223.Mendez MF, Shapira JS, Woods RJ, et al. Psychotic symptoms in frontotemporal dementia: prevalence and review. Dement Geriatr Cogn Disord. 2008;25:206–11. doi: 10.1159/000113418. [DOI] [PubMed] [Google Scholar]
- 224.Larner AJ. Delusion of pregnancy in frontotemporal lobar degeneration with motor neurone disease (FTLD/MND) Behav Neurol. 2008;19:199–200. doi: 10.1155/2008/149086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Liscic RM, Kogoj A. Social behaviour versus psychiatric features of frontotemporal dementia – Clinical report of two cases. Psychiatr Danub. 2010;22:179–82. [PubMed] [Google Scholar]
- 226.Mendez MF, Lauterbach EC, Sampson SM. An evidence-based review of the psychopathology of frontotemporal dementia: a report of the ANPA Committee on Research. J Neuropsychiatry Clin Neurosci. 2008;20:130–49. doi: 10.1176/jnp.2008.20.2.130. [DOI] [PubMed] [Google Scholar]
- 227.Reischle E, Sturm K, Schuierer G, et al. A case of schizophreniform disorder in frontotemporal dementia (FTD). Psychiatr Prax. 2003;30:S78–82. [PubMed] [Google Scholar]
- 228.Reischle E, Sturm K, Schuierer G, et al. Frontotemporal dementia presenting as acute late onset schizophrenia. Psychiatr Prax. 2003;30:78–82. doi: 10.1055/s-2003-39774. [DOI] [PubMed] [Google Scholar]
- 229.Kerssens CJ, Pijnenburg YA, Schouws S, et al. The development of psychotic symptoms in later life: late-onset schizophrenia or frontotemporal dementia? A case study. Tijdschr Psychiatr. 2006;48:739–44. [PubMed] [Google Scholar]
- 230.Lillo P, Garcin B, Hornberger M, et al. Neurobehavioral features in frontotemporal dementia with amyotrophic lateral sclerosis. Arch Neurol. 2010;67:826–30. doi: 10.1001/archneurol.2010.146. [DOI] [PubMed] [Google Scholar]
- 231.Velakoulis D, Walterfang M, Mocellin R, et al. Frontotemporal dementia presenting as schizophrenia-like psychosis in young people: clinicopathological series and review of cases. Br J Psychiatry. 2009;194:298–305. doi: 10.1192/bjp.bp.108.057034. [DOI] [PubMed] [Google Scholar]
- 232.Tartaglia MC, Kertesz A, Ang LC. Delusions and hallucinations in frontotemporal dementia: a clinicopathologic case report. Cogn Behav Neurol. 2008;21:107–10. doi: 10.1097/WNN.0b013e3181799e19. [DOI] [PubMed] [Google Scholar]
- 233.Loy CT, Kril JJ, Trollor JN, et al. The case of a 48 year-old woman with bizarre and complex delusions. Nat Rev Neurol. 2010;6:175–9. doi: 10.1038/nrneurol.2010.3. [DOI] [PubMed] [Google Scholar]
- 234.Claassen DO, Parisi JE, Giannini C, et al. Frontotemporal dementia mimicking dementia with Lewy bodies. Cogn Behav Neurol. 2008;21:157–63. doi: 10.1097/WNN.0b013e3181864a09. [DOI] [PubMed] [Google Scholar]
- 235.Roeber S, Mackenzie IR, Kretzschmar HA, et al. TDP-43-negative FTLD-U is a significant new clinico-pathological subtype of FTLD. Acta Neuropathol. 2008;116:147–57. doi: 10.1007/s00401-008-0395-x. [DOI] [PubMed] [Google Scholar]
- 236.Neumann M, Rademakers R, Roeber S, et al. A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain. 2009;132:2922–31. doi: 10.1093/brain/awp214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Mackenzie IR, Munoz DG, Kusaka H, et al. Distinct pathological subtypes of FTLD-FUS. Acta Neuropathol. 2011;121:207–18. doi: 10.1007/s00401-010-0764-0. [DOI] [PubMed] [Google Scholar]
- 238.Howland RH. Schizophrenia and amyotrophic lateral sclerosis. Compr Psychiatry. 1990;31:327–36. doi: 10.1016/0010-440x(90)90039-u. [DOI] [PubMed] [Google Scholar]
- 239.Thiel A, Pilichowska ME, Hemmerlein B, et al. Dementia and psychotic symptoms in amyotrophic lateral sclerosis. Nervenarzt. 1993;64:618–22. [PubMed] [Google Scholar]
- 240.Dervaux A, Laine H. [The psychiatric forms of Creutzfeldt-Jakob disease] Presse Med. 2003;32:1466–8. [PubMed] [Google Scholar]
- 241.Wall CA, Rummans TA, Aksamit AJ, et al. Psychiatric manifestations of Creutzfeldt-Jakob disease: a 25-year analysis. J Neuropsychiatry Clin Neurosci. 2005;17:489–95. doi: 10.1176/jnp.17.4.489. [DOI] [PubMed] [Google Scholar]
- 242.Dunn NR, Alfonso CA, Young RA, et al. Creutzfeldt-Jakob disease appearing as paranoid psychosis (Letter to the Editor) Am J Psychiatry. 1999;156:2016–7. [PubMed] [Google Scholar]
- 243.Stevens EM, Lament R. Psychiatric presentation of Jakob-Creutzfeldt disease. J Clin Psychiatry. 1979;40:445–6. [PubMed] [Google Scholar]
- 244.Jardri R, DiPaola C, Lajugie C, et al. Depressive disorder with psychotic symptoms as psychiatric presentation of sporadic Creutzfeldt-Jakob disease: a case report. Gen Hosp Psychiatry. 2006;28:452–4. doi: 10.1016/j.genhosppsych.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 245.Zeidler M, Johnstone EC, Bamber RW, et al. New variant Creutzfeldt-Jakob disease: psychiatric features. Lancet. 1997;350:908–10. doi: 10.1016/s0140-6736(97)03148-6. [DOI] [PubMed] [Google Scholar]
- 246.Dervaux A, Vicart S, Lopes F, et al. Psychiatric manifestations of a new variant of Creutzfeldt-Jakob disease. Apropos of a case. Encephale. 2001;27:194–7. [PubMed] [Google Scholar]
- 247.Martindale JL, Geschwind MD, Miller BL. Psychiatric and neuroimaging findings in Creutzfeldt-Jakob disease. Curr Psychiatry Rep. 2003;5:43–6. doi: 10.1007/s11920-003-0008-2. [DOI] [PubMed] [Google Scholar]
- 248.Spalletta G, Baldinetti F, Buccione I, et al. Cognition and behaviour are independent and heterogeneous dimensions in Alzheimer’s disease. J Neurol. 2004;251:688–95. doi: 10.1007/s00415-004-0403-6. [DOI] [PubMed] [Google Scholar]
- 249.Senanarong V, Cummings JL, Fairbanks L, et al. Agitation in Alzheimer’s disease is a manifestation of frontal lobe dysfunction. Dement Geriatr Cogn Disord. 2004;17:14–20. doi: 10.1159/000074080. [DOI] [PubMed] [Google Scholar]
- 250.Zamboni G, Huey ED, Krueger F, et al. Apathy and disinhibition in frontotemporal dementia: insights into their neural correlates. Neurology. 2008;71:736–42. doi: 10.1212/01.wnl.0000324920.96835.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Fischer C, Bozanovic R, Atkins JH, et al. Treatment of delusions in dementia with Lewy bodies – response to pharmacotherapy. Dement Geriatr Cogn Disord. 2007;23:307–11. doi: 10.1159/000100901. [DOI] [PubMed] [Google Scholar]
- 252.Banerjee AK, Falkai PG, Savidge M. Visual hallucinations in the elderly associated with the use of levodopa. Postgrad Med J. 1989;65:358–61. doi: 10.1136/pgmj.65.764.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Francis PT, Perry EK. Cholinergic and other neurotransmitter mechanisms in Parkinson’s disease, Parkinson’s disease dementia, and dementia with Lewy bodies. Mov Disord. 2007;22:S351–7. doi: 10.1002/mds.21683. [DOI] [PubMed] [Google Scholar]
- 254.Goetz CG. New developments in depression, anxiety, compulsiveness, and hallucinations in Parkinson’s disease. Vol. 25. Mov Disord; 2010. pp. S104–9. [DOI] [PubMed] [Google Scholar]
- 255.Goetz CG, Pappert EJ, Blasucci LM, et al. Intravenous levodopa in hallucinating Parkinson’s disease patients: high-dose challenge does not precipitate hallucinations. Neurology. 1998;50:515–7. doi: 10.1212/wnl.50.2.515. [DOI] [PubMed] [Google Scholar]
- 256.Rabey JM. Hallucinations and psychosis in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:S105–10. doi: 10.1016/S1353-8020(09)70846-6. [DOI] [PubMed] [Google Scholar]
- 257.Court JA, Ballard CG, Piggott MA, et al. Visual hallucinations are associated with lower alpha bungarotoxin binding in dementia with Lewy bodies. Pharmacol Biochem Behav. 2001;70:571–9. doi: 10.1016/s0091-3057(01)00644-x. [DOI] [PubMed] [Google Scholar]
- 258.Cheng AV, Ferrier IN, Morris CM, et al. Cortical serotonin-S2 receptor binding in Lewy body dementia, Alzheimer’s and Parkinson’s diseases. J Neurol Sci. 1991;106:50–5. doi: 10.1016/0022-510x(91)90193-b. [DOI] [PubMed] [Google Scholar]
- 259.Kurita A, Murakami M, Takagi S, et al. Visual hallucinations and altered visual information processing in Parkinson disease and dementia with Lewy bodies. Mov Disord. 2010;25:167–71. doi: 10.1002/mds.22919. [DOI] [PubMed] [Google Scholar]
- 260.Mori T, Ikeda M, Fukuhara R, et al. Correlation of visual hallucinations with occipital rCBF changes by donepezil in DLB. Neurology. 2006;66:935–7. doi: 10.1212/01.wnl.0000203114.03976.b0. [DOI] [PubMed] [Google Scholar]
- 261.Diederich NJ, Fenelon G, Stebbins G, et al. Hallucinations in Parkinson disease. Nat Rev Neurol. 2009;5:331–42. doi: 10.1038/nrneurol.2009.62. [DOI] [PubMed] [Google Scholar]
- 262.Goetz CG, Burke PF, Leurgans S, et al. Genetic variation analysis in parkinson disease patients with and without hallucinations: case-control study. Arch Neurol. 2001;58:209–13. doi: 10.1001/archneur.58.2.209. [DOI] [PubMed] [Google Scholar]
- 263.Piggott MA, Marshall EF, Thomas N, et al. Striatal dopaminergic markers in dementia with Lewy bodies, Alzheimer’s and Parkinson’s diseases: rostrocaudal distribution. Brain. 1999;122:1449–68. doi: 10.1093/brain/122.8.1449. [DOI] [PubMed] [Google Scholar]
- 264.Sweet RA, Hamilton RL, Healy MT, et al. Alterations of striatal dopamine receptor binding in Alzheimer disease are associated with Lewy body pathology and antemortem psychosis. Arch Neurol. 2001;58:466–72. doi: 10.1001/archneur.58.3.466. [DOI] [PubMed] [Google Scholar]
- 265.Langlais PJ, Thal L, Hansen L, et al. Neurotransmitters in basal ganglia and cortex of Alzheimer’s disease with and without Lewy bodies. Neurology. 1993;43:1927–34. doi: 10.1212/wnl.43.10.1927. [DOI] [PubMed] [Google Scholar]
- 266.Kiferle L, Ceravolo R, Petrozzi L, et al. Visual hallucinations in Parkinson’s disease are not influenced by polymorphisms of serotonin 5-HT2A receptor and transporter genes. Neurosci Lett. 2007;422:228–31. doi: 10.1016/j.neulet.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 267.Ballanger B, Strafella AP, van Eimeren T, et al. Serotonin 2A receptors and visual hallucinations in Parkinson disease. Arch Neurol. 2010;67:416–21. doi: 10.1001/archneurol.2010.35. [DOI] [PubMed] [Google Scholar]
- 268.Schneider JA, Arvanitakis Z, Bang W, et al. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 269.Fernando MS, Ince PG. Vascular pathologies and cognition in a population-based cohort of elderly people. J Neurol Sci. 2004;226:13–7. doi: 10.1016/j.jns.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 270.Kovacs GG, Alafuzoff I, Al-Sarraj S, et al. Mixed brain pathologies in dementia: the BrainNet Europe consortium experience. Dement Geriatr Cogn Disord. 2008;26:343–50. doi: 10.1159/000161560. [DOI] [PubMed] [Google Scholar]
- 271.Klugman A, Marshall J, Tabet N. Impact of cerebrovascular pathology on behavioural and neuropsychiatric symptoms in patients with Alzheimer’s dementia: findings from a retrospective, naturalistic study. Int J Clin Pract. 2009;63:1024–30. doi: 10.1111/j.1742-1241.2009.02079.x. [DOI] [PubMed] [Google Scholar]
- 272.Zekry D, Hauw JJ, Gold G. Mixed dementia: epidemiology, diagnosis, and treatment. J Am Geriatr Soc. 2002;50:1431–8. doi: 10.1046/j.1532-5415.2002.50367.x. [DOI] [PubMed] [Google Scholar]
- 273.Jellinger KA. Prevalence and impact of cerebrovascular lesions in Alzheimer and Lewy body diseases. Neurodegener Dis. 2010;7:112–5. doi: 10.1159/000285518. [DOI] [PubMed] [Google Scholar]
- 274.Woodward M, Mackenzie IR, Feldman H. High prevalence of multiple brain pathologies in demenmtia (abs.). Alzheimer’s & Dem. 2006;2:S426. [Google Scholar]
- 275.Jellinger KA. The enigma of mixed dementia. Alzheimers Dement. 2007;3:40–53. doi: 10.1016/j.jalz.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 276.Jellinger KA, Attems J. Neuropathological evaluation of mixed dementia. J Neurol Sci. 2007;257:80–7. doi: 10.1016/j.jns.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 277.Jellinger KA, Attems J. Prevalence of dementia disorders in the oldest-old: an autopsy study. Acta Neuropathol. 2010;119:421–33. doi: 10.1007/s00401-010-0654-5. [DOI] [PubMed] [Google Scholar]
- 278.Savva GM, Zaccai J, Matthews FE, et al. Prevalence, correlates and course of behavioural and psychological symptoms of dementia in the population. Br J Psychiatry. 2009;194:212–9. doi: 10.1192/bjp.bp.108.049619. [DOI] [PubMed] [Google Scholar]
- 279.Spalletta G, Musicco M, Padovani A, et al. Neuropsychiatric symptoms and syndromes in a large cohort of newly diagnosed, untreated patients with Alzheimer disease. Am J Geriatr Psychiatry. 2010;18:1026–35. doi: 10.1097/JGP.0b013e3181d6b68d. [DOI] [PubMed] [Google Scholar]
- 280.Snow RE, Arnold SE. Psychosis in neurodegenerative disease. Semin Clin Neuropsychiatry. 1996;1:282–93. doi: 10.1053/SCNP00100282. [DOI] [PubMed] [Google Scholar]


