Abstract
Our objective was to evaluate the diagnostic yield of rapid on-site evaluation (ROSE) on the differential diagnosis of non–small cell lung carcinoma, not otherwise specified (NSCLC-NOS). Biopsied cases diagnosed as NSCLC-NOS with ROSE during 2004 through 2008 were retrieved. Diagnostic confirmation was done with immunohistochemistry (IHC) involving thyroid transcription factor-1 and p63 immunostains. For the study, 106 cases were available. The final diagnoses rendered were squamous cell carcinoma (SqCC) (n=39) and adenocarcinoma (AC) (n=67). Cytologic, histologic, and IHC concordance for these diagnoses occurred in 75 cases (70.8%), of which 56 (52.8%) were AC and 19 (17.9%) were SqCC. Cytologic, histologic, and IHC discordance was found in 31 cases (29.2%). Of these 31 cases, 11 NSCLC-NOS diagnoses histologically corresponded to 1 SqCC plus 4 ACs, and 4 favor SqCC plus 2 ACs; the former 5 NSCLC-NOS cases classified correctly through cytology, as well as IHC. However, IHC was not available for the latter 6 NSCLC-NOS cases that were also classified correctly through cytology. In addition, only 3 NSCLC-NOS diagnoses cytologically corresponded to 3 favor SqCC histologically, in which IHC was not available, and for 2 cases that both corresponded to favor SqCC and favor AC histologically and cytologically. In the other 15 cases, histology labeled 4 cases NSCLC-NOS and misclassified 2 cases; cytology labeled 1 case NSCLC-NOS and misclassified 13 cases. ROSE has high diagnostic yield over subclassification of NSCLC-NOS. We recommend allocating a cytotechnologist for specimen adequacy and a cytopathologist for cytologic diagnosis.
Keywords: cytology, lung carcinoma, non–small cell lung carcinoma, on-site cytologic evaluation, subclassification
Background
Histopathologic distinction of lung carcinoma based on the 2004 World Health Organization classification of small cell lung carcinoma and non–small cell lung carcinoma (NSCLC) became inadequate in light of the genetic heterogeneity of NSCLC. The introduction of novel therapeutic agents (eg, bevacizumab, pemetrexed) for NSCLC in patients with nonsquamous histologic characteristics has forced pathologists to classify NSCLC into different subtypes (eg, adenocarcinoma vs squamous cell carcinoma [SqCC]) (1).
Since the recognition that 70% of patients with lung carcinoma present with advanced stage disease, the diagnosis and treatment of lung carcinoma largely depend on small biopsies, especially ultrasound-guided transthoracic fine-needle aspiration (FNA) biopsy (1,2). This procedure, besides being cost-effective, allows conventional and liquid-based smear preparation, which helps with rapid evaluation of specimen adequacy and diagnosis, cell block preparation, flow cytometry, and immunohistochemistry (IHC), as well as molecular analysis, for further classification of tumors (3).
The rapid on-site evaluation (ROSE) of tissue obtained by FNA was first introduced by Washington University investigators in St. Louis, Missouri (4). Since its introduction, publications on ROSE have discussed its advantages and disadvantages. Its major advantages have been improvements in health care resource utilization, reduction in the number of sites biopsied, shortening of anesthesia time, increase of sample adequacy rates, decrease of unsatisfactory or suspicious diagnoses, and decrease of potential morbidity related to the biopsy procedure (4). Major disadvantages have been the need for experienced on-site cytopathologists and a lack of proper billing for cytopathologist services in performing ROSE (3).
The objectives of the present study were to present our experience with cytologic ROSE and to compare its diagnostic yield on classification of NSCLC, not otherwise specified (NSCLC-NOS) when not enough tissue is available for ancillary histologic testing.
Materials and Methods
We retrospectively reviewed the 122 NSCLC-NOS cases obtained with the ROSE method from January 1, 2004, to December 31, 2008, at Mayo Clinic in Jacksonville, Florida. The data were retrieved after approval by the Mayo Clinic Institutional Review Board. For each case, cytologic and histologic slides were reviewed separately by 3 pathologists (B.C., A.K., and A.N.) to whom the diagnosis was masked. To increase diagnostic specificity and concordance, the immunostains thyroid transcription factor-1 (TTF-1) (clone 8G7G3/1; Dako) and p63 (catalog No. CM163C; Ventana Medical Systems Inc and Biocare Medical LLC) were applied to each case for tumor classification.
Cytologic and histologic diagnoses were made on the basis of guidelines from the International Association for the Study of Lung Cancer, the American Thoracic Society, and the European Respiratory Society. These diagnoses included definitely adenocarcinoma (AC), favor AC, definitely SqCC, favor SqCC, and NSCLC-NOS (5). In IHC evaluation, staining intensity was graded as weak, moderate, and strong. Percentage of cell staining was reported as 0, <5%, <50%, and ≥50%.
For each case, the final diagnosis was determined with p63 or TTF-1 immunostain. Only p63 immunoreactivity was accepted for an SqCC diagnosis, whereas either TTF-1 or p63 positivity, or both, was accepted for AC. When positivity for TTF-1 and p63 was seen together in the same biopsy but in different cells, the diagnosis was assigned as adenosquamous cell carcinoma. If neither TTF-1 nor p63 testing was positive, the diagnosis was made on the basis of histologic characteristics—clear-cut evidence of pearl formation, keratinization for SqCC differentiation, and glandular structure for AC differentiation. The same principle was applied to cases where no tissue was available or no malignant cells were left on the block.
If cytology is NSCLC-NOS but histology is SqCC or AC, then final diagnosis is accepted on the basis of histologic diagnosis. If histology is NSCLC-NOS but cytology is SqCC or AC, then final diagnosis is accepted on the basis of cytologic diagnosis. If cytology is NSCLC-NOS, histology is NSCLC-NOS, and IHC is not available, then the final diagnosis is NSCLC-NOS.
The ROSE procedure was performed by a cytotechnologist after staining the smears from the tissue obtained by a radiologist under guidance of CT scan. At least 2 smears were prepared from the tissue obtained, one stained immediately with a rapid Giemsa stain (Diff-Quik) and the other fixed in alcohol for Papanicolaou (PAP) staining later. The cytotechnologist determined specimen adequacy. Cytologic slides were then sent to the pathologist for immediate interpretation of whether the sample was negative (no malignant cells) or positive (definitive cytopathologic evidence of malignancy) (6). For each case, the remaining material was placed in 10% formaldehyde for routine histologic examination using cell block preparation. The pathologist both reviewed the biopsy and reached the relevant cystologic or histologic diagnosis.
Results
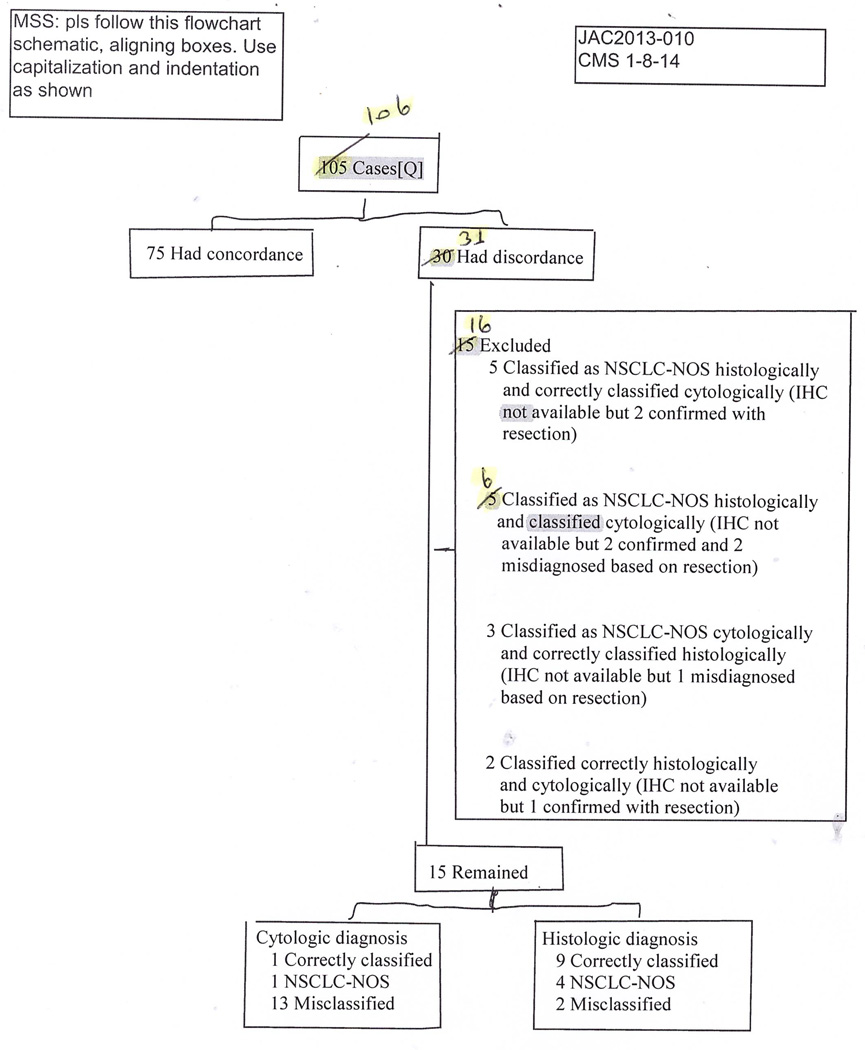
Cytology slides were not available for 5 of the 122 cases. There was diagnostic controversy for 1 case that had follow-up breast tumor biopsy with the same histologic characteristics. For 4 cases, there was a discrepancy between the cytologic and histologic diagnoses. The 4 cases were called favor AC cytologically and favor SqCC histologically. Since IHC was not available for these 4 cases, the cases were excluded from the study. One case in which IHC was not available and 1 case positive for p63 IHC were designated as NSCLC-NOS both histologically and cytologically; they also were excluded from the study. In addition, 4 cases—3 adenosquamous carcinoma and 1 small cell plus SqCC—were excluded from the study because cytology may not detect both components properly. These exclusions totaled 16 cases.
Findings of TTF-1 and p63 were negative for 11 of the 106 cases available for the study. IHC was not applied in 20 cases, because of limited tissue. Final diagnoses were possible in 19 of the 31 cases; these were based on pearl formation or keratinization and glandular structures seen in biopsy. Overall, final diagnoses rendered were 39 cases of SqCC and 67 cases of AC.
In total, the 106 cases had cytologic, histologic, and IHC concordance in 75 (70.8%) cases: 56 (52.8%) were AC and 19 (17.9%) were SqCC.
There was either cytologic, histologic, or IHC discordance in 31 of the 106 cases: 5 NSCLC-NOS diagnoses rendered through histology corresponded to 1 SqCC and 4 AC correctly classified through cytology, as well as IHC (Figure 1). IHC was not available for 6 NSCLC-NOS diagnoses rendered with histology that corresponded to 4 determinations of favor SqCC and 2 of AC through cytology; it also was not available for 3 NSCLC-NOS diagnoses rendered through cytology that corresponded to 3 determinations of favor SqCC on histology. In addition, IHC was not available for 2 cases that were both called favor SqCC and favor AC through histology and cytology.
Figure 1.
The Diagnoses Flow Shown Step by Step. IHC indicates immunohistochemistry; NSCLC-NOS, non–small cell lung carcinoma, not otherwise specified.
In the other 15 cases, histologic evaluation identified 4 cases as NSCLC-NOS, 8 as SqCC (one proved to be AC with IHC), and 3 as AC (one proved to be SqCC with IHC) (Table and Figure 1). Cytology identified 1 case as NSCLC-NOS, 3 as SqCC (all 3 proved to be AC with IHC), and 11 cases as AC (10 proved to be SqCC with IHC).
Table.
Final Diagnosis of 15 Cases in Which Cytologic and Histologic Discordance Observed
| IHCa |
|||||
|---|---|---|---|---|---|
| Case No. | Cytologic Diagnosis | Histologic Diagnosis | TTF-1 | p63 | Final Diagnosis |
| 9 | NSCLC-NOS | Fav AC | Neg | 3+ | SqCC |
| 14 | Fav AC | Fav SqCC | Neg | 3+ | SqCC |
| 15 | AC | Fav SqCC | Neg | 3+ | SqCC |
| 18 | Fav AC | Fav SqCC | 3+ | 3+ | AC |
| 21 | Fav AC | Fav SqCC | Neg | 3+ | SqCC |
| 22 | Fav AC | NSCLC-NOS | Neg | 3+ | SqCC |
| 27 | Fav SqCC | NSCLC-NOS | 3+ | 3+ | AC |
| 28 | Fav SqCC | Fav AC | 3+ | 3+ | AC |
| 29 | AC | Fav SqCC | Neg | 3+ | SqCC |
| 46 | Fav AC | SqCC | Neg | 3+ | SqCC |
| 49 | Fav AC | NSCLC-NOS | Neg | 3+ | SqCC |
| 79 | Fav AC | NSCLC-NOS | Neg | 3+ | SqCC |
| 80 | Fav AC | SqCC | Neg | 3+ | SqCC |
| 97 | AC | SqCC | Pearl formation | SqCC | |
| 109 | Fav SqCC | Fav AC | 3+ | 3+ | AC |
Abbreviations: AC, adenocarcinoma; fav, favor; IHC, immunohistochemistry; Neg, negative; NSCLC-NOS, non–small cell lung carcinoma, not otherwise specified; SqCC, squamous cell carcinoma, TTF-1, thyroid transcription factor-1.
3+ indicates strong immunostain observed in more than 50% of tumor cells.
Overall, there were 4 and 15 NSCLC-NOS diagnoses with cytology and histology and 13 and 2 cases misclassified with cytology and histology, respectively.
Discussion
Subclassification of NSCLC based on light microscopy is not a challenge when tumor cells have pearls, keratinization, or gland formation (ie, well-differentiated tumors). For moderately differentiated or less differentiated tumors, use of mucin stain, IHC stains, flow cytometry, and molecular analysis helps to delineate the classification. However, since such a small amount of tissue is obtained with minimally invasive methods (eg, FNA), the tissue left after routine light microscopic examination is usually not sufficient to apply these ancillary methods. Furthermore, pathologists face the challenge of maximizing the diagnostic capacity of this limited tissue. Despite those sophisticated analyses, NSCLC- NOS diagnosis in practice comprises 10% to 30% of small biopsies or cytologic specimens, or both (5).
Because of recent progress in molecular biology and oncology, pathologists need to minimize the use of the terms NSCLC and NSCLC-NOS and be more accurate in classifying lung tumors on the basis of limited samples. Tumors containing epidermal growth factor receptor mutations respond well to tyrosine kinase inhibitors in AC patients. In addition, although treatment with pemetrexed has efficacy over SqCC, it has toxicity when administered simultaneously with bevacizumab.
Up to 70% of patients with lung carcinoma present with advanced-stage disease, and the diagnosis and treatment largely depend on the findings from small biopsies. The FNA service has become an essential part of prompt clinical management in the current era of personalized medicine, and the inadequacy of a specimen is the major limitation of the procedure. Increasing the number of FNA passes has been an option in the past; FNA sensitivity for malignant diagnosis increased from 16.7% with 1 needle pass to 86.7% when more than 7 passes were performed (7). Since FNA complication is noted most frequently, secondary to concurrent biopsy (8), ROSE of specimen adequacy has been a promising solution. With this procedure, the percentage of cases with inadequate specimens and the FNA complication rates, as well as the number of passes, are reduced (8). ROSE also improves FNA effectiveness when the per-pass adequacy rate is low (9). Low FNA sensitivity has been associated with unavailability of ROSE (7), and factors associated in univariate and multivariate analyses with sensitivity greater than 80% have been related with ROSE availability. Without ROSE, the number of passes necessary for specimen adequacy may increase to 7 (7).
The most common reason for performing ROSE is to procure an adequate specimen, but it also may be used as a diagnostic test. Studies have shown that it increased malignant diagnosis from 35.2% to 39.2% and decreased atypical diagnosis from 6.9% to 5.3% (4). The accuracy rate of ROSE has been 93% (6,10).
Cytologic ROSE of specimens obtained through FNA has emerged as a highly effective tool in the diagnosis and staging of malignant tumors in the lung, especially for peripherally seated masses (3). Staging of lung carcinoma is performed with FNA in 31.4% (7), and ROSE has been reported to have 85% to 92% sensitivity and 100% specificity (11).
The main discussion on ROSE has been on specimen adequacy. Some of the results from randomized trials suggest that ROSE did not increase the diagnostic yield of FNA, and some results found that the possible avoidance of additional biopsy without loss in diagnostic yield was the most important benefit of ROSE (12,13).
Despite the advantages of ROSE, its utility is limited because of inadequate cytopathologist staffing and a lack of accurate coding for billing purposes. ROSE is used in 28% to 90% of diagnostic cases, but this use is mostly in academic centers. The main reason for its scarcity in other practices is the lack of on-staff cytopathologists. In the present study, we showed that an on-site cytotechnologist, a general pathologist, and 2 cytology smear slides (Figure 2) are adequate to give definitive diagnosis in 99% of cases. Our results were higher (86.8% concordance and 13.2% discordance with final pathology report) than a previous report where cytologic diagnosis with ROSE was concordant with the final diagnosis in 74% of cases and discordant in 26% of cases (14).
Figure 2.
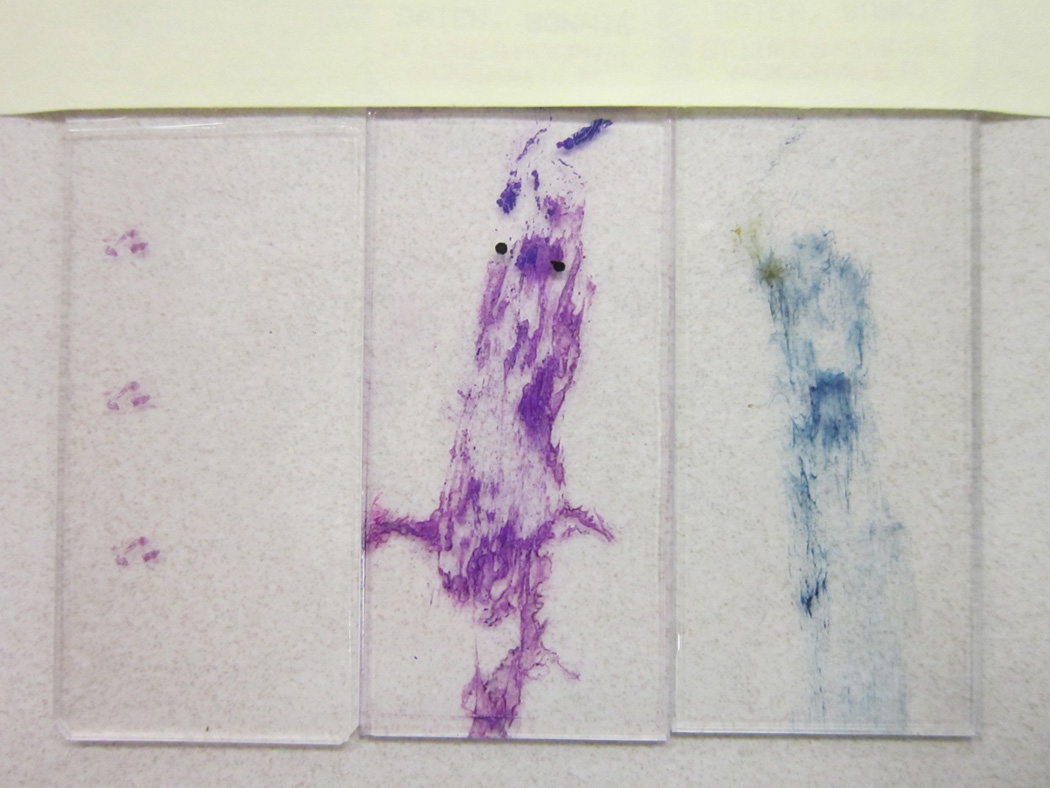
Smear Slides and Tissue Sample for Classification of Lung Carcinoma. Two properly prepared smear slides and 1 tiny tissue sample are usually enough to give a definitive diagnosis. The Papanicolaou stain is on the right and Diff-Quik is in the middle.
Diagnostic accuracy is found to be up to 97% and definite diagnostic yield may increase from 64.8% to 97.7% when an on-site cytopathologist is present (15). Nevertheless, most medical centers do not have provisions for on-site diagnosis of FNA (16). FNA evaluation was found to be done by general pathologists in 55.3% of cases (7). As in the present study, cytotechnologists must be in charge of deciding specimen adequacy, and after having an adequate specimen, the cytopathologist may evaluate and give a provisional diagnosis on the basis of cytologic evaluation.
We suggest that intraoperative frozen assessment and evaluation with ROSE may also help to justify the billing component. In the present study, although the 3 pathologists who reviewed the slides were senior pathologists, two of them were general pathologists. The third was a cytopathologist who reviewed less than 10% of the slides.
Small biopsies obtained from FNA are generally not cut in advance for further investigation (eg, mucin, IHC). Obtaining unstained slides is usually difficult because the tissue is typically too small and may be lost because of tissue trimming—and on occasion, only 1 remaining unstained slide is available. In the present study, we examined the diagnostic yield of cytology to differentiate AC and SqCC. To our knowledge, ROSE cannot be used to differentiate these 2 entities. Although cytology misclassified 13 cases, overall it successfully classified 11 cases that were called NSCLC-NOS histologically (Figure 1).
We could not label the 13 cytologic misclassifications as misdiagnosis. Their correct diagnosis was given the next day. Instead, we could indicate that histology or biopsy was not able to classify 15 cases, and cytology aided the correct classification.
We observed that most of the misclassified cases were called favor AC, instead of SqCC, in which cellular characteristics are more pronounced with PAP stain. Although pathologists generally are more confident while diagnosing with cytology (in the present study, only 4 cases were called NSCLC-NOS), they are more conservative when diagnosing with histology (15 cases in the present study were called NSCLC-NOS, and all of these were correctly classified with cytology) (17). The misclassification that was seen on cytology does not reflect interpretive errors; instead, it reflects specimen quality. All NSCLC-NOS cases where clear differentiation was difficult to appreciate on light microscopic examination were successfully classified with cytology, and most of the misclassification on cytology was due to suboptimal staining of the PAP-stained slides. If these slides had been stained appropriately, higher classification rates would have been reached on cytology because PAP stain emphasizes different cell properties compared with rapid Giemsa stain (Diff-Quik). Moreover, the first pass of FNA biopsy on a slide contains more cells than the second. Consequently, the first slide is the one that is prepared with rapid Giemsa stain. We also point out that the study population consisted of cases of NSCLC-NOS that were based on the pathology report (eg, we did not exclude hypocellular cytology slides from the study).
Strategic use of small biopsy and cytology samples is very important. The pathologist must preserve as much tissue as possible for further potential IHC and molecular studies. In this situation, cytologic provisional diagnosis with ROSE allows for a limited number of unstained slides to be triaged for lung carcinoma classification with TTF-1 or napsin or with SqCC investigation through p63 or CK5/6, or a combination of these evaluations (2). Besides the effect of ROSE on lowering the number of passes from 29.6% to 2.21%, the total slide number also decreases (16); therefore, the workload of the cytology laboratory and the work of the pathologist are also reduced. Sometimes, only 2 slides are adequate to give a definitive diagnosis.
Our study has limitations. We have not evaluated the specimen adequacy or number of passes. We also have not compared small cell carcinoma or other subtypes of lung carcinoma. We did not check to see whether the cytology slides were adequate. We did not evaluate the radiologist’s performance, which is also a variable affecting the study. For example, a recent survey of graduating pulmonary fellows showed that 50% of them believed that their aspiration training was inadequate (18), which had an impact on specimen adequacy and study results. Although we had an opportunity to review PAP-stained slides, the lack of PAP-stained slides at the time of on-site evaluation could also be a limitation for tumor subclassification.
In the present study, we showed that cytologic on-site diagnosis has high diagnostic yield for the subclassification of NSCLC-NOS. We recommend allocating a cytotechnologist for specimen adequacy and a cytopathologic diagnosis for immediate treatment of the patient. The ROSE procedure may be treated as a frozen section and can be billed accordingly. Even after several attempts to perform special stains, 1 case among the initial 122 cases continued to be classified as NSCLC-NOS (1,17).
Abbreviations
- AC
adenocarcinoma
- FNA
fine-needle aspiration
- IHC
immunohistochemistry, immunohistochemical
- NSCLC
non–small cell lung carcinoma
- NSCLC-NOS
non–small cell lung carcinoma, not otherwise specified
- PAP
Papanicolaou
- ROSE
rapid on-site evaluation
- SqCC
squamous cell carcinoma
- TTF-1
thyroid transcription factor-1
Footnotes
Conflict of interest: None
Authors’ Contributions
Betul Celik conceived, designed, interpreted, and drafted the study; had full access to all data in the study; and takes full responsibility for the integrity of all data and the accuracy of the data analysis. Betul Celik, Andras Khoor, and Aziza Nassar reviewed slides and gave final approval of the manuscript. Tangul Bulut helped with final revision of the manuscript.
References
- 1.Travis WD, Rekhtman N, Riley GJ, Geisinger KR, Asamura H, Brambilla E, et al. Pathologic diagnosis of advanced lung cancer based on small biopsies and cytology: a paradigm shift. J Thorac Oncol. 2010 Apr;5(4):411–414. doi: 10.1097/JTO.0b013e3181d57f6e. [DOI] [PubMed] [Google Scholar]
- 2.Agackiran Y, Ozcan A, Akyurek N, Memis L, Findik G, Kaya S. Desmoglein-3 and Napsin A double stain, a useful immunohistochemical marker for differentiation of lung squamous cell carcinoma and adenocarcinoma from other subtypes. Appl Immunohistochem Mol Morphol. 2012 Jul;20(4):350–355. doi: 10.1097/PAI.0b013e318245c730. [DOI] [PubMed] [Google Scholar]
- 3.da Cunha Santos G, Ko HM, Saieg MA, Geddie WR. “The petals and thorns” of ROSE (rapid on-site evaluation) Cancer Cytopathol. 2013 Jan;121(1):4–8. doi: 10.1002/cncy.21215. Epub 2012 Jul 3. [DOI] [PubMed] [Google Scholar]
- 4.Collins BT, Chen AC, Wang JF, Bernadt CT, Sanati S. Improved laboratory resource utilization and patient care with the use of rapid on-site evaluation for endobronchial ultrasound fine-needle aspiration biopsy. Cancer Cytopathol. 2013 Oct;121(10):544–551. doi: 10.1002/cncy.21320. Epub 2013 Jul 3. [DOI] [PubMed] [Google Scholar]
- 5.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J Thorac Oncol. 2011 Feb;6(2):244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitson BA, Groth SS, Odell DD, Briones EP, Maddaus MA, D’Cunha J, et al. True negative predictive value of endobronchial ultrasound in lung cancer: are we being conservative enough? Ann Thorac Surg. 2013 May;95(5):1689–1694. doi: 10.1016/j.athoracsur.2012.09.057. Epub 2012 Dec 13. [DOI] [PubMed] [Google Scholar]
- 7.Dumonceau JM, Koessler T, van Hooft JE, Fockens P. Endoscopic ultrasonography-guided fine needle aspiration: relatively low sensitivity in the endosonographer population. World J Gastroenterol. 2012 May 21;18(19):2357–2363. doi: 10.3748/wjg.v18.i19.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oki M, Saka H, Kitagawa C, Kogure Y, Murata N, Adachi T, et al. Rapid on-site cytologic evaluation during endobronchial ultrasound-guided transbronchial needle aspiration for diagnosing lung cancer: a randomized study. Respiration. 2013;85(6):486–492. doi: 10.1159/000346987. Epub 2013 Apr 3. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt RL, Howard K, Hall BJ, Layfield LJ. The comparative effectiveness of fine-needle aspiration cytology sampling policies: a simulation study. Am J Clin Pathol. 2012 Dec;138(6):823–830. doi: 10.1309/AJCP8BYTCFI0XJZU. [DOI] [PubMed] [Google Scholar]
- 10.Khurana KK, Kovalovsky A, Wang D, Lenox R. Feasibility of dynamic telecytopathology for rapid on-site evaluation of endobronchial ultrasound-guided transbronchial fine needle aspiration. Telemed J E Health. 2013 Apr;19(4):265–271. doi: 10.1089/tmj.2012.0168. [DOI] [PubMed] [Google Scholar]
- 11.Karnak D, Ciledag A, Ceyhan K, Atasoy C, Akyar S, Kayacan O. Rapid on-site evaluation and low registration error enhance the success of electromagnetic navigation bronchoscopy. Ann Thorac Med. 2013 Jan;8(1):28–32. doi: 10.4103/1817-1737.105716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koegelenberg CF, Diacon AH, Irusen EM, von Groote-Bidlingmaier F, Mowlana A, Wright CA, et al. The diagnostic yield and safety of ultrasound-assisted transthoracic biopsy of mediastinal masses. Respiration. 2011;81(2):134–141. doi: 10.1159/000322005. Epub 2010 Dec 2. [DOI] [PubMed] [Google Scholar]
- 13.Trisolini R, Cancellieri A, Tinelli C, Paioli D, Scudeller L, Casadei GP, et al. Rapid on-site evaluation of transbronchial aspirates in the diagnosis of hilar and mediastinal adenopathy: a randomized trial. Chest. 2011 Feb;139(2):395–401. doi: 10.1378/chest.10-1521. Epub 2010 Oct 28. [DOI] [PubMed] [Google Scholar]
- 14.Griffin AC, Schwartz LE, Baloch ZW. Utility of on-site evaluation of endobronchial ultrasound-guided transbronchial needle aspiration specimens. Cytojournal. 2011;8:20. doi: 10.4103/1742-6413.90081. Epub 2011 Nov 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hebert-Magee S, Bae S, Varadarajulu S, Ramesh J, Frost AR, Eloubeidi MA, et al. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: a meta-analysis. Cytopathology. 2013 Jun;24(3):159–171. doi: 10.1111/cyt.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ecka RS, Sharma M. Rapid on-site evaluation of EUS-FNA by cytopathologist: an experience of a tertiary hospital. Diagn Cytopathol. 2013 Dec;41(12):1075–1080. doi: 10.1002/dc.23047. Epub 2013 Oct 25. [DOI] [PubMed] [Google Scholar]
- 17.Nicholson AG, Gonzalez D, Shah P, Pynegar MJ, Deshmukh M, Rice A, et al. Refining the diagnosis and EGFR status of non-small cell lung carcinoma in biopsy and cytologic material, using a panel of mucin staining, TTF-1, cytokeratin 5/6, and P63, and EGFR mutation analysis. J Thorac Oncol. 2010 Apr;5(4):436–441. doi: 10.1097/JTO.0b013e3181c6ed9b. [DOI] [PubMed] [Google Scholar]
- 18.Hu Y, Puri V, Crabtree TD, Kreisel D, Krupnick AS, Patterson AG, et al. Attaining proficiency with endobronchial ultrasound-guided transbronchial needle aspiration. J Thorac Cardiovasc Surg. 2013 Dec;146(6):1387.e1–1392.e1. doi: 10.1016/j.jtcvs.2013.07.077. Epub 2013 Sep 24. [DOI] [PMC free article] [PubMed] [Google Scholar]