Abstract
Objectives
Functional dyspepsia is predominantly attributed to gastric sensorimotor dysfunctions. The contribution of intestinal chemosensitivity to symptoms is not understood. We evaluated symptoms and plasma hormones during enteral nutrient infusion and the association with impaired glucose tolerance and quality-of-life (QOL) scores in functional dyspepsia vs health.
Design
Enteral hormonal responses and symptoms were measured during isocaloric and isovolumic dextrose and lipid infusions into the duodenum in 30 patients with functional dyspepsia (n=27) or nausea and vomiting (n=3) and 35 healthy controls. Infusions were administered in randomized order over 120 minutes each, with a 120-minute washout. Cholecystokinin, glucose-dependent insulinotropic peptide, glucagonlike peptide 1 (GLP1), and peptide YY were measured during infusions.
Results
Moderate or more severe symptoms during lipid (4 controls vs 14 patients) and dextrose (1 control vs 12 patients) infusions were more prevalent in patients than controls (P≤.01), associated with higher dyspepsia symptom score (P=.01), worse QOL (P=.01), and greater plasma hormone concentrations (eg, GLP1 during lipid infusion). Moderate or more severe symptoms during enteral infusion explained 18%, and depression score explained 21%, of interpatient variation in QOL. Eight patients had impaired glucose tolerance, associated with greater plasma GLP1 and peptide YY concentrations during dextrose and lipid infusions, respectively.
Conclusions
Increased sensitivity to enteral dextrose and lipid infusions was associated with greater plasma enteral hormone concentrations, more severe daily symptoms, and worse QOL in functional dyspepsia. These observations are consistent with the hypothesis that enteral hormones mediate increased intestinal sensitivity to nutrients in functional dyspepsia.
Keywords: CCK, depression, dyspepsia, GLP1, hormones, sensation, symptoms
Introduction
A majority of patients with functional dyspepsia report postprandial symptoms (eg, epigastric pain, fullness, early satiation, nausea and vomiting), especially after meals rich in fat (1–3). The pathogenesis of meal-related symptoms is incompletely understood. Most studies have focused on gastric sensorimotor dysfunctions. Although hypersensitivity to postprandial distention and impaired gastric accommodation are correlated with the severity of meal-related symptoms in several studies, abnormal (accelerated or delayed) gastric emptying typically is not (2).
In the small intestine, the products of digestion stimulate release of enteral hormones (eg, cholecystokinin [CCK]) that act, through receptors on vagal afferents, to mediate postprandial sensations, such as satiety (4). Assessing proximal small intestinal nutrient sensitivity with orally-administered nutrients is suboptimal because ingested nutrients stimulate the stomach and duodenum. Moreover, gastric emptying, hence delivery of nutrients into the duodenum, may be normal, delayed, or rapid in functional dyspepsia (5). Hence, enteral nutrient infusion is necessary to evaluate enteral nutrient sensitivity. Four small studies, of which one was uncontrolled, suggest that duodenal lipid infusion increased the sensitivity to gastric distention in patients with functional dyspepsia (6–9). The sensitizing effect is blocked by a lipase inhibitor or a CCK-A receptor antagonist (10, 11), which suggests that CCK receptors mediate increased sensitivity to gastric distention during enteral lipid infusion.
However, several aspects are undetermined regarding duodenal chemosensitivity in functional dyspepsia. First, only two studies, with a total of 16 healthy subjects and 23 dyspepsia patients, evaluated duodenal nutrient sensitivity per se (ie, without gastric distention). One of these studies only infused 5 kcal of dextrose and lipid in the duodenum. In these studies, duodenal sensitivity during intestinal nutrient infusion without gastric distention was not increased in functional dyspepsia (8, 12). Second, in contrast to duodenal fat infusion, glucose infusion does not increase sensitivity to gastric distention in functional dyspepsia (6) despite the observations that dextrose also evokes dyspeptic symptoms (1). Third, the contribution of enteral hormones to symptoms in functional dyspepsia is unclear. Compared with healthy persons, patients with functional dyspepsia had higher plasma concentrations of CCK after a high-fat meal (13) but not during enteral lipid infusion (8). Other enteral hormones (eg, glucagonlike peptide 1 [GLP1], peptide YY [PYY]) that also inhibit gastric emptying and affect gastrointestinal sensation have not been evaluated during enteral nutrient infusions in functional dyspepsia. Fourth, the relation between symptoms during enteral nutrient infusion and day-to-day symptoms evoked by orally ingested meals is unknown in patients with functional dyspepsia.
Normally, small intestinal delivery of nutrients evokes neurohumoral duodenogastric feedback mechanisms that inhibit gastric emptying by modulating gastric motor activity (4). CCK, GLP1, and PYY induce satiety and delay gastric emptying by vagally-mediated mechanisms. GLP1 and glucose-dependent insulinotropic peptide (GIP) also regulate glycemia.
Hence, the broad aims of the present study were to compare sensitivity to duodenal nutrient infusion in functional dyspepsia and healthy persons. We also evaluated the relation between nutrient sensitivity and day-to-day symptoms and, separately, plasma enteral hormone concentrations in functional dyspepsia and healthy persons. Our hypotheses were that (i) patients with functional dyspepsia have more severe symptoms during enteral nutrient infusion, (ii) the severity of symptoms during enteral infusion is correlated with higher plasma levels of enteral hormones (eg, CCK and GLP-1), and (iii) more severe daily symptoms and worse QOL.
Methods
Study Participants
The present study involved 35 healthy asymptomatic persons (mean [standard error] [SE] age, 41 [3] years; 24 women) with a mean (SE) body mass index (BMI) of 26.4 (0.7) kg/m2 and 30 patients with functional upper gastrointestinal (GI) symptoms (dyspepsia or nausea and vomiting) by Rome III criteria (mean [SE] age, 40 [3] years; 26 women) with a mean (SE) BMI of 26.4 (0.7) kg/m2 (Table 1). Recruitment of participants was made through public advertisement (controls) and from the clinical practice (patients). None of these participants had previously participated in intubation studies. Exclusion criteria for all participants were age <18 or >70 years; a structural disorder affecting the GI tract; diabetes mellitus; clinically significant systemic (eg, cardiovascular, respiratory, renal) disease that may interfere with study objectives or pose safety concerns, or both; GI surgery other than appendectomy, cholecystectomy, hysterectomy, tubal ligation, or inguinal hernia repair; medications likely to affect GI motility; or a hemoglobin level <12.9 g/dL in men and <11.5 g/dL in women. Since age and BMI affect nutrient-induced hormone (eg, CCK, ghrelin) release (14), the age, sex distribution, and BMI of patients and healthy controls were matched. All women of child-bearing potential had to have a negative pregnancy test within 48 hours of study participation. The Mayo Clinic Institutional Review Board approved the study, and all participants signed informed consent.
Table 1.
Demographic and Baseline Clinical Characteristics
| Characteristic | Controlsa (n=35) |
Patients With Functional Dyspepsiaa (n=35) |
P Valueb for Association With Group Status |
|---|---|---|---|
| Age, y | 41 (3) | 40 (3) | .72 |
| BMI, kg/m2 | 26.4 (0.7) | 26.2 (0.9) | .55 |
| Female sex, No. (%) | 24 (69) | 20 (67) | >.99 |
| HADS score borderline, definite anxiety |
2, 0 | 4, 5 | .006c |
| HADS score borderline, definite depression |
0, 0 | 3, 2 | .052c |
| Mean NDI dyspepsia symptom severity score, median (IQR) |
0 (0–0.2) | 1.9 (1.2–2.6) | <.001 |
| Mean NDI QOL score, median (IQR) |
100 (100–100) | 40 (30–58) | <.001 |
| Gastric emptying of solids, min | |||
| 30 | 11 (1) | 15 (5) | .02 |
| 60 | 23 (2) | 27 (3) | .24 |
| 120 | 58 (4) | 58 (4) | .82 |
| 240 | 95 (2) | 93 (3) | .36 |
| Small-bowel transit time, min | 240 (14) | 240 (15) | >.99 |
Abbreviations: BMI, body mass index; HADS, Hospital Anxiety and Depression Scale; IQR, interquartile range; NDI, Nepean Dyspepsia Index; QOL, quality of life; SE, standard error.
Values are presented as mean (SE) unless specified otherwise.
Fisher exact test or Wilcoxon rank sum test.
For definite anxiety and depression
Assessment of Dyspepsia Symptoms
The self-administered Nepean Dyspepsia Index questionnaire was used to assess symptom severity and quality of life (QOL) related to dyspepsia in the past 3 months (15, 16). Responses were summarized with the mean symptom severity score, calculated by first averaging the frequency, intensity, and degree of bothersomeness for each of 15 symptoms and subsequently averaging across all 15 symptoms. Thereafter, this score is subtracted from 13 to obtain a mean symptom severity score; hence, lower scores correspond to increased symptoms. QOL was averaged for 5 domains to obtain an overall QOL that was then subtracted from 100; lower scores reflect poorer QOL. The Hospital Anxiety and Depression Scale (HADS) questionnaire was used to identify anxiety and depression (17).
Gastric and Small-Bowel Transit
Study procedures (ie, enteral nutrient infusion and gastric emptying) were performed on 2 separate days, which were 8 days apart on average. Gastric emptying of solids and liquids and small-bowel transit were simultaneously assessed with established scintigraphic techniques in 30 of 35 healthy persons and all 30 patients (18). In the first 5 healthy persons, we sought to ensure that enteral infusions were well tolerated; hence, transit was not evaluated. The meal (296 kcal; 32% protein, 35% fat, and 33% carbohydrate) consisted of 2 eggs labeled with technetium Tc 99m sulfur colloid (1 mCi) served on 1 slice of bread with milk (240 mL; 1%) labeled with indium In 111 diethylenetriaminepentaacetate [0.1 mCi]). Anterior and posterior gamma camera images were obtained immediately after ingestion of the radiolabeled meal, every 15 minutes for the first 2 hours, every 30 minutes for the next hour, and at 6 hours. Gastric emptying and small-bowel transit were analyzed by quantifying counts in the stomach and colon, respectively, corrected as necessary for isotope decay, tissue attenuation, and down-scatter of In 111 counts in the Tc 99m window.
In patients, both rapid and delayed gastric emptying were defined relative to 10th through 90th percentile values in controls from the present study. Rapid gastric emptying was defined as emptying >90th percentile value at 30 or 60 minutes or at half-time (t50) less the 10th percentile value. Delayed gastric emptying was defined as emptying <10th percentile value at 2 or 4 hours or t50 longer than the 90th percentile value. Rapid gastric emptying of liquids was defined as emptying >90th percentile value in controls at 15, 30, or 60 minutes. Small-bowel transit time was calculated as the time for 10% of the activity to arrive at the cecum, after correcting for gastric emptying (19).
Enteral Nutrient Infusions
On the enteral nutrient infusion day, an 8-Fr nasoduodenal feeding tube (Abbott Nutrition) was placed under fluoroscopic guidance, with its tip in the second part of the duodenum. Thereafter, hormonal responses to dextrose and lipids were assessed, in randomized order, because nutrient composition influences hormonal release—for example, lipids and carbohydrates are more potent stimuli for CCK and GIP, respectively (20)—and we sought to characterize oral glucose tolerance. Thus, isocaloric (300 kcal) and isovolumic (222 mL) dextrose (Limeondex; Therma Fisher Scientific Inc) (75 g) and lipid infusions (Microlipid; Covidean AG) (66.7 mL diluted to 222 mL, for 0.5 g/mL) were administered in randomized order over 120 minutes, separated by a 120-minute washout period (Figure 1). The 75-g dextrose load was similar to an oral glucose tolerance test. As previously described (21), the enteral glucose dosing regimen was designed to mimic the normal rate of systemic delivery of glucose after glucose ingestion (22–24).
Figure 1.
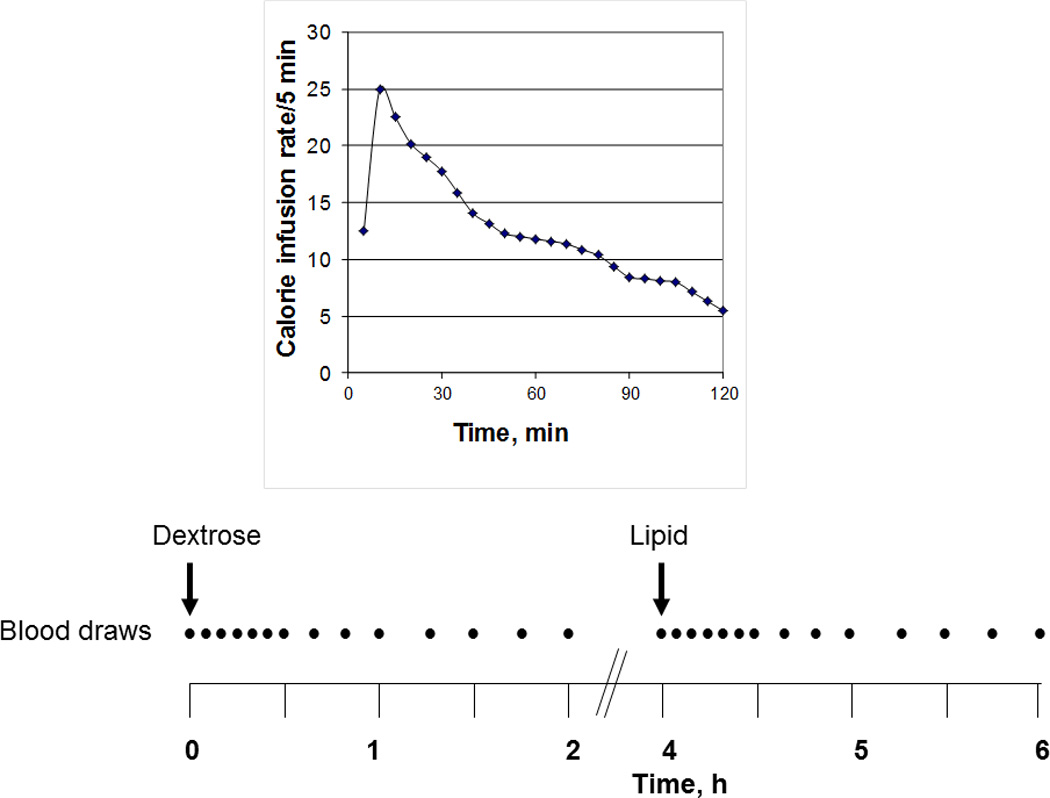
Study Design. Plasma hormones and gastrointestinal symptoms were assessed during enteral dextrose and lipid infusions, which were each administered over 2 hours in randomized order. The variable caloric infusion rate was designed to mimic systemic delivery of glucose after oral ingestion of glucose.
Symptoms During Enteral Nutrient Infusion
During enteral nutrient infusion, participants reported the severity of 6 symptoms—nausea, fullness, bloating, abdominal pain, belching, and burning—at 15-minute intervals on a Likert scale with 0 to 4 descriptors (absent, 0; light, 1; moderate, 2; severe, 3; and intolerable, 4). The proportion of participants with any symptom of moderate severity or worse was used for data analysis.
Glycemia and Enteral Hormonal Measurements
Blood samples for measurement of plasma glucose, C-peptide, glucagon, GLP1, CCK, GIP, ghrelin, and PYY were collected at 5-minute intervals for 30 minutes, at 10-minute intervals from 30 to 60 minutes, and at 15-minute intervals from 60 to 120 minutes. Arterialized venous plasma samples were obtained from a retrograde hand or forearm vein and were placed in a Perspex hot box heated to 55°C. Samples were placed in ice, centrifuged at 4°C, separated, and stored at −20°C until assayed. Glucose was measured by the Hitachi 912 assay (Roche Diagnostics).
GLP1 enzyme-linked immunosorbent assay (ELISA) (Linco Research, Inc) measures biologically active GLP1(7–36, 7–37) amide levels with no cross-reactivity to GLP1-(9–36) amide, GLP2, or glucagon; the threshold of detection is 3 pM. Immediately after collection, a DPP-IV inhibitor was added to these tubes.
CCK immunoassay (Alpco Diagnostics) uses rabbit antiserum to a synthetic cholecystokinin 26–33 sulphate (CCK 8 sulphate), binds to most biological active forms with nearly equimolar potency, (25) and has no cross-reactivity with gastrin. To prevent degradation, the tubes contain aprotinin.
GIP is measured with an ELISA (Linco Research, Inc) that uses a monoclonal capture antibody with a 100% cross-reactivity to intact human GIP(1–42) and human GIP(3–42) and has no measureable cross-reactivity with glucagon, oxyntomodulin, GLP1, or GLP2.
The PYY radioimmunoassay (Linco Research, Inc) detects 2 molecular physiologically active forms (1–36 and 3–36) and has no measureable cross-reactivity with glucagon, ghrelin, insulin, or GLP1
Glucagon was measured with a direct, double-antibody. radioimmunoassay (Linco Research, Inc) with no measurable cross-reactivity to insulin, proinsulin, C-peptide, GLP1, or somatostatin.
C-peptide levels are measured with a 2-site immunometric (sandwich) assay using electrochemiluminescence detection (Cobas e411; Roche Diagnostics).
Total ghrelin is measured with radioimmunoassay (Linco Research, Inc) that has no measurable cross-reactivity with glucagon, GLP1(7–36), or insulin.
Statistical Analysis
The associations between symptoms during nutrient infusion and participant status (controls vs patients) and separately with plasma hormone concentrations (ie, mean levels) was evaluated by Fisher exact test or Wilcoxon rank sum test. The association between symptoms during enteral dextrose vs lipid infusion was assessed by McNemar’s test for paired data. The Wilcoxon rank sum test was used to assess the associations between symptoms during enteral infusion separately with mean plasma hormone concentrations, daily symptoms, and QOL assessed with the Nepean Dyspepsia Index. Associations between subject status (ie, between patients with normal and impaired glucose tolerance) and plasma hormone concentrations were assessed with the Wilcoxon rank sum test. Multiple linear regression models assessed whether anxiety, depression, and severe symptoms during either dextrose infusion or lipid infusion could predict mean symptom severity and QOL determined with the Nepean Dyspepsia Index instrument.
Only 1 prior small study evaluated sensation during enteral nutrient infusion studies from our laboratory (8). Hence, the sample size estimate was based on a study which evaluated CCK concentrations after comparable enteral lipid infusions. Based on those observations, a sample size of 30 healthy participants vs 30 patients provided ∼80% power (2-sided alpha =0.05) to detect an association of subject status with peak CCK values corresponding to a difference of 32% relative to the overall mean postprandial CCK concentrations.
Results
Participants, Study Conduct, and Completion
The GI transit was evaluated in 30 controls and 30 patients. Two controls and 5 patients did not receive either infusion because of inability to place a nasojejunal tube (1 control and 4 patients), positive pregnancy test (1 control), and concurrent illness (1 patient). Hence, 33 of 35 controls and 25 of 30 patients received at least one enteral infusion. Of these, 6 controls and 1 patient did not receive the second infusion because of adverse effects during the first infusion. A total of 27 controls and 24 patients received both infusions.
Demographic and Clinical Characteristics
By study design, the sex distribution, BMI, and age were not significantly different between the control group and the patient group (Table 1). The predominant upper GI symptoms among patients were functional dyspepsia (ie, postprandial distress alone [n=17], epigastric pain alone [n=2], and both [n=8]) or functional nausea and vomiting (n=3). Twenty patients also had bowel symptoms—constipation alone (n=6), diarrhea alone (n=9), or both (n=4). A greater proportion of patients than controls had definite anxiety (ie, HADS anxiety score >8) (P=.006) and depression (P=.052). In 23 patients, the upper gastrointestinal endoscopy was essentially unremarkable (ie, normal except perhaps for a small hiatal hernia or cystic fundic polyps). In the remaining patients, upper gastrointestinal endoscopy disclosed antral erythema or minor erosions that were not deemed sufficient to explain symptoms in 5 patients, mild esophagitis (1 patient), and retained food (1 patient). No controls and 4 patients had a cholecystectomy.
Gastric Emptying and Small-Bowel Transit
On the basis of 10th to 90th percentile range for controls, the gastric emptying of solids and liquids was normal in 14 (47%) and rapid in 4 patients (13%). The other patients had rapid emptying of solids only (n=6, 20%), rapid emptying of liquids only (n=1, 3%), or delayed emptying of solids only (n=5, 17%). Among patients with rapid gastric emptying of solids, mean (SE) gastric emptying from the stomach was 24% (3%) at 30 minutes, 39% (4%) at 60 minutes, and 75% (3%) at 120 minutes. At 30 minutes, this proportion was more than twice that in controls (Table 1). Gastric emptying of liquids at 15 minutes was similar (42 ± 3%) in controls and patients. At 30 minutes (61 ± 3% [controls], 56 ± 3% [patients]) and 60 minutes (78 ± 3% [controls], 72 ± 3% [patients]) differences between controls and patients were not statistically significant. Four patients had accelerated small-bowel transit.
GI Symptoms During Enteral Nutrient Infusion
More patients than controls reported at least 1 symptom of moderate severity or worse during lipid (4 controls vs 14 patients; P≤.01) and dextrose (1 control vs 12 patients; P≤.01) infusions (Figure 2). Patients who had moderate symptoms or worse during lipid infusion were also more likely (P<.001) to have symptoms during dextrose infusion. Thus, 11 of 18 participants (61%) who reported, but only 1 of 30 participants (3%) who did not report, moderate or more severe symptoms during lipid infusion also reported these symptoms during dextrose infusion.
Figure 2.
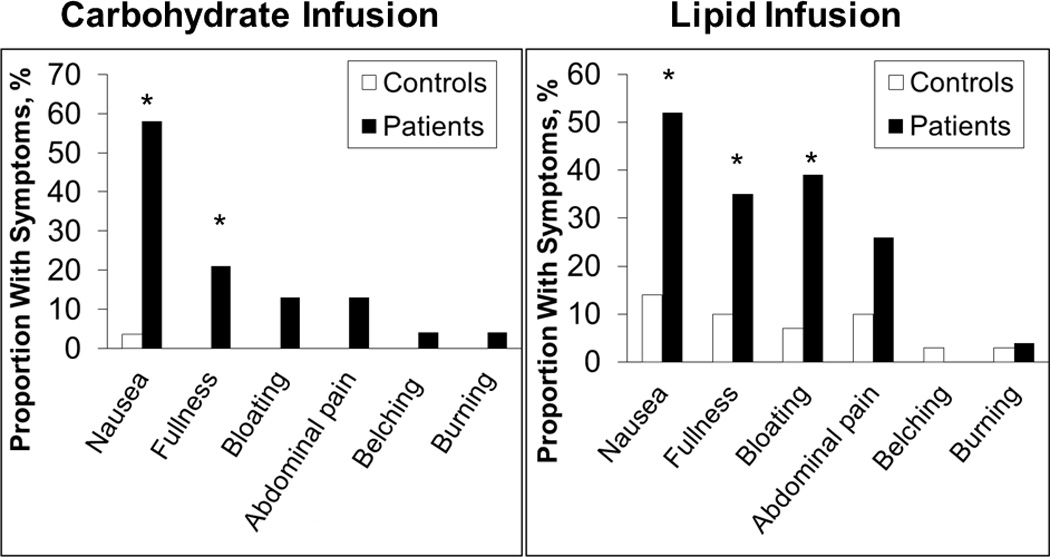
Gastrointestinal Symptoms During Enteral Nutrient Infusion. A greater proportion of patients than controls reported symptoms of moderate severity or worse during enteral dextrose and lipid infusions. Asterisk indicates p < 0.05 vs controls
Individual Symptoms
Nausea was the most frequently reported severe symptom during dextrose and lipid infusions. A greater proportion of patients had moderate or more severe nausea (P<.001) and fullness (P<.02) during dextrose infusions and lipid infusions (P<.006 and P<.05, respectively), as well as bloating (P<.01) during lipid infusions. The prevalence of moderate or more severe fullness (P=.025), bloating (P=.008), and abdominal pain (P=.06) was also higher during lipid infusion than dextrose infusion.
Timing of Severe Symptoms
Patients who reported moderate symptoms or worse generally reported them within 15 minutes (8 patients [dextrose] and 7 patients [lipid]) or 30 minutes (11 patients [dextrose] and 12 patients [lipid]) after infusions were started. Only 2 patients reported the onset of moderate or more severe symptoms at 45 and 60 minutes respectively after infusions were started. These symptoms persisted thereafter; the greatest proportion of participants who reported “significant” symptoms occurred at 45 minutes into the infusion for controls and 75 minutes into the infusion for patients. Even at 120 minutes into the infusion, 12 patients reported symptoms during both infusions and 3 patients reported symptoms during lipid infusions only.
Glucose Tolerance and Plasma Hormone Concentrations During Enteral Nutrient Infusion
Compared to controls, blood glucose concentration during dextrose infusion, as well as plasma GIP, GLP-1, and C-peptide concentrations during lipid infusion were all higher (P≤.03) in patients than controls (Table 2).
Table 2.
Comparison of Hormonal Responses to Dextrose and Lipid Infusions in Healthy Controls vs Dyspepsia Patients
| Patient Groupa | ||||
|---|---|---|---|---|
| Hormone | Controls | Patients | ||
| Dextrose Infusion | Lipid Infusion | Dextrose Infusion | Lipid Infusion | |
| Glucose | 19,455 (17,880–22,885) P<.001b |
11,663 (11,304–12,518) |
23,363 (19,650–27,988) P≤.03c |
11,429 (10,810–12,446) |
| C-peptide | 381 (271–534)P<.001b | 85 (67–121) | 566 (258–821) | 145 (88–299) P≤.01c |
| Glucagon | 2,776 (2,226–3,244) P=.02b |
3,300 (2,603–3,963) |
3,125 (2,514–3,862) |
3,773 (2,847–5,071) |
| GIP | 6,010 (5,040–8,232) P<.001b |
2,933 (2,279–4,392) |
6,369 (5,158–8,005) |
4,118 (3,241–6,553)P≤.03c |
| GLP1 | 2,534 (1,243–3,331) |
1,768 (1,063–2,909) |
2,857 (1,826–3,586) |
2,840 (1,740–3,930) P≤.03c |
| CCK | 430 (296–602) | 926 (619–1,130) | 465 (279–618) | 804 (571–1087) |
| Ghrelin | 68,255 (62,400–76,103) |
83,930 (71,508–95,208) |
72,123 (57,835–82,475) |
78,808 (69,323–87,563) |
| PYY | 5,400 (3,988–7,311) |
5,406 (3,733–6,500) |
4,773 (3,746–6,155) |
5,963 (4,817–7,577) |
Abbreviations: CCK, cholecystokinin; GIP, glucose-dependent insulinotropic peptide; GLP1, glucagonlike peptide 1; IGT, impaired glucose intolerance; NGT, normal glucose tolerance; PYY, protein YY.
Values are presented (area under the curve) as pmol/L per min (CCK, glucagon, GIP, GLP1, and PYY), mg/dL per min (glucose), pg/mL per min (ghrelin) and as nmol/L (C-peptide)
vs lipids in controls
vs corresponding nutrient, controls
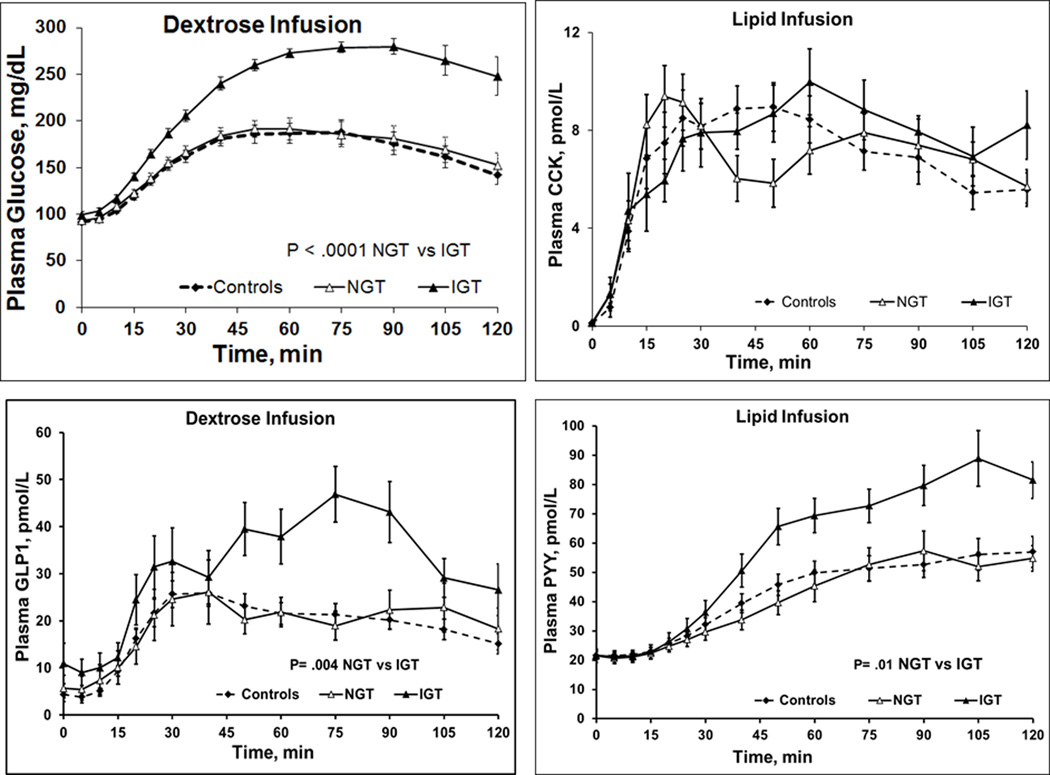
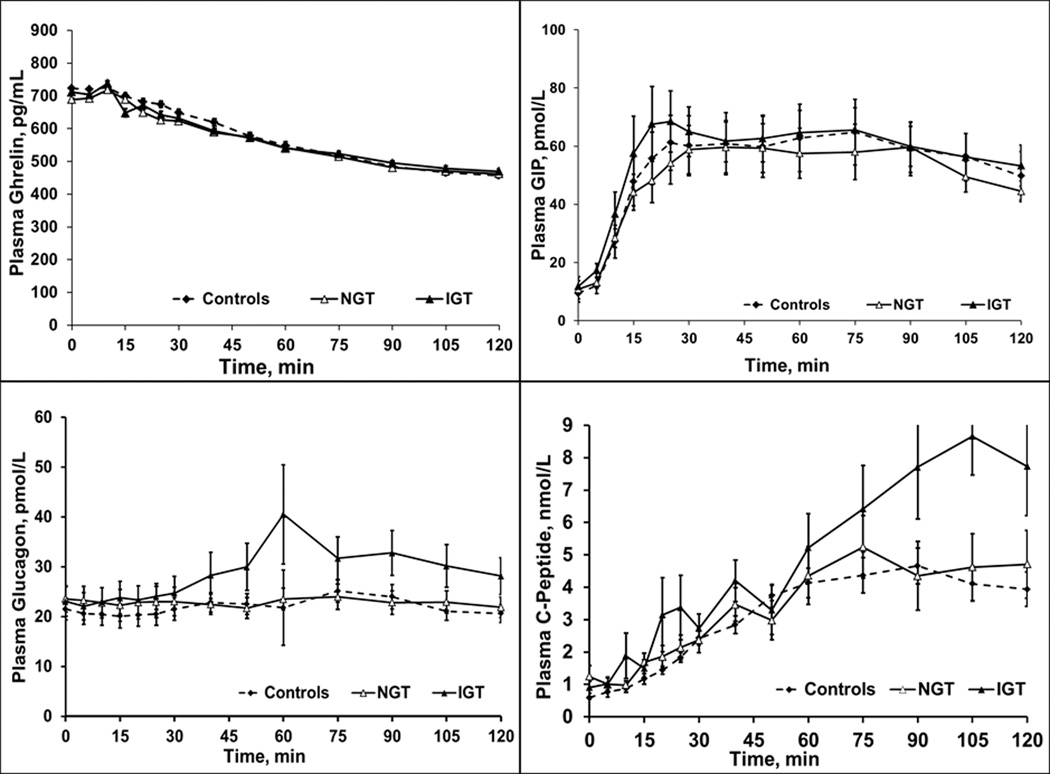
Indeed, the 90th percentile values for blood glucose concentration (area under the curve) during the 2-hour dextrose infusion in 35 controls showed that 8 of 25 patients (32%) who completed the dextrose infusion had impaired glucose tolerance (Table 3). Age and BMI were not significantly different (P≥.6) in dyspeptic patients with normal (38 ± 5y, 26 ± 1 kg/m2) and impaired glucose tolerance (40 ± 6y, 27 ± 2 kg/m2). Plasma levels of GLP1 (P=.004) during dextrose infusion, and PYY (P=.01) during dextrose and lipid infusions, were higher in patients with impaired glucose tolerance than normal glucose tolerance (Table 3 and Figure 3). In contrast, plasma concentrations of C-peptide, glucagon, and GIP, among other hormones, were not significantly associated with glucose tolerance status (Table 3 and Figure 4).
Table 3.
Comparison of Hormonal Responses to Dextrose and Lipid Infusions in Healthy Controls vs Dyspepsia Patients With NGT or IGT
| Hormone | NGT (n=16) | IGT (n=8) | ||
|---|---|---|---|---|
| Dextrose Infusion | Lipid Infusion | Dextrose Infusion | Lipid Infusion | |
| Glucose | 20,450 (19,530–23,110) |
11,184 (10,464–11,933) |
29,491 (28,151–31,774) P≤.004a |
12,278 (11,221–14,001) |
| C-peptide | 559 (172–800) |
145 (88–305) |
611 (497–837) |
105 (85–244) |
| Glucagon | 2,623 (2,257–4,079) |
3,379 (2,817–5,527) |
3,374 (3,032–3,687) |
4,151 (3,148–4,767) |
| GIP | 5,916 (4,807–7,304) |
3,900 (3,034–5,980) |
6,810 (5,959–10,327) |
5,392 (3,998–8,187) |
| GLP1 | 2,298 (1,721–3,312) |
2,658 (1,250–3,466) |
3,811 (3,353–4,953) P≤.004a |
3,248 (2,363–4,351) |
| CCK | 459 (255–618) | 652 (507–1,019) | 519 (335–612) | 940 (790–1,106) |
| Ghrelin | 67,260 (57,835–82,475) | 79,248 (73,053– 90,054) |
72,765 (51,208– 81,203) |
71,379 (63,698– 84,026) |
| PYY | 4,090 (3,622–5,552) |
5,502 (4,095–6,897) |
6,645 (5,451–7,836) P=.01a |
7,577 (6,580–9,008)P=.01a |
Abbreviations: CCK, cholecystokinin; GIP, glucose-dependent insulinotropic peptide; GLP1, glucagonlike peptide 1; IGT, impaired glucose intolerance; NGT, normal glucose tolerance; PYY, protein YY.
Values are presented (area under the curve) as pmol/L per min (CCK, glucagon, GIP, GLP1, and PYY), mg/dL per min (glucose), pg/mL per min (ghrelin) and as nmol/L (C-peptide)
P values are vs corresponding nutrient, NGT.
Figure 3.
Mean Plasma Concentrations of Glucose and Selected Hormones During Enteral Lipid and Dextrose Infusions. During dextrose infusion, plasma concentrations of glucose and glucagonlike peptide 1 (GLP1) were greater in patients with impaired glucose tolerance (IGT) than normal glucose tolerance (NGT). During lipid infusion, plasma concentration of peptide YY (PYY) but not cholecystokinin (CCK) was greater in patients with IGT than those with NGT. Error bars indicate SEM for all hormones.
Figure 4.
Plasma Concentrations of Selected Hormones During Enteral Dextrose Infusion. Plasma concentrations of ghrelin, glucose-dependent insulinotropic peptide (GIP), glucagon, and C-peptide were not significantly different in patients with impaired vs normal glucose tolerance.
Except for ghrelin, the plasma concentrations of all hormones increased during enteral nutrient infusions (Figures 3 and 4). Some plasma hormone levels (eg, CCK) peaked early and stayed high for the duration of the infusion. The levels of others (eg, GLP1, PYY) increased and also peaked later in the postprandial period. Among controls, the levels of plasma glucose, C-peptide, and GIP were higher (P<.001) during dextrose infusion than lipid infusion, but glucagon levels were higher (P=.02) during lipid infusion (Table 2).
With the exception of blood glucose concentration in controls, which was higher (P=0.01) when the dextrose infusion was given after the lipid infusion, plasma hormone concentrations in controls and patients, as well as the blood glucose concentration in patients, was not significantly associated with the order of dextrose vs lipid infusion.
Relation Between Symptoms, Plasma Hormone Concentrations During Enteral Nutrient Infusion
During dextrose infusion, symptom status was associated with plasma glucagon (P<.05), glucose (P=0.06), and GLP1 (P=.09) concentrations and was more severe in participants with, than without, moderate or more severe symptoms (Table 4). Moreover, a higher proportion (P=.09) of participants with than without moderate or more severe symptoms had plasma GLP1 concentrations greater than the 90th percentile value in controls (Figure 5). Seven of 17 patients (41%) with normal and 5 of 7 (71%) with impaired glucose tolerance had moderate or more severe symptoms during carbohydrate infusion; however differences were not significant (P=0.37).
Table 4.
Comparison of Plasma Hormone Concentrations With Symptom Severity During Enteral Nutrient Infusions
| Occurrence of Any Moderate or More severe Symptoms a | ||||
|---|---|---|---|---|
| Dextrose Infusion | Lipid Infusion | |||
| Hormone | No | Yes | No | Yes |
| Glucose | 20,130 (18,598– 23,935) |
25,030 (19,553– 27,988) P=.06 |
11,496 (11,098–12,538) |
11,856 (10,885– 12,446) |
| C-peptide | 391 (256–613) | 566 (376–748) | 90 (67–171) | 118 (82–243) |
| Glucagon | 3,283 (2,768– 52,185) |
3,568, P<.05 (2,940–3,862) |
3,384 (2,616–3,975) |
3,814 (2,796–4,594) |
| GIP | 5,954 (5025–8387) |
6,302 (5,080–7,076) |
3,200 (2,528–4,207) |
4,321, P<.05 (2,898–6,584) |
| GLP1 | 2,066 (1,087–2,857) |
2,732, P=.09 (2,183–4,206) |
1,564 (1,016–2,904) |
2,730, P<.05 (2,073–3,466) |
| CCK | 362 (196–451) |
525 (279–682) |
753 (529–1,092) |
991 (571–1,258) |
| Ghrelin | 78,808 (65,459– 92,040) |
69,323 (76,873– 86,063) |
78,368 (63,683–91,275) |
81,733 (69,403–98,718) |
| PYY | 5,198 (3,170–7,262) |
5,383 (3,530–7,135) |
5,515 (2,873–6,500) |
5,891 (5,087–7,522) |
Abbreviations: CCK, cholecystokinin; GIP, glucose-dependent insulinotropic peptide; GLP1, glucagonlike peptide 1; PYY, protein YY.
Values are presented (area under the curve) as pmol/L per min (CCK, glucagon, GIP, GLP1, and PYY), mg/dL per min (glucose), pg/mL per min (ghrelin) and as nmol/L (C-peptide)
All P values are vs no moderate or more severe symptoms in corresponding infusion.
Figure 5.
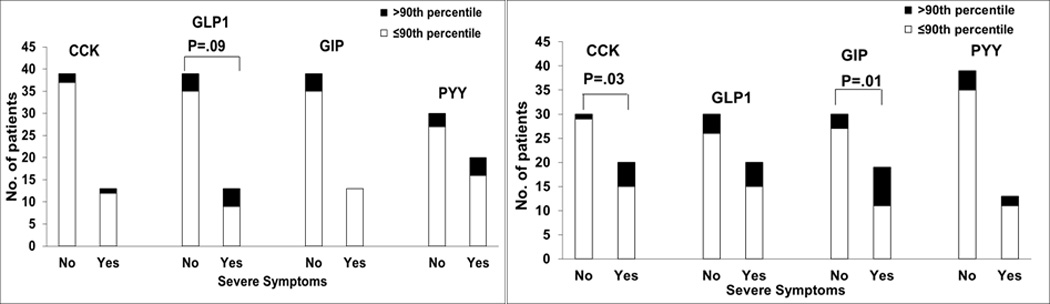
Relation Between Moderate or More severe Symptoms and Plasma Enteral Hormone Concentrations During Dextrose (A) and Lipid (B) Infusions. Moderate or more severe symptoms were associated with high plasma hormone levels of glucagonlike peptide 1 (GLP1) during dextrose infusion and of cholecystokinin (CCK) and glucose-dependent insulinotropic peptide (GIP) during lipid infusion. PYY indicates peptide YY.
During lipid infusion, plasma GIP (P<.05) and GLP1 (P<.05) concentrations were associated with moderate or more severe symptoms, being greater in patients with than without these symptoms. Moreover, a higher proportion of participants with than without moderate or more severe symptoms had plasma CCK (P=.03) and GIP (P=.01) concentrations greater than the 90th percentile in controls (Figure 5). Other hormones—PYY, ghrelin, glucagon, and C-peptide—were not associated with these symptoms during the infusions.
Relation Between Symptoms of Dyspepsia and Sensitivity During Enteral Nutrient Infusion
Moderate or more severe symptoms during enteral dextrose or lipid infusion were associated with worse upper GI symptoms, as identified by the Nepean Dyspepsia Index: abdominal pain (P=.01), postprandial distress (P=.001), and mean symptom score (P=.01). Moderate or more severe symptoms during infusions were also associated with worse QOL (P=.02).
Relation Between GI Transit and Plasma Hormone Concentrations
Two of 6 patients (33%) with normal gastric emptying, 6 of 16 (38%) with rapid gastric emptying, and none of 3 (0%) with delayed gastric emptying had impaired glucose tolerance. The proportions were not different among these gastric emptying groups.
Plasma PYY concentrations were inversely correlated with small-bowel transit time during dextrose infusion (r= −0.43; P=.004) and lipid infusions (r= −0.30; P<.05). Other plasma hormone concentrations were not correlated with small-bowel transit time.
Relation Among Anxiety, Depression, Enteral Nutrient Sensitivity, and GI Symptoms
Mean (SD) depression scores were higher in patients with (4.2 [1.1]) than patients without (2.2 [0.4]) moderate or more severe symptoms during dextrose infusion (P=.04). Otherwise, anxiety and depression scores were not significantly associated with these symptoms during enteral infusions.
In the multiple linear regression models, the predictor variables (HADS anxiety and depression scores and moderate or more severe symptoms during dextrose or lipid infusion) explained 24% and 33% of the interparticipant variation in mean symptom severity and QOL, respectively. Moderate or more severe symptoms during either dextrose infusion or lipid infusion were the strongest predictor of the mean symptom score and explained 18% of the interparticipant variation. The HADS depression score explained 21% of the interparticipant variation in QOL.
Discussion
The contribution of intestinal chemosensitivity and hormones to symptoms in functional dyspepsia is unclear. To avoid the confounding effect of delayed or accelerated gastric emptying, we evaluated intestinal chemosensitivity by directly infusing nutrients into the duodenum at the same controlled rate in controls and patients. Three important and original observations were made. First, patients with functional dyspepsia reported more severe symptoms during enteral lipid and dextrose infusions. Second, increased sensitivity was associated with greater plasma concentrations of enteral hormones during lipid infusion. Third, patients with increased sensitivity during enteral infusion also had more severe dyspeptic symptoms and reported a more pronounced impact of dyspepsia on QOL. After adjustment for anxiety and depression, symptoms during either dextrose or lipid infusion were the strongest and only independent predictor of the mean symptom score and explained 18% of the interparticipant variation in symptom severity. Taken together, these findings implicate a role for intestinal nutrient sensitivity to symptoms in functional dyspepsia. Fourth, nearly one -third of patients with dyspepsia with normal fasting glucose had impaired glucose tolerance during enteral nutrient infusion. Impaired glucose tolerance was associated with higher plasma concentrations of GLP1 during dextrose and PYY during dextrose and lipid infusions.
Duodenal infusion of lipids increases gastric accommodation and sensitivity to gastric distension (6–9, 26). In the present study, patients with functional dyspepsia were more sensitive to duodenal lipid and dextrose infusions without gastric distention, which is suggestive of increased intestinal chemosensitivity. While a relatively small volume (222 mL over 2 hours) was infused, we cannot be certain if symptoms during enteral nutrient infusion were related to distention per se or due to chemosensitivity. However, duodenal infusion of saline (216 mL over 90 minutes) did not affect perception of gastric distention in humans (27). Hence, differences between patient and control groups are unlikely to be explained through increased mechanosensitivity. Because we sought to mimic the pattern of glucose delivery into the circulation during an oral glucose tolerance test, the infusion rate over the first 15 minutes in this study was at the higher end of the rate in previous studies that evaluated the effects of duodenal lipid infusion (ie, 4 kcal/min) (28). Conceivably, the intestinal caloric delivery rate in the present study approximates that in patients with rapid gastric emptying.
During enteral infusion, CCK and GIP increased first, followed by increase in GLP1 and, finally, increase in PYY. This pattern is consistent with the site of release of these hormones in the small intestine: duodenum for CCK and GIP, jejunum and ileum for GLP1, and ileum for PYY. The 3-fold increase in plasma GLP1 concentration is similar to the increase observed during enteral infusion, at 6 kcal/minute for 10 minutes (29). The observed associations between moderate or more severe symptoms and greater plasma concentrations of hormones (ie, CCK, GLP-1, GIP, and glucagon) during nutrient infusion are consistent with the effects of CCK and GLP1, which mediate satiation and nausea by stimulating receptors on vagal afferents and centrally mediated effects (8, 30). CCK release during intraduodenal fat infusion also relaxed the lower esophageal sphincter and increased gastroesophageal acid reflux, which may partly explain why symptoms of dyspepsia and reflux occur concurrently (31). Indeed, the CCK antagonist dexloxiglumide inhibited upper GI symptoms during duodenal lipid perfusion (8). Neither physiological nor pharmacological concentrations of GIP regulate satiety in humans (32).
We were surprised to uncover impaired glucose tolerance in one-third of patients with functional dyspepsia. Differences in glycemic exposure between patients with normal glucose tolerance and impaired glucose tolerance were striking. Moreover, patients with impaired glucose tolerance also had higher plasma levels of GLP1 and PYY, reflecting greater secretion, during dextrose and lipid infusions.
Marked hyperglycemia (blood glucose ∼ 270 mg/dL) increases symptoms (eg, nausea, fullness) during gastric distention even in healthy subjects (33). One possible limitation is that hyperglycemia may contribute to symptoms in patients with dyspepsia and impaired glucose tolerance. While a greater proportion (71% vs 41%) of patients with impaired than normal glucose tolerance had moderate or more severe symptoms during carbohydrate infusion, differences were not significant. Moreover, hyperglycemia cannot be invoked to explain severe symptoms during lipid infusion. Six of 8 patients with impaired glucose tolerance also had rapid gastric emptying, which can cause hyperglycemia and exaggerated hormonal responses (eg, GLP1, GIP, insulin) after nutrient ingestion (34–37). In the present study, however, impaired glucose tolerance cannot be explained by rapid gastric emptying because the enteral nutrients were delivered at the same rate in all participants. The correlation between plasma PYY concentrations and small-bowel transit time is suggestive that rapid intestinal transit with increased delivery of nutrients to the lower small intestine might explain, at least partly, the exaggerated release of PYY.
Normally, GLP1 improves glucose tolerance by stimulating glucose-dependent insulin release and inhibiting glucagon secretion, among other mechanisms. In contrast, patients with impaired glucose tolerance had increased plasma glucose concentrations despite increased GLP1 release. Moreover, increased GLP1 release was not accompanied by increased plasma C-peptide or reduced plasma glucagon concentrations. Taken together, these findings suggest reduced beta islet cell responsiveness or insulin sensitivity, or both, but they need to be confirmed with mathematical modeling of glucose disposition and with additional prospective studies. Conceivably, interindividual differences in response to GLP1 may be explained by genetic variations that reduce the function of GLP1 receptors (38). Since GLP1 contributes to postprandial gastric accommodation (39, 40), it is tempting to speculate that decreased responsiveness to GLP1 might explain impaired gastric accommodation, which underlies functional dyspepsia, and impaired glucose tolerance. Moreover, patients had rapid gastric emptying despite increased plasma concentrations of GLP1 and PYY, suggestive that rapid gastric emptying cannot be explained by impaired secretion of these hormones, which are mediators of the gastric brake. To the contrary, they suggest the possibility that the end-organ mechanisms in the stomach are resistant to these hormones.
The severity of symptoms after a meal is associated with postprandial sensitivity to balloon distention and, in some studies, with impaired gastric accommodation (2, 41, 42). Selected symptoms (eg, postprandial fullness, vomiting) recorded over a longer duration, typically a few weeks, were also associated with delayed gastric emptying in some studies of functional dyspepsia (5). In the present study, increased nutrient sensitivity was associated not only with increased severity of day-to-day symptoms, but also with poorer QOL in functional dyspepsia, which confirms the criterion validity of these measurements. Moreover, in the multiple variable models, symptoms resulting from infusion of nutrients and depression were the only significant predictors of overall symptom severity and QOL in functional dyspepsia, respectively. Taken together, these findings are aligned with the biopsychosocial model of dyspepsia (43) and highlight the greater contribution of enteral nutrient sensitivity to symptom severity and of depression to QOL. Of particular interest, patients with functional dyspepsia had duodenal mucosal inflammation, altered expression of mucosal tight junction proteins, and increased mucosal permeability (44), which might conceivably predispose to increased visceral sensitivity. Likewise, other studies have implicated a role for eosinophils in functional dyspepsia (45).
However, duodenal mucosal biopsies were not obtained in this study. Hence, we do not know if these observations (eg, increased enteral sensitivity to nutrient infusions) are associated with duodenal mucosal inflammation. While we cannot exclude tertiary care bias, the prevalence of anxiety and depression and dyspepsia symptom severity scores argue against the same. In summary, increased sensitivity to enteral dextrose and lipid infusions was associated with greater plasma concentrations of enteral hormone, more severe daily symptoms, and worse QOL in functional dyspepsia. These observations are consistent with the hypothesis that enteral hormones mediate increased intestinal sensitivity to nutrients in functional dyspepsia.
What is current knowledge?
Most patients with functional dyspepsia report postprandial symptoms, especially after meals rich in fat
Duodenal lipid infusion increased the sensitivity to gastric distention in patients with functional dyspepsia.
It is unknown if duodenal nutrient sensitivity per se (ie, without gastric distention) is increased in functional dyspepsia
What is new here?
Sensitivity to duodenal lipid and dextrose infusion is greater in functional dyspepsia than in controls.
Increased nutrient sensitivity is associated with increased day-to-day symptoms of dyspepsia, worse dyspepsia-related QOL, and higher plasma hormone (ie, GLP1, CCK, and GIP) concentrations during duodenal nutrient infusion.
One third of patients with dyspepsia, not due to diabetes mellitus, had impaired glucose tolerance, which was associated with greater GLP1 and peptide YY concentrations during dextrose and lipid infusions, respectively.
How might it impact on clinical practice in the foreseeable future?
Taken together with recent studies (eg, Gut 2014;63:262-71), these findings, contrary to current concepts, implicate the duodenum in the pathophysiology of symptoms in functional dyspepsia, and prompt consideration of GLP-1 and CCK antagonists to reduce enteral nutrient sensitivity and improve symptoms in functional dyspepsia.
Acknowledgments
Funding disclosure: This study was supported by US Public Health Service National Institutes of Health grant R01 DK68055. This project was supported by grant number UL1 TR000135 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Financial support:
This study was supported by US Public Health Service National Institutes of Health grant P01 DK68055. This project was supported by grant number UL1 TR000135 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- BMI
body mass index
- CCK
cholecystokinin
- ELISA
enzyme-linked immunosorbent assay
- GI
gastrointestinal
- GIP
glucose-dependent insulinotropic peptide
- GLP1
glucagonlike peptide 1
- HADS
Hospital Anxiety and Depression Scale
- PYY
peptide YY
- QOL
quality of life
Footnotes
Guarantor of the article:
Adil E. Bharucha, MD
Specific author contributions:
Adil E. Bharucha—Study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; approved the final draft submitted
Michael Camilleri—Analysis and interpretation of data; critical revision of the manuscript for important intellectual content; approved the final draft submitted
Duane Burton, Shannon Thieke, Kelly Feuerhak—Acquisition of data; approved the final draft submitted
Ananda Basu—Study design, critical revision of the manuscript for important intellectual content; approved the final draft submitted
Alan R. Zinsmeister—Statistical analysis; approved the final draft submitted
Conflicts of interest:
None
Contributor Information
Adil E. Bharucha, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota.
Michael Camilleri, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota.
Duane D. Burton, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota.
Shannon L. Thieke, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota
Kelly J. Feuerhak, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota.
Ananda Basu, Division of Endocrinology, Diabetes, Metabolism and Nutrition, Mayo Clinic, Rochester, Minnesota.
Alan R. Zinsmeister, Division of Biomedical Statistics and Informatics, Mayo Clinic, Rochester, Minnesota.
References
- 1.Feinle-Bisset C, Azpiroz F. Dietary and lifestyle factors in functional dyspepsia. Nature Reviews Gastroenterology & Hepatology. 2013;10:150–157. doi: 10.1038/nrgastro.2012.246. [DOI] [PubMed] [Google Scholar]
- 2.Vanheel H, Farre R. Changes in gastrointestinal tract function and structure in functional dyspepsia. Nature Reviews Gastroenterology & Hepatology. 2013;10:142–149. doi: 10.1038/nrgastro.2012.255. [DOI] [PubMed] [Google Scholar]
- 3.Camilleri M, Stanghellini V. Current management strategies and emerging treatments for functional dyspepsia. Nature Reviews Gastroenterology & Hepatology. 2013;10:187–194. doi: 10.1038/nrgastro.2013.11. [DOI] [PubMed] [Google Scholar]
- 4.Camilleri M. Integrated upper gastrointestinal response to food intake. Gastroenterology. 2006;131:640–658. doi: 10.1053/j.gastro.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Lacy BE. Functional dyspepsia and gastroparesis: one disease or two? American Journal of Gastroenterology. 2012;107:1615–1620. doi: 10.1038/ajg.2012.104. [DOI] [PubMed] [Google Scholar]
- 6.Barbera R, Feinle C, Read NW. Nutrient-specific modulation of gastric mechanosensitivity in patients with functional dyspepsia. Digestive Diseases and Sciences. 1995;40:1636–1641. doi: 10.1007/BF02212683. [DOI] [PubMed] [Google Scholar]
- 7.Barbera R, Feinle C, Read NW. Abnormal sensitivity to duodenal lipid infusion in patients with functional dyspepsia. European Journal of Gastroenterology and Hepatology. 1995;7:1051–1057. doi: 10.1097/00042737-199511000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Feinle C, Meier O, Otto B, et al. Role of duodenal lipid and cholecystokinin A receptors in the pathophysiology of functional dyspepsia. Gut. 2001;48:347–355. doi: 10.1136/gut.48.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjornsson E, Sjoberg J, Ringstrom G, et al. Effects of duodenal lipids on gastric sensitivity and relaxation in patients with ulcer-like and dysmotility-like dyspepsia. Digestion. 2003;67:209–217. doi: 10.1159/000072059. [DOI] [PubMed] [Google Scholar]
- 10.Feinle C, D'Amato M, Read NW. Cholecystokinin-A receptors modulate gastric sensory and motor responses to gastric distension and duodenal lipid. Gastroenterology. 1996;110:1379–1385. doi: 10.1053/gast.1996.v110.pm8613041. [DOI] [PubMed] [Google Scholar]
- 11.Feinle C, Rades T, Otto B, et al. Fat digestion modulates gastrointestinal sensations induced by gastric distention and duodenal lipid in humans. Gastroenterology. 2001;120:1100–1107. doi: 10.1053/gast.2001.23232. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz MP, Samsom M, Smout AJ. Chemospecific alterations in duodenal perception and motor response in functional dyspepsia. American Journal of Gastroenterology. 2001;96:2596–2602. doi: 10.1111/j.1572-0241.2001.04103.x. [DOI] [PubMed] [Google Scholar]
- 13.Pilichiewicz AN, Feltrin KL, Horowitz M, et al. Functional dyspepsia is associated with a greater symptomatic response to fat but not carbohydrate, increased fasting and postprandial CCK, and diminished PYY. American Journal of Gastroenterology. 2008;103:2613–2623. doi: 10.1111/j.1572-0241.2008.02041.x. [DOI] [PubMed] [Google Scholar]
- 14.MacIntosh CG, Andrews JM, Jones KL, et al. Effects of age on concentrations of plasma cholecystokinin, glucagon-like peptide 1, and peptide YY and their relation to appetite and pyloric motility. American Journal of Clinical Nutrition. 1999;69:999–1006. doi: 10.1093/ajcn/69.5.999. [DOI] [PubMed] [Google Scholar]
- 15.Talley NJ, Haque M, Wyeth JW, et al. Development of a new dyspepsia impact scale: the Nepean Dyspepsia Index. Alimentary Pharmacology and Therapeutics. 1999;13:225–235. doi: 10.1046/j.1365-2036.1999.00445.x. [DOI] [PubMed] [Google Scholar]
- 16.Talley NJ, Verlinden M, Jones M. Validity of a new quality of life scale for functional dyspepsia: a United States multicenter trial of the Nepean Dyspepsia Index. American Journal of Gastroenterology. 1999;94:2390–2397. doi: 10.1111/j.1572-0241.1999.01363.x. [DOI] [PubMed] [Google Scholar]
- 17.Zigmond A, Snaith R. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 18.Camilleri M, Zinsmeister AR, Greydanus MP, et al. Towards a less costly but accurate test of gastric emptying and small bowel transit. Digestive Diseases & Sciences. 1991;36:609–615. doi: 10.1007/BF01297027. [DOI] [PubMed] [Google Scholar]
- 19.Rao SSC, Camilleri M, Hasler WL, et al. Evaluation of gastrointestinal transit in clinical practice: position paper of the American and European Neurogastroenterology and Motility Societies. Neurogastroenterology and Motility. 2011;23:8–23. doi: 10.1111/j.1365-2982.2010.01612.x. [DOI] [PubMed] [Google Scholar]
- 20.Frost G, Brynes AE, Ellis S, et al. Nutritional influences on gut hormone release. Current Opinion in Endocrinology and Diabetes. 2006;13:42–48. [Google Scholar]
- 21.Alzaid AA, Dinneen SF, Turk DJ, et al. Assessment of insulin action and glucose effectiveness in diabetic and nondiabetic humans. Journal of Clinical Investigation. 1994;94:2341–2348. doi: 10.1172/JCI117599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Firth RG, Bell PM, Marsh HM, et al. Postprandial hyperglycemia in patients with noninsulin-dependent diabetes mellitus. Role of hepatic and extrahepatic tissues. Journal of Clinical Investigation. 1986;77:1525–1532. doi: 10.1172/JCI112467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMahon MM, Schwenk WF, Haymond MW, et al. Underestimation of glucose turnover measured with [6-3H]- and [6,6-2H]- but not [6-14C]glucose during hyperinsulinemia in humans. Diabetes. 1989;38:97–107. doi: 10.2337/diab.38.1.97. [DOI] [PubMed] [Google Scholar]
- 24.Butler PC, Rizza RA. Contribution to postprandial hyperglycemia and effect on initial splanchnic glucose clearance of hepatic glucose cycling in glucose-intolerant or NIDDM patients. Diabetes. 1991;40:73–81. [PubMed] [Google Scholar]
- 25.Rehfeld JF. Accurate measurement of cholecystokinin in plasma. Clinical Chemistry. 1998;44:991–1001. [see comment] [PubMed] [Google Scholar]
- 26.Caldarella MP, Azpiroz F, Malagelada JR. Selective effects of nutrients on gut sensitivity and reflexes. Gut. 2007;56:37–42. doi: 10.1136/gut.2004.062869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feinle C, Christen M, Grundy D, et al. Effects of duodenal fat, protein or mixed-nutrient infusions on epigastric sensations during sustained gastric distension in healthy humans. Neurogastroenterology & Motility. 2002;14:205–213. doi: 10.1046/j.1365-2982.2002.00318.x. [DOI] [PubMed] [Google Scholar]
- 28.Pilichiewicz AN, Papadopoulos P, Brennan IM, et al. Load-dependent effects of duodenal lipid on antropyloroduodenal motility, plasma CCK and PYY, and energy intake in healthy men. American Journal of Physiology - Regulatory Integrative & Comparative Physiology. 2007;293:R2170–R2178. doi: 10.1152/ajpregu.00511.2007. [DOI] [PubMed] [Google Scholar]
- 29.Chaikomin R, Doran S, Jones KL, et al. Initially more rapid small intestinal glucose delivery increases plasma insulin, GIP, and GLP-1 but does not improve overall glycemia in healthy subjects. American Journal of Physiology - Endocrinology & Metabolism. 2005;289:E504–E507. doi: 10.1152/ajpendo.00099.2005. [DOI] [PubMed] [Google Scholar]
- 30.Dailey MJ, Moran TH. Glucagon-like peptide 1 and appetite. Trends in Endocrinology & Metabolism. 2013;24:85–91. doi: 10.1016/j.tem.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lacy BE, Carter J, Weiss JE, et al. The effects of intraduodenal nutrient infusion on serum CCK, LES pressure, and gastroesophageal reflux. Neurogastroenterology and Motility. 2011;23 doi: 10.1111/j.1365-2982.2011.01701.x. 631-e256. [DOI] [PubMed] [Google Scholar]
- 32.Edholm T, Degerblad M, Gryback P, et al. Differential incretin effects of GIP and GLP-1 on gastric emptying, appetite, and insulin-glucose homeostasis. Neurogastroenterology and Motility. 2010;22:1191–1200. doi: 10.1111/j.1365-2982.2010.01554.x. e315. [DOI] [PubMed] [Google Scholar]
- 33.Rayner CK, Samsom M, Jones KL, et al. Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care. 2001;24:371–381. doi: 10.2337/diacare.24.2.371. [DOI] [PubMed] [Google Scholar]
- 34.Lawaetz O, Blackburn AM, Bloom SR, et al. Gut hormone profile and gastric emptying in the dumping syndrome. A hypothesis concerning the pathogenesis. Scandinavian Journal of Gastroenterology. 1983;18:73–80. doi: 10.3109/00365528309181562. [DOI] [PubMed] [Google Scholar]
- 35.Andreasen JJ, Orskov C, Holst JJ. Secretion of glucagon-like peptide-1 and reactive hypoglycemia after partial gastrectomy. Digestion. 1994;55:221–228. doi: 10.1159/000201151. [DOI] [PubMed] [Google Scholar]
- 36.Gebhard B, Holst JJ, Biegelmayer C, et al. Postprandial GLP-1, norepinephrine, and reactive hypoglycemia in dumping syndrome. Digestive Diseases & Sciences. 2001;46:1915–1923. doi: 10.1023/a:1010635131228. [DOI] [PubMed] [Google Scholar]
- 37.Tack J, Arts J, Caenepeel P, et al. Pathophysiology, diagnosis and management of postoperative dumping syndrome. Nature Reviews Gastroenterology & Hepatology. 2009;6:583–590. doi: 10.1038/nrgastro.2009.148. [DOI] [PubMed] [Google Scholar]
- 38.Koole C, Wootten D, Simms J, et al. Polymorphism and ligand dependent changes in human glucagon-like peptide-1 receptor (GLP-1R) function: allosteric rescue of loss of function mutation. Molecular Pharmacology. 2011;80:486–497. doi: 10.1124/mol.111.072884. [Erratum appears in Mol Pharmacol. 2012 Jul;82(1):142] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delgado-Aros S, Kim DY, Burton DD, et al. Effect of GLP-1 on gastric volume, emptying, maximum volume ingested, and postprandial symptoms in humans. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2002;282:G424–G431. doi: 10.1152/ajpgi.2002.282.3.G424. [DOI] [PubMed] [Google Scholar]
- 40.Andrews CN, Bharucha AE, Camilleri M, et al. Nitrergic contribution to gastric relaxation induced by glucagon-like peptide-1 (GLP-1) in healthy adults. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2007;292:G1359–G1365. doi: 10.1152/ajpgi.00403.2006. [DOI] [PubMed] [Google Scholar]
- 41.Farre R, Vanheel H, Vanuytsel T, et al. In functional dyspepsia, hypersensitivity to postprandial distention correlates with meal-related symptom severity. Gastroenterology. 2013;145:566–573. doi: 10.1053/j.gastro.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 42.Delgado-Aros S, Camilleri M, Cremonini F, et al. Contributions of gastric volumes and gastric emptying to meal size and postmeal symptoms in functional dyspepsia. Gastroenterology. 2004;127:1685–1694. doi: 10.1053/j.gastro.2004.09.006. [see comment] [DOI] [PubMed] [Google Scholar]
- 43.Van Oudenhove L, Aziz Q. The role of psychosocial factors and psychiatric disorders in functional dyspepsia. Nature Reviews Gastroenterology & Hepatology. 2013;10:158–167. doi: 10.1038/nrgastro.2013.10. [DOI] [PubMed] [Google Scholar]
- 44.Vanheel H, Vicario M, Vanuytsel T, et al. Impaired duodenal mucosal integrity and low-grade inflammation in functional dyspepsia. Gut. 2014;63:262–271. doi: 10.1136/gutjnl-2012-303857. [DOI] [PubMed] [Google Scholar]
- 45.Walker MM, Warwick A, Ung C, et al. The role of eosinophils and mast cells in intestinal functional disease. Current Gastroenterology Reports. 2011;13:323–330. doi: 10.1007/s11894-011-0197-5. [DOI] [PubMed] [Google Scholar]