Abstract
Up-regulation of insulin-like growth factor 2 receptor (IGF-2R) involved in angiotensin II–induced cell apoptosis in cardiomyoblasts, and correlated with cardiomyocyte apoptosis in hypertensive rat hearts. Here, we detected IGF-2R levels and explored the possible underlying implications in end-stage heart failure (HF) patients before and after heart transplantation. Western blot and immunohistochemistry were used to measure cardiac IGF-2R levels. ELISA was used to detect serum IGF-2R and CD8 levels. Labelling of DNA strand breaks and dihydroethidium detection were used to determine cellular apoptosis and reactive oxygen species, respectively. Cardiac IGF-2R levels increased in end-stage HF patients (n = 11) compared with non-failing control subjects. Leu27-IGF-2, an IGF-2 analogue to activate specially the IGF-2R, could induce apoptosis and reactive oxygen species production in neonatal rat ventricular myocytes. The serum IGF-2R levels were significantly higher in HF patients than those in non-failing control subjects. An unexpected observation is that the serum IGF-2R levels further increased after heart transplantation, peaked at the first month, and gradually reduced close to the levels before heart transplantation at the 6th months after heart transplantation. Serum CD8, a marker of acute rejection, had no change after heart transplantation, but IGF-2R and Granzyme B, as a ligand for the IGF-2R and a marker for CD8 T lymphocyte activation, coexisted in the transplanted hearts. Our preliminary studies suggest that elevation of IGF-2R may participate in pathological process of end-stage HF and involved in the acute cellular rejection after heart transplantation.
Keywords: end-stage heart failure, insulin-like growth factor 2 receptor, apoptosis, heart transplantation, acute cellular rejection
Introduction
The IGF-2R, also known as the cation-independent mannose-6-phosphate receptor (M6PR), is a single-pass transmembrane glycoprotein containing a large extracellular domain and a small cytoplasmic tail, which binds IGF-2 with higher affinity than IGF-1 and does not bind insulin [1]. The receptor also binds, via distinct sites, with M6P-bearing lysosomal enzymes and a variety of other ligands, such as apoptosis/growth-inhibitory factor-retinoic acid (RA) [2], growth and differentiation factor-leukaemia inhibitory factor (LIF) [3], angiogenic peptide proliferin [4], transforming growth factor-β (TGF-β) [5], prorenin [6], and so forth. A majority of IGF-2R molecules are located in Golgi and pre-endosomal compartments, in which they segregate newly synthesized lysosomal enzymes for subsequent sorting to endosomes and lysosomes. IGF-2R are also present in the plasma membrane to regulate endocytosis of lysosomal enzymes and RA, mediates internalization and subsequent degradation of IGF-2, LIF, and proliferin, and potentiates activation of latent TGF-β and prorenin.
IGF-2R is a multifunctional protein and its expression level in the heart has a vital role in the regulation of cardiac development, growth and survival either in embryo or in the adult [7, 8]. The expression of IGF-2R is developmentally regulated, with the receptor being highly expressed in foetal and neonatal tissues and the expression declining postnatally [9]. The knockout of the IGF-2R gene in transgenic mice results in over proliferation of myocardial cells in ventricular hyperplasia and impairs cardiac development [10]. An increased expression of IGF-2R was detected in cardiomyocytes that had undergone pathological hypertrophy [11] and in the myocardial infarction scars that had undergone myocardial remodelling fibrosis [8], and IGF-2R may trigger intracellular signalling cascades involved in the progression of pathologically cardiac hypertrophy [12] and myocardial remodelling [8]. Up-regulation of IGF-2R involved in angiotensin II–induced cell apoptosis in H9c2 cardiomyoblasts, and correlated with promoting the cardiomyocyte apoptosis in hypertensive rat hearts [13]. The disruption of IGF-2R protein mediated by ribozyme leads to cellular protection against cardiomyocyte apoptosis [7]. In addition to its cardiovascular roles, IGF-2R also functions as a tumour suppressor gene involved in apoptosis and tumourigenesis [14].
To our knowledge, very limited information was available about the levels of IGF-2R in end-stage HF patients and heart transplant patients. The major purpose of this study is to demonstrate whether IGF-2R levels would increase in end-stage HF patients and heart transplant patients, and if so, to investigate the possible underlying implications of elevated IGF-2R in the process of HF and the acute rejection after heart transplantation.
Materials and methods
Patients and sample collection
For Western blot and histological analyses of cardiac IGF-2R, human LV myocardial samples were obtained from 11 patients diagnosed with end-stage HF due to dilated cardiomyopathy (DCM) during cardiac surgery at the time of heart transplantation and 11 age- and sex-matched non-failing control subjects died from accident with no history of heart disease. Table 1 presents the clinical and pathological features of the 11 HF patients. For ELISA, blood samples (5 ml) were collected at baseline (before heart transplantation) and at 1, 3, 6, and 12 months after heart transplantation. Normal blood samples were from the non-failing control subjects. Serum was prepared by clotting blood at 4°C for 2 hrs followed by centrifugation at 3000 r.p.m. for 15 min., and subsequently subdivided and stored at −70°C until test. All the patients before heart transplantation had a standard therapeutic regimen, including diuretics, digoxin, or intravenous inotropes, and ACE inhibitors, and none of them had other organ failures or detected diseases. The transplantation was performed according to the standard procedure developed at Fuwai hospital. All the heart transplant patients received immunosuppressive therapy with basiliximab (20 mg, twice) before transplantation and methylprednisolone (500 mg, twice) during transplant. Maintenance immunosuppressive therapy consisted of methylprednisolone (1 mg/kg/day tapered to 0.3 mg/kg/day at day 30), azathioprine (1 mg/kg/day), and cyclosporine after transplantation. The dose of cyclosporine (3–6 mg/kg/day) was adjusted to maintain whole blood cyclosporine concentration between 150 and 250 ng/ml. Acute rejection was diagnosed by histological assessment of myocardial biopsies and graded according to the guidelines of the International Society for Heart Transplantation (ISHT) [15]. Patients with rejection grade ≥2 were considered to have an acute rejection (AR+) and received additional immunosuppressive treatment. Table 1 also presents the biopsy grading at 1, 3, 6 and 12 months after heart transplantation. All patients and control subjects gave written informed consent for this investigation, which was approved by the Institutional Ethical Review Board of Fuwai Hospital. The investigation also conforms to the principles outlined in the Declaration of Helsinki.
Table 1.
Clinical and pathological features of patients with end-stage heart failure due to dilated cardiomyopathy before and after heart transplantation
| Before Htx | Months after Htx (Biopsy grading) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heart transplant patients | Sex | Age (years) | NYHA class | LVEDD | LVEF | 1 | 3 | 6 | 12 | |||||||||
| #1 | M | 55 | IV | 81 | 25 | 0 | 0 | 0 | 0 | |||||||||
| #2 | M | 48 | III | 94 | 15 | I | I | I | I | |||||||||
| #3 | M | 53 | III | 90 | 15 | 0 | 0 | 0 | 0 | |||||||||
| #4 | M | 64 | III | 72 | 24 | I | II | II | II | |||||||||
| #5 | M | 52 | III | 92 | 27 | 0 | 0 | 0 | 0 | |||||||||
| #6 | M | 61 | III | 102 | 26 | 0 | III | I | 0 | |||||||||
| #7 | M | 53 | IV | 79 | 14 | I | II | II | 0 | |||||||||
| #8 | M | 51 | III | 83 | 26 | 0 | 0 | 0 | I | |||||||||
| #9 | M | 46 | III | 80 | 29 | 0 | I | 0 | 0 | |||||||||
| #10 | M | 51 | III | 68 | 25 | II | 0 | 0 | 0 | |||||||||
| #11 | M | 46 | IV | 86 | 31 | I | I | 0 | 0 | |||||||||
M: male; Htx: heart transplantation; NYHA class: New York Heart Association functional class; LVEDD: LV end-diastolic dimension (mm); LVEF: LV ejection fraction (%). Biopsy grading: 0, no rejection; I, focal or diffuse but sparse infiltrate without necrosis; II, 2 or more foci of infiltrate with associated myocyte damage; III, diffuse infiltrate with multifocal myocyte damage.
Western blot
The relative abundance of IGF-2R was examined in LV myocardial extracts using standard immunoblotting procedures as described previously [16]. Primary antibodies used included goat anti-human IGF-2R antibody (0.1 μg/ml, Cat. No. AF2447; R&D System, Inc., MN, USA), and mouse anti-human antibody for Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:2000, Cat. No. ab9484; Abcam Inc., MA, USA). GAPDH was used as a loading control to normalize protein samples.
Histopathology and immunohistochemistry
For histopathological analysis, samples were fixed in 10% neutral buffered formalin. Dehydration was accomplished through alcohol and xylene gradients, followed by embedding in paraffin. Serial 5-μm sections were prepared and stained with haematoxylin and eosin to assess morphologic feature.
For immunohistochemical analysis, sections were fixed for 10 min. in 4% paraformaldehyde, permeabilized with 0.2% Triton X-100 for 5 min., and blocked in 5% bovine serum albumin. Sections were then incubated with primary antibodies for 1 hr at room temperature and washed in PBS buffer for 10 min., followed by incubation with IgG-peroxidase conjugated secondary antibody (Sigma-Aldrich, St. Louis, MO, USA) for 1 hr at room temperature, washed in PBS buffer for 10 min., and incubated with 0.5 mg/ml diaminobenzidine tetrahydrochloride 2-hydrate plus 0.05% H2O2 for 5 min. Primary antibodies used included goat anti-human IGF-2R antibody (2 μg/ml, Cat. No. AF2447; R&D System Inc., MN, USA) and rabbit anti-human granzyme B polyclonal antibodies (1:150, Cat. No. Z2145; ZETA Corporation, CA, USA).
ELISA
The commercial human IGF-2R ELISA Kit (Cat. No. ESBL2286; Ever Systems Biology Laboratory Inc., Sacramento, CA, USA) and human sCD8 ELISA kit (Cat. No. ESBL2296; Ever Systems Biology Laboratory Inc.) were used in this study to determine serum IGF-2R and serum CD8 levels according to the manufacturer’s protocols. The resultant reaction was read at a wavelength of 450 nm on 96-well Microplate Rader (Bio Rad, Mode 680, Tokyo, Japan).
Small interfering RNA (siRNA) and transfection
Double-stranded siRNA targeting IGF-2R (GenBank accession no. NM_012756.1, 2099 nucleotides downstream of the start codon; sense: 5’-GGA AUG GCA CGC UGU AUA Att-3’; antisense: 5’-UUA UAC AGC GUG CCA UUC Ctt-3’) was designed on the siRNA Design Guidelines web site (http://www.ambion.com/techlib/misc/siRNA_design.html) and synthesized by GeneChem (Shanghai, China). A non-silencing-siRNA-duplex (sense: 5’-UUC UCC GAA CGU GUC ACG Utt-3’; antisense: 5’-ACG UGA CAC GUU CGG AGA Att-3’, GeneChem) was used as a negative control. The silencing effect of IGF-2R-siRNA was confirmed by checking its mRNA expression levels via real-time RT-PCR. IGF-2R mRNA expression was reduced by 60% in cultured neonatal rat ventricular myocytes transfected with IGF-2R-siRNA (40 nM) for 12 hrs using Lipofecttamine™ 2000 (Cat. No. 11668-027).
Neonatal rat ventricular myocyte (NRVM) culture and treatment
Neonatal rat ventricular myocytes were isolated and cultured as described previously [17]. After the cells started beating 48 hrs post-culture, they were cultured in serum-free medium for 24 hrs and then treated with or without Leu27-IGF-2 (10−8 M; GroPep, Adelaid, Australia) for 24 hrs. For IGF-2R inhibition study, after transfection with IGF-2R-siRNA (40 nM) for 12 hrs, NRVMs were treated with Leu27-IGF-2 (10−8 M) for 24 h, then the cells were harvested for immunocytochemical detection and quantification of apoptosis and measurement of reactive oxygen species (ROS). Valinomycin (10−7M, Cat. no. V3639; Sigma-Aldrich, Inc., MO, USA) treated cells were used as positive control for apoptosis [18] and ROS [19] detection.
The procedure using rats for primary NCVM culture was approved by the Animal Laboratory Use and Care Committee at Fuwai Hospital. Studies also conformed to the Guide for the Care and Use of Laboratory Animals (NIH Publication 85-23, revised 1996).
Immunocytochemical detection and quantification of apoptosis
Immunocytochemical detection and quantification of NRVM apoptosis was detected using an In Situ Cell Death Detection Kit (Cat. No. 11-684-817-910; Roche, Mannheim, Germany), based on labelling of DNA strand breaks (TUNEL technology), according to the manufacturer’s protocol. Six fields of three independent experiments were randomly selected under light microscopy and the percentage of apoptosis was calculated by the ratio of TUNEL-positive cells to total cells per field of vision (100χ; Olympus BX61, Olympus, Tokyo, Japan). Parallel cultured cells were stained with DAPI for nuclear labelling according to the method previously described [18] to count the total cells per field of vision.
Measurement of ROS
ROS was measured using ROS Fluorescent Probe-Dihydroethidium (DHE) Detection Kit (Cat. No. R001; Vigorous Biotechnology, Beijing, China) according to the manufacturer’s protocol. Imaging was acquired using a blue excitation fluorescence filter under fluorescent microscope. Six fields of three independent experiments were randomly selected under fluorescent microscopy and the percentage of ROS positive cells was calculated by the ratio of ROS-positive cells to total cells per field of vision (40χ; Olympus IX70). Parallel cultured cells were stained with DAPI for nuclear labelling according to the method previously described [18] to count the total cells per field of vision.
Statistical analysis
All data are presented as mean ± S.D. SPSS software (SPSS Inc., Chicago, IL, USA) for windows 11.0 was used for all statistical analyses. Differences between two groups were compared using a one-way ANOVA. A values of P < 0.05 was considered statistically significant.
Results
Expression analyses of IGF-2R in failing hearts
Five DCM failing hearts (DCM-HF) selected randomly from the patient cohort shown in Table 1 and five matched non-failing control hearts (NF) were analysed by Western blot analysis. The results showed an apparent increase in IGF-2R protein levels in DCM-HF when compared with NF (P < 0.01; Fig. 1).
Fig 1.
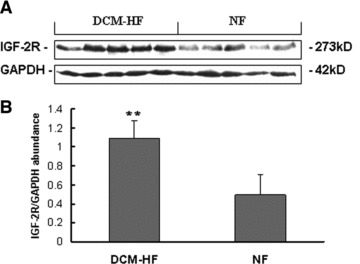
Western blot analysis of cardiac IGF-2R. (A) Representative Western blot analysis of cardiac IGF-2R expression. (B) Densitometric quantification of cardiac IGF-2R expression. The IGF-2R protein levels related to the internal standard protein GAPDH were calculated as the relative abundance. Densitometric analyses of the blots showed an apparent increase in IGF-2R in DCM failing hearts (DCM-HF, n = 5) compared with non-failing control hearts (NF, n = 5; **P < 0.01). Data are presented as mean ± S.D.
Interstitial fibrosis in DCM-HF was observed compared with NF in the HE staining (Fig. 2A). In the immunohistochemical staining, the control specimens from NF exhibited few and weak IGF-2R immunoreactivity. In contrast, more and strong IGF-2R immunoreactivity was observed in DCM-HF (Fig. 2B).
Fig 2.
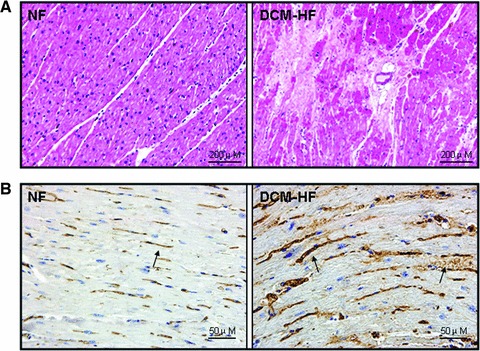
Histopathological analysis of IGF-2R. (A) Representative light microscopic findings in haematoxylin and eosin staining. The normal appearance of myocardial fibres with central nuclei is seen in non-failing control hearts (NF) and interstitial fibrosis replacement is found in DCM failing hearts (DCM-HF). (B) Representative light microscopic IGF-2R immunoreactivity using a monoclonal antibody, which recognizes IGF-2R. Few and weak immunoreactivity of IGF-2R is observed in non-failing control hearts (NF), but more and strong IGF-2R immunoreactivity can be seen in DCM failing hearts (DCM-HF). Arrows indicated the positive staining.
Effects of IGF-2R on apoptosis and ROS in cultured NRVMs
To know whether IGF-2R might involve in the pathological progress of HF, we observed the effects of IGF-2R on apoptosis and ROS in cultured NRVMs. Leu27-IGF-2, an IGF-2 analogue to activate specially the IGF-2R, was used to specifically stimulate IGF-2R.
In TUNEL assay (Fig. 3), Leu27-IGF-2 (10−8M) could increase the percentage of TUNEL-positive NRVMs (P < 0.01 versus control), and siRNA-IGF-2R could partly reduce the Leu27-IGF-2–induced cell apoptosis (#P < 0.05 versus Leu27-IGF-2-treated cells).
Fig 3.
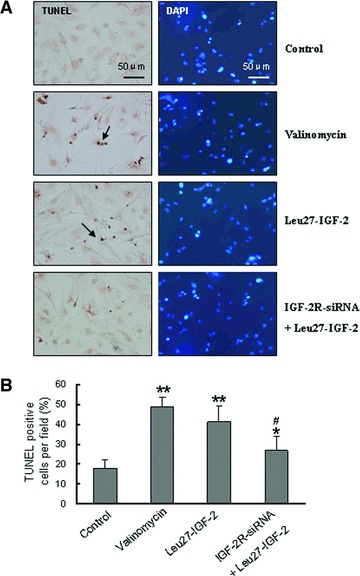
Apoptosis induced by Leu27-IGF-2. (A) Apoptotic cells were detected by TUNEL assay in NRVMs. Parallel cultured cells were stained with DAPI for nuclear labelling to count total cells per field of vision. Valinomycin treatment served as the positive control for apoptosis. (B) The percentage of apoptotic cells was calculated by the ratio of TUNEL-positive cells to total cells per field of vision. The data were obtained from six different visual fields of three independent experiments. Data are presented as mean ± S.D. **P < 0.01 and *P < 0.05 versus untreated control. #P < 0.05 versus Leu27-IGF-2–treated cells.
Figure 4 showed that Leu27-IGF-2 (10−8M) could increase the percentage of ROS-positive NRVMs (P < 0.01 versus control), and that the siRNA-IGF-2R could partly reduce the Leu27-IGF-2-induced ROS generation (#P < 0.01 versus Leu27-IGF-2-treated cells).
Fig 4.
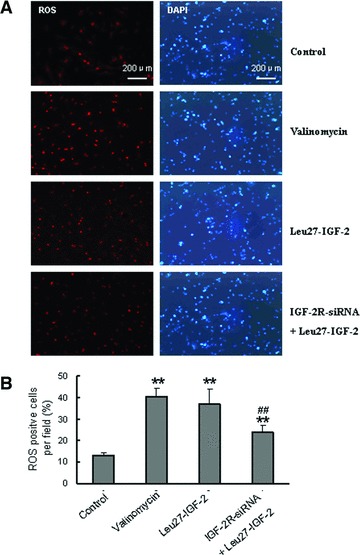
ROS generation induced by Leu27-IGF-2. (A) ROS-positive cells were detected by fluorescent probe-Dihydroethidium (DHE) in NRVMs. Parallel cultured cells were stained with DAPI for nuclear labelling to count total cells per field of vision. Valinomycin treatment served as the positive control for ROS. (B) The percentage of ROS-positive cells was calculated by the ratio of ROS-positive cells to total cells per field of vision. The data were obtained from six different visual fields of three independent experiments. Data are presented as mean ± S.D. **P < 0.01 versus untreated control. ##P < 0.01 versus Leu27-IGF-2–treated cells.
Serum IGF-2R levels in end-stage HF patients before and after heart transplantation
Figure 5A showed that the mean level of serum IGF-2R in DCM HF patients before heart transplantation (Bef-Htx, 849.44 ± 385.24 pg/ml, n = 11) was significantly higher than that in non-failing control subjects (NF, 328.01 ± 20.35 pg/ml, n = 11; P < 0.01). After heart transplantation, serum IGF-2R levels further increased, peaked at the first month (1439.40 ± 491.15 pg/ml), and gradually reduced close to the pre-operative level at the 6th months, but remained to be higher than that in NF (P < 0.01).
Fig 5.
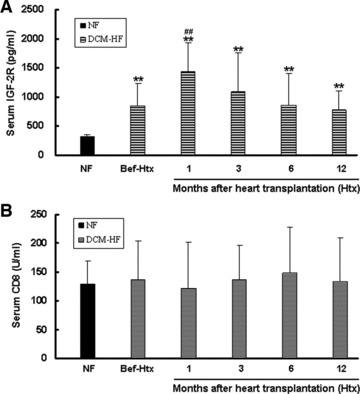
ELISA of serum IGF-2R and serum CD8. (A) The mean level of serum IGF-2R in DCM heart failure patients before heart transplantation (Bef-Htx, n = 11) was significantly higher than that in non-failing control subjects (NF, n = 11). After heart transplantation, serum IGF-2R levels further increased, peaked at the first month, and gradually reduced close to the pre-operative level at the 6th months, but remained to be higher than that in non-failing controls. **P < 0.01 versus NF. ##P < 0.01 versus Bef-Htx. (B) The mean level of serum CD8 in DCM heart failure patients before heart transplantation (Bef-Htx, n = 11) had no increase when compared with that in non-failing control subjects (NF, n = 11). Mean levels of serum CD8 also had no change at different time point after heart transplantation compared with that before heart transplantation (Bef-Htx). Data are presented as mean ± S.D.
To know the relationship of serum IGF-2R with serum CD8, which acts as the marker for T lymphocyte activation and acute rejection, we determined the circulating concentrations of CD8 in the same cohort before and after heart transplantation (Fig. 5B). The mean level of serum CD8 in DCM HF patients (DCM-HF, n = 11) had no increase compared with that in non-failing control subjects (NF, n = 11). Mean level of serum CD8 also had no change at different time point after heart transplantation when compared with that before heart transplantation (Bef-Htx). We failed to find any correlation between serum IGF-2R and serum CD8.
Coexistence of IGF-2R with granzyme B-positive T lymphocytes in transplanted hearts with acute rejection
To explore the relationship between IGF-2R and acute rejection after heart transplantation, endomyocardial biopsies with rejection grade ≥ 2 was studied by immunohistochemistry on serial sections. Representative immunohistochemical staining revealed that IGF-2R and granzyme B-positive T lymphocytes coexisted at the same site in biopsies with rejection grade 3 from patients #6 (Fig. 6A) and grade 2 from patient #4 (Fig. 6B) at the 3rd month after heart transplantation, the corresponding HE staining indicated mononuclear cell infiltration with myocyte damage in the same sites.
Fig 6.
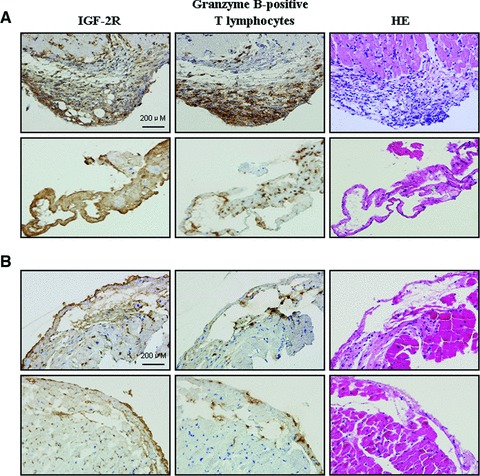
Immunohistochemical detection of IGF-2R and granzyme B-positive T lymphocytes. Representative immunohistochemical staining on the serial sections from biopsies with rejection grade 3 from patients #6 (A) and from biopsies with rejection grade 2 from patient #4 (B) at the 3rd month after heart transplantation showed coexistence of IGF-2R and granzyme B-positive T lymphocytes, accompanied by mononuclear cell infiltration with myocyte damage in the same sites in haematoxylin and eosin (HE) staining.
Discussion
IGF-2R dependently correlated with the progression of pathological hypertrophy after complete abdominal aorta ligation [18]. Up-regulation of IGF-2R involved in angiotensin II–induced cell apoptosis in H9c2 cardiomyoblasts, and correlated with promoting the cardiomyocyte apoptosis in hypertensive rat hearts [13]. A significant association was observed between IGF-2R overexpression and infracted myocardium in human cardiovascular tissue array by immunohistochemistry [12]. The earlier results gave us some hint and urged us to examine the levels of IGF-2R and explore the possible underlying implications of IGF-2R in end-stage HF patients and heart transplant patients.
IGF-2R in end-stage HF patients
In our previous studies, we detected an increase in levels of cardiac IGF-2R in end-stage HF patients using protein antibody array technology [20]. In this study, we further confirmed our previous results in another cohort of end-stage HF patients by Western blot and immunohistochemistry. We also observed for the first time that serum IGF-2R levels increased in end-stage HF patients compared with non-failing control subjects. In addition to intracellular and membrane-associated IGF-2R, a circulating form of soluble IGF-2R has been identified in serum in humans, and the release of the soluble receptor may be a major mechanism determining the turnover of intracellular and membrane-associated IGF-2R [21]. Therefore, our findings of elevated levels of serum IGF-2R might reflect the increase of intracellular and membrane-associated IGF-2R in the failing hearts. Our preliminary results suggested that circulating IGF-2R might act as a biomarker of HF, which is worthy of being further investigated.
It was recently reported that IGF-2R activation might trigger its downstream cascades and promote the cardiomyocyte apoptosis [18]. Apoptosis has been identified in a wide variety of cardiovascular disorders, including HF [23]. In our study, we got the same results with previous reports [13, 18] that IGF-2R activation could induce apoptosis of cultured cardiomyocytes, suggesting that elevated levels of IGF-2R in the failing hearts might contribute to the progression of HF by IGF-2R–induced apoptosis. The molecular mechanism behind IGF-2R–induced apoptosis is that IGF-2R could function as a G protein–coupled receptor to modulate calcineurin together with Gαq, contributing to the impairment of MOMP and induce apoptosis [18]. In view of the fact of IGF-2R activation inducing cardiomyocyte apoptosis, suppression of IGF-2R signalling pathways might be a good strategy for both the protection against myocardiocyte apoptosis and the prevention of HF progression.
Experimental and clinical studies have demonstrated the increased ROS production in the failing heart, regardless of the aetiology [23-26]. ROS have long been proposed as contributing to the deterioration of cardiac function in HF patients [23, 27]. ROS may act in some instances as second messengers downstream of specific ligands, such as TGF-β1, PDGF, angiotensin II, FGF-2, endothelin, tumour necrosis factor-α and others [24, 27] to induce cardiomyocyte apoptosis, by which ROS may be implicated in experimental and clinical HF [24, 25, 27]. In this study, we demonstrated for the first time that IGF-2R activation also promoted the generation of ROS in cultured cardiomyocytes, which might act as one of the mechanisms that IGF-2R–induced cardiomyocyte apoptosis, because ROS were reported to induce cardiomyocyte apoptosis by a variety of mechanisms [27]. Our results suggest that elevated levels of cardiac IGF-2R might be involved in the pathological process of end-stage HF by inducing cardiomyocyte apoptosis via the increased generation of cardiac ROS. We have to acknowledge that our result is preliminary and descriptive, and further investigations are required to clarify the signal pathways involved in IGF-2R-stimulated ROS generation and determine the intracellular source of ROS generation stimulated by IGF-2R.
IGF-2R in heart transplant patients
We thought that the elevated levels of serum IGF-2R in end-stage HF patients would decrease after heart transplantation. Unexpectedly, serum IGF-2R levels further increased at the first month after heart transplantation compared with those before heart transplantation, and thereafter gradually decreased. It attracted our interest to further investigate the possible implications of increased IGF-2R after heart transplantation. Although the source of IGF-2R in blood is not clear, the circulating IGF-2R may represent a truncated receptor that has lost its cytosolic and transmembrane domain and the release of soluble receptor fragments may be a major mechanism determining the turnover of intracellular and membrane-associated IGF-2R [21]. Therefore, elevated circulating IGF-2R might mirror at least in part the increase of cardiac IGF-2R in our heart transplant patients. We hypothesized that enhanced cardiac IGF-2R might be involved in acute rejection after heart transplantation.
Acute rejection after heart transplantation is a significant cause of morbidity and mortality [28]. Although the incidence of acute cardiac allograft rejection varies from centre to centre, transplant recipients have an average of two to three episodes in the first year after transplantation with 50–80% of patients having at least one rejection episode [28]. Several lines of evidence have established granzyme B released by activated CD8 T lymphocytes as main mediators of acute cellular rejection [29, 30]. Gramzyme B-positive CD8 T lymphocytes in cardiac allograft infiltrates during acute cellular rejection are the main cell type engaged in rejection [29], which is crucial in acute rejection of allografts by granzyme B–mediated target cell apoptosis and death through several pathways [31, 32]. To test our hypothesis that increased IGF-2R in transplanted hearts might involve in acute rejection, we used endomyocardial biopsies to perform HE staining to observe crisis of cardiac cellular rejection and immunohistochemical staining to observe whether granzyme B-positive CD8 T lymphocytes and IGF-2R coexisted in transplanted hearts on the serial sections. Our results showed that obvious coexistence of imunoreactive granzyme B-positive CD8 T lymphocytes and IGF-2R in the biopsies with rejection grades 2 and 3. Although we have no evidence to show the direct association between IGF-2R and granzyme B, previous studies have demonstrated that IGF-2R is the cell surface receptor that can bind and internalize granzyme B [31, 32]. Inhibition of the granzyme B–IGF-2R interaction prevented granzyme B cell surface binding, uptake and the induction of apoptosis and necrosis, indicating that expression of the IGF-2R was essential for granzyme B–mediated death of target cell in vitro and for the rejection of allogeneic cells in vivo [31]. Therefore, our results of the coexistence of immunoreactive IGF-2R and granzyme B-positive DC8 T lymphocyte in transplanted hearts provide the indirect evidence that IGF-2R might act as receptor for granzyme B and involved in acute rejection by inducing granzyme B–mediated necrosis of transplanted hearts.
Acute cellular rejection occurs most frequently during the first 3 months after transplantation, with the incidence declining significantly thereafter [33]. In our study, serum IGF-2R levels further increased after heart transplantation compared with those before heart transplantation, peaked at the first month, and gradually reduced to the close pre-operative levels at the 6th months after heart transplantation, but remained to be higher than that in non-failing controls. Our results showed that the time course of serum IGF-2R levels after heart transplantation is similar with that of acute cellular rejection, which reminded us that serum IGF-2R level might act as a marker for acute cellular rejection. Several lines of evidence suggested that serum CD8 in blood may act as a marker for T lymphocyte activation and acute cellular rejection [34, 35]. Activated T lymphocytes release soluble forms of CD8 antigen that migrate from the inflammation site to the circulatory system [34]. In our study, to observe the correlation of serum IGF-2R with serum CD8 antigen, we determined the abundance of serum CD8 antigen at different time point after heart transplantation in the same patient cohort. Unlike apparent elevation of serum IGF-2R levels, serum CD8 levels have no changes after heart transplantation, which might be because of the immunosuprssive therapy to inhibit the response of serum CD8. Although further investigations are necessary to confirm the correlation between IGF-2R and acute cellular rejection, a fundamental prediction of our preliminary results is that circulating IGF-2R might act as a hopeful candidate for markers to predict acute rejection in patients with early cellular rejection crisis. One advantage over serum CD8 to select serum IGF-2R as a marker for acute rejection in heart transplantation is that immunosuppressive therapy had no interference on the serum IGF-2R levels.
Conclusion
Our preliminary studies suggest that elevated levels of cardiac IGF-2R might involved in the pathological process of end-stage HF by inducing cardiac cell apoptosis via the increased generation of cardiac ROS, and participate in the acute cellular rejection by granzyme B–mediated myocyte damage after heart transplantation. Further investigations need to be performed to confirm our findings, especially in animal models of HF and heart transplantation to avoid interference of drug therapy.
Acknowledgments
This study was supported by the National 973 Program [2010CB529506 to Y.J.W]; the National 863 Program [2006AA02Z4B8 to Y.J.W] and the National science and technology major project [2008ZX09312-018 to Y.S.L].
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Hawkes C, Jhamandas JH, Harris KH, et al. Single transmembrane domain insulin-like growth factor-II/mannose-6-phosphate receptor regulates central cholinergic function by activating a G-protein sensitive, protein kinase C-dependent pathway. J Neurosci. 2006;26:585–96. doi: 10.1523/JNEUROSCI.2730-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kang JX, Bell J, Beard RL, et al. Mannose 6-phosphate/insulin-like growth factor II receptor mediates the growth-inhibitory effects of retinoids. Cell Growth Differ. 1999;10:591–600. [PubMed] [Google Scholar]
- 3.Blanchard F, Duplomb L, Raher S, et al. Mannose 6-phosphate/insulin-like growth factor II receptor mediates internalization and degradation of leukemia inhibitory factor but not signal transduction. J Biol Chem. 1999;274:24685–93. doi: 10.1074/jbc.274.35.24685. [DOI] [PubMed] [Google Scholar]
- 4.Lee SJ, Nathans D. Proliferin secreted by cultured cells binds to mannose 6-phosphate receptors. J Biol Chem. 1988;263:3521–7. [PubMed] [Google Scholar]
- 5.Dennis PA, Rifkin DB. Cellular activation of latent transforming growth factor beta requires binding to the cation-independent mannose 6-phosphate/insulin-like growth factor type II receptor. Proc Natl Acad Sci U S A. 1991;88:580–4. doi: 10.1073/pnas.88.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Kesteren CAM, Danser AHJ, Derkx FHM, et al. Mannose 6-phosphate receptor-mediated internalization and activation of prorenin by cardiac cells. Hypertension. 1997;30:1389–96. doi: 10.1161/01.hyp.30.6.1389. [DOI] [PubMed] [Google Scholar]
- 7.Chen Z, Ge Y, Kang JX. Down-regulation of the M6P/IGF-II receptor increases cell proliferation and reduces apoptosis in neonatal rat cardiac myocytes. BMC Cell Biol. 2004;5:15. doi: 10.1186/1471-2121-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang MH, Kuo WW, Chen RJ, et al. IGF-II/Mannose 6-phosphate receptor activation induces metalloproteinase-9 matrix activity and increases plaminogen activator expression inH9c2 cardiomyoblast cells. J Mol Endocrinol. 2008;41:65–74. doi: 10.1677/JME-08-0051. [DOI] [PubMed] [Google Scholar]
- 9.Nissley P, Kiess W, Skler M. Developmental expression of the IGF-II/Mannose 6-phosphate receptor. Mol Reprod Dev. 1993;35:408–13. doi: 10.1002/mrd.1080350415. [DOI] [PubMed] [Google Scholar]
- 10.Lau MM, Stewart CE, Liu Z, et al. Loss of the imprinted IGF2/cation-independent mannose 6-phosphate receptor results in foetal overgrowth and perinatal lethality. Genes Dev. 1994;8:2953–63. doi: 10.1101/gad.8.24.2953. [DOI] [PubMed] [Google Scholar]
- 11.Huang CY, Hao LY, Buetow DE. Hypertrophy of cultured adult rat ventricular cardiomyocytes induced by antibodies against the insulin-like growth factor (IGF)-I or the IGF-I receptor is IGF-II-dependent. Mol Cell Biochem. 2002;233:65–72. doi: 10.1023/a:1015514324328. [DOI] [PubMed] [Google Scholar]
- 12.Chu CH, Tzang BS, Chen LM, et al. IGF-II/mannose-6-phosphate receptor signaling induced cell hypertrophy and atrial natriuretic peptide/BNP expression via Gaq interaction and protein kinase C-a/CaMKII activation in H9c2 cardiomyoblast cells. J Endocrinol. 2008;197:381–90. doi: 10.1677/JOE-07-0619. [DOI] [PubMed] [Google Scholar]
- 13.Lee SD, Chu CH, Huang EJ, et al. Roles of insulin-like growth factor II in cardiomyoblast apoptosis and in hypertensive rat heart with abdominal aorta ligation. Am J Physiol Endocrinol Metab. 2006;291:E306–14. doi: 10.1152/ajpendo.00127.2005. [DOI] [PubMed] [Google Scholar]
- 14.Motyka B, Korbutt G, Pinkoski MJ, et al. Mannose 6-phosphate/insulin-like growth factor II receptor is a death receptor for granzyme B during cytotoxic T cell-induced apoptosis. Cell. 2000;103:491–500. doi: 10.1016/s0092-8674(00)00140-9. [DOI] [PubMed] [Google Scholar]
- 15.Billingham ME, Cary NR, Hammond ME, et al. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group. The International Society for Heart Transplantation. J Heart Transplant. 1990;9:587–93. [PubMed] [Google Scholar]
- 16.Seeland U, Selejan S, Engelhardt S, et al. Interstitial remodeling in β1-adrenergic receptor transgenic mice. Basic Res Cardiol. 2007;102:183–93. doi: 10.1007/s00395-006-0635-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeyaseelan R, Poizat C, Baker RK, et al. A novel cardiac-restricted target for doxorubicin. J Biol Chem. 1997;272:22800–8. doi: 10.1074/jbc.272.36.22800. [DOI] [PubMed] [Google Scholar]
- 18.Chu CH, Tzang BS, Chen LM, et al. Activation of insulin-like growth factor II receptor induces mitochondrial-dependent apoptosis through G_q and downstream calcineurin signaling in myocardial cells. Endocrinology. 2009;150:2723–31. doi: 10.1210/en.2008-0975. [DOI] [PubMed] [Google Scholar]
- 19.Andrukhiv A, Costa AD, West IC, et al. Opening mitoKATP increases superoxide generation from complex I of the electron transport chain. Am J Physiol Heart Circ Physiol. 2006;291:H2067–74. doi: 10.1152/ajpheart.00272.2006. [DOI] [PubMed] [Google Scholar]
- 20.Wei Y, Cui C, Lainscak M, et al. Type-specific dysregulation of matrix metalloproteinases and their tissue inhibitors in end-stage heart failure patients. J Cell Mol Med. 2011;15:773–82. doi: 10.1111/j.1582-4934.2010.01049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Causin C, Waheed A, Braulke T, et al. Mannose 6-phosphate/insulin-like growth factor-II binding proteins in human serum and urine. Biochem J. 1988;252:795–9. doi: 10.1042/bj2520795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang PM, Izumo S. Apoptosis and heart failure: a critical review of the literature. Circ Res. 2000;86:1107–13. doi: 10.1161/01.res.86.11.1107. [DOI] [PubMed] [Google Scholar]
- 23.Grieve DJ, Shah AM. Oxidative stress in heart failure. Eur Heart J. 2003;24:2161–3. doi: 10.1016/j.ehj.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 24.Shizukuda Y, Buttrick PM. Oxygen free radicals and heart failure: new insight into an old question. Am J Physiol Lung Cell Mol Physiol. 2002;283:L237–8. doi: 10.1152/ajplung.00111.2002. [DOI] [PubMed] [Google Scholar]
- 25.Byrne JA, Grieve DJ, Cave AC, et al. Oxidative stress and heart failure. Arch Mal Coeur Vaiss. 2003;96:214–21. [PubMed] [Google Scholar]
- 26.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–8. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singal PK, Khaper N, Palace V, et al. The role of oxidative stress in the genesis of heart disease. Cardiovas Res. 1998;40:426–32. doi: 10.1016/s0008-6363(98)00244-2. [DOI] [PubMed] [Google Scholar]
- 28.Hunt SA. Complications of heart transplantation. J Heart Transplant. 1983;3:70–8. [Google Scholar]
- 29.Hameed A, Truong LD, Price V, et al. Immunohistochemical localization of granzyme B antigen in cytotoxic cells in human tissues. Am J Pathol. 1991;138:1069–75. [PMC free article] [PubMed] [Google Scholar]
- 30.Clement MV, Haddad P, Soulie A, et al. Perforin and granzyme B as markers for acute rejection in heart transplantation. Int Immunol. 1991;3:1175–81. doi: 10.1093/intimm/3.11.1175. [DOI] [PubMed] [Google Scholar]
- 31.Bruce Motyka B, Korbutt G, Pinkoski MJ, et al. Mannose 6-phosphate/insulin-like growth factor II receptor is a death receptor for granzyme B during cytotoxic t cell–induced apoptosis. Cell. 2000;103:491–500. doi: 10.1016/s0092-8674(00)00140-9. [DOI] [PubMed] [Google Scholar]
- 32.Veugelers K, Motyka B, Goping IS, et al. Granule-mediated killing by granzyme B and perforin requires a mannose 6-phosphate receptor and is augmented by cell surface heparan sulfate. Mol Biol Cell. 2006;17:623–33. doi: 10.1091/mbc.E05-07-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kubo SH, Naftel DC, Mills RM, Jr, et al. Risk factors for late recurrent rejection after heart transplantation: a multiinstitutional, multivariable analysis. J Heart Lung Transplant. 1995;14:409–18. [PubMed] [Google Scholar]
- 34.Ho AD, Maruyama M, Maghazachi A, et al. Corringham RET: soluble CD4, soluble CD8,soluble CD25, lymphopoetic recovery and endogenous cytokines after high dose chemotherapy and blood stem cell transplantation. Blood. 1994;84:3550–7. [PubMed] [Google Scholar]
- 35.Willsie SK, Herndon BL, Miller L, et al. Soluble versus cell-bound CD4, CD8 from bronchoalveolar lavage: correlation with pulmonary diagnoses in human immunodeficiency virus-infected individuals. J Leukocyte Biol. 1996;59:813–6. doi: 10.1002/jlb.59.6.813. [DOI] [PubMed] [Google Scholar]


