Abstract
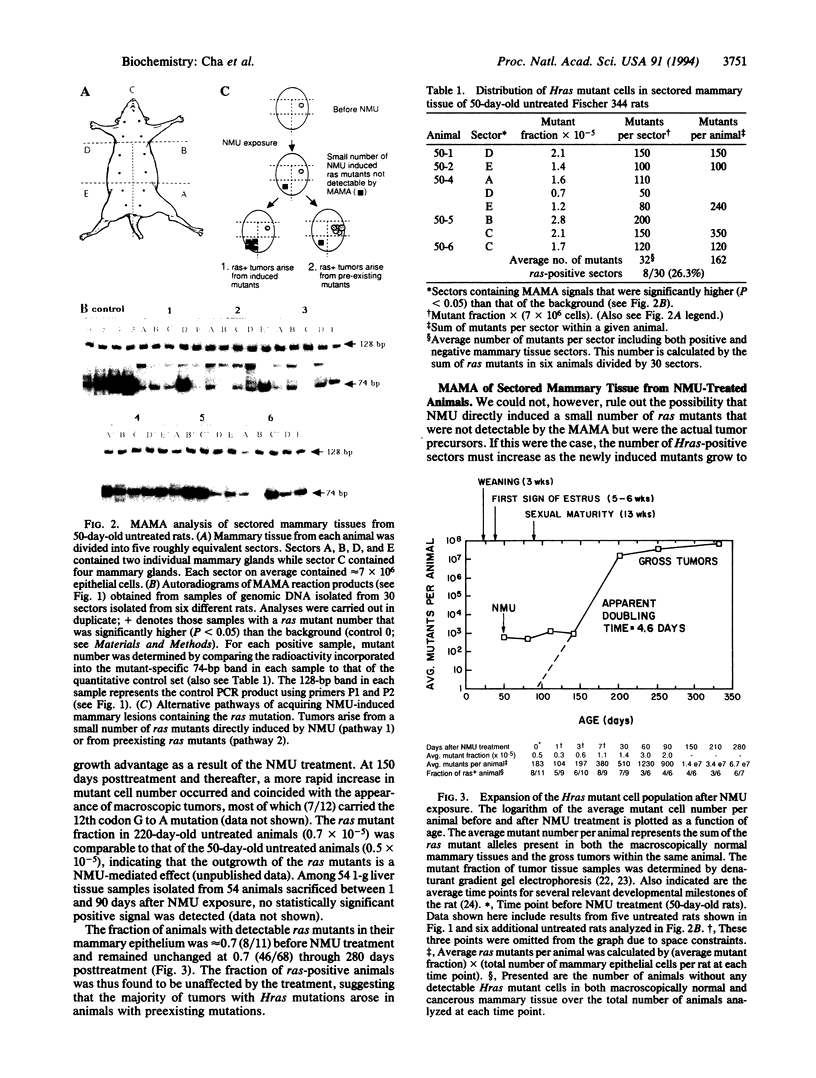
GGA to GAA mutations in the 12th codon of the Hras gene are frequently observed in rat mammary tumors induced by N-nitroso-N-methylurea (NMU). We developed an assay to measure point mutations present in tissues at a frequency of 10(-5) and have now applied this assay to measure the specific G to A transition of the Hras gene in rat mammary epithelium. We find that (i) 70% of untreated rats contain detectable levels of Hras mutants; (ii) these mutants are clustered within the gland as sectors in a manner consistent with their origin as a mutation arising during early organ development; and (iii) treatment with a carcinogenic dose of NMU did not result in a significant increase in the number of such mutants, the fraction of organ sectors with mutant cells, or the fraction of animals containing detectable levels of ras mutants. We conclude that the NMU-induced mammary tumors carrying the G to A transition at the 12th codon of the Hras gene arose from preexisting ras mutants and that an independent effect of NMU was directly or indirectly responsible for tumor formation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aidoo A., Lyn-Cook L. E., Mittelstaedt R. A., Heflich R. H., Casciano D. A. Induction of 6-thioguanine-resistant lymphocytes in Fischer 344 rats following in vivo exposure to N-ethyl-N-nitrosourea and cyclophosphamide. Environ Mol Mutagen. 1991;17(3):141–151. doi: 10.1002/em.2850170302. [DOI] [PubMed] [Google Scholar]
- Ames B. N. Endogenous DNA damage as related to cancer and aging. Mutat Res. 1989 Sep;214(1):41–46. doi: 10.1016/0027-5107(89)90196-6. [DOI] [PubMed] [Google Scholar]
- Bains W., Bains J. Rate of base substitution in mammalian nuclear DNA is dependent on local sequence context. Mutat Res. 1987 Jul;179(1):65–74. doi: 10.1016/0027-5107(87)90042-x. [DOI] [PubMed] [Google Scholar]
- Balmain A., Brown K. Oncogene activation in chemical carcinogenesis. Adv Cancer Res. 1988;51:147–182. doi: 10.1016/s0065-230x(08)60222-5. [DOI] [PubMed] [Google Scholar]
- Belinsky S. A., Devereux T. R., Maronpot R. R., Stoner G. D., Anderson M. W. Relationship between the formation of promutagenic adducts and the activation of the K-ras protooncogene in lung tumors from A/J mice treated with nitrosamines. Cancer Res. 1989 Oct 1;49(19):5305–5311. [PubMed] [Google Scholar]
- Bird A. P. DNA methylation and the frequency of CpG in animal DNA. Nucleic Acids Res. 1980 Apr 11;8(7):1499–1504. doi: 10.1093/nar/8.7.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm T. L., Drahovsky D. Hypomethylation of DNA in Raji cells after treatment with N-methyl-N-nitrosourea. Carcinogenesis. 1981;2(1):39–42. doi: 10.1093/carcin/2.1.39. [DOI] [PubMed] [Google Scholar]
- Cha R. S., Zarbl H., Keohavong P., Thilly W. G. Mismatch amplification mutation assay (MAMA): application to the c-H-ras gene. PCR Methods Appl. 1992 Aug;2(1):14–20. doi: 10.1101/gr.2.1.14. [DOI] [PubMed] [Google Scholar]
- Chuang R. Y., Laszlo J., Keller P. Effects of nitrosoureas on human DNA polymerase activities from acute and chronic granulocytic leukemia cells. Biochim Biophys Acta. 1976 Apr 2;425(4):463–468. doi: 10.1016/0005-2787(76)90010-1. [DOI] [PubMed] [Google Scholar]
- Cox R., Irving C. C. O6-methylguanine accumulates in DNA of mammary glands after administration of N-methyl-N-nitrosourea to rats. Cancer Lett. 1979 Apr;6(4-5):273–278. doi: 10.1016/s0304-3835(79)80045-2. [DOI] [PubMed] [Google Scholar]
- Dipple A., Pigott M., Moschel R. C., Costantino N. Evidence that binding of 7,12-dimethylbenz(a)anthracene to DNA in mouse embryo cell cultures results in extensive substitution of both adenine and guanine residues. Cancer Res. 1983 Sep;43(9):4132–4135. [PubMed] [Google Scholar]
- DuBridge R. B., Tang P., Hsia H. C., Leong P. M., Miller J. H., Calos M. P. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol Cell Biol. 1987 Jan;7(1):379–387. doi: 10.1128/mcb.7.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney R. E., Bishop J. M. Predisposition to neoplastic transformation caused by gene replacement of H-ras1. Science. 1993 Jun 4;260(5113):1524–1527. doi: 10.1126/science.8502998. [DOI] [PubMed] [Google Scholar]
- Fischer S. G., Lerman L. S. DNA fragments differing by single base-pair substitutions are separated in denaturing gradient gels: correspondence with melting theory. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1579–1583. doi: 10.1073/pnas.80.6.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Kohn K. W., Kann H. E., Jr Inhibition of the ligase step of excision repair by 2-chloroethyl isocyanate, a decomposition product of 1,3-bis(2-chloroethyl)-1-nitrosourea. Cancer Res. 1978 Apr;38(4):1064–1069. [PubMed] [Google Scholar]
- Guerrero I., Villasante A., Corces V., Pellicer A. Activation of a c-K-ras oncogene by somatic mutation in mouse lymphomas induced by gamma radiation. Science. 1984 Sep 14;225(4667):1159–1162. doi: 10.1126/science.6474169. [DOI] [PubMed] [Google Scholar]
- Jensen J. C., Thilly W. G. Spontaneous and induced chromosomal aberrations and gene mutations in human lymphoblasts: mitomycin C, methylnitrosourea, and ethylnitrosourea. Mutat Res. 1986 Apr;160(2):95–102. doi: 10.1016/0027-5107(86)90033-3. [DOI] [PubMed] [Google Scholar]
- Keohavong P., Thilly W. G. Fidelity of DNA polymerases in DNA amplification. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9253–9257. doi: 10.1073/pnas.86.23.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Sukumar S., Barbacid M. Activation of ras oncogenes preceding the onset of neoplasia. Science. 1990 Jun 1;248(4959):1101–1104. doi: 10.1126/science.2188364. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Momand J., Finlay C. A. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- Loeb L. A. Endogenous carcinogenesis: molecular oncology into the twenty-first century--presidential address. Cancer Res. 1989 Oct 15;49(20):5489–5496. [PubMed] [Google Scholar]
- Lu S. J., Archer M. C. Ha-ras oncogene activation in mammary glands of N-methyl-N-nitrosourea-treated rats genetically resistant to mammary adenocarcinogenesis. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1001–1005. doi: 10.1073/pnas.89.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S. J., Chaulk E. J., Archer M. C. Formation and repair of O6-methylguanine and methylation of the Ha-ras proto-oncogene by N-methyl-N-nitrosourea are not associated with mammary tumor resistance in the Copenhagen rat. Carcinogenesis. 1992 May;13(5):857–861. doi: 10.1093/carcin/13.5.857. [DOI] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov N. M., Bleicher F., Martel-Planche G., Montesano R. Nonrandom distribution of O6-methylguanine in H-ras gene sequence from DNA modified with N-methyl-N-nitrosourea. Mutat Res. 1993 Aug;288(2):197–205. doi: 10.1016/0027-5107(93)90085-t. [DOI] [PubMed] [Google Scholar]
- Newcomb E. W., Diamond L. E., Sloan S. R., Corominas M., Guerrerro I., Pellicer A. Radiation and chemical activation of ras oncogenes in different mouse strains. Environ Health Perspect. 1989 May;81:33–37. doi: 10.1289/ehp.898133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanilla M., Brown K., Ramsden M., Balmain A. Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature. 1986 Jul 3;322(6074):78–80. doi: 10.1038/322078a0. [DOI] [PubMed] [Google Scholar]
- Reynolds S. H., Stowers S. J., Patterson R. M., Maronpot R. R., Aaronson S. A., Anderson M. W. Activated oncogenes in B6C3F1 mouse liver tumors: implications for risk assessment. Science. 1987 Sep 11;237(4820):1309–1316. doi: 10.1126/science.3629242. [DOI] [PubMed] [Google Scholar]
- Richardson K. K., Richardson F. C., Crosby R. M., Swenberg J. A., Skopek T. R. DNA base changes and alkylation following in vivo exposure of Escherichia coli to N-methyl-N-nitrosourea or N-ethyl-N-nitrosourea. Proc Natl Acad Sci U S A. 1987 Jan;84(2):344–348. doi: 10.1073/pnas.84.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield V. C., Cox D. R., Lerman L. S., Myers R. M. Attachment of a 40-base-pair G + C-rich sequence (GC-clamp) to genomic DNA fragments by the polymerase chain reaction results in improved detection of single-base changes. Proc Natl Acad Sci U S A. 1989 Jan;86(1):232–236. doi: 10.1073/pnas.86.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha Y. N., Tucker H. A. Mammary gland growth of rats between 10 and 100 days of age. Am J Physiol. 1966 Mar;210(3):601–605. doi: 10.1152/ajplegacy.1966.210.3.601. [DOI] [PubMed] [Google Scholar]
- You M., Candrian U., Maronpot R. R., Stoner G. D., Anderson M. W. Activation of the Ki-ras protooncogene in spontaneously occurring and chemically induced lung tumors of the strain A mouse. Proc Natl Acad Sci U S A. 1989 May;86(9):3070–3074. doi: 10.1073/pnas.86.9.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You M., Wang Y., Lineen A. M., Gunning W. T., Stoner G. D., Anderson M. W. Mutagenesis of the K-ras protooncogene in mouse lung tumors induced by N-ethyl-N-nitrosourea or N-nitrosodiethylamine. Carcinogenesis. 1992 Sep;13(9):1583–1586. doi: 10.1093/carcin/13.9.1583. [DOI] [PubMed] [Google Scholar]
- You M., Wang Y., Stoner G., You L., Maronpot R., Reynolds S. H., Anderson M. Parental bias of Ki-ras oncogenes detected in lung tumors from mouse hybrids. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5804–5808. doi: 10.1073/pnas.89.13.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbl H., Sukumar S., Arthur A. V., Martin-Zanca D., Barbacid M. Direct mutagenesis of Ha-ras-1 oncogenes by N-nitroso-N-methylurea during initiation of mammary carcinogenesis in rats. 1985 May 30-Jun 5Nature. 315(6018):382–385. doi: 10.1038/315382a0. [DOI] [PubMed] [Google Scholar]
- Zhang R., Haag J. D., Gould M. N. Reduction in the frequency of activated ras oncogenes in rat mammary carcinomas with increasing N-methyl-N-nitrosourea doses or increasing prolactin levels. Cancer Res. 1990 Jul 15;50(14):4286–4290. [PubMed] [Google Scholar]