Abstract
Objective
Obese women experience worse reproductive outcomes compared to normal weight women, specifically infertility, pregnancy loss, fetal malformations and developmental delay. The objective of this study was to use a genetic mouse model of obesity in order to recapitulate the human reproductive phenotype and further examine potential mechanisms and therapies.
Methods
New inbred, polygenic Type 2 diabetic TallyHO mice and age matched control C57BL/6 mice were superovulated to obtain morulae or blastocysts stage embryos which were cultured in human tubal fluid media. Deoxyglucose uptake was performed on insulin-stimulated individual blastocysts. Apoptosis was detected by confocal microscopy using TUNEL assay and Topro-3 nuclear dye. Embryos were scored for %TUNEL positive/total nuclei. AMPK activation, TNFα expression, and adiponectin expression were analyzed by western immunoblot and confocal immunofluorescent microscopy. Lipid accumulation was assayed by Bodipy. Finally all measured parameters were compared between TallyHO mice in morulaes cultured to blastocyst embryos in either human tubal fluid (HTF) media or HTF with 25ug/ml metformin added.
Results
TallyHo mice developed whole body abnormal insulin tolerance, decreased litter number and increased NEFA. Blastocysts demonstrated increased apoptosis, decreased insulin sensitivity, and decreased activation of AMP activated protein-kinase (AMPK). As a possible cause of the insulin resistance/abnormal P-AMPK, we found that Tumor necrosis Factor (TNFα) expression and lipid accumulation as detected by BODIPY were increased in TallyHO blastocysts and adiponectin was decreased. Culturing TallyHO morulae with the AMPK activator, metformin lead to a reversal of all abnormal findings, including increased p-AMPK, improved insulin-stimulated glucose uptake and normalization of lipid accumulation.
Conclusions
Women with obesity and insulin resistance experience poor pregnancy outcomes. Previously we have shown in mouse models of insulin resistance that AMPK activity is decreased and that activators of AMPK reverse the poor embryo outcomes. Here, we show for the first time using a genetically altered obese model, not a diet-induced model, that metformin reverses many of the adverse effects of obesity at the level of the blastocyst. Expanding on this we determine that activation of AMPK via metformin reduces lipid droplet accumulation, presumably by eliminating the inhibitory effects of TNFα, resulting in normalization of fatty acid oxidation and HADH2 activity. Metformin exposure in vitro was able to partially reversing these effects, at the level of the blastocyst and thus may be effective in preventing the adverse effects of obesity on pregnancy and reproductive outcomes.
Introduction
Over the last two decades there has been a dramatic increase in the incidence of obesity both in the U.S and globally (Hammond, 2009, Ogden, et al., 2012). Obesity plays a key role in the insulin resistance syndrome that includes hyperinsulinemia, hyperlipidemia, hypertension, type 2 diabetes and increased cardiovascular risks (Steinberger, et al., 2003). Obesity and insulin resistance during pregnancy carry a higher risk for birth defects and poor pregnancy outcomes, as well as significant maternal risks during pregnancy (Waller, et al., 2007). Infants of obese women experience higher rates of birth defects and these are responsible for 20% of all infant deaths (Blackmore and Ozanne, 2013). Furthermore, the usual concurrent insulin resistance experienced by these women can cause polycystic ovary syndrome, infertility, spontaneous abortion and significant intrauterine growth abnormalities (Allemand, et al., 2009, Cocksedge, et al., 2008, Street, et al., 2009). Polycystic ovary syndrome (PCOS) incidence is much higher in obese and insulin resistant women, and insulin resistance is a common feature in patients with this syndrome, affecting approximately 65% of obese and 25% of lean PCOS patients (Dale, et al., 1998) but the pathogenesis remains unknown.
Obesity and type-2 diabetes initiate chronic inflammatory responses leading to abnormal cytokine production that is linked with dysregulated adipokine release and systemic insulin resistance (Sweet, et al., 2009, Yoo and Choi, 2014). Secretion of the cytokine TNFα in adipose tissue alters the differentiation of preadipocytes, and reduces adiponectin secretion (Fain, et al., 2008). TNFα is a pleiotropic cytokine that can exert a variety of effects including growth promotion and inhibition, cytotoxicity, and inflammation (Balkwill, 2009). In addition, TNFα is implicated in the pathogenesis of insulin resistance because it is elevated in circulation, skeletal muscle and adipose tissue of patients with diabetes (Li, et al., 2009). Impaired insulin signaling results from TNFα promotion of serine phosphorylation of insulin receptor substrate 1 (IRS-1) and reduced expression of Glucose Transporter 4 (GLUT 4). Furthermore, elevated levels of TNFα observed in obesity, suppress AMPK signaling and are reversed in mice null for both TNFR1 and 2 or following treatment with a TNFα neutralizing antibody (Li, Yang, Shi, Yang, Liu and Boden, 2009).
AMPK is an evolutionary conserved energy sensing protein that has been correlated with insulin resistant embryo models (Dzamko and Steinberg, 2009). ATP generation during catabolic processes is switched on by AMPK while switching off ATP requiring pathways. Originally AMPK was classified as a cellular energy gauge however; evidence indicates that AMPK regulates whole-body energy homeostasis, acting in metabolic tissues in response to nutrient and hormonal signals (Xu, et al., 2014). In skeletal muscle, the adipokines leptin and adiponectin, as well as exercise, activate AMPK, thus stimulating fatty acid oxidation (Claret, et al., 2007, Shibata, et al., 2005, Yamauchi, et al., 2002). In embryos and embryonic cells there is crosstalk between AMPK and Insulin signaling. AMPK activation stimulates glucose-uptake, meiotic maturation in mouse oocytes, improves insulin signaling and rescues blastocyst from apoptosis due to insulin resistance (Eng, et al., 2007, Louden, et al., 2008). Thus, in peripheral tissues, AMPK regulates substrate oxidation and fuel storage, maintaining the appropriate partitioning of metabolites. However, the mechanisms between decreased AMPK activation and insulin resistance remain unclear.
As the largest endocrine organ in the body, adipose tissue plays an important role in the regulation of metabolism and inflammation (Antuna-Puente, et al., 2008). Adiponectin is the most abundant adipokine secreted by fat cells. Adiponectin is positively correlated with insulin sensitivity and enhances insulin action (Tschritter, et al., 2003). In contrast to leptin, adiponectin levels in plasma vary little between feeding and fasting. The mechanism by which adiponectin can modulate insulin action is largely unknown. Conversely, during euglycemic hyperinsulinemic clamps, a 10% to 15% fall in circulating adiponectin levels has been reported (Isakson, et al., 2009). Full-length adiponectin, down-regulates genes involved in gluconeogenesis through AMPK in the liver and has been shown to reduce serum glucose concentrations via glucose uptake, stimulate fatty acid oxidation, and lactate production through AMPK activation in C2C12 myocytes and skeletal muscle (Barb, et al., 2007). Decreased plasma adiponectin concentrations are associated with insulin resistance, type 2 diabetes, and atherosclerosis.
Previous models of insulin resistance, exhibiting high circulating levels of insulin or IGF-I, have shown a detrimental effect on murine preimplantation embryos both in vitro and in vivo. Insult to the insulin metabolic pathway results in apoptosis, abnormal AMPK activation, and decreased glucose uptake which is critical to embryo development and survival (Chi, et al., 2000). Thus, the outcomes are higher rates of miscarriages and lower implantation rates (Riley, et al., 2006). Women with PCOS, type 2 diabetes, and obesity all experience increased rates of early pregnancy loss similar to the animal findings. We have previously shown that there is significant crosstalk between the AMPK and insulin signaling pathways in embryonic cells from hyperinsulinemic models and that AMPK activation is altered (Louden, Chi and Moley, 2008). However, these effects can be reversed by activation of the AMPK pathway by metformin or phenformin. Our studies sought to elucidate the mechanisms in the preimplantation embryo responsible for decreased AMPK activation and insulin resistance using a novel mouse model of type 2 diabetes, the TallyHO mouse (Kim, et al., 2001, Kim, et al., 2005). We hypothesize that elevated TNFα levels in murine embryonic models of hyperinsulinemia causes perturbations to the insulin signaling pathway through suppression of AMPK activation, decreased fatty acid oxidation and increased lipid accumulation.
Materials & Methods
Insulin tolerance tests
For the insulin tolerance test (ITT), 10 week old C57Bl/6 females mice (The Jackson Laboratories, Bar Harbor, ME, USA) (n = 17) and age matched TallyHO female mice (The Jackson Laboratories, Bar Harbor, ME, USA) (n = 15) mice were fasted 3 h prior to an intraperitoneal injection of bovine insulin (0.75 mU/g body wt; Sigma, St. Louis, MO USA). Blood glucose was measured at 0, 15, 30, 45, and 60 min via the tail vein. The percentage decrease in blood glucose from the 0 min time point was calculated.
Non-esterified fatty acids (NEFA) were measured in serum using an in vitro enzymatic colorimetric method assay (Wako HR series NEFA-HR; Richmond VA, USA).
Litter Size
Beginning at 10 weeks of age following confirmation of insulin intolerance by ITT, TallyHO and control mice were housed with males and allowed to mate spontaneously. Over a further 10 weeks, the total number of pups per litter were calculated and tracked.
Embryo recovery and culture
Embryos were recovered as previously described. Briefly, 3-week-old female mice (C57Bl/J or hyperinsulinemic TallyHO (TH) mice) were given free access to food and water and were maintained on a 12-h light/dark cycle. Female mice were superovulated with an intraperitoneal injection of 10 IU/animal pregnant mare serum gonadotropin (Sigma, St. Louis, MO, USA), followed 48 h later by 10 IU/animal human chorionic gonadotropin (hCG) (Sigma, St. Louis, MO USA). To count the number of MII oocytes, mice were sacrificed 12 to 14 hours later, and cumulus enclosed oocytes were collected from the ampulla. To collect embryos, female mice were mated with males of proven fertility overnight after the hCG injection. Mating was confirmed by identification of a vaginal plug. Two-cell embryos were obtained by flushing the oviducts 46h post-hCG. To recover embryos at the morula or blastocyst embryo stage, mice were killed 72 or 96 h after hCG injection respectively. Embryos were recovered by flushing dissected uterine horns with human tubal fluid (HTF) medium (Irvine Scientific, Santa Ana, CA) containing 0.25% BSA (fraction V; Sigma). C57Bl/6 embryos were then cultured in vitro in the following treatment media for 24 h until blastocyst stage: 1) control HTF medium; 2) HTF with added metformin (25µg/ml). These experiments were conducted at least three times each. All procedures described above were reviewed and approved by the animal studies committee at Washington University and were performed in accordance with the institutional animal care and use committee's approval.
TUNEL Assay
Apoptosis was assayed in blastocysts and morulae from C57Bl/6 or TallyHO mice after culturing for 24h in HTF ± 25µg/ml Metformin (Sigma; St. Louis, MO, USA), using terminal dUTP nick end labeling (TUNEL). Blastocysts were fixed in 2% paraformaldehyde (Sigma; St. Louis, MO, USA) for 1 h and permeabilized in 0.1% Triton X-100 (Sigma; St. Louis, MO, USA) for 2 min. Apoptosis was assessed using the In Situ Cell Death Detection Kit, TMR (Roche Diagnostics; Indianapolis, IN, USA). After the TUNEL assay was performed the nuclei of the blastocyst were stained using 4 µM To-Pro-3-iodide (Molecular Probes, Eugene, OR, USA) for 20 min. Pictures were taken using a Nikon laser-scanning confocal microscope (Eclipse C1 Plus) at 20×.
Insulin-stimulated 2-deoxyglucose uptake in blastocysts
Nonradioactive insulin-stimulated 2-deoxyglucose uptake into single blastocysts was performed using microfluorometric assays combined with enzymatic cycling reactions as previously described (Chi, et al., 2002). Briefly, two separate types of experiments were conducted. First, C57Bl/6 blastocysts or TallyHO blastocyst were isolated. Alternatively, C57Bl/6 or TallyHO embryos were collected at morula stage and cultured for 24 h in HTF media with added 25µg/ml Metformin. Next, blastocysts from each condition were incubated in HTF medium with or without a final glucose concentration of 5.6mmol/l with 500nmol/l insulin (bovine pancreas; Sigma, St. Louis, MO USA) for 30 min. 2-Deoxyglucose uptake was then measured as described previously (Chi, Hoehn and Moley, 2002) and expressed as millimoles per kilogram wet weight over a 15-min time interval. The rate is expressed as the difference between insulin-stimulated and basal values. This assay was conducted at least 3 times with each set of experiments. Fifteen to 20 blastocysts were used in each assay.
Embryo Western immunoblot analysis/immunofluorescence
An average of 75 TallyHO or C57Bl/6 blastocysts were pooled, added directly to Laemmli sample buffer, subjected to SDS-PAGE, and transferred to nitrocellulose. Samples were subjected to SDS-PAGE and transferred to nitrocellulose. Blots were blocked for 1 h at RT in 5% milk in TBS-T. The blots were probed overnight at 4°C in 1% milk in TBS-T with the indicated antibodies: TNFα, AMPK, p-AMPK, (Cell Signaling, Danvers, MA, USA); adiponectin [Covance, Princeton, NJ, USA). The HRP-conjugated secondary antibody (either goat anti-rabbit or goat anti-mouse) (Sigma; St. Louis, MO, USA) was used for detection and visualized using SuperSignal West Dura (Thermo Scientific, Rockford, IL, USA). For the immunofluorescence, blastocysts from the two culture conditions were fixed on slides in 3% paraformaldehyde (Sigma, St. Louis, MO USA) for 20 min and permeabilized in a 0.1% Tween-20 (Sigma, St. Louis, MO USA) solution for 20 min. The embryos were blocked with 20% normal donkey serum in PBS with 2% BSA (Sigma, St. Louis, MO USA) at room temperature for 60 min, washed three times with PBS/BSA, followed by 1-h incubation with primary antibodies noted above. Following three washes with PBS/BSA the embryos were incubated for 20 min in the appropriate fluorescein-tagged secondary antibody. The nuclei were stained with To-Pro-3-iodide (Molecular Probes, Eugene, OR, USA) for 20 min. Three final washes with PBS/BSA were performed, and the embryos were mounted in VectaShield (Vector Labs, Burlingame, CA, USA), covered with cover slips, and sealed. The slides were examined with an Olympus laser-scanning confocal microscope (FV1200) at 20×. All experiments, Western immunoblots and immunofluorescence were conducted at least three times on separate pools of blastocysts to generate the figures shown.
Hydroxyacyl-CoA dehydrogenase (Hadha), metabolic analysis
The TallyHO embryos were freeze-dried as described by Chi et al (Chi, Hoehn and Moley, 2002). Briefly, they were transferred with 0.5–1 µl of the incubation medium onto a glass slide with a braking pipette, frozen by dipping the slide into isopentane, brought to −150°C with liquid nitrogen, and then freeze-dried on the slide in a glass vacuum tube in a cryostat at −35°C. The dry medium and crystals surrounding the ova were removed with a stiff hair point, and specimens were stored at −70°C in individual wells, measuring 0.5-mm deep and 3-mm wide, drilled into the surface of a microscopic glass slide. Each embryo was added to 1 µl of a special extraction medium (Chi, Hoehn and Moley, 2002) that permits storage at −70°C and repeated freezing and thawing without appreciable loss of any of the Hadha reported here. A single embryo was used to extract aliquots obtained in nanoliter volumes under oil using constriction glass pipettes, as previously described for the assay of enzymes and metabolites from oocytes and embryos. The specific methods were followed as previously published procedures: hydroxyacyl-CoA dehydrogenase (Hadha) (previously referred to as BOAC) from Passonneau & Lowry (Passonneau and Lowry, 1993) and (Jimenez, et al., 2013).
Lipid Accumulation
Lipid accumulation was detected by staining paraformaldehyde-fixed TallyHO and C57Bl/6 treated blastocysts with the neutral lipid specific dye Bodipy 493/503 (Molecular probes, Eugene, OR, USA) at 1mg/ml as previously described (Jimenez, Frolova, Chi, Grindler, Willcockson, Reynolds, Zhao and Moley, 2013). Microscopic images were obtained using a Nikon fluorescent microscope (Nikon Instruments; Melville, NY, USA) at 20× and fluorescence intensity was measured in an image of an entire blastocyst with Image J software (NIH).
Statistical Analysis
All experiments were repeated at least three times. Results are expressed as mean ±S.D. of three separate experiments. The deoxyglucose uptake Assay, Bodipy Fluorescence and Hadha activity were analyzed statistically by ANOVA with Fisher post-hoc test. The pups per litter and the metabolic parameters such as glucose levels and NEFAs were compared using Students T test. Significance was defined as p<0.05.
Results
TallyHO females experience impaired insulin tolerance, elevated free fatty acids levels and decreased litter size
By 10 weeks of age TallyHO female mice (n=15) display impaired insulin tolerance as compared to C57Bl/6 controls (n=17) as assayed by an insulin tolerance test (ITT) (Figure 1A) (*p<0.01; **p<0.05). Serum levels of free fatty acids were significantly elevate (Figure 1B) (*p<0.001). In addition the average number of pups per litter was significantly lower in the 10–13 week old TallyHO females as compared to age matched controls (Figure 1C) (*p<0.01). The conclusion from these experiments is that this animal model accurately recapitulated the obesity phenotype seen in the DIO mouse model (Luzzo, et al., 2012)
Figure 1. TallyHO females experience impaired insulin tolerance, elevated free fatty acids and decreased litter size.
A. Insulin tolerance test on TallyHO mice (dashed line; n=15) vs Control C57Bl/6 mice (solid line; n=17). T0 (time zero); T15 (15 minutes after insulin injection); T30 (30 minutes after insulin injection); T45 (45 minutes after insulin injection); T60 (60 minutes after insulin injection). * p<0.01; ** p< 0.05. B. Nonesterified Fatty acids were also measured in sera from the same mice as A. * p< 0.001. C. Five TallyHO female and five control female mice were individually placed in breeding cages with control males of proven fertility. The males were 8 weeks and the females 10 weeks. Over the next 10 weeks, the numbers of pups per litter were recorded and averages obtained. *p< 0.01.
Blastocyst stage embryos from TallyHO females exhibit increased apoptosis and decreased insulin-stimulated glucose uptake
Due to the low litter number, we examined the number of ovulated oocytes in response to hyperstimulation with PMSG and ovulation with hCG. The number of MII oocytes did not differ between control mice and TallyHO females (Data not shown). Next we examined the number of 2-cell embryos and we found that the total number did not differ, however, more of the 2-cells from the TallyHO looked morphologically abnormal meaning misshapen and asymmetrical (Data not shown). We next examined the blastocyst stage embryos recovered 72 hours after hCG and mating. The blastocysts from the TallyHO mice (n=49) appear morphologically abnormal (misshapen and fragmented) vs blastocysts from control mice (n=61) and thus TUNEL was performed to measure apoptosis. Blastocysts recovered from TallyHO females had significantly higher rates of apoptosis than C57Bl/6 blastocysts (Figure 2A) (*p<0.05). Next, since we had previously shown hyperinsulinemic conditions resulted in decreased insulin-stimulated glucose uptake, we measured this rate in individual blastocysts from control vs TallyHO mice. Blastocysts recovered from TallyHO females (n=33) had significantly lower insulin-stimulated glucose uptake rates vs control blastocysts (n=52) suggesting an insulin-resistant phenotype (Figure 2B) (p<0.01). We concluded that decreased glucose metabolism due to impaired insulin signaling may be responsible for the poor reproductive outcome in these mice.
Figure 2. Blastocyst stage embryos from TallyHO females exhibit increased apoptosis and decreased insulin-stimulated glucose uptake.
A. Blastocysts from 10 control mice (61 blastocysts) and 10 TallyHO mice (49 blastocysts) were subjected to TUNEL staining and the percent of TUNEL positive nuclei (pink) of total nuclei (blue nuclear stain-ToPro, Molecular Probes, Eugene OR, USA) was recorded. These cohorts were significantly different with a P value of < 0.05. B. Individual blastocyst deoxyglucose uptake in pmoles/pmkg wet weight/10 min was measured using enzymatic cycling reactions. Blastocysts from 10 control mice (52) and 10 TallyHO mice (33) were used and assayed. *p<0.01.
Blastocyst stage embryos from TallyHO females express increased protein levels of TNFα and no change in TNFα1R
Next we decided to investigate the potential mechanism responsible for blastocyst insulin-resistance. TNFα is a cytokine which is commonly elevated in insulin-resistant conditions. We discovered an elevation in blastocyst TNFα levels in TallyHO derived embryos compared to control, both by Western immunoblot and by confocal immunofluorescent microscopy (Figure 3A). In addition, expression of TNFα1R was not significantly different between control and TallyHO blastocysts (Figure 3A). We conclude from this experiment that one potentially detrimental alteration in TallyHO embryos may be the changes in TNFα seen in these blastocysts.
Figure 3. Blastocyst stage embryos from TallyHO females express increased protein levels of TNFα and no change in TNFα1R, as well as decreased phosphorylation of AMPK and decreased adiponectin protein expression.
A. Western immunoblot demonstrating significant increase in TNFα protein expression and no change in TNFα1R, both normalized to actin, in 50 blastocysts from control mice vs 50 blastocysts from TallyHO (TH) mice. Representative confocal image of blastocysts from control vs TallyHO blastocysts. Green is TNFαprotein; Blue is a nuclear dye (To-Pro). B. Western immunoblot demonstrating significantly higher pAMPK normalized to total AMPK, in 50 blastocysts from control mice vs 50 blastocysts from TallyHO mice, thus indicating activation of this component. Representative confocal image reveals less phosphor-protein in the TallyHO blastocysts, specifically excluding the Inner cell mass. C. Western immunoblot demonstrating decreased adiponectin. Representative confocal images depict less adiponectin expression (green labeling) in the TallyHO blastocyst.
Blastocysts stage embryos from TallyHO females express decreased phosphorylation of AMPK and decreased adiponectin protein expression
TNFα suppresses AMPK activation and expression of adiponectin (Li, et al., 2013, Zhang, et al., 2009). Thus we examined levels of phosphorylated AMPK (p-AMPK) in blastocysts from both groups. P-AMPK was significantly lower in the TallyHO blastocyst, whereas total AMPK did not differ Figure 3B). TNFα has also been shown to decrease expression of adiponectin, a protein primarily secreted by adipocytes, but recently shown to be expressed in preimplantation embryos (Kim, et al., 2011). Adiponectin was similarily decreased in TallyHO blastocyst embryos (Figure 3C). We conclude from these studies that elevated TNFα levels may be responsible for the changes in AMPK activity and adiponectin expression. From previous studies in our laboratory (Kim, Marquard, Stephens, Louden, Allsworth and Moley, 2011, Louden, Chi and Moley, 2008), it is clear that both these factors play key roles in embryo metabolism and survival and thus may be a mechanism to explain the poor reproductive outcomes.
Blastocyst stage embryos from TallyHO females have increased lipid droplet accumulation and compensatory increased in HADHA activity; however this phenotype, and the blastocyst insulin resistance and apoptosis are all reversed with metformin
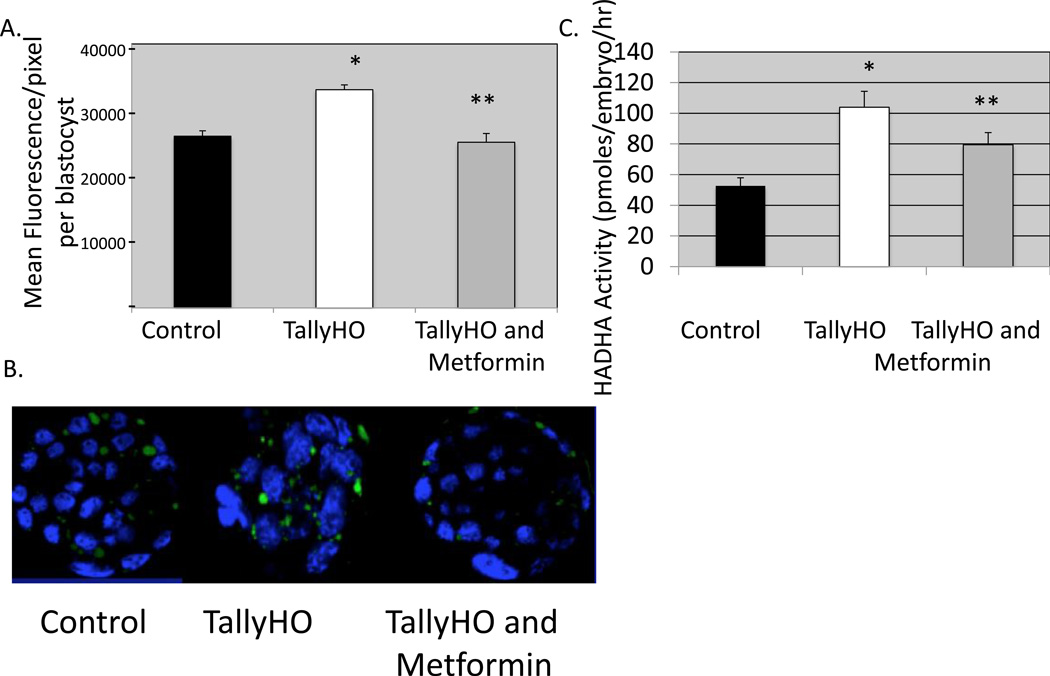
Since it was found that glucose utilization is impaired in TallyHO blastocyst, it is possible that fatty acid oxidation may compensate for this aberrant metabolic switch and thus normalize energy production. However, in several insulin-resistant states it has been shown that instead of burning fat, some cell types accumulate fatty acids in lipid droplets, leading to a pathological condition known as lipotoxicity ADD ROBKER (Sam and Mazzone, 2014). We next examined the storage of lipid in the TallyHO blastocyst and found that lipid droplets are more abundant in these blastocysts as compared to their C57Bl/6 counterparts (Figure 4a and b). This difference is significant. Other studies have demonstrated that elevated TNFα leads to inhibition of PPARδ, a less understood PPAR but one that has significant reproductive and developmental repercussions (Zhang and Kim, 1995). PPARδ is a transcription factor which activated fatty acid oxidation via increasing expression of key enzymes including ACAA2 which converts Ketoacyl CoA to acetyl CoA in the final step of oxidation. Since HADHA is the enzyme directly upstream from ACAA2, we measured its activity in anticipation that it would be elevated due to the downstream block by elevated TNFα. HADHA activity in individual blastocysts from the TallyHO mice was significantly increased as compared to individual control blastocysts using microanalytic enzyme cycling assays specifically designed to measure this activity. We conclude from these experiments that lipid accumulation is significantly greater in TallyHO blastocysts vs control due to elevated circulating NEFAs; and that fatty acid oxidation is decreased as a result of increased TNFα, blocked downstream fatty acid oxidation via ACAA2, and a compensatory increase in upstream HADH2 in compensation which has been shown in other tissue types.
Figure 4. Blastocyst stage embryos from TallyHO females have increased lipid droplet accumulation and compensatory increased in HADHA activity, however this is reversed with metformin.
A. Morulae from control (10 mice) or TallyHO mice (20 mice) were recovered and cultured for 24 hrs in either control media (approximately 50 blastocysts from control mice and 50 blastocysts from TallyHO mice) or in media with added metformin (25µg/ml) (50 blasts from TallyHO mice). The number of lipid droplets (stained green) were counted per blastocyst in each of the three groups of blastocysts. B. Representative confocal images of embryos cultured from the morulae to blastocyst stage (24hr) in control media +/− metformin. *p< 0.01 control vs Tally HO; **p<0.01 TallyHO vs TallyHO plus metformin. C. HADHA activity was calculated in individual blastocysts cultured from the morulae to blastocyst stage in control media or media with added metformin. Values expressed as pmoles/embryo/hr. Control n=47 blastocysts; TallyHO n=49 blastocysts; TallyHO +metformin n=51 blastocysts; *p<0.001 TallyHO vs control; **p< 0.01 TallyHO plus metformin vs TallyHO.
Since activators of AMPK have been identified and shown in blastocysts to reverse high IGF-1 induced inhibition (Eng, Sheridan, Wyman, Chi, Bibee, Jungheim and Moley, 2007), we next examined the effect of culturing TallyHO morulae for 24 hr in metformin. This incubation lead to a reversal in the lipid droplet accumulation (Figure 4a and b) and reversal of the HADHA activity (Figure 4c). Moreover this 24 hr incubation resulted in decreased TUNEL positive nuclei, a reversal in TallyHO decreased DG uptake phenotype, and finally significantly higher P-AMPK levels in TallyHO blastocysts treated with metformin vs TallyHO untreatead (Figure 5a–c). We conclude from these experiments that activation of AMPK was sufficient to reverse the blastocyst insulin resistance and improve survival. It also appears that normalizing pAMPK activity lead to a normalization of lipid utilization.
Figure 5. Blastocyst stage embryos from TallyHO mice when treated with metformin experience a reversal of their insulin resistance and apoptosis phenotype.
A. Apoptosis as measured by TUNEL assay in TallyHO morulae culture in control media or media with metformin for 24 hrs to a blastocyst stage. TallyHO in control media; n=42 blastocysts with 27.9±6% vs TallyHO in media with added metformin; n=38 blastocysts with 10.1+3% TUNEL positive nuclei (pink)/total nuclei (blue). B. Insulin stimulated deoxyglucose (DG) uptake in individual embryos culture from a morula to blastocyst stage in control media vs media with added metformin expressed as pmoles/kg wet weight/10min. Control embryos in control media, n=32 blastocysts; TallyHO embryos in control media, n=41; TallyHO in media with added metformin, n=39. *p< 0.01 TallyHO vs Control; **p< 0.01 TallyHO plus metformin vs TallyHO. C. Western immunoblot showing pAMPK and total AMPK in TallyHO embryos cultured from a morula to blastocyst stage in metformin (n=54 embryos) or control media (n=51 embryos).
Discussion
In this paper, we demonstrate for the first time using a congenic strain of obese mice, the TallyHO mouse instead of a diet-induced obesity model, that maternal obesity, characterized by insulin resistance and elevated fatty acids, results in an abnormal reproductive phenotype, specifically a smaller litter size. Moreover, we determine that blastocyst stage embryos from these mice experience decreased insulin-stimulated glucose uptake and decreased fatty acid oxidation leading to increased lipid droplet accumulation at this stage of embryo development. The lipid accumulation is due to two factors: increased circulating NEFA levels in these mice and decreased oxidation due to the TNFα inhibition of the final step in fatty acid oxidation and compensatory increase in HADHA. These embryos also demonstrate decreased expression of adiponectin and subsequently less activation of AMPK, both of which result in insulin resistance in the blastocyst. We attribute this resistance to elevated TNFα expression at the blastocyst stage, which we confirm by Western immunoblot and confocal immunofluorescent microscopy. Elevated TNFα also explains the block in beta oxidation at the level of ACAA2, and the upstream increase in HADHA activity due to the downstream block. The resulting phenotype of decreased litter size is most likely due to the combination of decreased insulin stimulated glucose uptake and abnormal fatty acid oxidation resulting in increased blastocyst apoptosis. Adding an AMPK activator, metformin, results in reversal of the pAMPK inactivation and leads to decreased lipid accumulation, decreased apoptosis and a normalization of insulin sensitivity with increased insulin stimulated glucose uptake.
Population studies estimate that more than 1 billion people are overweight globally and the rate of the development of obesity is increasing at an astonishing rate (Flegal, et al., 2012, Ogden, Carroll, Kit and Flegal, 2012). The obesity epidemic has a grave impact on human reproduction and infertility. Nearly 60% of women desiring pregnancy are overweight and/or obese. A well- known linear trend exists between BMI and subfertility, however the mechanism responsible has not been elucidated. Obesity is known to lead to ovulatory disorders, decreased spontaneous rates of conception, increased rates of spontaneous miscarriage, infertility, early pregnancy loss, fetal loss, congenital abnormalities, and neonatal adverse conditions (Jungheim and Moley, 2010). The results of this study support obesity-associated subfertility, with decreased numbers of pups/mother in the mouse model of obesity used here, the Tally HO mouse. Moreover this study focuses on the blastocyst stage of development and attempts to find metabolic abnormalities at this critical developmental stage to explain the lower numbers of live pups.
Prior studies have looked at the effect of in vitro exposure of the preimplantation embryo to either elevated IGF-1 concentrations or elevated palmitic acid, in an attempt to mimic the obese maternal environment (Eng, Sheridan, Wyman, Chi, Bibee, Jungheim and Moley, 2007, Jungheim, et al.). Both conditions resulted in insulin resistance and increased apoptosis at a blastocyst stage, similar to our finding here. In both of these studies, 2-cell embryos were exposed in vitro to the abnormal conditions and cultured for 72 hours. During this time in development, the embryo transitions from the cleavage stage metabolism, primarily fueled by oxidative phosphorylation, to a glycolytic metabolism and with a dramatic conversion to an insulin sensitive embryo and an increase in glucose uptake. This timing in development may be a particularly vulnerable period, due to the metabolic changes that must occur for normal transitions to occur.
Several studies have also shown that exposure to maternal high fat diet in vivo adversely affects the developing oocyte, prior to fertilization (Jungheim, et al., 2010, Luzzo, Wang, Purcell, Chi, Jimenez, Grindler, Schedl and Moley, 2012). Our group as well as others have demonstrated that a high fat diet results in abnormal oocytes with impaired mitochondria (Igosheva, et al., 2010, Luzzo, Wang, Purcell, Chi, Jimenez, Grindler, Schedl and Moley, 2012, Wu, et al., 2010). These oocytes have mitochondria with fewer but disarrayed cristae, decreased electron density of the mitochondrial matrix, increased internal membrane swelling, and a greater number of vacuoles compared to mice on the control diet. Most likely, the blastocysts in this study represent embryos with defective mitochondria, which may explain in part the phenotype seen, however, the oocytes in the TallyHO mice have not been examined. It is still most likely, given the high levels of TNFα at the blastocyst stage and the reversibility of this effect with metformin, that the mitochondrial defect in the TallyHO oocytes may be less severe in this model of obesity as compared to the acute onset high fat fed mouse model. Future studies will attempt to determine if the mitochondrial effect occurs as early as the oocyte in the TallyHO and if this blastocyst phenotype can be affected by earlier treatment of the mice with metformin, prior to fertilization suggesting an oocyte contribution to the subfertility.
Figure 6. Overall conclusion cartoon.
The TallyHO mice have elevated TNFα expression in blastocyst stage embryos which leads to decreased Adiponectin and activation of AMPK via phosphorylation. As a result insulin-stimulated glucose uptake is lower in TallyHO blastocysts. TNFα also inhibits PPARδ leading to inhibition of ACAA2 and fatty acid oxidation, however HADHA activity increases due to the downstream blockade. Activation of AMPK and possibly PPARδ metformin reversed the insulin resistant phenotype.
ACKNOWLEDGEMENTS
This work was supported by NIH grants T32 HDO49305 (EDL), T32 HD040135 (to PTJ and KML), and R01 HD065435 (to KHM) and by an ADA research grant (KHM).
References
- Allemand MC, Irving BA, Asmann YW, Klaus KA, Tatpati L, Coddington CC, Nair KS. Effect of testosterone on insulin stimulated IRS1 Ser phosphorylation in primary rat myotubes--a potential model for PCOS-related insulin resistance. PloS one. 2009;4:e4274. doi: 10.1371/journal.pone.0004274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antuna-Puente B, Feve B, Fellahi S, Bastard JP. Adipokines: the missing link between insulin resistance and obesity. Diabetes & metabolism. 2008;34:2–11. doi: 10.1016/j.diabet.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Balkwill F. Tumour necrosis factor and cancer. Nature reviews Cancer. 2009;9:361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- Barb D, Williams CJ, Neuwirth AK, Mantzoros CS. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. The American journal of clinical nutrition. 2007;86:s858–s866. doi: 10.1093/ajcn/86.3.858S. [DOI] [PubMed] [Google Scholar]
- Blackmore HL, Ozanne SE. Maternal diet-induced obesity and offspring cardiovascular health. Journal of developmental origins of health and disease. 2013;4:338–347. doi: 10.1017/S2040174412000761. [DOI] [PubMed] [Google Scholar]
- Chi MM, Hoehn A, Moley KH. Metabolic changes in the glucose-induced apoptotic blastocyst suggest alterations in mitochondrial physiology. American journal of physiology Endocrinology and metabolism. 2002;283:E226–E232. doi: 10.1152/ajpendo.00046.2002. [DOI] [PubMed] [Google Scholar]
- Chi MM, Pingsterhaus J, Carayannopoulos M, Moley KH. Decreased glucose transporter expression triggers BAX-dependent apoptosis in the murine blastocyst. The Journal of biological chemistry. 2000;275:40252–40257. doi: 10.1074/jbc.M005508200. [DOI] [PubMed] [Google Scholar]
- Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LG, Clements M, Al-Qassab H, Heffron H, Xu AW, et al. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. The Journal of clinical investigation. 2007;117:2325–2336. doi: 10.1172/JCI31516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocksedge KA, Li TC, Saravelos SH, Metwally M. A reappraisal of the role of polycystic ovary syndrome in recurrent miscarriage. Reproductive biomedicine online. 2008;17:151–160. doi: 10.1016/s1472-6483(10)60304-5. [DOI] [PubMed] [Google Scholar]
- Dale PO, Tanbo T, Haug E, Abyholm T. The impact of insulin resistance on the outcome of ovulation induction with low-dose follicle stimulating hormone in women with polycystic ovary syndrome. Human reproduction. 1998;13:567–570. doi: 10.1093/humrep/13.3.567. [DOI] [PubMed] [Google Scholar]
- Dzamko NL, Steinberg GR. AMPK-dependent hormonal regulation of whole-body energy metabolism. Acta physiologica. 2009;196:115–127. doi: 10.1111/j.1748-1716.2009.01969.x. [DOI] [PubMed] [Google Scholar]
- Eng GS, Sheridan RA, Wyman A, Chi MM, Bibee KP, Jungheim ES, Moley KH. AMP kinase activation increases glucose uptake, decreases apoptosis, and improves pregnancy outcome in embryos exposed to high IGF-I concentrations. Diabetes. 2007;56:2228–2234. doi: 10.2337/db07-0074. [DOI] [PubMed] [Google Scholar]
- Fain JN, Buehrer B, Tichansky DS, Madan AK. Regulation of adiponectin release and demonstration of adiponectin mRNA as well as release by the non-fat cells of human omental adipose tissue. International journal of obesity. 2008;32:429–435. doi: 10.1038/sj.ijo.0803745. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA : the journal of the American Medical Association. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- Hammond RA. Complex systems modeling for obesity research. Preventing chronic disease. 2009;6:A97. [PMC free article] [PubMed] [Google Scholar]
- Igosheva N, Abramov AY, Poston L, Eckert JJ, Fleming TP, Duchen MR, McConnell J. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PloS one. 2010;5:e10074. doi: 10.1371/journal.pone.0010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakson P, Hammarstedt A, Gustafson B, Smith U. Impaired preadipocyte differentiation in human abdominal obesity: role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes. 2009;58:1550–1557. doi: 10.2337/db08-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez PT, Frolova AI, Chi MM, Grindler NM, Willcockson AR, Reynolds KA, Zhao Q, Moley KH. DHEA-mediated inhibition of the pentose phosphate pathway alters oocyte lipid metabolism in mice. Endocrinology. 2013;154:4835–4844. doi: 10.1210/en.2012-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungheim ES, Louden ED, Chi MM, Frolova AI, Riley JK, Moley KH. Preimplantation exposure of mouse embryos to palmitic acid results in fetal growth restriction followed by catch-up growth in the offspring. Biology of reproduction. 85:678–683. doi: 10.1095/biolreprod.111.092148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungheim ES, Moley KH. Current knowledge of obesity's effects in the pre- and periconceptional periods and avenues for future research. American journal of obstetrics and gynecology. 2010;203:525–530. doi: 10.1016/j.ajog.2010.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungheim ES, Schoeller EL, Marquard KL, Louden ED, Schaffer JE, Moley KH. Diet-induced obesity model: abnormal oocytes and persistent growth abnormalities in the offspring. Endocrinology. 2010;151:4039–4046. doi: 10.1210/en.2010-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Sen S, Avery CS, Simpson E, Chandler P, Nishina PM, Churchill GA, Naggert JK. Genetic analysis of a new mouse model for non-insulin-dependent diabetes. Genomics. 2001;74:273–286. doi: 10.1006/geno.2001.6569. [DOI] [PubMed] [Google Scholar]
- Kim JH, Stewart TP, Zhang W, Kim HY, Nishina PM, Naggert JK. Type 2 diabetes mouse model TallyHo carries an obesity gene on chromosome 6 that exaggerates dietary obesity. Physiological genomics. 2005;22:171–181. doi: 10.1152/physiolgenomics.00197.2004. [DOI] [PubMed] [Google Scholar]
- Kim ST, Marquard K, Stephens S, Louden E, Allsworth J, Moley KH. Adiponectin and adiponectin receptors in the mouse preimplantation embryo and uterus. Human reproduction. 2011;26:82–95. doi: 10.1093/humrep/deq292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Chen X, Guan L, Qi Q, Shu G, Jiang Q, Yuan L, Xi Q, Zhang Y. MiRNA-181a regulates adipogenesis by targeting tumor necrosis factor-alpha (TNF-alpha) in the porcine model. PloS one. 2013;8:e71568. doi: 10.1371/journal.pone.0071568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Yang G, Shi S, Yang M, Liu H, Boden G. The adipose triglyceride lipase, adiponectin and visfatin are downregulated by tumor necrosis factor-alpha (TNF-alpha) in vivo. Cytokine. 2009;45:12–19. doi: 10.1016/j.cyto.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Louden E, Chi MM, Moley KH. Crosstalk between the AMP-activated kinase and insulin signaling pathways rescues murine blastocyst cells from insulin resistance. Reproduction. 2008;136:335–344. doi: 10.1530/REP-08-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzo KM, Wang Q, Purcell SH, Chi M, Jimenez PT, Grindler N, Schedl T, Moley KH. High fat diet induced developmental defects in the mouse: oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PloS one. 2012;7:e49217. doi: 10.1371/journal.pone.0049217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009–2010. NCHS data brief. 2012:1–8. [PubMed] [Google Scholar]
- Passonneau JV, Lowry OH. Enzymatic Analysis: A practical guide. Humana Press; 1993. [Google Scholar]
- Riley JK, Carayannopoulos MO, Wyman AH, Chi M, Moley KH. Phosphatidylinositol 3-kinase activity is critical for glucose metabolism and embryo survival in murine blastocysts. The Journal of biological chemistry. 2006;281:6010–6019. doi: 10.1074/jbc.M506982200. [DOI] [PubMed] [Google Scholar]
- Sam S, Mazzone T. Adipose tissue changes in obesity and the impact on metabolic function. Translational research : the journal of laboratory and clinical medicine. 2014 doi: 10.1016/j.trsl.2014.05.008. [DOI] [PubMed] [Google Scholar]
- Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nature medicine. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberger J, Daniels SR American Heart Association Atherosclerosis H, Obesity in the Young C and American Heart Association Diabetes C. Obesity, insulin resistance, diabetes, and cardiovascular risk in children: an American Heart Association scientific statement from the Atherosclerosis, Hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young) and the Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism) Circulation. 2003;107:1448–1453. doi: 10.1161/01.cir.0000060923.07573.f2. [DOI] [PubMed] [Google Scholar]
- Street ME, Volta C, Ziveri MA, Viani I, Bernasconi S. Markers of insulin sensitivity in placentas and cord serum of intrauterine growth-restricted newborns. Clinical endocrinology. 2009;71:394–399. doi: 10.1111/j.1365-2265.2009.03533.x. [DOI] [PubMed] [Google Scholar]
- Sweet IR, Gilbert M, Maloney E, Hockenbery DM, Schwartz MW, Kim F. Endothelial inflammation induced by excess glucose is associated with cytosolic glucose 6-phosphate but not increased mitochondrial respiration. Diabetologia. 2009;52:921–931. doi: 10.1007/s00125-009-1272-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschritter O, Fritsche A, Thamer C, Haap M, Shirkavand F, Rahe S, Staiger H, Maerker E, Haring H, Stumvoll M. Plasma adiponectin concentrations predict insulin sensitivity of both glucose and lipid metabolism. Diabetes. 2003;52:239–243. doi: 10.2337/diabetes.52.2.239. [DOI] [PubMed] [Google Scholar]
- Waller DK, Shaw GM, Rasmussen SA, Hobbs CA, Canfield MA, Siega-Riz AM, Gallaway MS, Correa A National Birth Defects Prevention S. Prepregnancy obesity as a risk factor for structural birth defects. Archives of pediatrics & adolescent medicine. 2007;161:745–750. doi: 10.1001/archpedi.161.8.745. [DOI] [PubMed] [Google Scholar]
- Wu LL, Dunning KR, Yang X, Russell DL, Lane M, Norman RJ, Robker RL. High-fat diet causes lipotoxicity responses in cumulus-oocyte complexes and decreased fertilization rates. Endocrinology. 2010;151:5438–5445. doi: 10.1210/en.2010-0551. [DOI] [PubMed] [Google Scholar]
- Xu XJ, Valentine RJ, Ruderman NB. AMP-activated Protein Kinase (AMPK): Does This Master Regulator of Cellular Energy State Distinguish Insulin Sensitive from Insulin Resistant Obesity? Current obesity reports. 2014;3:248–255. doi: 10.1007/s13679-014-0095-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nature medicine. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- Yoo HJ, Choi KM. Adipokines as a novel link between obesity and atherosclerosis. World journal of diabetes. 2014;5:357–363. doi: 10.4239/wjd.v5.i3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Kim KH. TNF-alpha inhibits glucose-induced insulin secretion in a pancreatic beta-cell line (INS-1) FEBS letters. 1995;377:237–239. doi: 10.1016/0014-5793(95)01272-9. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhao M, Li Q, Zhao H, Wang J, Li Y. Acetyl-l-carnitine inhibits TNF-alpha-induced insulin resistance via AMPK pathway in rat skeletal muscle cells. FEBS letters. 2009;583:470–474. doi: 10.1016/j.febslet.2008.12.053. [DOI] [PubMed] [Google Scholar]