Abstract
The Sirtuins are a phylogenetically conserved family of NAD+ dependent protein deacetylases that consume one molecule of NAD+ for every deacetylated lysine side chain. Their requirement for NAD+ potentially makes them prone to regulation by fluctuations in NAD+ or biosynthesis intermediates, thus linking them to cellular metabolism. The Sir2 protein from Saccharomyces cerevisiae is the founding sirtuin family member and has been well characterized as a histone deacetylase that functions in transcriptional silencing of heterochromatin domains and as a pro longevity factor for replicative lifespan, defined as the number of times a mother cells divides (buds) before senescing. Deleting SIR2 shortens replicative lifespan, while increased gene dosage causes extension. Furthermore, Sir2 has been implicated in mediating the beneficial effects of caloric restriction on lifespan, not only in yeast, but also higher eukaryotes. While this paradigm has had its share of disagreements and debate, it has also helped rapidly drive the aging research field forward. S. cerevisiae has four additional sirtuins, Hst1, Hst2, Hst3, and Hst4. This review discusses the function of Sir2 and the Hst homologs in replicative aging and chronological aging, and also addresses how the sirtuins are regulated in response to environmental stresses such as caloric restriction.
Keywords: lifespan, Sir2, NAD+, caloric restriction, silencing, metabolism
Introduction
The sirtuins are a highly conserved group of NAD+ dependent protein deacetylases found in eukaryotes ranging from yeast to humans. Given their dependence on and sensitivity to the metabolite NAD+ for their catalytic activity, and the variety of protein and genetic targets they have been found to regulate, sirtuins are in a unique position to translate different metabolic states into global cellular changes. Potential processes affected range from stress responses, energy metabolism, and longevity extension. The founding member of the sirtuin family, Sir2, was originally identified in Saccharomyces cerevisiae for its role in silencing at the cryptic mating type loci, HML and HMR (Rine & Herskowitz 1987; Ivy et al. 1986), though at the time, nobody recognized that these proteins were deacetylases. Four additional Sir2 homologs were eventually identified from yeast (Hst1, Hst2, Hst3, and Hst4) through a combination of cloning and database searches (Brachmann et al. 1995; Derbyshire et al. 1996). However, individual deletions of these genes did not cause loss of silencing at HML and HMR (Brachmann et al. 1995; Derbyshire et al. 1996). Hst1 and Sir2 are paralogs derived from an ancient genome duplication that have functionally diverged, yet retained a certain level of redundancy (Hickman et al. 2007). For example, overexpression of HST1 was shown early on to partially suppress the silencing defect of a sir2 deletion in MATα cells (Brachmann et al. 1995). Hst1 forms a complex with the DNA binding protein Sum1 and represses transcription of specific genes involved in middle sporulation (Xie et al. 1999), de novo NAD+ biosynthesis (Bedalov et al. 2003), and thiamine biosynthesis (Li et al. 2010). Hst2 is the most mysterious yeast sirtuin and localizes primarily in the cytoplasm, though it likely shuttles into the nucleus, and has been implicated in repression of subtelomeric genes (Halme et al. 2004) and negatively affecting rDNA silencing (Perrod et al. 2001). Hst3 and Hst4 together function as histone deacetylases specific for H3K56 that affect cell cycle progression, transcriptional silencing, and promote genome stability (Brachmann et al. 1995; Celic et al. 2006). Over the last 13 years, since the discovery of NAD+-dependent deacetylation activity (Imai et al. 2000; Landry et al. 2000), there has been tremendous interest in this protein family because of its potential for linking metabolism with multiple cellular processes. This review will focus on aging-related functions of the sirtuins and ways they respond to different metabolic states of the cell and how this in turn regulates either yeast replicative or chronological lifespan. For a review of mammalian sirtuins and their effect on metabolism and lifespan please refer to (Houtkooper et al. 2012).
Sir2 function in transcriptional silencing
For all eukaryotes, including S. cerevisiae, regulating chromatin structure both locally and globally is essential to controlling cellular processes through transcriptional activation and, more importantly in terms of the sirtuins, through transcriptional repression. Transcriptional repression can be highly localized and transient, such as at the promoters of specific genes, or more widespread across large regions of the genome that remain in a repressive and condensed state for extended periods. These latter domains tend to be heterochromatic and stable, sometimes even through multiple generations. In budding yeast, HML, HMR, and telomeres are generally considered to be the heterochromatin equivalents in this organism (Rusche et al. 2003), and are characterized by hypoacetylation of the N-terminal tails of histones H3 and H4 (Braunstein et al. 1993). The repetitive ribosomal DNA (rDNA) in yeast also has characteristics of heterochromatin, including transcriptional silencing of Pol II transcription within the tandem array and suppression of homologous recombination between the repeats (Gottlieb & Esposito 1989; Fritze et al. 1997; Smith & Boeke 1997; Bryk et al. 1997). The suppression of rDNA recombination plays a significant role in lifespan control (Sinclair & Guarente 1997; Kaeberlein et al. 1999), which will be discussed further below.
Sir2 functions in transcriptional silencing at HML, HMR, and telomeres as part of a multiprotein silencing factor known as the SIR holocomplex, which consists of Sir2, Sir3, and Sir4 (Moretti et al. 1994; Ghidelli et al. 2001) (Fig. 1). This complex is responsible for the establishment, spreading, and maintenance of silent chromatin across these heterochromatin-like domains (Hecht et al. 1996; Strahl-Bolsinger et al. 1997). It was eventually determined that Sir2 was functioning in this complex as a histone deacetylase responsible for the histone hypoacetylation that was earlier shown to be required for the silent chromatin conformation. Specifically, Sir2 deacetylates histones through an NAD+ driven reaction in which the cleavage of a molecule of NAD+ into nicotinamide (NAM) and O-acetyl-ADP-ribose is coupled to deacetylation of a single lysine side chain (Landry et al. 2000; Imai et al. 2000; Tanny et al. 2001). The deacetylation activity of Sir2 and other sirtuins is strongly inhibited by NAM in vitro (Landry et al. 2000), and growing yeast in culture with 5 mM NAM inhibits silencing at the rDNA, telomeres, and mating type loci (Bitterman et al. 2002).
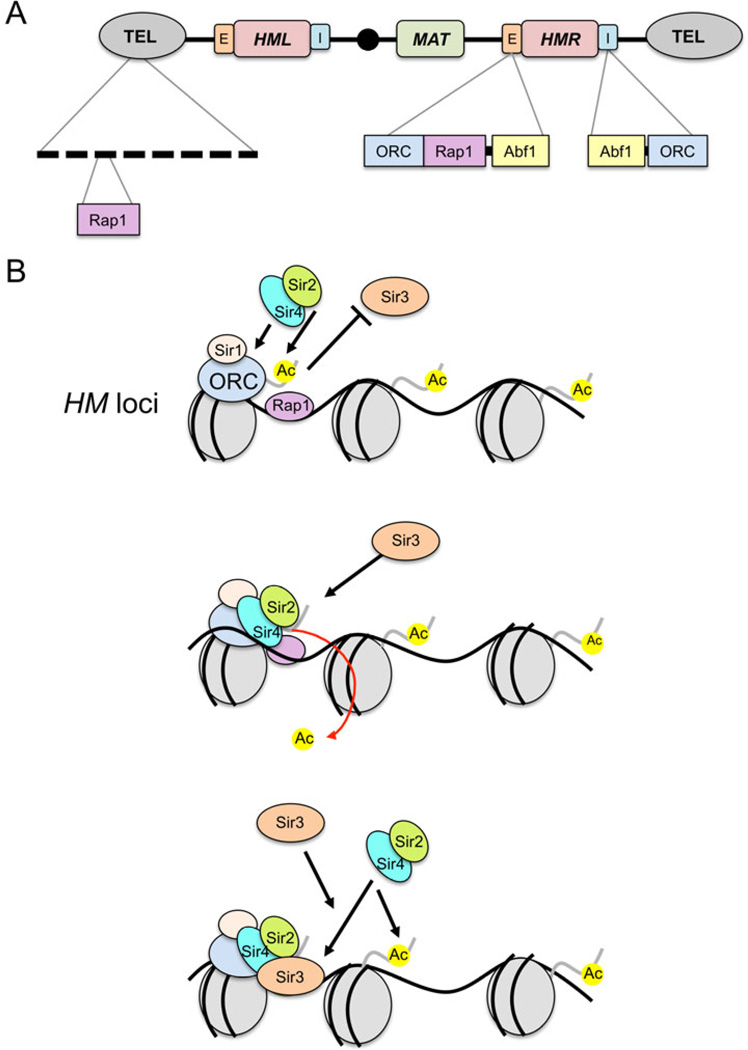
Fig. 1.
Classic silencing targets of Sir2 on chromosome III. A) Schematic diagram showing Rap1 binding sites at the telomeric repeats. The Rap1, origin recognition complex (ORC), and Abf1 binding sites within the E and I silencer elements flanking HMR are also shown. B) Updated model for Sir2-mediated histone deacetylation at silent chromatin (via the SIR complex). A Sir2/Sir4 sub-complex is recruited by ORC, Sir1, and Rap1 at the silencers. H4K16 is then deacetylated, which promotes Sir3 binding to form a SIR holocomplex, and induce spreading. Silencing spreads as more Sir2/4 is recruited to adjacent nucleosomes, resulting in further histone deacetylation and the binding/stabilization of additional Sir3 units.
At HML and HMR, the SIR complex is recruited to cis-acting sequences flanking the silent domains known as E and I silencers (Fig. 1A). The silencer elements contain binding sites for Rap1, ORC, and Abf1, which together act as binding platforms for SIR (reviewed in Rusche et al. 2003). Following the recruitment to silencers, the general model for yeast heterochromatin formation is that targeted Sir2 locally deacetylates an adjacent nucleosome on H3 and H4 tails (Fig. 1B), which then promotes continual spreading of the SIR complex across the silenced domains in between the silencers (Hecht et al. 1996; Hoppe et al. 2002; Rusche et al. 2002). Spreading outside the silencers is blocked by boundary/insulator elements, one of which is well defined as a tRNAThr gene (Donze et al. 1999). For telomeric silencing, Sir4 in complex with Sir2 is thought to initially be recruited through interactions with the Rap1 protein associated with terminal telomeric repeat sequences. This recruitment of Sir2/Sir4 then triggers histone deacetylation and spreading of telomeric chromatin, including Sir3 across subtelomeric regions (Hecht et al. 1996; Tanny et al. 2001; Hoppe et al. 2002), typically up to 1 or 2 kb. Multiple lysines on the histone tails are deacetylated in silent chromatin (Suka et al. 2001). However, H4K16 is particularly important in the regulation of silenced chromatin. It has a dual role in silencing such that the acetylated state recruits Sir2-4 and repels Sir3 (Fig. 1B), but deacetylation of H4K16ac by Sir2 actively promotes high-affinity binding of the SIR holocomplex (Oppikofer et al. 2011). The degree of spreading is controlled dynamically by the opposing histone acetylation activity of several HATs, most importantly Sas2 (Shia et al. 2005).
For silencing at the rDNA repeats, histone deacetylation by Sir2 is also essential (Bryk et al. 1997; Smith & Boeke 1997); however, it does not function at this location as part of the SIR complex. In early experiments, it was shown that although deleting other components of the Sir complex (Sir3 or Sir4) eliminated silencing at telomeres and the silent mating type loci, silencing at the rDNA increased, implying that cofactors for silencing at the rDNA were distinct from the other silent loci (Smith et al. 1998; Smith & Boeke 1997). These other cofactors were found to be Net1 and Cdc14, and together they form the RENT complex (Fig. 2A and B), which is critical for formation of silent chromatin at the rDNA (Shou et al. 1999; Straight et al. 1999; Ghidelli et al. 2001). The RENT complex is recruited to the intergenic spacer through interactions with Fob1 at IGS1 and Pol I at the rDNA promoter in IGS2 (Huang et al. 2003) (Fig. 2A). Consequently, loss of Fob1 or Pol I causes severe rDNA silencing defects (Buck et al. 2002; Huang 2003; Cioci et al. 2003). Furthermore, Sir2-dependent silencing spreads downstream of rDNA repeats in the same direction as Pol I transcription (Buck et al. 2002).
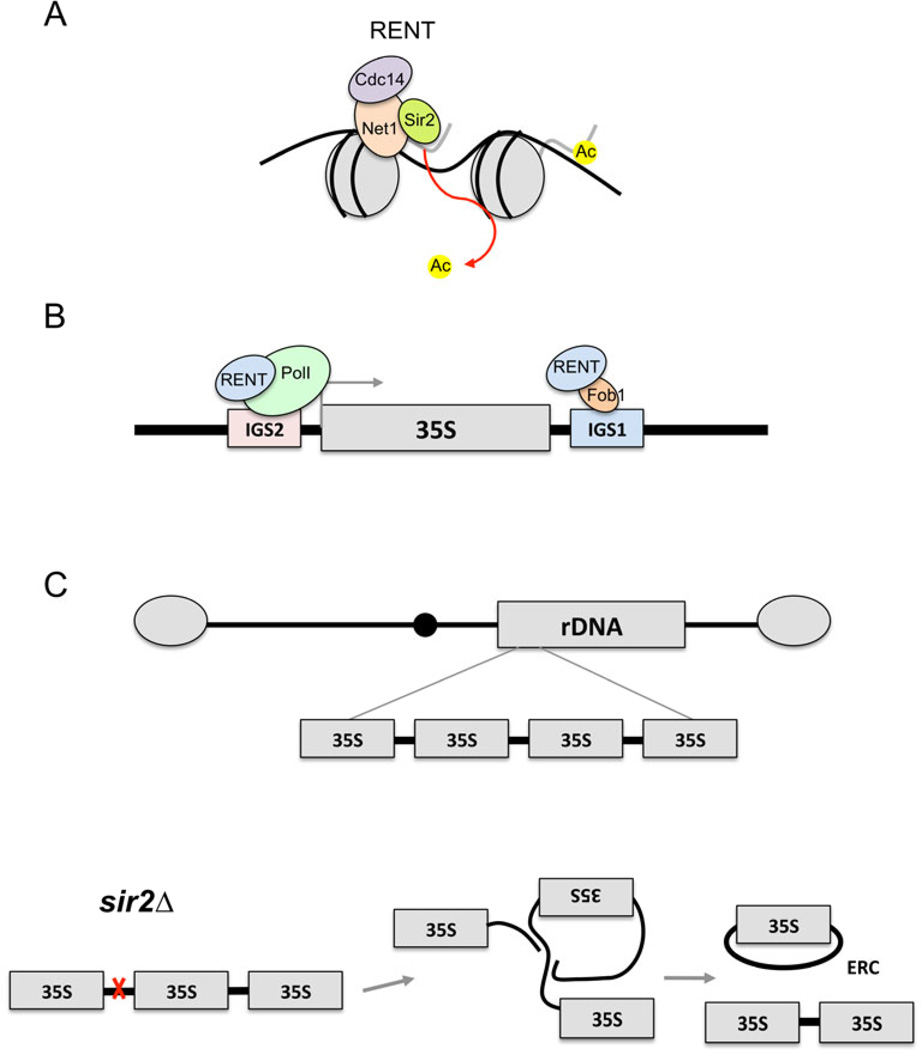
Fig. 2.
The RENT complex, recruitment to the rDNA and rDNA stability in yeast aging. A) The RENT complex consists of Sir2, Net1, and Cdc14, and functions as a histone H3 and H4 deacetylase within the nucleolus. B) RENT is recruited to the intergenic spacers of the rDNA repeats at either IGS1 via interactions with Fob1, or at IGS2 via interactions with RNA polymerase I at the rDNA gene promoter (Pol I). C) The rDNA genes are organized as a large tandem array on the right arm of chromosome XII. Double-strand DNA breaks that occur at the replication fork block site in IGS1 (red x) are repaired through homologous recombination. In the absence of SIR2, the rDNA tandem array is destabilized and unequal intra-chromatid exchange results in the formation of extrachromosomal rDNA circles (ERCs), which are self-replicating and asymmetrically segregated into mother cells.
Fob1 also recruits Tof2 and the cohibin complex (Lrs4 and Csm1) to IGS1, where they act synergistically with RENT in silencing and suppressing rDNA recombination between the rDNA repeats, most likely by maintaining the repetitive rDNA genes within proper register to prevent unequal sister chromatid recombination (Huang et al. 2006; Chan et al. 2011). Mechanistically, Sir2-dependent silencing of a non-coding Pol II-transcribed promoter (E-pro) within IGS1 is required for cohesin association with the IGS (Kobayashi et al. 2005). Transcription of this promoter in a sir2Δ mutant prevents cohesin association, thus allowing the rDNA genes on opposite sister chromatids to become misaligned and increasing the frequency of unequal exchange events. Cohibin association with nuclear envelope proteins may also assist in maintaining proper rDNA alignment (Chan et al. 2011). This control of rDNA stability is one of the Sir2 activities that promotes replicative lifespan, and is described in the next section.
Sir2 as a longevity factor
Aging can be modeled in S. cerevisiae in two ways: replicatively or chronologically. Replicative lifespan (RLS) is defined by the number of times a mother cell divides and produces a daughter before it senesces (Mortimer & Johnston 1959), and is traditionally measured using a micromanipulation procedure on agar plates where the buds are removed from mother cells after each division (Steffen et al. 2009). Chronological lifespan (CLS) is the length of time that yeast cells survive in a post-mitotic state (Werner-Washburne et al. 1993; Fabrizio et al. 2003). For CLS studies, yeast are usually grown in rich or synthetic media into stationary phase, and then either transferred to water or maintained in the original expired growth medium (MacLean et al. 2001; Fabrizio et al. 2005; Smith Jr. et al. 2007). Aliquots are then taken every few days and the cells tested for viability by measuring either the proportion of cells capable of reentering the cell cycle and forming a colony when plated on fresh media plates, or by staining with fluorescent vital dyes such as FUN-1 (Fabrizio et al. 2003; Essary et al. 2009). There has been some debate over which aging model is more relevant to aging in metazoans. It has been reported that the number of conserved genes that impact RLS and also impact the lifespan of C. elegans is higher than those involved in CLS (Burtner et al. 2011). However, it has also been argued that chronological aging is the more relevant model given that the main nutrient signaling pathways affecting aging across eukaryotes affect chronological lifespan, and the majority of aging cells in higher eukaryotes exist in a post-mitotic state (Parrella & Longo 2010). Given that replicative and chronological lifespan can both be extended by repressing the same nutrient sensing pathways (either genetically or environmentally) or by manipulating mitochondria function, the mechanisms that regulate these two forms of aging are not mutually exclusive (Kaeberlein et al. 2005; Piper 2006; Wei et al. 2008).
The importance of Sir2 toward RLS was initially found indirectly through a screen for stress resistant mutants that also extend RLS (Kennedy et al. 1995). Several different mutants were isolated and the affected genes named UTH1 through UTH4. The uth2-42 mutation turned out to be a dominant allele of SIR4 that was renamed SIR4-42 (Kennedy et al. 1995). The SIR4-42 allele encodes a C-terminal truncation that specifically prevents recruitment of the SIR complex to HML, HMR, and telomeres (Kennedy et al. 1997). The Sir proteins released from these formerly silenced domains in the SIR4-42 mutant, including Sir2, are redistributed to the nucleolus/rDNA where rDNA silencing is subsequently strengthened and the frequency of rDNA recombination reduced, thus extending RLS (Kennedy et al. 1995; Kennedy et al. 1997). This redistribution of Sir proteins to the nucleolus also occurs in normal replicatively aging mother cells, and likely causes the sterility of these old cells due to the loss of silencing at HML and HMR (Smeal et al. 1996).
The link between rDNA recombination and replicative aging was primarily centered on the formation of extrachromosomal rDNA circles (ERCs) (Fig. 2C). ERCs consist of individual or multiple rDNA genes that have been excised from the tandem array through homologous recombination, or more specifically, through unequal intra-chromatid exchange. Each rDNA gene contains a functional ARS element within the IGS2 region, making the ERCs self-replicating. However, since they do not have centromeres, ERCs act like typical ARS-containing plasmids that are asymmetrically segregated into mother cells during mitosis (Murray & Szostak 1983; Sinclair & Guarente 1997). This is caused by a septin-dependent lateral diffusion barrier between mother and daughter (Shcheprova et al. 2008). ERCs are duplicated during each S-phase, resulting in exponential accumulation in older mother cells (Sinclair & Guarente 1997). Deleting SIR2 results in hyper-recombination within the rDNA (Gottlieb & Esposito 1989), thus increasing rDNA instability and ERC formation (Kaeberlein et al. 1999) (Fig. 2C). Furthermore, increasing SIR2 gene dosage suppresses rDNA recombination/ERC formation and extends RLS (Kaeberlein et al. 1999). The accumulating ERCs were originally thought to prevent further cell divisions by diluting the pool of proteins responsible for efficient DNA replication and maintenance (Sinclair & Guarente 1997); however, more recent data have shown that rDNA instability in the mother cells is sufficient to limit RLS regardless of the absolute number of ERCs that are formed (Falcon & Aris 2003; Ganley et al. 2009). It has been proposed that nonfunctional DNA repair proteins could be preferentially segregated to aging mother cells, and damage in the mother’s rDNA could then accumulate and perhaps lead to decreased quality in the ribosomes produced (Ganley et al. 2009). Regardless of whether through ERC formation or a reduction in the quality of proteins produced from the rDNA itself, recombination and increased instability at the rDNA has a limiting effect on RLS. A central role for the rDNA in RLS regulation was recently confirmed by two traditional QTL genetic mapping studies of natural lifespan variation showing the SIR2 and rDNA loci to be major sources of variation (Stumpferl et al. 2012; Kwan et al. 2013). This predominant role of rDNA in yeast lifespan regulation is further described in another review from this special FEMS Yeast Research issue (Kobayashi & Ganley, 2013).
With rDNA instability being a major source of replicative aging in the yeast system, this can potentially cause problems when attempting to identify or study other sources of lifespan regulation (Kaeberlein et al. 2004), or when studying Sir2-mediated processes outside of rDNA silencing or recombination suppression. For example, changes in rDNA recombination/instability can mask the phenotypes induced by other longevity gene mutations (Delaney et al. 2011). Deletion of the FOB1 gene is typically used to suppress rDNA recombination (Defossez et al. 1999). Fob1 binds to IGS1 of each rDNA gene, where it blocks rightward moving DNA replication forks initiated at the origin in IGS2 from colliding with the Pol I transcriptional machinery moving in the opposing direction (Kobayashi et al. 1998). Blocked replication forks at this site in IGS1 induce double strand DNA breaks that trigger rDNA recombination/instability. Deleting FOB1 prevents the fork blocks, thus acting to preserve rDNA stability, suppress ERC formation, and extend RLS (Defossez et al. 1999). Conversely, deleting SIR2 or treating cells with nicotinamide dramatically increases the frequency of rDNA recombination and significantly shortens RLS (Kaeberlein et al. 1999; Bitterman et al. 2002). Deleting FOB1 suppresses the short RLS of a sir2Δ mutant (Kaeberlein et al. 1999), but viewed differently, deleting SIR2 still suppresses the extended RLS of a fob1Δ mutant, strongly suggesting that Sir2 has additional functions in promoting longevity that are independent of the rDNA. One of these rDNA-independent functions for Sir2 in longevity is its role in the asymmetric segregation of oxidatively damaged proteins, mitochondria, and repair machinery between mother and daughter cells during mitosis (Aguilaniu et al. 2003; Erjavec & Nystrom 2007; McFaline-Figueroa et al. 2011). Accumulating oxidative damage has been known to negatively impact RLS (Nestelbachera et al. 2000; Laun et al. 2001). Budding yeast’s asymmetric cell division, in which certain components of the cell that limit RLS are kept within the mother cell, allows the daughter to retain full replicative potential (Sinclair & Guarente 1997; Shcheprova et al. 2008). This process is Sir2 dependent and appears to be independent of rDNA silencing (Aguilaniu et al. 2003; Orlandi et al. 2010). Specifically, daughter cells inherit fewer ROS, higher levels of the cytosolic catalase Ctt1, and mitochondria with higher redox potential (Aguilaniu et al. 2003; Erjavec & Nystrom 2007; McFaline-Figueroa et al. 2011). Sir2 may facilitate this asymmetric inheritance by promoting actin-folding activity of the chaperonin CCT ring complex, potentially through direct deacetylation (Liu et al. 2010). This, in turn, impacts the polarisome-dependent retrograde transport of aggregates containing damaged cellular components away from the daughter cell (Liu et al. 2010).
Sir2 and telomeres
In higher eukaryotes, telomere length plays a major role in regulating cellular senescence. The telomeres of primary cells such as fibroblasts that do not express telomerase become shorter during each cell division. Once telomeres become short enough, the cells reach a point of crisis (the Hayflick limit) and either senesce, or occasionally repair the telomeres through a recombination-mediated alternative lengthening of telomere (ALT) mechanism that allows them to survive the crisis (Draskovic et al. 2013). Yeast telomerase is constitutively expressed, so the telomeres do not progressively shorten in mitotic cells, including aging mothers and their daughters (D’Mello & Jazwinski 1991). When telomerase is inactivated by mutations in subunits such as the Est2, the telomeres do progressively shorten in mother and daughter cells, ultimately leading to widespread senescence within the population (Lundblad & Szostak 1989; Lingner et al. 1997). In a telomerase deficient est2Δ rad52Δ mutant that can’t repair its telomeres through homologous recombination, deleting SIR2 further accelerates senescence within the population (Maicher et al. 2012). Mechanistically, the lack of Sir2 and resulting loss of telomeric silencing, increases the transcription of non-coding TElomeric Repeat containing RNAs (TERRA), which has been proposed to somehow interfere with proper telomere maintenance (Maicher et al. 2012).
The effect of SIR2 on telomere length and senescence of telomerase-deficient cells in a population (see above) should not be confused with the role of SIR2 in mother cell RLS. A separate telomere-related function for Sir2 in mother cell RLS has been discovered. Sir2 protein levels drop significantly in aging mother cells, and this decrease correlates with dramatically increased H4K16 acetylation and loss of silencing at specific X core (XC) subtelomeric regions, more so than the increase observed at silenced rDNA Sir2 target regions (Dang et al. 2009). SAS2 encodes an H4K16 acetyltransferase that counteracts Sir2-mediated H4K16 deacetylation (Suka et al. 2002). A sas2Δ mutant shows reduced H4K16 acetylation at telomeric XC domains and extends RLS. H4K16Q or H4K16R mutations also shorten RLS in a fob1Δ background, suggesting that their effects on lifespan are occurring independent of ERCs or rDNA instability (Dang et al. 2009). The mechanism by which Sir2-dependent H4K16 deacetylation promotes RLS remains unclear, and additional non-telomeric domains could also be involved. Indeed, Sir2, Sir3, and Sir4 have been shown to associate with numerous loci outside the typical silenced domains in yeast (Lieb et al. 2001; Li et al. 2013; Radman-Livaja et al. 2011; Dubarry et al. 2011). Perhaps some of the telomere-related activity of Sir2 in RLS could be related to its recruitment to telomeres via the cohibin complex, which was originally defined for its function in maintaining rDNA stability (Huang et al. 2006). However, cohibin also associates with subtelomeric regions where it contributes to Sir2 recruitment and telomere stability (Chan et al. 2011). Cohibin mutants interfere with localization of telomeres toward the nuclear periphery, and shorten lifespan even when the rDNA is stabilized by deletion of FOB1 (Chan et al. 2011). Similarly, the Mediator complex associates with telomeres, and knocking out specific subunits required for the telomere association results in a shift of the boundary between Sir2 and Sas2 such that telomeric H4K16ac levels are increased, silencing is decreased, and RLS is decreased (Zhu et al 2011). There is significant competition for a limiting amount of Sir2 between telomeres and the rDNA (Smith et al. 1998). The telomere regulatory protein Rif1 was recently shown to help maintain a proper balance of Sir2 levels at rDNA for RLS by preventing excessive recruitment to telomeres and the HM loci (Salvi et al. 2013).
Sirtuin regulation by NAD+ metabolism
As mentioned above, the sirtuins are NAD+-dependent protein deacetylases, which potentially makes their activity sensitive to changes in the intracellular NAD+ concentration. In yeast, NAD+ is synthesized from tryptophan and the three vitamin precursors of NAD+, nicotinic acid (NA), nicotinamide (NAM), and nicotinamide riboside (NR) (Fig. 3). NAD+ homeostasis is maintained by a combination of biosynthesis and salvage pathways, and balanced secretion/import of the vitamin precursors (Belenky et al. 2011; Lu & Lin 2011). In the absence of any vitamin precursors in the growth medium, NAD+ is synthesized de novo from tryptophan using the Bna1-Bna6 proteins (Kucharczyk et al. 1998), which ultimately produce nicotinic acid mononucleotide (NAMN). The NAMN is then adenylated by the redundant nicotinic acid/nicotinamide adenylyltransferases Nma1 or Nma2 to generate nicotinic acid adenine dinucleotide (NaAD) (Anderson et al. 2002), followed by conversion to NAD+ by the NAD synthetase (Qns1) (Bieganowski et al. 2003; Suda et al. 2003). NA is imported from the growth medium by the NA permease Tna1 and then converted by nicotinic acid phosphoribosyltransferase (Npt1) into NAMN, thus merging with the last two steps of the de novo pathway (Fig. 3). This is historically known as the Preiss-Handler pathway. In commonly used yeast growth media containing NA as the vitamin precursor, Npt1 is the rate-limiting step for NAD+ production, and deleting NPT1 results in a 2- to 3-fold reduction in the intracellular NAD+ concentration (Smith et al. 2000; Lin et al. 2000). This decrease in NAD+ is sufficient to inhibit Sir2 function in transcriptional silencing and shorten RLS (Smith et al. 2000; Sandmeier et al. 2002; Belenky et al. 2007). The Npt1, Nma1, and Nma2 proteins are each concentrated in the nucleus (Sandmeier et al. 2002; Anderson et al. 2002), and their overexpression extends RLS without increasing the overall NAD+ level (Anderson et al. 2002), suggesting that flux through the Preiss-Handler pathway is important for maintaining proper Sir2 activity in the nucleus (Sandmeier et al. 2002; Anderson et al. 2002).
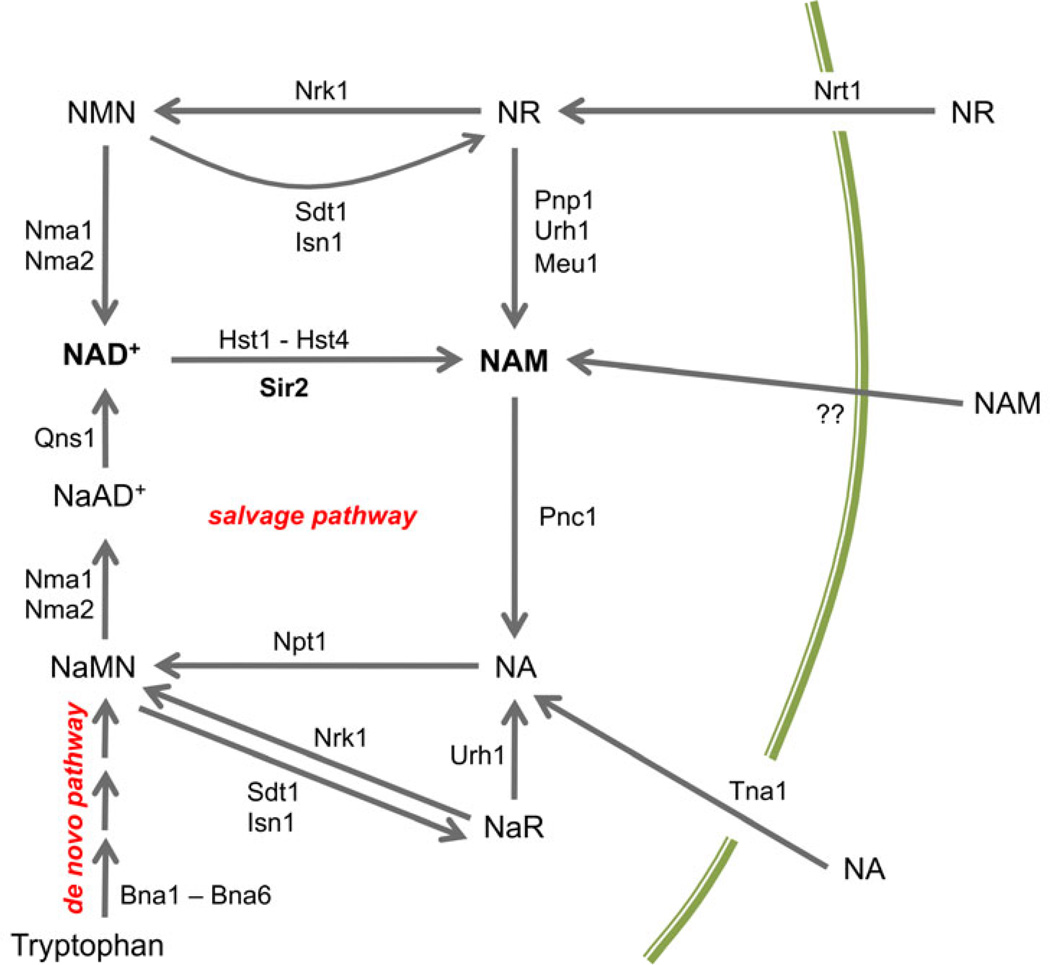
Fig. 3.
Overview of NAD+ biosynthesis and metabolism in Saccharomyces cerevisiae. NAD+ is synthesized de novo by the Bna1-Bna6 enzymes using tryptophan as the starting substrate. The vitamin precursors nicotinic acid (NA), nicotinamide (NAM), and nicotinamide riboside (NR) are imported and then enter a set of salvage pathways that ultimately feed into nicotinic acid mononucleotide (NaMN) or nicotinamide mononucleotide (NMN). These mononucleotides are further adenylated to by Nma1 or Nma2 to form the dinucleotide forms, which for nicotinamide, is actually NAD+. Precursors from the nicotinamide branch of the salvage pathways can be shifted to the nicotinic acid branch through deamidation of nicotinamide by Pnc1. The sirtuins produce nicotinamide during the deacetylation reaction. The mechanism of nicotinamide import is unknown. Other abbreviations: NaAD, deamido NAD; NaR, nicotinic acid mononucleotide.
NR is imported by the thiamine/NR transporter Nrt1 (Belenky et al. 2008), and then phosphorylated by nicotinamide riboside kinase (Nrk1) to produce nicotinamide mononucleotide (NMN), which is adenylated by Nma1 or Nma2 to produce NAD+ (Bieganowski & Brenner 2004). Supplementing YPD or SC medium with NR suppresses the short RLS and transcriptional silencing defects of an npt1Δ mutant by restoring normal NAD+ concentration (Belenky et al. 2007). NR can also be degraded into NAM by several nucleoside hydrolases and phosphorylases (Belenky et al. 2007). The nicotinamidase Pnc1 scavenges any NAM generated by NR hydrolysis, sirtuin activity, or other NAD+ consuming reactions by deamidating it into NA. This not only prevents the accumulation of NAM to high concentrations that could inhibit sirtuins, but also pushes it into the Preiss-Handler pathway to be recycled into NAD+ via Npt1 (Anderson et al. 2003; Gallo et al. 2004). High NAM concentrations (5 mM) in the growth medium are inhibitory for sirtuin activity, and result in shortened RLS that is similar to the effects of a sir2Δ mutant (Bitterman et al. 2002). Overexpressing PNC1 suppresses this inhibition by detoxifying the NAM through deamidation (Gallo et al. 2004), and PNC1 overexpression extends RLS even when NAM is not added to the growth medium (Anderson et al. 2003), probably because of its dual role in promoting flux through the Preiss-Handler pathway and preventing NAM accumulation. The downstream effect is most likely Sir2-mediated enhancement of rDNA silencing and stability, though other Sir2 and sirtuin-dependent processes could certainly also be at play.
Sir2 and caloric restriction
Caloric restriction (CR) is a dietary regimen defined by reducing an organism’s calorie intake while maintaining proper nutrition, and has been shown to extend the lifespan of almost all model organisms in which it has been tested. In S. cerevisiae, CR is typically modeled by reducing glucose concentration in the growth medium from the standard non-restricted 2% (NR) to 0.5% (moderate CR) or 0.05% (extreme CR). Below 0.05% glucose, yeast cell growth is significantly impaired, so RLS cannot be easily tested. CLS assays monitor the viability of nondividing cells, so even water can be used to represent extreme CR in that lifespan system (Fabrizio et al. 2005). An early study from the Guarente lab (Lin et al. 2000) showed that SIR2 was required for the extension of RLS when CR was genetically mimicked by deleting hexokinase (hxk2Δ), which reduces the entry of glucose into glycolysis. Impairing the Ras/cAMP/PKA signaling pathway that responds to changes in glucose levels, also extended RLS (Lin et al. 2000). SIR2 deletion was initially shown to block the extension of RLS induced by moderate CR in a fob1Δ mutant (Lin et al. 2002), but later studies found that SIR2 was not required for the extension, especially during extreme CR (Kaeberlein et al. 2004). Inhibition of the TOR signaling pathway either through deletion of TOR1 or by low doses of rapamycin, also extends RLS (Kaeberlein et al. 2005). However, this pathway appears to work on lifespan through effects on ribosome maturation and translation, rather than through Sir2. While Sir2 may not be absolutely required for the RLS extension by CR or attenuation of nutrient signaling pathways, it likely still plays a role. For example, nutrient deprivation or rapamycin inhibition of TOR signaling results in condensin association with the rDNA (Tsang et al. 2007), as well as increased Sir2 binding to the rDNA (Ha & Huh 2011). This becomes intriguing because Sir2 and Hst1 were recently shown to be required for efficient condensin loading onto various chromatin targets, including the rDNA (Li et al. 2013), raising the question of whether Sir2-directed chromatin structural conformations could be involved in lifespan regulation.
CR is considered a form of nutrient stress, and like inhibition of the various nutrient signaling pathways linked to RLS extension (cAMP/PKA, TOR, Sch9), it also results in upregulation of Pnc1 from the NAD+ salvage pathway (Anderson et al. 2003; Medvedik et al. 2007). Furthermore, PNC1 is required for the RLS extension induced by CR (Anderson et al 2003). PNC1 and other stress-induced genes are activated by the transcription factors Msn2 and Msn4 (Medvedik et al. 2007). CR and the other stresses such as TOR inhibition appear to shuttle Msn2/4 into the nucleus where they activate PNC1 expression, among many other genes. Increased Pnc1 in this context has been proposed to stimulate Sir2 activity by reducing the intracellular NAM concentration, or alternatively, by increasing flux through the NAD+ salvage pathway (Anderson et al. 2003; Medvedik et al. 2007). Consistent with this model, Msn2/4 is also activated in response to inhibition of the mitochondrial translation control (MTC) module, which extends RLS in a Sir2-dependent manner (Caballero et al. 2011). Importantly, PNC1 was required for this RLS extension even in the absence of respiration, and rDNA silencing was enhanced.
The role that upregulation of the NAD+ salvage pathway plays in activating sirtuins during CR has been debated. For example, PNC1 upregulation during moderate CR is not always observed, yet this condition extends RLS. Some labs have shown that growing cells in 0.5% glucose is sufficient to increase Pnc1 levels while others do not see an increase in Pnc1 unless glucose levels are reduced to 0.1% and lower (extreme CR) (Anderson et al. 2003; Rahat et al. 2011). In addition, CR was still able to extend RLS in a pnc1Δ mutant when NAM was cleared through an independent mechanism (Lin et al. 2004), suggesting the existence of a NAM-independent (and presumably Sir2-independent) mechanism of lifespan extension. The most popular alternative model has been modulation of the NAD+/NADH ratio (see below).
During exponential growth in glucose-containing media, S. cerevisiae primarily ferments the glucose into ethanol, even in the presence of oxygen. As glucose becomes depleted, the cells undergo a massive reconfiguration of their transcription profile and metabolism toward ethanol/acetate utilization and mitochondrial respiration, a process called the diauxic shift (Gray et al. 2004). This change prepares the cells for survival during extended periods of time in stationary phase (the chronological lifespan). Moderate CR increases the expression of genes involved in respiration (Lin et al. 2002; Rahat et al. 2011), and increases the rate of respiration (Lin et al. 2002). More recently, yeast grown under moderate CR were shown to undergo the shift to respiration earlier and more efficiently than yeast grown in non-restricted conditions (Tahara et al. 2013), suggesting they are better primed for survival. The transition to respiration results in an increase of the NAD+/NADH ratio by lowering the concentration of NADH without altering the overall level of NAD+ (Lin et al. 2002). While NADH is a relatively weak inhibitor of Sir2 activity, the increased NAD+/NADH ratio has been proposed to stimulate Sir2 activity by relieving NADH inhibition (Lin et al. 2004). This view is supported by the observation that CR does not extend RLS when the malate-aspartate NADH shuttle is defective and unable to balance the NAD+/NADH ratio between the mitochondrial and cytosolic/nuclear pools, while overexpressing shuttle components extends RLS (Easlon et al. 2008). Additionally, raising the NAD+/NADH ratio through overexpression of alcohol dehydrogenase, ADH1, also extends RLS (Reverter-Branchat et al. 2007).
Not all data directly supports this NAD+/NADH model of sirtuin activation during CR. Other labs have found that in vitro, NADH does not exert enough of an inhibitory effect on Sir2 activity to be physiologically relevant without altering the concentration of NAD+ (Schmidt et al. 2004). Steady state NAD+ levels were also found to decrease during CR, as compared to nonrestricted yeast (Anderson et al. 2003; Evans et al. 2010). However, it is still possible that CR leads to a greater flux through the NAD+ salvage pathways, which has been proposed to enhance Sir2 function without measurably changing NAD+ levels (Anderson et al. 2002, Sandmeier et al. 2002). Alternatively, specific NAD+ biosynthesis intermediates, rather than NAD+ directly, could be changed in certain cellular compartments where sirtuins are active. Consistent with this hypothesis is the observation that NAMN levels are higher in CR yeast (Evans et al. 2010). Further emphasizing the importance of flux in the NAD+ biosynthesis pathways in activating Sir2, the RLS of cells unable to utilize endogenous NR is not extended by CR (Lu et al. 2009). This result could also potentially indicate the importance of maintaining NAD+ homeostasis for RLS. The NAM analog, isonicotinamide (INAM) promotes NAD+ homeostasis when added to the growth medium at high concentrations, especially during the diauxic shift (McClure et al. 2012). Similar to NR, INAM also suppresses the short RLS of an npt1Δ mutant.
While the Pnc1 and NAD+/NADH models for Sir2-mediated RLS extension are attractive, there is considerable evidence of Sir2-independent mechanisms at play. Analysis of rDNA and telomeric silencing concluded that Sir2 activity at these locations was not enhanced by CR growth conditions (Smith Jr et al. 2009; Riesen & Morgan 2009). Additionally, there have been several studies where sir2Δ did not prevent CR-mediated RLS extension, either with moderate or extreme CR (Jiang et al. 2002; Kaeberlein et al. 2004). It has been argued that the SIR2-independent lifespan extension by CR is due to partial redundancy with HST2 and HST1 (Lamming et al. 2005), but other studies found that a strain lacking all 5 sirtuins still lived longer with moderate or extreme CR conditions (Kaeberlein et al. 2006; Tsuchiya et al. 2006). In hindsight, the existence of SIR2-independent mechanisms for CR-mediated lifespan extension was probably not unexpected, especially given the complexity of aging. For example, mutations in the dihydrolipoamide acetyltransferase E2 subunit of the mitochondrial pyruvate dehydrogenase complex is required for CR-induced RLS extension independent of Sir2 (Easlon et al. 2006).
Sir2 and chronological lifespan
While Sir2 has an anti-aging role in RLS, most evidence thus far has pointed toward a proaging role in CLS. Depending on the strain background, sir2Δ either has no effect or modestly increases CLS when cells are grown in YPD or SC medium (Fabrizio et al. 2005; Smith Jr et al. 2007; Wu et al. 2011). SIR2 is also not required for CR-induced CLS extension when the cells age chronologically in their expired growth medium (Smith Jr et al. 2007). When cells are transferred to water after reaching stationary phase they have a long CLS (considered an extreme form of CR), and deleting SIR2 dramatically extends the lifespan even more (Fabrizio et al. 2005). Similar extreme CLS extension is observed when sir2Δ is combined with sch9Δ or Ras/cAMP/PKA mutations that reduce nutrient signaling (Fabrizio et al. 2005). Given that both of these pathways extend CLS through the activation of stress response genes (Wei et al. 2008), it is possible that Sir2 may prevent full CLS extension by partially repressing these same genes. This would be consistent with the observation that sir2Δ also confers greater resistance to oxidative and heat stresses (Fabrizio et al. 2005).
Though the exact mechanisms remain unclear, the accumulation of acetic acid and ethanol in the media of aging yeast cultures can decrease CLS (Fabrizio et al. 2005; Burtner et al. 2009). Sir2 has been found to play a role in decreasing the rate of consumption of these two substrates through regulation of the alcohol dehydrogenase Adh2 (Fabrizio et al. 2005), as well as the deacetylation and subsequent inactivation of phosphoenolpyruvate carboxykinase (Pck1) (Casatta et al. 2013; Lin et al. 2009). In short, the loss of Sir2 activity pushes cellular metabolism from glycolysis towards gluconeogenesis and glycogen and trehalose production during chronological aging (Casatta et al. 2013). Both of these pathways contribute to the survival by increasing stress resistance of the non-dividing cells and allowing them to quickly reenter mitosis upon reintroduction of nutrients to the media (Gray et al. 2004; Shi et al. 2010).
CLS is notoriously susceptible to variations in strain and media conditions (Fabrizio et al. 2005; Burtner et al. 2009; Matecic et al. 2010). For example, despite clear evidence of a proaging role for Sir2 during post-mitotic aging in standard laboratory growth media and water, deleting SIR2 was recently found to actually shorten CLS when cells are grown under wine making conditions in grape juice (Orozco et al. 2012), which is probably a more natural growth substrate. Consistent with this idea, our lab recently discovered that Sir2 and Hst1 collaborate to repress glycolytic and fermentation genes during onset of the diauxic shift (Li et al. 2013). In the absence of SIR2 and HST1, glucose fermentation genes such as pyruvate decarboxylase (PDC1) fail to be efficiently repressed with the proper timing. The genes are eventually repressed in stationary phase, but they may not be properly primed for long-term survival. Therefore, the effect of deleting SIR2 on CLS appears to depend on how the cells have been grown and in what medium. Sir2 acts in concert with Hst1 to regulate such key metabolic genes during the diauxic shift (Li et al. 2013), making it possible that simple single-gene knockout experiments are insufficient to determine the full extent of Sir2’s role in chronological aging.
Other yeast sirtuins and their effects on aging
In addition to Sir2, there are four other members of the sirtuin protein family in S. cerevisiae: Hst1, Hst2, Hst3, and Hst4. All five share a similar core domain required for the NAD+ dependent deacetylation reaction, but the variation among their terminal domains allows for differences in their specific functions, protein interactions, and cellular localizations (Brachmann et al. 1995). Hst1 forms a complex with Sum1 and Rfm1 to repress transcription of specific genes by deacetylating histones at their promoters (Xie et al. 1999; McCord et al. 2003). Some of the specific genes known to be regulated by this Sum1 complex include meiosis genes containing middle sporulation elements (MSE), genes involved in NAD+ biosynthesis, as well as genes involved in thiamine biosynthesis (Xie et al. 1999; Bedalov et al. 2003; McCord et al. 2003; Li et al. 2010). From ChIP-seq analysis, Hst1, Sum1, and Sir2 bind to the open reading frames and repress multiple genes involved in glycolysis, glucose fermentation, and translation (Li et al. 2013). Strains lacking Hst1 do not properly repress these genes during the diauxic shift, which could be related to the slightly shorter CLS of an hst1Δ mutant (Smith Jr et al. 2007). Strains lacking HST1 or SUM1 also upregulate the de novo NAD+ biosynthesis genes, which raises the intracellular NAD+ concentration (Bedalov et al. 2003). This effect on NAD+ homeostasis could potentially impact RLS or CLS under certain growth conditions, though it has not yet been demonstrated. Finally, Hst1 has been shown to weakly suppress rDNA recombination in response to CR when SIR2 and HST1 are deleted (Lamming et al. 2005).
Hst2 is the only primarily cytosolic yeast sirtuin (Perrod et al. 2001). It contains a leucine-rich nuclear export sequence that causes it to be actively excluded from the nucleus by the action of the exportin Crm1 (Wilson et al. 2006). However, overexpression of Hst2 leads to decreased Sir2-dependent silencing at telomeres, as well as increased silencing and reduced recombination at the rDNA (Perrod et al. 2001; Lamming et al. 2005). Hst2 may compete with Sir2 for another factor that is necessary for telomeric silencing, and decrease in the telomeric Sir2 pool would result in an increased presence at the rDNA (Perrod et al. 2001). Not all of the observed decrease in the rate of recombination by HST2 overexpression can be attributed to increased Sir2 at the rDNA. In some strains, CR can still extend RLS and reduce recombination at the rDNA even with sir2Δ fob1Δ (Kaeberlein et al. 2004). Deleting HST2 in addition to SIR2 and FOB1 prevents CR from extending RLS and reducing rDNA recombination (Lamming et al. 2005). However, this does not hold true for extreme CR (0.05% glucose), which appears to extend RLS through alternative sirtuin independent mechanisms (Kaeberlein et al. 2006).
In the cytoplasm, Hst2 may play a role in CLS through its association with another nuclear histone deacetylase Hos2 and various stress-related proteins during stationary phase (Liu et al. 2012). After cells have ceased dividing, these proteins assemble into stationary phase granules (SPGs) that can be quickly dispersed upon the addition of new nutrients. Though hst2Δ does not appear to have an effect on CLS, hos2Δ decreases CLS and prevents cells from reentering mitosis from stationary phase upon the addition of nutrients (Liu et al. 2012). The precise role Hst2 may be playing with Hos2 and the other proteins in these SPGs and their resulting impact on regulation of cellular quiescence is unclear at this time (Liu et al. 2012).
Hst3 and Hst4 have somewhat redundant roles in stabilizing the genome during the cell cycle (Brachmann et al. 1995; Celic et al. 2006). Cells lacking both HST3 and HST4 are unable to progress normally through the cell cycle (Brachmann et al. 1995). This is primarily due to the hyperacetylation of H3K56, the target of Hst3 and Hst4 deacetylation (Celic et al. 2006; Maas et al. 2006). Hyperacetylation of H3K56 due to a lack of Hst3 and Hst4 leads to increased thermo sensitivity and genotoxic stress susceptibility as well as higher level of spontaneous DNA damage (Brachman et al. 1995; Celic et al. 2006). These effects can be rescued by also knocking down Asf1, the chaperone responsible for depositing the acetylated histones, or by genetically altering H3K56 to an arginine (Celic et al. 2006). Consistent with their overall increased genomic instability, hst3Δ hst4Δ yeast display silencing defects at the telomeres and increased recombination at the rDNA (Brachmann et al. 1995; Ide et al. 2013). Hst3 is the predominant H3K56 deacetylase, and hst3Δ mutants have a shortened RLS (Tsuchiya et al. 2006). While RLS of an hst4Δ mutant is close to normal, an hst3Δ hst4Δ double mutant has a very short RLS reflective of its poor overall viability and genomic instability (Tsuchiya et al. 2006). Interestingly, Hst3 and Hst4 appear to be more resistant to NAM than Sir2 or Hst1, as it takes 25 mM NAM to phenocopy an hst3Δ hst4Δ mutant (Tsuchiya et al. 2006).
The overall effects of deleting HST3 and HST4 on CLS are similar to the effects on RLS. An hst3Δ mutation decreases CLS, while an hst4Δ mutation has little effect (Smith Jr et al. 2007). Surprisingly, reducing H3K56ac by deleting ASF1 and the H3K56 acetyltransferase gene RTT109, has no effect on CLS (Hachinohe et al. 2013). Instead, the decreased CLS of hst3Δ hst4Δ cells is most likely due to an observed change in glucose metabolism. Reducing gluconeogenesis without impacting the rate of glycolysis by deleting TDH2 (Glyceraldehyde-3-phosphate dehydrogenase/GAPDH) suppressed the short CLS of the hst3Δ hst4Δ mutant (Hachinohe et al. 2013). Like Sir2 deacetylation of Pck1, perhaps Hst3 and Hst4 also have nonhistone targets that participate in glucose metabolism. A clear take home message up to this point in yeast sirtuin research is that all five yeast enzymes impact lifespan one way or another, and future work on identifying additional deacetylation targets, both chromatin and non-chromatin, is critical.
Conclusions
The discovery of Sir2 as an NAD+-dependent histone deacetylase energized the aging research field because it provided a mechanistic link between a known longevity factor (in yeast at the time) and metabolism. Since then, sirtuins in more complex eukaryotes (e.g. mammals) have been found to deacetylate and regulate numerous non-histone proteins related to age-associated disease. As described in this review, most Sir2 research in yeast has focused on its role as a histone deacetylase, especially in transcriptional silencing and maintaining rDNA stability. Sir2 and the other yeast sirtuins also likely deacetylate numerous non-histone proteins, as there are a large number of acetylated proteins in yeast (Henriksen et al. 2012), including many proteins involved in intermediary metabolism, just as in mammals. Furthermore, genetic and physical interaction maps have identified a ‘metabolism-centric’ Sir2 interaction network, highlighting the integration of Sir2 with metabolism (Ralser et al. 2012). The identification of Sir2 and Hst1 regulation of glycolysis and ribosomal protein genes during the diauxic shift parallels similar findings for mouse SIRT6 (Li et al. 2013; Zhong & Mostoslavsky 2010), suggesting metabolism as an emerging area of conservation for sirtuin activity between yeast and mammals. Yeast will surely remain a powerful model system for further elucidating these connections to aging.
Acknowledgements
This work was supported by NIH RO1 grant GM075240 and R21 grant AG042686.
Footnotes
Author’s contribution
M.B.W. wrote most of the manuscript and J.S.S. contributed additional writing and editing.
References
- Aguilaniu H, Gustafsson L, Rigouet M, Nystrom T. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science. 2003;299:1751–1753. doi: 10.1126/science.1080418. [DOI] [PubMed] [Google Scholar]
- Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Cohen H, Lin SS, Manchester JK, Gordon JI, Sinclair DA. Manipulation of a nuclear NAD+ salvage pathway delays aging without altering steady-state NAD+ levels. J Biol Chem. 2002;277:18881–18890. doi: 10.1074/jbc.M111773200. [DOI] [PubMed] [Google Scholar]
- Anderson RM, Latorre-Esteves M, Rute Neves A, Lavu S, Medvedik O, Taylor C, Howitz KT, Santos H, Sinclair DA. Yeast life-span extension by calorie restriction is independent of NAD fluctuation. Science. 2003;302:2124–2126. doi: 10.1126/science.1088697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, Bitterman KJ, Wood JG, Sinclair DA. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature. 2003;423:181–185. doi: 10.1038/nature01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedalov A, Hirao M, Posakony J, Nelson M, Simon JA. NAD+-dependent deacetylase Hst1p controls biosynthesis and cellular NAD+ levels in Saccharomyces cerevisiae. 2003;23:7044–7054. doi: 10.1128/MCB.23.19.7044-7054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenky P, Racette FG, Bogan KL, McClure JM, Smith JS, Brenner C. Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+ Cell. 2007;129:473–484. doi: 10.1016/j.cell.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Belenky P, Stebbins R, Bogan KL, Evans CR, Brenner C. Nrt1 and Tna1-independent export of NAD+ precursor vitamins promotes NAD+ homeostasis and allows engineering of vitamin production. PLoS One. 2011;6:e19710. doi: 10.1371/journal.pone.0019710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenky PA, Moga TG, Brenner C. Saccharomyces cerevisiae YOR071C encodes the high affinity nicotinamide riboside transporter Nrt1. J Biol Chem. 2008;283:8075–8079. doi: 10.1074/jbc.C800021200. [DOI] [PubMed] [Google Scholar]
- Bieganowski P, Pace HC, Brenner C. Eukaryotic NAD+ synthetase Qns1 contains an essential, obligate intramolecular thiol glutamine amidotransferase domain related to nitrilase. J Biol Chem. 2003;278:33049–33055. doi: 10.1074/jbc.M302257200. [DOI] [PubMed] [Google Scholar]
- Bieganowski P, Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell. 2004;117:495–502. doi: 10.1016/s0092-8674(04)00416-7. [DOI] [PubMed] [Google Scholar]
- Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast Sir2 and human SIRT1. J Biol Chem. 2002;277:45099–45107. doi: 10.1074/jbc.M205670200. [DOI] [PubMed] [Google Scholar]
- Brachmann CB, Sherman JM, Devine SE, Cameron EE, Pillus L, Boeke JD. The SIR2 gene family, conserved from bacteria to humans, functions in silencing, cell cycle progression, and chromosome stability. Genes Dev. 1995;9:2888–2902. doi: 10.1101/gad.9.23.2888. [DOI] [PubMed] [Google Scholar]
- Braunstein M, Rose AB, Holmes SG, Allis CD, Broach JR. Transcriptional silencing in yeast is associated with reduced nucleosome acetylation. Genes Dev. 1993;7:592–604. doi: 10.1101/gad.7.4.592. [DOI] [PubMed] [Google Scholar]
- Bryk M, Banerjee M, Murphy M, Knudsen KE, Garfinkel DJ, Curcio MJ. Transcriptional silencing of Ty1 elements in the RDN1 locus of yeast. Genes Dev. 1997;11:255–269. doi: 10.1101/gad.11.2.255. [DOI] [PubMed] [Google Scholar]
- Buck SW, Sandmeier JJ, Smith JS. RNA polymerase I propagates unidirectional spreading of rDNA silent chromatin. Cell. 2002;111:1003–1014. doi: 10.1016/s0092-8674(02)01193-5. [DOI] [PubMed] [Google Scholar]
- Burtner CR, Murakami CJ, Kennedy BK, Kaeberlein M. A molecular mechanism of chronological aging in yeast. Cell Cycle. 2009;8:1256–1270. doi: 10.4161/cc.8.8.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtner CR, Murakami CJ, Olsen B, Kennedy BK, Kaeberlein M. A genomic analysis of chronological longevity factors in budding yeast. Cell Cycle. 2011;10:1385–1396. doi: 10.4161/cc.10.9.15464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero A, Ugidos A, Liu B, Oling D, Kvint K, Hao X, Mignat C, Nachin L, Molin M, Nystrom T. Absence of mitochondrial translation control proteins extends life span by activating sirtuin-dependent silencing. Mol Cell. 2011;42:390–400. doi: 10.1016/j.molcel.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Casatta N, Porro A, Orlandi I, Brambilla L, Vai M. Lack of Sir2 increases acetate consumption and decreases extracellular pro-aging factors. Biochim Biophy Acta. 2013;1833:593–601. doi: 10.1016/j.bbamcr.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Celic I, Masumoto H, Griffith WP, Meluh P, Cotter RJ, Boeke JD, Verreault A. The Sirtuins Hst3 and Hst4p preserve genome integrity by controlling histone H3 lysine 56 deacetylation. Curr Biol. 2006;16:1280–1289. doi: 10.1016/j.cub.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Chan JN, Poon BP, Salvi J, Olsen JB, Emili A, Mekhail K. Perinuclear cohibin complexes maintain replicative life span via roles at distinct silent chromatin domains. Dev Cell. 2011;20:867–879. doi: 10.1016/j.devcel.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Cioci F, Vu L, Eliason K, Oakes M, Siddiqi IN, Nomura M. Silencing in yeast rDNA chromatin. Mol Cell. 2003;12:135–145. doi: 10.1016/s1097-2765(03)00262-4. [DOI] [PubMed] [Google Scholar]
- D'Mello NP, Jazwinski SM. Telomere length constancy during aging of Saccharomyces cerevisiae. J Bact. 1991;173:6709–6713. doi: 10.1128/jb.173.21.6709-6713.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang W, Steffen KK, Perry R, Dorsey JA, Johnson FB, Shilatifard A, Kaeberlein M, Kennedy BK, Berger SL. Histone H4 lysine 16 acetylation regulates cellular lifespan. Nature. 2009;459:802–807. doi: 10.1038/nature08085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defossez PA, Prusty R, Kaeberlein M, Lin S-J, Ferrigno P, Silver PA, Keil RL, Guarente L. Elimination of replication block protein Fob1 extends the life span of yeast mother cells. Mol Cell. 1999;3:447–455. doi: 10.1016/s1097-2765(00)80472-4. [DOI] [PubMed] [Google Scholar]
- Delaney JR, Sutphin GL, Dulken B, et al. Sir2 deletion prevents lifespan extension in 32 long-lived mutants. Aging Cell. 2011;10:1089–1091. doi: 10.1111/j.1474-9726.2011.00742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire MK, Weinstock KG, Strathern JN. HSTl, a new member of the SIR2 family of genes. Yeast. 1996;12:631–640. doi: 10.1002/(SICI)1097-0061(19960615)12:7%3C631::AID-YEA960%3E3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Donze D, Adams CR, Rine J, Kamakaka RT. The boundaries of the silenced HMR domain in Saccharomyces cerevisiae. Genes Dev. 1999;13:698–708. doi: 10.1101/gad.13.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draskovic I, Londono-Vallejo A. Telomere recombination and alternative telomere lengthening mechanisms. 18 ed. Frontiers in Biosciences; 2013. [DOI] [PubMed] [Google Scholar]
- Dubarry M, Loiodice I, Chen CL, Thermes C, Taddei A. Tight protein-DNA interactions favor gene silencing. Genes Dev. 2011;25:1365–1370. doi: 10.1101/gad.611011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easlon E, Tsang F, Dilova I, Wang C, Lu SP, Skinner C, Lin SJ. The dihydrolipoamide acetyltransferase is a novel metabolic longevity factor and is required for calorie restriction-mediated life span extension. J Biol Chem. 2006;282:6161–6171. doi: 10.1074/jbc.M607661200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easlon E, Tsang F, Skinner C, Wang C, Lin SJ. The malate-aspartate NADH shuttle components are novel metabolic longevity regulators required for calorie restriction-mediated life span extension in yeast. Genes Dev. 2008;22:931–944. doi: 10.1101/gad.1648308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erjavec N, Nystrom T. Sir2p-dependent protein segregation gives rise to a superior reactive oxygen species management in the progeny of Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2007;104:1087710881. doi: 10.1073/pnas.0701634104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essary BD, Marshall PA. Assessment of FUN-1 vital dye staining: Yeast with a block in the vacuolar sorting pathway have impaired ability to form CIVS when stained with FUN-1 fluorescent dye. J Microbiol Meth. 2009;78:208–212. doi: 10.1016/j.mimet.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Evans C, Bogan KL, Song P, Burant CF, Kennedy RT, Brenner C. NAD+ metabolite levels as a function of vitamins and calorie restriction: evidence for different mechanisms of longevity. BMC Chem Biol. 2010;10:2. doi: 10.1186/1472-6769-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio P, Gattazzo C, Battistella L, Wei M, Cheng C, McGrew K, Longo VD. Sir2 blocks extreme life-span extension. Cell. 2005;123:655–667. doi: 10.1016/j.cell.2005.08.042. [DOI] [PubMed] [Google Scholar]
- Fabrizio P, Liou L-L, Moy VN, Diaspro A, Selverstone Valentine J, Longo VD, Gralla EB. SOD2 functions downstream of Sch9 to extend longevity in yeast. Genetics. 2003;163:35–46. doi: 10.1093/genetics/163.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon AA, Aris JP. Plasmid accumulation reduces life span in Saccharomyces cerevisiae. J Biol Chem. 2003;278:41607–41617. doi: 10.1074/jbc.M307025200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritze CE, Verschueren K, Strich R, Esposito RE. Direct evidence for SIR2 modulation of chromatin structure in yeast rDNA. EMBO J. 1997;16:6495–6509. doi: 10.1093/emboj/16.21.6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo CM, Smith DL, Jr, Smith JS. Nicotinamide clearance by Pnc1 directly regulates Sir2-mediated silencing and longevity. Mol Cell Biol. 2004;24:1301–1312. doi: 10.1128/MCB.24.3.1301-1312.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganley AR, Ide S, Saka K, Kobayashi T. The effect of replication initiation on gene amplification in the rDNA and its relationship to aging. Mol Cell. 2009;35:683–693. doi: 10.1016/j.molcel.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Ghidelli S, Donze D, Dhillon N, Kamakaka RT. Sir2p exists in two nucleosome-binding complexes with distinct deacetylase activities. EMBO J. 2001;20:4522–4535. doi: 10.1093/emboj/20.16.4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb S, Esposito RE. A new role for a yeast transcriptional silencer gene, SIR2, in regulation of recombination in ribosomal DNA. Cell. 1989;56:771–776. doi: 10.1016/0092-8674(89)90681-8. [DOI] [PubMed] [Google Scholar]
- Gray JV, Petsko GA, Johnston GC, Ringe D, Singer RA, Werner-Washburne M. “Sleeping Beauty”: Quiescence in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2004;68:187–206. doi: 10.1128/MMBR.68.2.187-206.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha CW, Huh WK. Rapamycin increases rDNA stability by enhancing association of Sir2 with rDNA in Saccharomyces cerevisiae. Nucleic Acids Res. 2011;39:1336–1350. doi: 10.1093/nar/gkq895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachinohe M, Yamane M, Akazawa D, Ohsawa K, Ohno M, Terashita Y, Masumoto H. A reduction in age-enhanced gluconeogenesis extends lifespan. PLoS One. 2013;8:e54011. doi: 10.1371/journal.pone.0054011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halme A, Bumgarner S, Styles C, Fink GR. Genetic and epigenetic regulation of the FLO gene family generates cell-surface variation in yeast. Cell. 2004;116:405–415. doi: 10.1016/s0092-8674(04)00118-7. [DOI] [PubMed] [Google Scholar]
- Hecht A, Strahl-Bolsinger S, Grunstein M. Spreading of transcriptional repressor SIR3 from telomeric heterochromatin. Nature. 1996;385:92–96. doi: 10.1038/383092a0. [DOI] [PubMed] [Google Scholar]
- Henriksen P, Wagner SA, Weinert BT, Sharma S, Bacinskaja G, Rehman M, Juffer AH, Walther TC, Lisby M, Choudhary C. Proteome-wide analysis of lysine acetylation suggests its broad regulatory scope in Saccharomyces cerevisiae. Mol Cell Proteomics. 2012;11:1510–1522. doi: 10.1074/mcp.M112.017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman MA, Rusche LN. Substitution as a mechanism for genetic robustness: The duplicated deacetylases Hst1p and Sir2p in Saccharomyces cerevisiae. PLoS Genet. 2007;3:e126. doi: 10.1371/journal.pgen.0030126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe GJ, Tanny JC, Rudner AD, Gerber SA, Danaie S, Gygi SP, Moazed D. Steps in assembly of silent chromatin in yeast: Sir3-independent binding of a Sir2/Sir4 complex to silencers and role for Sir2-dependent deacetylation. Mol Cell Biol. 2002;22:4167–4180. doi: 10.1128/MCB.22.12.4167-4180.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012;13:225–238. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Moazed D. Association of the RENT complex with nontranscribed and coding regions of rDNA and a regional requirement for the replication fork block protein Fob1 in rDNA silencing. Genes Dev. 2003;17:2162–2176. doi: 10.1101/gad.1108403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Brito IL, Villen J, Gygi SP, Amon A, Moazed D. Inhibition of homologous recombination by a cohesin-associated clamp complex recruited to the rDNA recombination enhancer. Genes Dev. 2006;20:2887–2901. doi: 10.1101/gad.1472706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide S, Saka K, Kobayashi T. Rtt109 prevents hyper-amplification of ribosomal RNA genes through histone modificaiton in budding yeast. PLOS Genetics. 2013;9:1–14. doi: 10.1371/journal.pgen.1003410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- Ivy JM, Klar AJ, Hicks JB. Cloning and characterization of four SIR genes of Saccharomyces cerevisiae. Mol Cell Biol. 1986;6:688–702. doi: 10.1128/mcb.6.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JC, Wawryn J, Shantha Kumara HM, Jazwinski SM. Distinct roles of processes modulated by histone deacetylases Rpd3p, Hda1p, and Sir2p in life extension by caloric restriction in yeast. Experimental Gerontology. 2002;37:1023–1030. doi: 10.1016/s0531-5565(02)00064-5. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, Steffen KK, Hu D, Dang N, Kerr EO, Tsuchiya M, Fields S, Kennedy BK. Comment on “HST2 mediates SIR2-independent life-span extension by calorie restriction”. Science. 2006;312:1312. doi: 10.1126/science.1124608. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, Powers RW, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, Kirkland KT, Fields S, Kennedy BK. Sir2-independent life span extension by calorie restriction in yeast. PLoS Biol. 2004;2:e296. doi: 10.1371/journal.pbio.0020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BK, Gotta M, Sinclair DA, Mills K, McNabb DS, Murthy M, Pak SM, Laroche T, Gasser SM, Guarente L. Redistribution of silencing proteins from telomeres to the nucleolus is associated with extension of life span in S. cerevisiae. Cell. 1997;89:381–391. doi: 10.1016/s0092-8674(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Kennedy BK, Austriaco NR, Jr, Zhang J, Guarente L. Mutation in the silencing gene SIR4 can delay aging in S. cerevisiae. Cell. 1995;80:485–496. doi: 10.1016/0092-8674(95)90499-9. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Heck DJ, Nomura M, Horiuchi T. Expansion and contraction of ribosomal DNA repeats in Saccharomyces cerevisiae: requirement of replication fork blocking (Fob1) protein and the role of RNA polymerase I. Genes Dev. 1998;12:3821–3830. doi: 10.1101/gad.12.24.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Ganley AR. Recombination regulation by transcription-induced cohesin dissociation in rDNA repeats. Science. 2005;309:1581–1584. doi: 10.1126/science.1116102. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Ganley AR. Ribosomal DNA and cellular senescence: new evidence supporting the connection between rDNA and aging. FEMS Yeast Res. 2013 doi: 10.1111/1567-1364.12133. (in press). [DOI] [PubMed] [Google Scholar]
- Kucharczyk R, Zagulski M, Rytka J, Herbert CJ. The yeast gene YJR025c encodes a 3-hydroxyanthranilic acid dioxygenase and is involved in nicotinic acid biosynthesis. FEBS Lett. 1998;424:127–130. doi: 10.1016/s0014-5793(98)00153-7. [DOI] [PubMed] [Google Scholar]
- Kwan EX, Foss EJ, Tsuchiyama S, Alvino GM, Kruglyak L, Kaeberlein M, Raghuraman MK, Brewer BJ, Kennedy BK, Bedalov A. A natural polymorphism in rDNA replication origins links origin activation with calorie restriction and lifespan. PLoS Genet. 2013;9:e1003329. doi: 10.1371/journal.pgen.1003329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming DW, Lattore-Esteves M, Medvedik O, Wong SN, Tsang FA, Wang C, Lin S, Sinclair DA. HST2 mediates SIR2-independent life-span extension by calorie restriction. Science. 2005;309:1861–1864. doi: 10.1126/science.1113611. [DOI] [PubMed] [Google Scholar]
- Landry J, Slama JT, Sternglanz R. Role of NAD+ in the deacetylase activity of the SIR2- like proteins. Biochem Biophys Res Comm. 2000;278:685–690. doi: 10.1006/bbrc.2000.3854. [DOI] [PubMed] [Google Scholar]
- Laun P, Pichova A, Madeo F, Fuchs J, Ellinger A, Kohlwein S, Dawes I, Frohlich KU, Breitenbach M. Aged mother cells of Saccharomyces cerevisiae show markers of oxidative stress and apoptosis. Mol Microbiol. 2001;39:1166–1173. [PubMed] [Google Scholar]
- Li M, Petteys BJ, McClure JM, Valsakumar V, Bekiranov S, Frank EL, Smith JS. Thiamine biosynthesis in Saccharomyces cerevisiae is regulated by the NAD+-dependent histone deacetylase Hst1. Mol Cell Biol. 2010;30:3329–3341. doi: 10.1128/MCB.01590-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Valsakumar V, Poorey K, Bekiranov S, Smith JS. Genome-wide analysis of functional sirtuin chromatin targets in yeast. Genome Biol. 2013;14:R48. doi: 10.1186/gb-2013-14-5-r48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb JD, Liu X, Botstein D, Brown PO. Promoter-specific binding of Rap1 revealed by genome-wide maps of protein-DNA association. Nat Genet. 2001;28:327–334. doi: 10.1038/ng569. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Ford E, Haigis M, Liszt G, Guarente L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev. 2004;18:12–16. doi: 10.1101/gad.1164804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SJ, Kaeberlein M, Andalis AA, Sturtz LA, Defossez PA, Culotta VC, Fink GR, Guarente L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Defossez P, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- Lin YY, Lu JY, Zhang J, Walter W, Dang W, Wan J, Tao SC, Qian J, Zhao Y, Boeke JD, Berger SL, Zhu H. Protein acetylation microarray reveals that NuA4 controls key metabolic target regulating gluconeogenesis. Cell. 2009;136:1073–1084. doi: 10.1016/j.cell.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingner J, Hughes TR, Shevchenko A, Mann M, Lundblad V, Cech TR. Reverse transcriptase motifs in the catalytic subunit of telomerase. Science. 1997;276:561–567. doi: 10.1126/science.276.5312.561. [DOI] [PubMed] [Google Scholar]
- Liu B, Larsson L, Caballero A, Hao X, Öling D, Grantham J, Nyström T. The polarisome is required for segregation and retrograde transport of protein aggregates. Cell. 2010;140:257–267. doi: 10.1016/j.cell.2009.12.031. [DOI] [PubMed] [Google Scholar]
- Liu IC, Chiu SW, Lee HY, Leu JY. The histone deacetylase Hos2 forms an Hsp42- dependent cytoplasmic granule in quiescent yeast cells. Mol Biol Cell. 2012;23:1231–1242. doi: 10.1091/mbc.E11-09-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SP, Kato M, Lin SJ. Assimilation of endogenous nicotinamide riboside is essential for calorie restriction-mediated life span extension in Saccharomyces cerevisiae. J Biol Chem. 2009;284:17110–17119. doi: 10.1074/jbc.M109.004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SP, Lin SJ. Phosphate-responsive signaling pathway is a novel component of NAD+ metabolism in Saccharomyces cerevisiae. J Biol Chem. 2011;286:14271–14281. doi: 10.1074/jbc.M110.217885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundblad V, Szostak JW. A mutant with a defect in telomere elongation leads to senescence in yeast. Cell. 1989;57:633–643. doi: 10.1016/0092-8674(89)90132-3. [DOI] [PubMed] [Google Scholar]
- Maas NL, Miller KM, DeFazio LG, Toczyski DP. Cell cycle and checkpoint regulation of histone H3 K56 acetylation by Hst3 and Hst4. Mol Cell. 2006;23:109–119. doi: 10.1016/j.molcel.2006.06.006. [DOI] [PubMed] [Google Scholar]
- MacLean M, Harris N, Piper PW. Chronological lifespan of stationary phase yeast cells; a model for investigating the factors that might influence the ageing of postmitotic tissues in higher organisms. Yeast. 2001;18:499–509. doi: 10.1002/yea.701. [DOI] [PubMed] [Google Scholar]
- Maicher A, Kastner L, Dees M, Luke B. Deregulated telomere transcription causes replication-dependent telomere shortening and promotes cellular senescence. Nucleic Acids Res. 2012;40:6649–6659. doi: 10.1093/nar/gks358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matecic M, Smith DL, Pan X, Maqani N, Bekiranov S, Boeke JD, Smith JS. A microarray-based genetic screen for yeast chronological aging factors. PLoS Genet. 2010;6:e1000921. doi: 10.1371/journal.pgen.1000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure JM, Wierman MB, Maqani N, Smith JS. Isonicotinamide enhances Sir2 protein-mediated silencing and longevity in yeast by raising intracellular NAD+ concentration. J Biol Chem. 2012;287:20957–20966. doi: 10.1074/jbc.M112.367524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord R, Pierce M, Xie J, Wonkatal S, Mickel C, Vershon AK. Rfm1, a novel tethering factor required to recruit the Hst1 histone deacetylase for repression of middle sporulation genes. Mol Cell Biol. 2003;23:1–8. doi: 10.1128/MCB.23.6.2009-2016.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFaline-Figueroa JR, Vevea J, Swayne TC, Zhou C, Liu C, Leung G, Boldogh IR, Pon LA. Mitochondrial quality control during inheritance is associated with lifespan and mother-daughter age asymmetry in budding yeast. Aging Cell. 2011;10:885–895. doi: 10.1111/j.1474-9726.2011.00731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedik O, Lamming DW, Kim KD, Sinclair DA. MSN2 and MSN4 link calorie restriction and TOR to sirtuin-mediated lifespan extension in Saccharomyces cerevisiae. Plos Biol. 2007;5:e261. doi: 10.1371/journal.pbio.0050261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti P, Freeman K, Coodly L, Shore D. Evidence that a complex of SIR proteins interacts with the silencer and telomere-binding protein RAP1. Genes Dev. 1994;8:2257–2269. doi: 10.1101/gad.8.19.2257. [DOI] [PubMed] [Google Scholar]
- Mortimer RK, Johnston JR. Life span of individual yeast cells. Nature. 1959:1751–1752. doi: 10.1038/1831751a0. [DOI] [PubMed] [Google Scholar]
- Murray AW, Szostak JW. Pedigree analysis of plasmid segregation in yeast. Cell. 1983;34:961–970. doi: 10.1016/0092-8674(83)90553-6. [DOI] [PubMed] [Google Scholar]
- Nestelbachera R, Laun P, Vondrakova D, Pichova A, Schuller C, Breitenbach M. The influence of oxygen toxicity on yeast mother cell-specific aging. Exp Gerontol. 2000;35:63–70. doi: 10.1016/s0531-5565(99)00087-x. [DOI] [PubMed] [Google Scholar]
- Oppikofer M, Kueng S, Martino F, Soeroes S, Hancock SM, Chin JW, Fischle W, Gasser SM. A dual role of H4K16 acetylation in the establishment of yeast silent chromatin. EMBO J. 2011;30:2610–2621. doi: 10.1038/emboj.2011.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi I, Bettiga M, Alberghina L, Nyström T, Vai M. Sir2-dependent asymmetric segregation of damaged proteins in ubp10 null mutants is independent of genomic silencing. Biochim Biophys Acta. 2010;1803:630–638. doi: 10.1016/j.bbamcr.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Orozco H, Matallana E, Aranda A. Wine yeast sirtuins and Gcn5p control aging and metabolism in a natural growth medium. Mech Ageing Dev. 2012;133:348–358. doi: 10.1016/j.mad.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Parrella E, Longo VD. Insulin/IGF-I and related signaling pathways regulate aging in nondividing cells: from yeast to the mammalian brain. Scientific World Journal. 2010;10:161–177. doi: 10.1100/tsw.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrod S, Cockell MM, Laroche T, Renauld H, Ducrest A, Bonnard C, Gasser SM. A cytosolic NAD-dependent deacetylase, Hst2p, can modulate nucleolar and telomeric silencing in yeast. EMBO J. 2001;20:197–209. doi: 10.1093/emboj/20.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper PW. Long-lived yeast as a model for ageing research. Yeast. 2006;23:215–226. doi: 10.1002/yea.1354. [DOI] [PubMed] [Google Scholar]
- Radman-Livaja M, Ruben G, Weiner A, Friedman N, Kamakaka R, Rando OJ. Dynamics of Sir3 spreading in budding yeast: secondary recruitment sites and euchromatic localization. EMBO J. 2011;30:1012–1026. doi: 10.1038/emboj.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahat O, Maoz N, Cohen HY. Multiple Pathways Regulating the Calorie Restriction Response in Yeast. J Gerontol A Biol Sci Med Sci. 2011;66:163–169. doi: 10.1093/gerona/glq165. [DOI] [PubMed] [Google Scholar]
- Ralser M, Michel S, Breitenbach M. Sirtuins as regulators of the yeast metabolic network. Front Pharmacol. 2012;3:32. doi: 10.3389/fphar.2012.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reverter-Branchat G, Cabiscol E, Tamarit J, Sorolla MA, Angeles de la Torre M, Ros J. Chronological and replicative life-span extension in Saccharomyces cerevisiae by increased dosage of alcohol dehydrogenase 1. Microbiology. 2007;153:3667–3676. doi: 10.1099/mic.0.2007/009340-0. [DOI] [PubMed] [Google Scholar]
- Riesen ML, Morgan A. Calorie restriction reduces rDNA recombination independently of rDNA silencing. Aging Cell. 2009;8:624–632. doi: 10.1111/j.1474-9726.2009.00514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rine J, Herskowitz I. Four genes responsible for a position effect on expression from HML and HMR in Saccharomyces cerevisiae. Genetics. 1987;116:9–22. doi: 10.1093/genetics/116.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusche LN, Kirchmaier AL, Rine J. The establishment, inheritance, and function of silencing chromatin in. Annu. Rev. Biochem. 2003;72:481–516. doi: 10.1146/annurev.biochem.72.121801.161547. [DOI] [PubMed] [Google Scholar]
- Rusche LN, Kirchmaier AL, Rine J. Ordered nucleation and spreading of silenced chromatin in Saccharomyces cerevisiae. Mol Biol Cell. 2002;13:2207–2222. doi: 10.1091/mbc.E02-03-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi JS, Chan JN, Pettigrew C, Liu TT, Wu JD, Mekhail K. Enforcement of a lifespan-sustaining distribution of Sir2 between telomeres, mating-type loci, and rDNA repeats by Rif1. Aging Cell. 2013;12:67–75. doi: 10.1111/acel.12020. [DOI] [PubMed] [Google Scholar]
- Sandmeier JJ, Celic I, Boeke JD, Smith JS. Telomeric and rDNA Silencing in Saccharomyces cerevisiae are dependent on a nuclear NAD+ salvage pathway. Genetics. 2002;160:877–889. doi: 10.1093/genetics/160.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MT, Smith BC, Jackson MD, Denu JM. Coenzyme specificity of Sir2 protein deacetylases: implications for physiological regulation. J Biol Chem. 2004;279:40122–40129. doi: 10.1074/jbc.M407484200. [DOI] [PubMed] [Google Scholar]
- Shcheprova Z, Baldi S, Frei SB, Gonnet G, Barral Y. A mechanism for asymmetric segregation of age during yeast budding. Nature. 2008;454:728–734. doi: 10.1038/nature07212. [DOI] [PubMed] [Google Scholar]
- Shi L, Sutter BM, Ye X, Tu BP. Trehalose is a key determinant of the quiescent metabolic state that fuels cell cycle progression upon return to growth. Mol Biol Cell. 2010;21:1982–1990. doi: 10.1091/mbc.E10-01-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shia WJ, Osada S, Florens L, Swanson SK, Washburn MP, Workman JL. Characterization of the yeast trimeric-SAS acetyltransferase complex. J Biol Chem. 2005;280:11987–11994. doi: 10.1074/jbc.M500276200. [DOI] [PubMed] [Google Scholar]
- Shou W, Seol JH, Shevchenko A, Baskerville C, Moazed D, Chen ZW, Jang J, Shevchenko A, Charbonneau H, Deshaies RJ. Exit from mitosis is triggered by Tem1-dependent release of the protein phosphatase Cdc14 from nucleolar RENT complex. Cell. 1999;97:233–244. doi: 10.1016/s0092-8674(00)80733-3. [DOI] [PubMed] [Google Scholar]
- Sinclair DA, Guarente L. Extrachromosomal rDNA circles— A cause of aging in yeast. Cell. 1997;91:1033–1042. doi: 10.1016/s0092-8674(00)80493-6. [DOI] [PubMed] [Google Scholar]
- Smeal T, Claus J, Kennedy B, Cole F, Guarente L. Loss of transcriptional silencing causes sterility in old mother cells of S. cerevisiae. Cell. 1996;84:1–10. doi: 10.1016/s0092-8674(00)81038-7. [DOI] [PubMed] [Google Scholar]
- Smith DL, Jr, Li C, Matecic M, Maqani N, Bryk M, Smith JS. Calorie restriction effects on silencing and recombination at the yeast rDNA. Aging Cell. 2009;8:633–642. doi: 10.1111/j.1474-9726.2009.00516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DL, Jr, McClure JM, Matecic M, Smith JS. Calorie restriction extends the chronological lifespan of Saccharomyces cerevisiae independently of the Sirtuins. Aging Cell. 2007;6:649–662. doi: 10.1111/j.1474-9726.2007.00326.x. [DOI] [PubMed] [Google Scholar]
- Smith JS, Boeke JD. An unusual form of transcriptional silencing in yeast ribosomal DNA. Genes Dev. 1997;11:241–254. doi: 10.1101/gad.11.2.241. [DOI] [PubMed] [Google Scholar]
- Smith JS, Brachmann CB, Celic I, et al. A phylogenetically conserved NAD+-dependent protein deacetylase activity in the Sir2 protein family. Proc Nat Acad Sci USA. 2000;97:6658–6663. doi: 10.1073/pnas.97.12.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JS, Brachmann CB, Pillus L, Boeke JD. Distribution of a limited Sir2 protein pool regulates the strength of yeast rDNA silencing and is modulated by Sir4p. Genetics. 1998;149:1205–1219. doi: 10.1093/genetics/149.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen KK, Kennedy BK, Kaeberlein M. Measuring replicative life span in the budding yeast. J Vis Exp. 2009;28:1029. doi: 10.3791/1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl-Bolsinger S, Hecht A, Luo K, Grunstein M. SIR2 and SIR4 interactions differ in core and extended telomeric heterochromatin in yeast. Genes Dev. 1997;11:83–93. doi: 10.1101/gad.11.1.83. [DOI] [PubMed] [Google Scholar]
- Straight AF, Shou W, Dowd GJ, Turck CW, Deshaies RJ, Johnson AD, Moazed D. Net1, a Sir2-associated nucleolar protein required for rDNA silencing and nucleolar integrity. Cell. 1999;97:245–256. doi: 10.1016/s0092-8674(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Stumpferl SW, Brand SE, Jiang JC, Korona B, Tiwari A, Dai J, Seo JG, Jazwinski SM. Natural genetic variation in yeast longevity. Genome Res. 2012;22:1963–1973. doi: 10.1101/gr.136549.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda Y, Tachikawa H, Yokota A, Nakanishi H, Yamashita N, Miura Y, Takahashi N. Saccharomyces cerevisiae QNS1 codes for NAD+ synthestase that is functionally conserved in mammals. Yeast. 2003;20:995–1005. doi: 10.1002/yea.1008. [DOI] [PubMed] [Google Scholar]
- Suka N, Luo K, Grunstein M. Sir2p and Sas2p opposingly regulate acetylation of yeast histone H4 lysine16 and spreading of heterochromatin. Nat Genet. 2002;32:378–383. doi: 10.1038/ng1017. [DOI] [PubMed] [Google Scholar]
- Suka N, Suka Y, Carmen AA, Wu J, Grunstein M. Highly specific antibodies determine histone acetylation site usage in yeast heterochromatin and euchromatin. Mol Cell. 2001;8:473–479. doi: 10.1016/s1097-2765(01)00301-x. [DOI] [PubMed] [Google Scholar]
- Tahara EB, Cunha FM, Basso TO, Bianca Della BE, Gombert AK, Kowaltowski AJ. Calorie restriction hysteretically primes aging Saccharomyces cerevisiae toward more effective oxidative metabolism. PLoS One. 2013;8:e56388. doi: 10.1371/journal.pone.0056388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanny JC, Moazed D. Coupling of histone deacetylation to NAD breakdown by the yeast silencing protein Sir2: Evidence for acetyl transfer from substrate to an NAD breakdown product. Proc Natl Acad Sci USA. 2001;98:415–420. doi: 10.1073/pnas.031563798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang CK, Li H, Zheng XS. Nutrient starvation promotes condensin loading to maintain rDNA stability. EMBO J. 2007;26:1–11. doi: 10.1038/sj.emboj.7601488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya M, Dang N, Kerr EO, Hu D, Steffen KK, Oakes JA, Kennedy BK, Kaeberlein M. Sirtuin-independent effects of nicotinamide on lifespan extension from calorie restriction in yeast. Aging Cell. 2006;5:505–514. doi: 10.1111/j.1474-9726.2006.00240.x. [DOI] [PubMed] [Google Scholar]
- Wei M, Fabrizio P, Hu J, Ge H, Cheng C, Li L, Longo VD. Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, Sch9. PLoS Genet. 2008;4:e13. doi: 10.1371/journal.pgen.0040013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner-Washburne M, Braun E, Johnston GC, Singer RA. Stationary phase in the yeast Saccharomyces cerevisiae. Microbiol Biol Rev. 1993;57:383–401. doi: 10.1128/mr.57.2.383-401.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM, Le VQ, Zimmerman C, Marmorstein R, Pillus L. Nuclear export modulates the cytoplasmic Sir2 homologue Hst2. EMBO Rep. 2006;7:1247–1251. doi: 10.1038/sj.embor.7400829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Song L, Liu SQ, Huang D. A high throughput screening assay for determination of chronological lifespan of yeast. Exp Gerontol. 2011;46:915–922. doi: 10.1016/j.exger.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Xie J, Pierce M, Gailus-Durner V, Wagner M, Winter E, Vershon AK. Sum1 and Hst1 repress middle sporulation-specific gene expression during mitosis in Saccharomyces cerevisiae. EMBO J. 1999;18:6448–6454. doi: 10.1093/emboj/18.22.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Mostoslavsky R. SIRT6: a master epigenetic gatekeeper of glucose metabolism. Transcription. 2010;1:17–21. doi: 10.4161/trns.1.1.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Liu B, Carlsten JO, Beve J, Nystrom T, Myers LC, Gustafsson CM. Mediator influences telomeric silencing and cellular life span. Mol Cell Biol. 2011;31:2413–2421. doi: 10.1128/MCB.05242-11. [DOI] [PMC free article] [PubMed] [Google Scholar]