Abstract
Objectives
PAK5 and PAK6 are protein kinases highly expressed in the brain. Previously, we observed that Pak6 knockout mice gained significantly more weight during development than Pak5 knockout mice as well as wild-type controls and double-knockout mice lacking both Pak5 and Pak6. In this study, we assessed the effects of exercise on food intake and weight gain of these mice as well as their sensitivity to the stimulant effects of amphetamine.
Methods
Mice of each genotype were placed in cages with free access to run wheel exercise or in cages without run wheels for a total of 74 days. Food and fluid intake as well as body weight of each mouse were measured on a weekly basis. Finally, mice were given a high dose of amphetamine and activity levels were observed immediately thereafter for 90 minutes. Brains and testes of mice were assayed for protein levels of the estrogen alpha and progesterone receptors.
Results
While run wheel mice consumed significantly more food, they weighed less than non-run wheel mice. In addition, although Pak6 knockout mice consumed the same amount of food as wild-type mice, they were significantly heavier regardless of run wheel condition. Pak5 knockout mice were found to be more active than other genotypes after amphetamine treatment. Finally, protein levels of the progesterone and estrogen alpha receptors were altered in brain and testes of the Pak6 knockout mice.
Discussion
Collectively, these data suggest that PAK6 play a role in weight gain unrelated to exercise and caloric intake and that Pak5 knockout mice are more sensitive to the stimulant effects of amphetamine.
Keywords: PAK, Run wheel, Exercise, Body Weight, Amphetamine
Introduction
P21-activated kinases (PAKs) are serine/threonine kinases that bind to Rho GTPases Rac and Cdc42.1–3 In mammals, two families of PAKs (A and B) each contain three subtypes. Group A PAKs (PAK1, PAK2, and PAK3) are differentiated from group B PAKs (PAK4, PAK5, and PAK6) in that they contain several unique regulatory domains in addition to the common amino-terminal GTP-binding and carboxyl-terminal kinase domains.1–3 For group B PAKs, PAK4 is highly expressed in the prostate, testes and colon of humans, and lung, kidney, smooth muscle, testes, and pituitary of mice. PAK5 is expressed preferentially in the brain and to a lesser extent in adrenals, pancreas, prostate, and testes of humans and eyes, lung, and testes of mice. PAK6 is also highly expressed in brain, prostate, testes, thyroid, kidney, and placenta of humans and testes of mice.4–9
Signaling through steroid receptors has been shown to be important in controlling body weight.10,11 PAK6 appears to interact with androgen receptor signaling through a pathway independent of Rho GTpases.4,12 Binding of a ligand to the androgen receptor dissociates heat shock proteins from the complex and the receptor translocates to the nucleus.13 PAK6 antagonizes the transcriptional activity of both androgen and estrogen receptors (ERs) in a kinase-dependent manner that is independent of Cdc42 activation.4,12,14
To further investigate the function of these genes, knockout mice lacking Pak5, Pak6, and both Pak5 and Pak6 were generated. In a battery of behavioral tests, it was observed that Pak5/Pak6 double knockout (DKO) mice exhibited significantly lower activity levels as well as subtle deficits in learning and memory compared to their wild-type (WT) counterparts.9 Interestingly, Pak6 knockout mice became significantly heavier than the other genotypes. This suggests that deletion of the Pak6 gene may play a critical role in weight management and obesity.
We have previously shown that exercise alters body weight composition, increasing muscle mass, and decreasing body fat and that the mice that exercise will consume more food than non-exercising controls.15,16 In addition, voluntary run wheel exercise decreased omental fat pad and retroperitoneal fat weight compared to non-exercise controls.16 Exercise in some cases can override a genetic predisposition to obesity as it was shown for MC4R knockout mice.17 In that study, mice given access to run wheels had lower body weights after two weeks than knockout mice without run wheels. In addition, the knockout mice with exercise had significantly lower body fat mass than knockout mice without exercise.17 Because the Pak5/Pak6 double knockout mice and the Pak6 knockout mice have been shown to have increased body weight compared to WT mice, the first objective of this study was to assess the effects of exercise on food consumption and body weight gain of Pak5, Pak6, and Pak5/Pak6 double knockout mice relative to non-exercising controls. This was done to determine whether the cause of the increased body weight could be due to increased food consumption or a lack of movement and exercise and to determine whether the increased body weight of the Pak6 and Pak5/Pak6 knockout mice could be ameliorated by exercise. In addition, we have previously assessed the interaction between exercise, body weight, and sensitivity to the stimulant effects of amphetamine in BALBc mice.18 While exercise was not found to protect against the toxic effects of amphetamine, only one strain of mouse was evaluated. Following exposure to amphetamine mice have been shown to have increased self-injurious behavior and stereotypic behaviors.19 Differential effects of amphetamine and/or exercise has not yet been evaluated in PAK knockout mice. Given that the double knockout mice appear to have altered brain functioning and locomotor deficits, as seen in prior publications, a second objective was to assess the sensitivity of these genotypes to the activity-increasing effects induced by high-dose administration of amphetamine and determine whether the deficits in locomotion can be affected by amphetamine. Finally, in an effort to explore a possible underlying mechanism that might account for the increased weight gain observed in Pak6 knockout mice, protein levels of the progesterone and estrogen alpha receptors were examined in brain and testes of these mice.
Materials and methods
Genotyping
Verification of genotypes of mice was carried out by polymerase chain reaction (PCR) of samples of genomic DNA isolated from the tails. Genotyping by PCR for Pak5 allele was completed as described by Li and Minden8 and for Pak6 allele as described in Nekrasova et al.9
Mice
Pak5 knockout, Pak6 knockout, and Pak5/ Pak6 DKO mice were bred in the Laboratory for Cancer Research, Rutgers University.7,9 Pak5 and Pak6 knockout mice were backcrossed to C57BL/6J strain nine times and contained 99.8% of C57BL/6J genes. DKO and WT mice were generated from the intermediate cross and both contained 87.5% of C57BL/ 6J background genes. All mice are now available from the Jackson Laboratory: Pak5 KO mice – stock 015827; Pak6 KO mice – stock 015826; Pak5/Pak6 DKO mice – stock 015825. Mice of each genotype (Pak5−/−, Pak6−/−, Pak5−/−/Pak6−/−, and WT) were maintained as a separate colony and were randomly intercrossed. Forty male mice were evaluated; 7 WT, 10 Pak5 knockouts, 12 Pak6 knockouts, and 11 double knockouts. All mice were individually housed in a temperature-controlled colony room on a 12-hour on/12-hour off light cycle in polypropylene cages (48 cm × 27 cm × 20 cm) with or without run wheels. Body weights were recorded weekly. All data were collected between 49 and 140 days of age.
Food and fluid consumption
Subjects had free access to food and water throughout the course of the study. The amount of food consumed was measured once a week and the amount of fluid consumed was measured twice per week.
Run wheel
Twenty mice were housed in run wheel cages: four WTs, five Pak5 knockouts, six Pak6 knockouts, and five double knockouts. The mice were placed in run wheel cages at 7 weeks of age and continued in those cages throughout testing to 20 weeks of age. Run wheels were 7.0 cm in width and 16.5 cm in diameter for an inner circumference of 51.8 cm. Each run wheel was attached to a counter that registered the number of wheel rotations. Mice in run wheel cages had free access to run wheels. Every morning the number of wheel rotations was recorded and the counter was reset.
Amphetamine challenge
A separate set of mice of each genotype, 1-year-old, +/−1 month, were used for the amphetamine challenge. They were administered a subcutaneous injection of either saline or amphetamine at 50 mg/kg. Four WT mice, three Pak5 knockout mice, three Pak6 knockout mice, and three Pak5/Pak6 double knockout mice received amphetamine, while three of each genotype received saline. They were placed in activity chambers with infrared sensors placed approximately 7 cm apart and 2.5 cm above the floor of the chamber. Beam breaks were recorded for 90 minutes after injection.
Western blot analysis
Western blot analysis was performed on lysates of mouse tissues as described in Nekrasova et al.9 For detection of proteins tissues were isolated from three different mice of the same genotype and pooled together. Rabbit monoclonal antibodies for ER alpha detection (clone SP1, 1:1000) and rabbit monoclonal antibodies for progesterone receptor (PR) detection (clone SP2, 1:1000) were from Lab Vision. Anti-actin mouse monoclonal antibodies were from Sigma (clone AC-40, 1:1000). Protein quantification was performed by densitometry of bands using ImageJ software and data were normalized by beta-actin loading for the same samples. Quality of the lysates was confirmed by the presence of actin protein. Sharp bands of 67 KDa (size of murine ERalpha) and 81KDa (size of murine PR) detected in the testes of mice served as a quality control for expression and detection of ER) alpha and PR in tested tissues.
Statistical analysis
The results of the experiments were analyzed using repeated measures analysis of variance (ANOVA) for all behavioral experiments. When significant main effects were found, Fisher’s least significant difference post hoc comparisons were also conducted.
Results
Weight
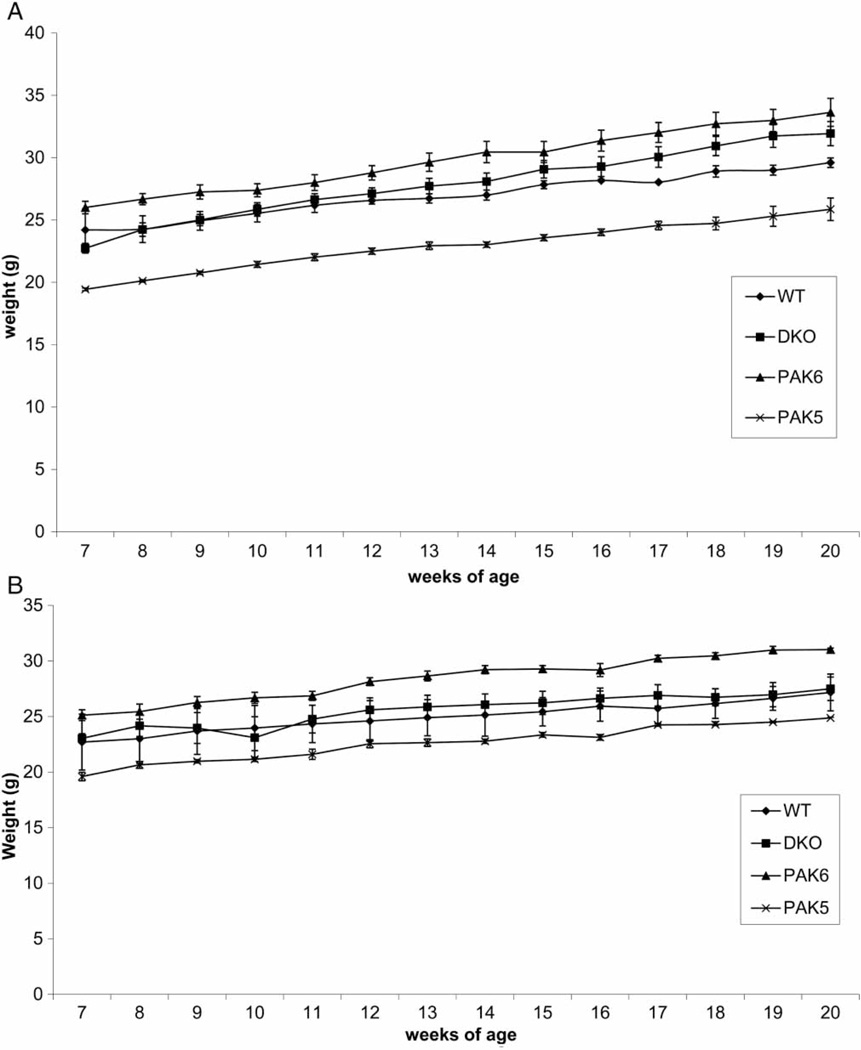
Body weights were taken weekly starting at 7 weeks of age and ending at 20 weeks of age. In the no run wheel condition, a repeated measures one-way ANOVA revealed an overall effect of genotype on weight (F(3,39) = 25.899, P < 0.0001) (Fig. 1a). Post hoc analysis revealed that Pak5 knockout mice weighed significantly less than DKO (P = 0.0002), Pak6 (P < 0.0001), and WT mice (P = 0.0069). In the run wheel condition, a repeated measures one-way ANOVA revealed a significant effect of genotype on weight (F(3,39) = 10.988, P = 0.0012) (Fig. 1b). Post hoc tests revealed that the Pak6 knockout mice weighed significantly more than the Pak5 knockout mice (P = 0.0013).
Figure 1.
(A) Body weight – no run wheel: Body weight in grams over 14 weeks of the study. In the no run wheel condition, there was an overall effect of genotype, where Pak5-knockout mice weighed significantly less than Pak6 and double-knockout (DKO) mice as well as wild type (WT). (B) Body weight – run wheel condition: In the run wheel condition, the Pak6 knockout mice were shown to be significantly heavier than the Pak5 knockout mice over the length of the study.
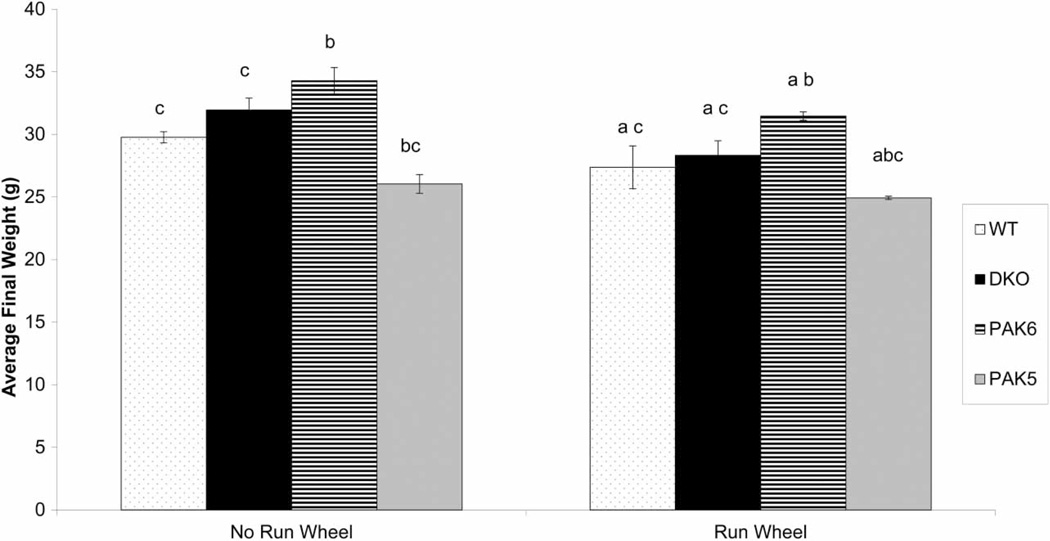
Final body weights were also taken on the day of sacrifice. All genotypes in the run wheel condition weighed significantly less than the non-run wheel controls (F(1,28) = 13.420, P = 0.001). In addition, there was an effect of genotype on weight (F(3,28) = 23.694, P ≤ 0.0001). Post hoc analysis revealed that Pak6 knockout mice were significantly heavier than all the other genotypes (DKO (P = 0.0072), WT (P = 0.0001), and Pak5 (P ≤ .0001)). The DKO mice weighed significantly more than the Pak5 mice (P < 0.0001) and significantly less than Pak6 knockout mice; however, there was no significant difference between the WT mice and the DKO mice weights. The Pak5 knockout mice weighed significantly less than all other genotypes (Pak6 (P < 0.0001), DKO (P < 0.0001), WT (P = 007)) (Fig. 2).
Figure 2.
Final body weight of all subjects. The mice in the run wheel condition weighed significantly less than those in the no run wheel condition (a = P < 0.05 compared to no run wheel). The Pak6 and Pak5 knockout mice weighed significantly different than the WT mice (b = P < 0.05 compared to WT). The WT, DKO, and Pak5 mice all weighed significantly less than the Pak6 knockout mice (c = P < 0.05 compared to Pak6).
Food consumption
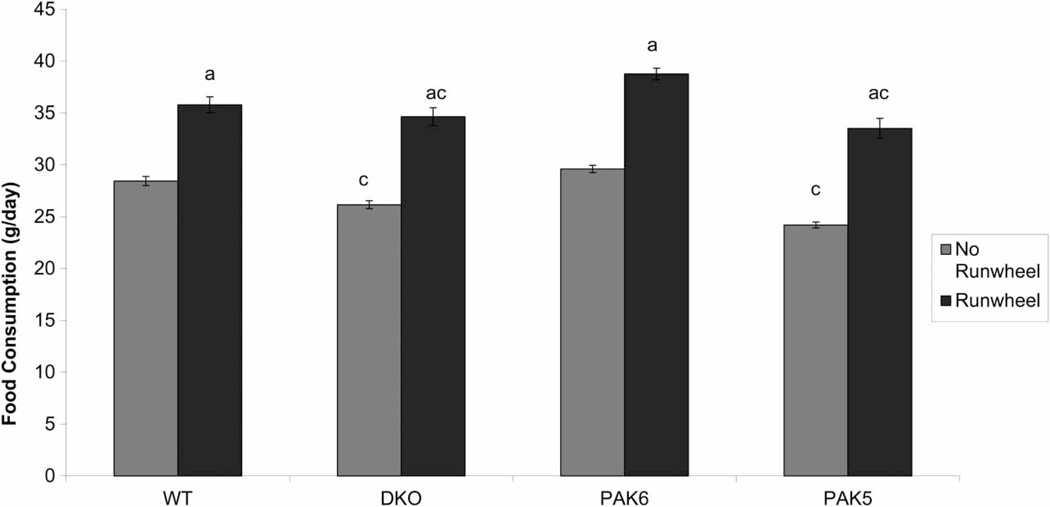
A repeated measures ANOVA was run on food consumption data. All genotypes in run wheel cages consumed significantly more food than the non-run wheel controls (F(1,227) = 101.593, P ≤ 0.0001). There was an effect of genotype on overall food consumption (F(3,27) = 8.711, P = 0.0003). Post hoc tests showed the Pak6 mice ate significantly more than the Pak5 (P = 0.0004) and the DKO (P = 0.0019), but did not differ in the amount of food they consumed as compared to the WT mice (Fig. 3).
Figure 3.
Comparison of food intake between run wheel and no run wheel condition across genotypes. The animals in the run wheel condition ate significantly more than those in the no run wheel condition (a = P < 0.05 compared to no run wheel). The DKO and Pak5 mice ate significantly less than the Pak6 mice (c = P < 0.05 compared to PAK6).
Fluid consumption
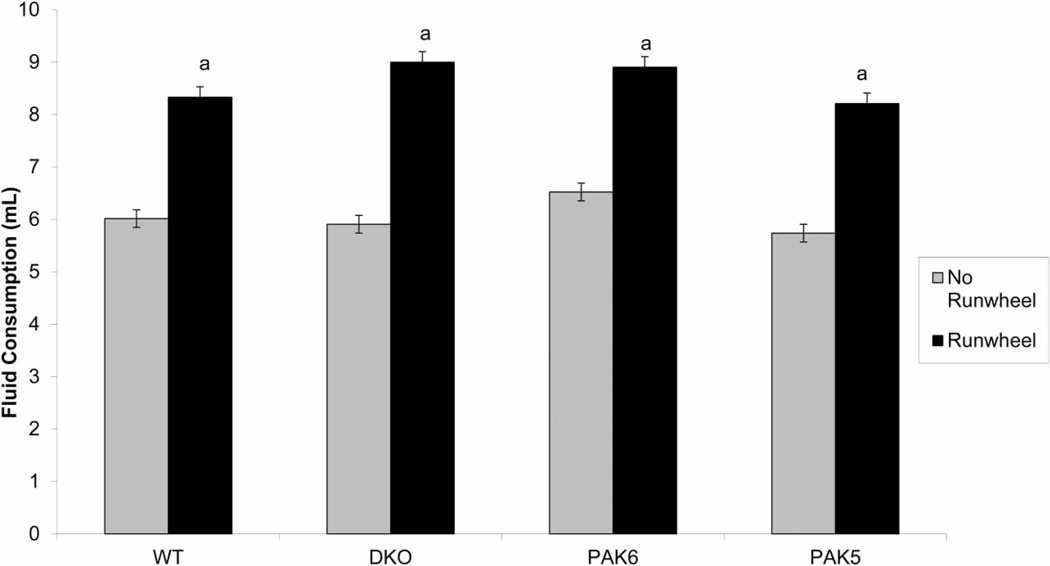
A repeated measures ANOVA was run on the fluid consumption data. All genotypes in run wheel cages consumed significantly more water than their non-run wheel counterparts (F(1, 29) = 82.903, P ≤ 0.001). There was no significant difference of fluid consumption between genotypes (Fig. 4).
Figure 4.
Comparison of fluid consumption between run wheel and no run wheel condition. The mice in the run wheel condition consumed significantly more water than those in the no run wheel condition (a = P < 0.05 compared to no run wheel).
Run wheel
A repeated measures ANOVA was run on the run wheel data. The data were averaged over 9 weeks of run wheel recordings. There was no significant difference in the number of run wheel rotations across genotypes (F(3,584) = 0.081, P = 0.9687). The WT mice ran on average 10.7 (±0.6) km per 24 hours; Pak6 mice ran on average 10.0 (±0.9) km per 24 hours; Pak5 mice ran on average 9.7 (±0.6) km per 24 hours; and the double knockout mice ran on average 9.9 (±1.2) km per 24 hours.
Amphetamine challenge
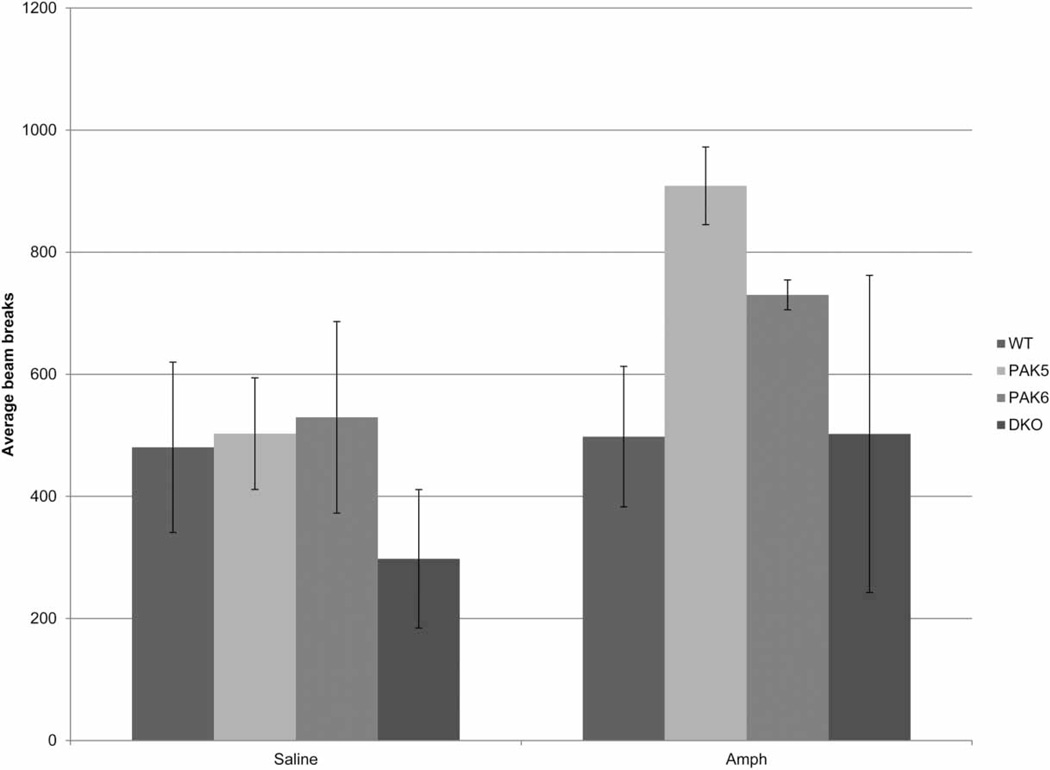
A repeated measures ANOVA for the average total activity over 90 minutes following amphetamine challenge revealed no overall effect of genotype (F(3,15) = 1.570, P = 0.2380), no overall effect of treatment (F(1,15) = 3.853, P = 0.0685), and no overall interaction of genotype by treatment (F(3,15) = 0.0586, P = 0.6334) (Fig. 5).
Figure 5.
Amphetamine-induced increase in locomotor activity. Pak5 mice were significantly more active following amphetamine administration as compared to following saline.
Expression of steroid receptors in the tissues of WT and Pak6−/− mice
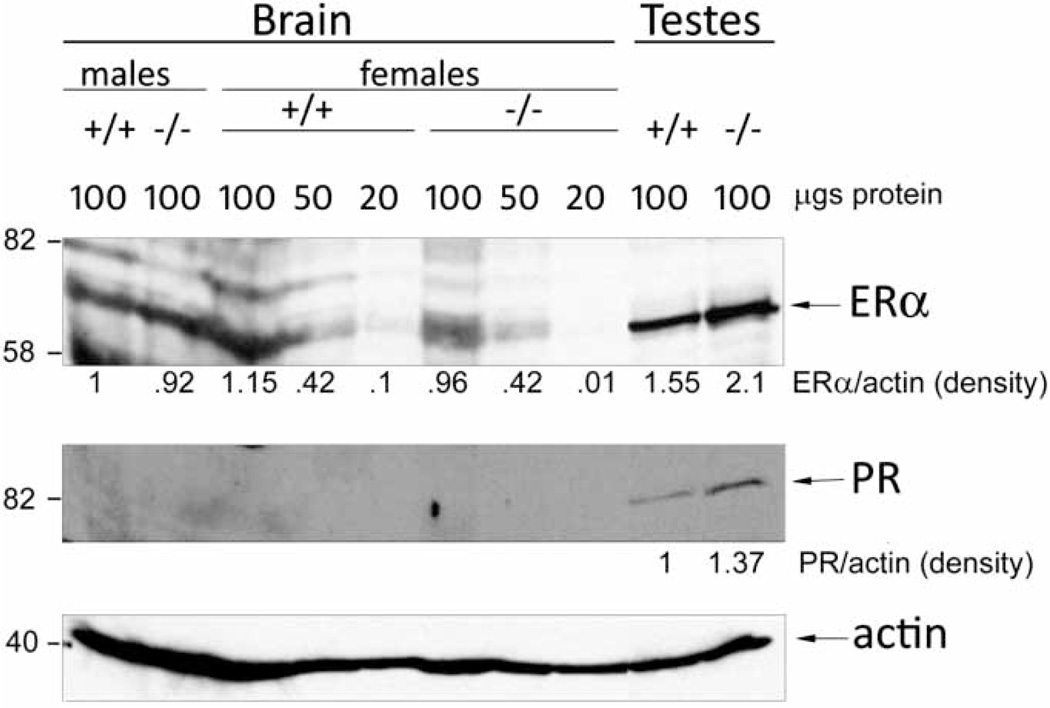
To gain an insight into the mechanism of increased body weight in Pak6−/− mice, we analyzed protein levels of ER alpha and PR in the tissues of mice of both genotypes by western blotting. expression of ER alpha was decreased in the brains of Pak6−/− mice; however, in the testes of Pak6 knockout mice amounts of ER alpha and PR proteins were increased (Fig. 6). Tissues from three mice of the same genotype were pooled together for western blot analysis therefore our results represent average expression of the protein in the brains and testes for three male WT and three male Pak6 null mice, and for three female WT and three female Pak6null mice. N = 3 animals for every lane.
Figure 6.
Western blot analysis of estrogen receptor alpha (ERα) and progesterone receptor (PR) expression in the brains and testes of adult wild type (+/+) and Pak6null (−/−) mice. Lysates were prepared from three mice of the same genotypes. Pooled tissue samples of the same genotype were separated on 7% acrylamide gel, transferred to immobilon membrane and probed with antibodies. Quantification data were normalized by beta-actin loading for the same samples. Sizes of protein markers are indicated on the left side of each blot.
Discussion
In our prior study, Pak6 knockout mice were shown to be significantly heavier than Pak5 knockouts, Pak5/Pak6 double knockouts and WT mice.9 In this study, this finding was replicated. Previous studies in our lab have shown that body weight and fat composition can be reduced with exercise.16,18 In this study, groups of mice from each of the four genotypes were given free access to run wheels and their activity levels were recorded daily. As we had previously observed using other strains, mice of all four genotypes in the run wheel condition weighed significantly less than their non-run wheel counterparts. Although there was no significant difference in the amount of exercise, Pak6 null mice, weighed significantly more than mice of all other genotypes including those in the non-run wheel conditions. While there was a significant difference in water consumption between the run wheel and non-run wheel conditions, there were no differences in water consumption across genotypes. Furthermore, although the Pak6 knockout mice consumed significantly more food than Pak5 knockout and DKO mice, they consumed the same amount of food as WT animals, though they remained heavier throughout the study. Therefore, food consumption, fluid consumption, and total amount of run wheel activity cannot account for the overall increase in weight seen in Pak6 knockout mice. Finally, in this study, we observed that Pak5 knockout mice consumed significantly less food than Pak6 knockout and WT animals. The Pak5 knockout mice did not differ in food consumption from the DKO mice. The Pak5 knockout mice were also significantly lighter than all other genotypes, even though they had the same water consumption and run wheel use. It is interesting to note that the double knockout mice show a normal body weight, while the Pak6 single knockout mice show an increase in weight. This may indicate a regulatory balancing of body weight normally controlled by PAK5 and PAK6. However, this awaits further investigation as the underlying mechanism of the differences observed remains unknown.
Subjects in this study were all males. PAK6 has been shown to have an important role in androgen receptor signaling, inhibiting its transcriptional activity.4,12 The inhibition of the transcriptional activity alters testosterone’s effect on body fat content.10,11 In obese men, serum testosterone levels are lower. Androgen receptor knockout out mice have been shown to have increased body weight caused by enhanced adiposity.11 Thus, PAK6’s ability to inhibit the androgen receptor may be a factor in the increased weight observed in the Pak6 knockout mice. Interestingly, male and female mice with a targeted deletion in the ERα gene also have increased adiposity. While the cause of the obesity is unclear, overeating does not appear to be essential and the increased adiposity in ER alpha knockout mice is attributed primarily to decreased energy expenditure.20,21 In this study, we found a decrease in the amount of ER alpha in the brains of Pak6 knockout mice, which may have contributed to the weight increase in these mice.
We had previously demonstrated that the Pak5/Pak6 double knockout mice showed alteration in locomotor activity.9 Here we sought to determine whether amphetamine-induced hyperactivity would be altered in the single knockout mice. While no significant effect of amphetamine was found in any of the genotypes, the effect approached significance. There was a general trend whereby amphetamine-treated mice had an increase in activity compared to the saline-treated mice. The WT mice were not shown to have an increase in activity following the high dose of amphetamine. The activity chambers that were used evaluate horizontal movement; however, stereotypic behaviors induced by amphetamine are not recorded by these chambers because the movement occurs without horizontal movement. Therefore, the WT mice may have had increased activity levels as a result of amphetamine treatment, but that activity was stereotypic rather than horizontal. Further evaluation of the effects of amphetamine in the Pak5, Pak6, and Pak5/Pak6 knockout mice should be done to determine whether there is a differential sensitivity. These tests should include a measure of self-injurious behavior and a neurochemical assay to determine whether the dose of amphetamine given caused long-term damage to the striatum as amphetamine has been reported to show in the literature.18,19
Obesity is a major health problem around the world. The Pak6 knockout mice may serve as a novel model of this prevalent disease. The full understanding of why these knockout mice remain heavier than other genotypes, even with exercise, may help answer some of questions of obesity in humans.
Acknowledgements
This study was supported, in part, by ES007148 and ES05022.
References
- 1.Sells MA, Chernoff J. Emerging from the Pak: the p21-activated protein kinase family. Trends Cell Biol. 1997;7:162–167. doi: 10.1016/S0962-8924(97)01003-9. [DOI] [PubMed] [Google Scholar]
- 2.Knaus UG, Bokoch GM. The p21Rac/Cdc42-activated kinases (PAKs) Int J Biochem Cell Biol. 1998;30:857–862. doi: 10.1016/s1357-2725(98)00059-4. [DOI] [PubMed] [Google Scholar]
- 3.Daniels RH, Bokoch GM. p21-activated protein kinase: a crucial component of morphological signaling? Trends Biochem Sci. 1999;24:350–355. doi: 10.1016/s0968-0004(99)01442-5. [DOI] [PubMed] [Google Scholar]
- 4.Lee SR, Ramos SM, Ko A, Masiello D, Swanson KD, Lu ML, et al. AR and ER interaction with a p21-activated kinase (PAK6) Mol Endocrinol. 2002;16:85–99. doi: 10.1210/mend.16.1.0753. [DOI] [PubMed] [Google Scholar]
- 5.Jaffer ZM, Chernoff J. p21-activated kinases: three more join the Pak. Int J Biochem Cell Biol. 2002;34:713–717. doi: 10.1016/s1357-2725(01)00158-3. [DOI] [PubMed] [Google Scholar]
- 6.Pandey A, Dan I, Kristiansen TZ, Watanabe NM, Voldby J, Kajikawa E, et al. Cloning and characterization of PAK5, a novel member of mammalian p21-activated kinase-II subfamily that is predominantly expressed in brain. Oncogene. 2002;21:3939–3948. doi: 10.1038/sj.onc.1205478. [DOI] [PubMed] [Google Scholar]
- 7.Qu J, Li X, Novitch BG, Zheng Y, Kohn M, Xie JM, et al. PAK4 kinase is essential for embryonic viability and for proper neuronal development. Mol Cell Biol. 2003;23:7122–7133. doi: 10.1128/MCB.23.20.7122-7133.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Minden A. Targeted disruption of the gene for the PAK5 kinase in mice. Mol Cell Biol. 2003;23:7134–7142. doi: 10.1128/MCB.23.20.7134-7142.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nekrasova T, Jobes ML, Ting JH, Wagner GC, Minden A. Targeted disruption of the PAK5 and PAK6 genes in mice leads to deficits in learning and locomotion. Dev Biol. 2008;322(1):95–108. doi: 10.1016/j.ydbio.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Zitzmann M, Gromoll J, von Eckardstein A, Nieschlag E. The CAG repeat polymorphism in the androgen receptor gene modulates body fat mass and serum concentrations of leptin and insulin in men. Diabetologia. 2003;46:31–39. doi: 10.1007/s00125-002-0980-9. [DOI] [PubMed] [Google Scholar]
- 11.Fan W, Yanase T, Nomura M, Okabe T, Goto K, Sato T, et al. Androgen receptor null male mice develop late-onset obesity caused by decreased energy expenditure and lipolytic activity, but show normal insulin sensitivity with high adiponectin secretion. Diabetes. 2005;54:1000–1008. doi: 10.2337/diabetes.54.4.1000. [DOI] [PubMed] [Google Scholar]
- 12.Yang F, Li X, Sharma M, Zarnegar M, Lim B, Sun Z. Androgen receptor specifically interacts with a novel p21-activated kinase, PAK6. J Biol Chem. 2001;276:15345–15353. doi: 10.1074/jbc.M010311200. [DOI] [PubMed] [Google Scholar]
- 13.Roy AK, Lavrovsky Y, Song CS, Chen S, Jung MH, Velu NK, et al. Regulation of androgen action. Vitam Horm. 1999;55:309–352. doi: 10.1016/s0083-6729(08)60938-3. [DOI] [PubMed] [Google Scholar]
- 14.Schrantz N, da Silva Correia J, Fowler B, Ge Q, Sun Z, Bokoch GM. Mechanism of p21-activated kinase 6-mediated inhibition nof androgen receptor signaling. J Biol Chem. 2004;279(3):1922–1931. doi: 10.1074/jbc.M311145200. [DOI] [PubMed] [Google Scholar]
- 15.Michna L, Lu YP, Lou YR, Wagner GC, Conney AH. Stimulatory effect of oral administration of green tea and caffeine on locomotor activity in SKH-1 mice. Life Sci. 2003;73:1383–1392. doi: 10.1016/s0024-3205(03)00468-5. [DOI] [PubMed] [Google Scholar]
- 16.Ju J, Nolan B, Cheh M, Bose M, Lin Y, Wagner GC, et al. Voluntary exercise inhibits intestinal tumorigenesis in ApcMin/+ mice and azoxymethane/dextran sulfate sodium-treated mice. BMC Cancer. 2008;8:316–323. doi: 10.1186/1471-2407-8-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haskell-Luevano C, Schaub JW, Andreasen A, Haskell KR, Moore MC, Koerper LM, et al. Voluntary exercise prevents the obese and diabetic metabolic syndrome of the melanocortin-4 receptor knockout mouse. FJ Express. 2008;10:1096. doi: 10.1096/fj.08-109686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlson KM, Wagner GC. Voluntary exercise and tail shock have differential effects on amphetamine-induced dopaminergic toxicity in adult BALB/c mice. Behav Pharmacol. 2006;17(5–6):475–484. doi: 10.1097/00008877-200609000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Kita T, Wagner GC, Nakashima T. Current research on methamphetamine-induced neurotoxicity: animal models of monoamine disruption. J Pharmacological Sci. 2003;92:178–195. doi: 10.1254/jphs.92.178. [DOI] [PubMed] [Google Scholar]
- 20.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci USA. 2000;97(23):12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohlsson C, Hellberg N, Parini P, Vidal O, Bohlooly YM, Rudling M, et al. Obesity and disturbed lipoprotein profile in estrogen receptor-alpha-deficient male mice. Biochem Biophys Res Commun. 2000;278(3):640–645. doi: 10.1006/bbrc.2000.3827. [DOI] [PubMed] [Google Scholar]