Abstract
This study determined if the pupillary light reflex (PLR) driven by brief stimulus presentations can be accounted for by the product of stimulus luminance and area (i.e., corneal flux density, CFD) under conditions biased toward the rod, cone, and melanopsin pathways. Five visually normal subjects participated in the study. Stimuli consisted of 1-s short- and long-wavelength flashes that spanned a large range of luminance and angular subtense. The stimuli were presented in the central visual field in the dark (rod and melanopsin conditions) and against a rod-suppressing short-wavelength background (cone condition). Rod- and cone-mediated PLRs were measured at the maximum constriction after stimulus onset whereas the melanopsin-mediated PLR was measured 5–7 s after stimulus offset. The rod- and melanopsin-mediated PLRs were well accounted for by CFD, such that doubling the stimulus luminance had the same effect on the PLR as doubling the stimulus area. Melanopsin-mediated PLRs were elicited only by short-wavelength, large (>16°) stimuli with luminance greater than 10 cd/m2, but when present, the melanopsin-mediated PLR was well accounted for by CFD. In contrast, CFD could not account for the cone-mediated PLR because the PLR was approximately independent of stimulus size but strongly dependent on stimulus luminance. These findings highlight important differences in how stimulus luminance and size combine to govern the PLR elicited by brief flashes under rod-, cone-, and melanopsin-mediated conditions.
Keywords: pupillometry, spatial summation, rods, cones, melanopsin
Introduction
Several factors affect human pupil size, including the level of retinal illuminance (Bouma, 1962; Crawford, 1936; McDougal & Gamlin, 2010), the accommodative state of the eye (Campbell, 1957; Marg & Morgan, 1949), and age (Watson & Yellott, 2012; Winn, Whitaker, Elliott, & Phillips, 1994) as well as emotional conditions (Bradley, Miccoli, Escrig, & Lang, 2008; Hess & Polt, 1960). The effect of illumination characteristics on pupil size has been most widely studied by varying the luminance, size, and wavelength of a steady adapting field. The effect of varying these characteristics has been described using various relationships that permit the diameter of the pupil to be predicted under conditions of steady illumination (see Watson & Yellott, 2012, for a review). There is general agreement that under steady illumination, the diameter of the pupil is primarily dependent on the product of adapting field luminance and area, referred to as corneal flux density (CFD) (Atchison et al., 2011; Crawford, 1936; Stanley & Davies, 1995). That is, doubling the area of the adapting field has the same effect on pupil diameter as doubling the luminance of the adapting field. However, the extent to which CFD accounts for pupil size under conditions in which retinal illuminance varies (e.g., brief flashes) has not been widely studied.
Recently, paradigms have been introduced that assess pupil size across a range of flash durations rather than only in response to a steady adapting field (e.g., McDougal & Gamlin, 2010; Park et al., 2011). These paradigms have been used to assess the contributions of the photoreceptor pathways that govern pupil size. Specifically, the response of the pupil, the pupillary light reflex (PLR), is largely mediated by intrinsically photosensitive retinal ganglion cells (ipRGCs) that contain the photopigment melanopsin (Guler et al., 2008). In addition to being intrinsically photosensitive, the ipRGCs receive input from rod and cone photoreceptors (Dacey et al., 2005). Consequently, the PLR can be a complex response with contributions from more than one receptor type (Barrionuevo et al., 2014; McDougal & Gamlin, 2010; Park et al., 2011).
The relative contributions of the three receptor types to the PLR have been examined by manipulating the characteristics of large-field (≈90°) flash stimuli and the adaptation conditions (light vs. dark adapted) (Park et al., 2011). For example, high-luminance, long-wavelength (red) flashes presented against a rod-suppressing adapting field elicit a PLR that is predominately cone-mediated whereas low-luminance, short-wavelength (blue) flashes presented to the dark-adapted eye elicit a PLR that is primarily rod-mediated. For high-luminance, short-wavelength flashes presented to the dark-adapted eye, there is an initial transient pupil constriction (rod- and cone-mediated) that is followed by a melanopsin-mediated sustained constriction that can last for more than 30 s after stimulus offset. The prolonged melanopsin-mediated constriction has been referred to as both the “sustained pupil response” (Gamlin et al., 2007; Kardon et al., 2009; Park et al., 2011) and the “postillumination pupil response” (Feigl et al., 2012; Kankipati, Girkin, & Gamlin, 2010), and this response has been used in clinical protocols to assess inner-retina function (Kawasaki, Collomb, Leon, & Munch, 2014; Kawasaki, Crippa, Kardon, Leon, & Hamel, 2012; Kawasaki, Munier, Leon, & Kardon, 2012; Moura et al., 2013; Park et al., 2011).
The goal of the present study was to examine the spatial summation characteristics of the PLR using briefly presented stimuli (1 s in duration) under rod-, cone-, and melanopsin-mediated conditions. PLRs mediated by the three receptor classes were elicited by manipulating the stimulus and adaptation conditions. In the present study, rod-, cone-, and melanopsin-mediated PLRs were obtained using stimuli of two different wavelengths, a wide range of luminance levels, and different angular subtenses. This allowed us to characterize fully the spatial summation of the PLR and how stimulus luminance and size interact to govern pupil size.
Methods
Subjects
Five subjects, three males and two females, ranging in age from 25 to 36 years participated in the study. All subjects had best-corrected visual acuity of 0 log MAR or better (equivalent to Snellen acuity of 20/20 or better), normal Pelli-Robson contrast sensitivity, and no history of visual abnormalities. Informed consent was obtained from all subjects before participation. Procedures adhered to the tenets of the Declaration of Helsinki, and the protocol was approved by an Institutional Review Board at the University of Illinois at Chicago.
Apparatus and stimuli
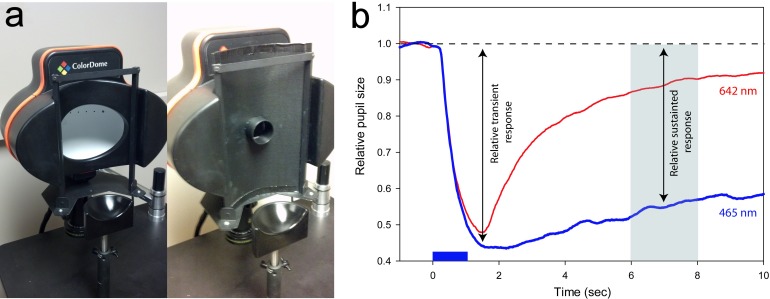
An LED-driven ganzfeld system was used for stimulus generation and display (Espion V6; ColorDome™ desktop ganzfeld, Diagnosys LLC, Lowell, MA). The stimuli were presented to one eye, and the pupil responses were recorded from the same eye using a ViewPoint EyeTrack® System (Arrington Research, Scottsdale, AZ) with the fellow eye patched. This system allows real-time pupillometry with high spatial resolution (<0.03 mm) at a 60-Hz sampling rate. The eye-tracking system consists of an infrared camera mounted on a plastic eye frame that does not obstruct the field of view. During the pupil recordings, the subject's head was stabilized with a chin rest, and a small spot of light was displayed for fixation. Stimuli consisted of short-wavelength (“blue”; dominant wavelength of 465 nm) and long-wavelength (“red”; dominant wavelength of 642 nm) pulses of light that were 1 s in duration. The photopic luminance of the stimuli ranged from −4.0 to 2.6 log cd/m2. The angular subtense of the stimuli was controlled using a light-blocking slide with a circular aperture (Figure 1a). Two different aperture sizes were used, and the distance between the subject's eye and the circular aperture was adjusted to achieve three different stimulus sizes (4°, 16°, and 32° in diameter). A funnel (2.5 cm in length) was inserted into the aperture to minimize light scatter (see Figure 1a). For the largest stimulus size, the light blocking slide and aperture were removed, and the subject's eye was positioned as close as possible to the ganzfeld stimulator; this produced a field of view that was approximately 90° (horizontal diameter) by 60° (vertical diameter), which is referred to as “90°” below. Of note, this stimulus has been referred to as “full field” in a previous report (Lei, Goltz, Chandrakumar, & Wong, 2014) and is the same as that used in our earlier study (Park et al., 2011). Stimulus wavelength and luminance were verified with a SpectraScan® 740 (Photo Research, Chatsworth, CA).
Figure 1.
(a) The standard ganzfeld stimulus source (left). The angular subtense of the stimulus was controlled by adjusting the size of an aperture (shown at right) and the viewing distance as described in the text. (b) An example PLR (relative to the baseline pupil size) and the definitions of the PLR parameters. The blue and red traces represent PLRs obtained in response to 2.6 log cd/m2 short- and long-wavelength stimuli, respectively. The rectangle along the abscissa marks the 1-s stimulus presentation, and the horizontal dashed line shows the relative baseline pupil size. The relative transient response was defined as the difference between the baseline and the minimum relative pupil size after stimulus onset. The relative sustained response was defined as the difference between the baseline and the median pupil size at 5 to 7 s after stimulus offset (gray region). The PLR elicited by a long-wavelength stimulus did not produce a significant relative sustained response and is shown here for comparison.
Procedure
As discussed elsewhere (Park et al., 2011), the rod-, cone-, and melanopsin-mediated PLR can be recorded by manipulating the stimulus luminance, wavelength, and adaptation conditions. In the present study, rod-, cone-, and melanopsin-mediated PLRs were measured for a series of stimulus luminances and sizes using short- and long-wavelength light. PLRs were measured after a 10-min dark adaption period (rod and melanopsin conditions) or after a 2-min exposure to a short-wavelength (465 nm), rod-suppressing, circular adapting field of 0.78 log cd/m2 (6 phot. cd/m2; 73 scot. cd/m2; cone condition). The adapting field was presented continuously throughout the session, and its size and shape matched the size and shape of the stimulus, which minimized possible effects of inhibition from the periphery that could occur with full-field adaptation. The scotopic luminance of the adapting field was approximately equal to the adapting field luminance recommended by the International Society for Clinical Electrophysiology of Vision (McCulloch et al., 2015) for suppressing rod pathway responses (i.e., approximately 75 scot. cd/m2). A long-wavelength pulse presented against a short-wavelength adapting field is thought to generate a PLR that is primarily cone-mediated as exposure to a short-wavelength adapting field effectively removes both the rod and melanopsin components of the PLR (Park et al., 2011). After dark adaption, a low-luminance, short-wavelength stimulus (≤−2 log cd/m2) will elicit a transient PLR that is primarily rod-pathway mediated whereas short-wavelength, higher luminance stimuli (>0.5 log cd/m2) will elicit a sustained pupil response after stimulus offset that is melanopsin-mediated. To provide additional evidence of the pathways mediating the PLR, PLRs elicited by photopically matched short- and long-wavelength stimuli were compared. For example, a robust sustained response is not expected in response to long-wavelength stimuli, even at high luminances, as the spectral sensitivity of melanopsin is over 3 log units lower for long-wavelength light (642 nm) compared to short-wavelength light (465 nm) (Gamlin et al., 2007). Similarly, the rod-mediated PLR is expected to be larger when elicited by short-wavelength stimuli compared to long-wavelength stimuli because the rod pathway is over 2 log units more sensitive to 465-nm light compared to 642-nm light.
Subjects were tested in a total of 16 conditions over two sessions: 4 stimulus sizes × 2 adaptation conditions × 2 wavelengths. In one session, the subject was dark-adapted, and measurements for both the rod and melanopsin conditions were obtained (approximately 60-min session). In the other session, the subject was light-adapted to the rod-suppressing adapting field, and measurements for the cone condition were obtained (approximately 40-min session). Stimulus luminance was increased sequentially in approximately 1 log unit steps from −4.0 to 2.6 log cd/m2 under the dark-adapted condition (rod and melanopsin conditions) and from −1.0 to 2.6 log cd/m2 under the light-adapted condition (cone condition). The interval between stimuli was increased from 15 to 60 s as luminance increased to ensure that the pupil size returned (dilated) to its baseline value between stimulus exposures. Stimuli were presented in order of increasing luminance and increasing angular subtense.
Data analysis
Data were analyzed offline using custom scripts programmed in MATLAB (MathWorks Inc., Natick, MA), which allowed for semiautomated analysis as follows: First, a median filter with a 300-ms time window was applied to remove eye blinks. In the case of long eye blinks (or eye closure) the filter failed, and the contamination was removed manually. The filtered PLRs were then normalized by the median pupil size during the 1 s prior to each stimulus onset (baseline pupil size). The relative pupil size was defined as Absolute pupil size (mm)/Baseline pupil size (mm). The relative pupil size was used to calculate the relative transient and relative sustained responses reported in the figures below. As shown in Figure 1b, the relative transient response was defined as the difference between the normalized baseline (dashed line) and minimum relative pupil sizes after stimulus onset whereas the relative sustained response was defined as the difference between the normalized baseline and median relative pupil size at 5 to 7 s following stimulus offset. The baseline pupil size was essentially constant under the dark-adapted condition but depended on adapting field size under the light-adapted condition. Expressing the pupil response in relative units accounts for the baseline differences under the light-adapted condition and also minimized the effects of the small differences in baseline pupil size among the five subjects.
Results
Dark-adapted (rod- and melanopsin-mediated) PLRs
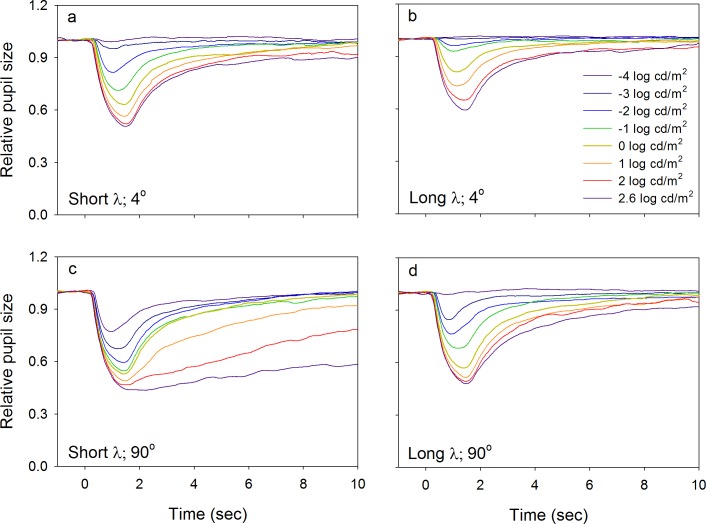
Figure 2 shows the mean PLRs (relative pupil size) for the five subjects obtained with short-wavelength (left column) and long-wavelength (right column) stimuli that subtended 4° (top row) and 90° (bottom row) of visual angle. Measurements were performed at a series of luminance levels (indicated by the key) at these two stimulus sizes. For both the short- and long-wavelength stimuli that subtended 4° (top row), the PLRs were characterized by a transient constriction followed by a relatively rapid return to baseline. The relative transient response increased as stimulus luminance increased for both wavelengths (Figure 2a, b). In general, for a given luminance, the relative transient response elicited by a short-wavelength stimulus was larger than that elicited by a photopically matched long-wavelength stimulus as expected for rod-mediated PLRs.
Figure 2.
Mean dark-adapted PLRs for the five subjects for a series of luminance levels (given by the key). PLRs are shown for stimuli that subtended 4° (a, b; top row) and 90° (c, d; bottom row) of visual angle that were either short wavelength (a, c; left column) or long wavelength (b, d right column).
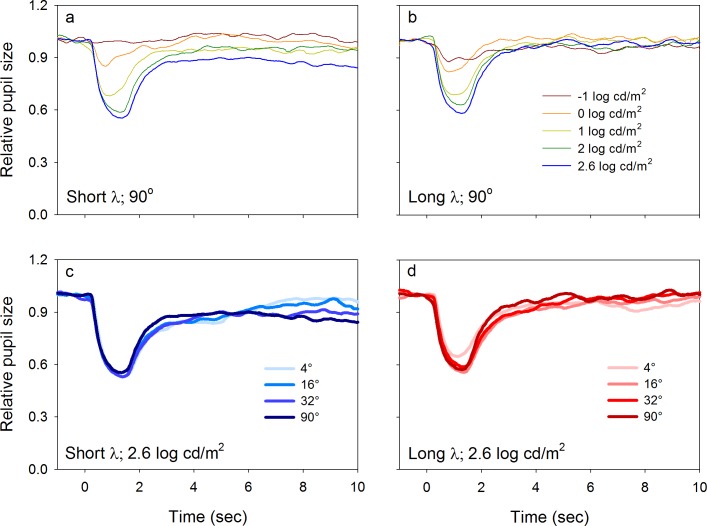
Data for the 90° stimulus size are shown in the bottom row of Figure 2. For low-to-moderate luminance levels (<1 log cd/m2), the PLRs elicited by both short-wavelength (Figure 2c) and long-wavelength (Figure 2d) stimuli were characterized by a transient constriction followed by a rapid return to baseline like those observed for the 4° stimuli. In contrast, for higher-luminance flashes (≥1 log cd/m2), the PLR elicited by the short-wavelength, 90° stimulus (Figure 2c) had a sustained constriction that persisted for several seconds or longer following stimulus offset (i.e., the relative sustained response). There was minimal or no relative sustained response for the long-wavelength, 90° stimulus (Figure 2d).
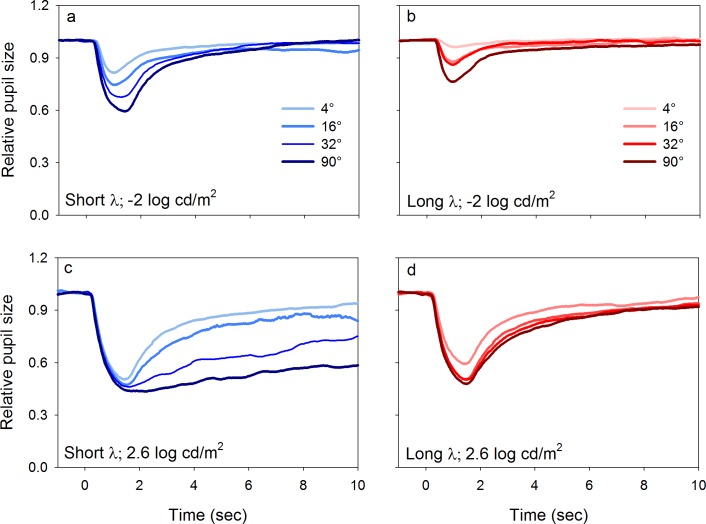
Figure 3 shows the mean PLRs (relative pupil size) for the five subjects obtained with short-wavelength (left column) and long-wavelength (right column) stimuli of different sizes that had a luminance of −2.0 log cd/m2 (top row) and 2.6 log cd/m2 (bottom row). Measurements were performed at a series of stimulus sizes (indicated by the key) at the two different luminance levels. At −2.0 log cd/m2 (Figure 3a, b), the relative transient response increased as stimulus size increased for both wavelengths, but the dependence on size was greater for the short-wavelength stimulus. The bottom row shows that for short-wavelength, high-luminance stimuli (2.6 log cd/m2) presented at moderate to large size (≥32°), a clear, relative sustained response was present, which was not observed for smaller stimulus sizes (Figure 3c). In comparison, high luminance, long-wavelength stimuli did not elicit a relative sustained response at any stimulus size (Figure 3d).
Figure 3.
Mean dark-adapted PLRs for the five subjects for a series of stimulus sizes (given by the key). PLRs are shown for luminance levels of −2 log cd/m2 (a, b; top row) and 2.6 log cd/m2 (c, d; bottom row) that were either short wavelength (a, c; left column) or long wavelength (b, d; right column).
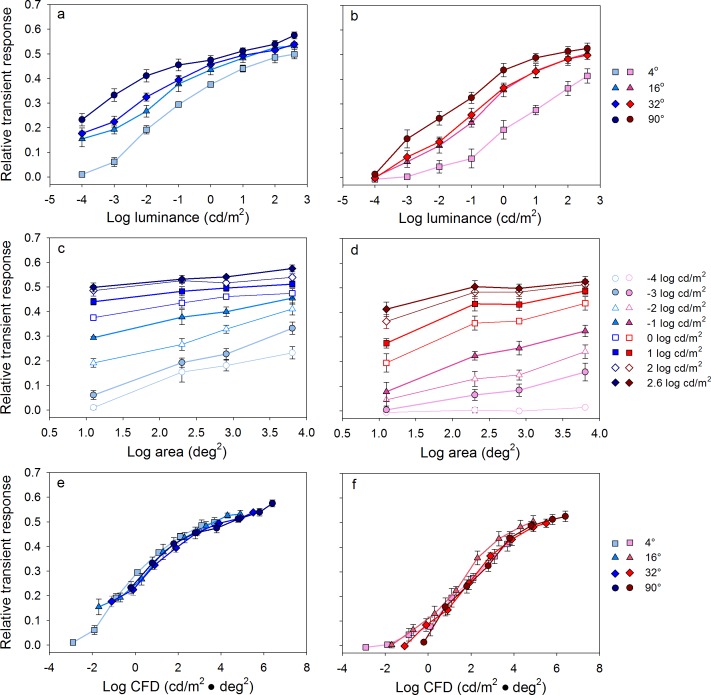
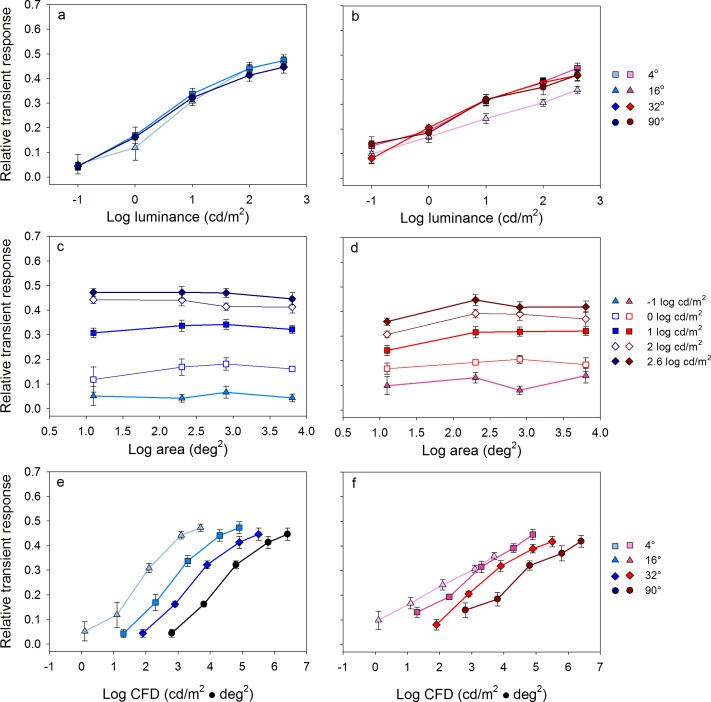
The relative transient responses for the different stimulus sizes and luminances shown in Figures 2 and 3 are quantified in Figure 4. Figure 4a and b shows the relative transient response as function of log luminance (cd/m2) for short- and long-wavelength stimuli, respectively. The data points represent the mean of the five subjects and the error bars show ±1 standard error of the mean. Each function represents a series of responses obtained for stimuli of different luminance at a constant size (indicated in the key). For a given stimulus size, the relative transient response depended on the stimulus luminance, such that higher luminance stimuli elicited greater pupil constrictions (i.e., each function had a positive slope). This was the case for both the short- and long-wavelength stimuli. Across most of the luminance range tested, the pupil constriction for the short-wavelength stimuli was greater than that of the long-wavelength stimuli of equal luminance. For example, the relative transient response for the short-wavelength, 90° stimulus of −4.0 log cd/m2 (Figure 4a, leftmost circle in dark blue) was approximately 0.23. To elicit a similar relative transient response using a 90°, long-wavelength stimulus, the luminance was required to be 2 log units higher (Figure 4b, the third circle from the left in dark red). This difference between the two wavelengths became smaller in the high-luminance range in which the cone pathway gains sensitivity, which can also be seen by comparing the relative transient responses in Figure 3c and d.
Figure 4.
Mean (±1 SEM) dark-adapted relative transient responses for the five subjects. (a, b; top row) The relative transient response as a function of log stimulus luminance (cd/m2) for stimuli of different size. (c, d; middle row) The relative transient response as a function of log stimulus area (deg2) for stimuli of different luminance. (e, f; bottom row) The relative transient response as a function of CFD (cd/m2 · deg2) for stimuli of different size. Responses elicited by short- and long-wavelength stimuli are shown in the left and right columns, respectively.
Figure 4c and d shows the relative transient response as a function of log stimulus area (deg2) for short- and long-wavelength stimuli, respectively. Each function represents a series of responses obtained for stimuli of different size at a constant luminance (indicated in the key). For a given stimulus luminance, the relative transient response depended on the stimulus size, such that larger stimuli elicited greater pupil constrictions (i.e., the functions tended to have positive slopes). This was the case for both the short- and long-wavelength stimuli although the pupil responses for the lowest luminance, long-wavelength stimulus were nearly absent due to relatively low rod sensitivity for long-wavelength stimuli.
The data of Figure 4a through d show that the relative transient response is jointly dependent on stimulus luminance and size. Given this dependence, the data were replotted in terms of CFD (the product of stimulus luminance and area; cd/m2 · deg2). The relative transient response is plotted as a function of CFD for the short- and long-wavelength stimuli in Figure 4e and f, respectively. When plotted in terms of CFD, the functions (i.e., measurements for the different sizes) superimposed, indicating that CFD can account for the relative transient response for all stimulus sizes. That is, doubling the stimulus luminance had the same effect on the relative transient response as doubling the stimulus area. Of note, the pupil constriction for the short-wavelength stimuli was greater than that for the long-wavelength stimuli of equal CFD in the low-to-middle CFD range, consistent with the data shown in Figure 4a through d. The difference between the short- and long-wavelength functions is likely due to the relative transient response being mediated primarily by the rod system in the low-to-middle CFD range (<1.5 log cd/m2 · deg2) as the rod system is over 2 log units more sensitive to the short-wavelength stimulus, compared to the photopically matched long-wavelength stimulus. In sum, for the rod-mediated PLR, CFD accounted well for the relative transient response because both stimulus size and luminance have approximately equal contributions to the PLR.
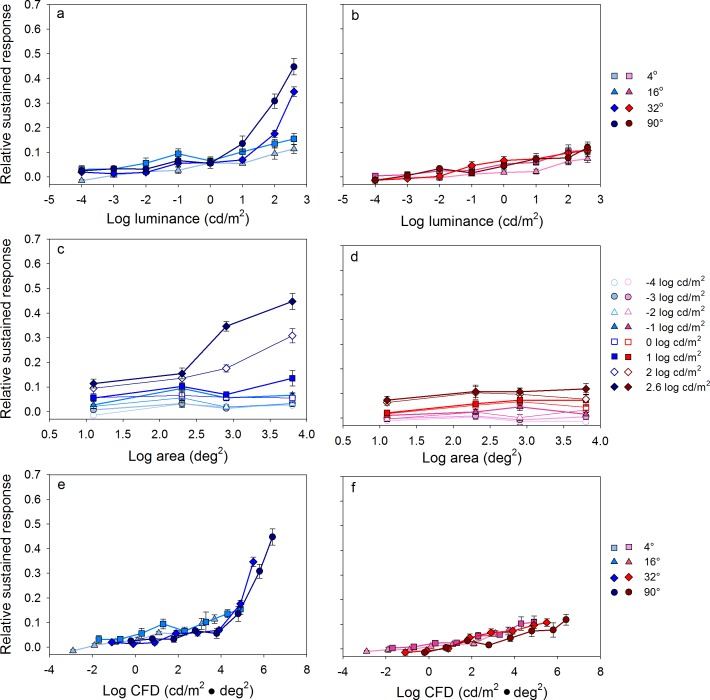
Figure 5a and b shows the relative sustained response as function of log luminance (cd/m2) for short- and long-wavelength stimuli, respectively. Each function represents a series of responses obtained for stimuli of different luminance at a constant size (indicated in the key). Minimal to no relative sustained response was observed at any size for short-wavelength stimuli less than approximately 1 log cd/m2 (Figure 5a). At higher luminances, however, relative sustained responses were observed that depended on the stimulus size; no relative sustained response was observed for the 4° or 16° stimulus sizes even in the highest luminance range. Figure 5b shows that the relative sustained response was essentially absent for all sizes and luminance levels for the long-wavelength stimuli.
Figure 5.
Mean (±1 SEM) dark-adapted relative sustained responses for the five subjects. Conventions as in Figure 4.
Figure 5c and d shows the relative sustained response as function of log stimulus area (deg2) for short- and long-wavelength stimuli, respectively. Each function represents a series of responses obtained for stimuli of different size at a constant luminance (indicated in the key). The relative sustained response elicited by the short-wavelength stimuli at the two highest luminance levels was dependent on the stimulus size, such that larger stimuli generated larger relative sustained responses (i.e., the high luminance functions in Figure 5c tended to have positive slopes). There was an increase in the relative sustained response as the stimulus size increased from 2.3 to 3.8 log deg2 (i.e., 16° to 90°) for the 2.0 and 2.6 log cd/m2 short-wavelength stimuli (open and filled diamonds). Figure 5d shows that there was no relative sustained response for long-wavelength stimuli of any size and thus no size effect.
The data of Figure 5a through d show that the relative sustained response is dependent on the luminance and size of the short-wavelength stimulus and that there is minimal or no relative sustained response for long-wavelength stimuli. The relative sustained response is plotted as a function of CFD for the short- and long-wavelength stimuli in Figure 5e and f, respectively. When plotted in terms of CFD, the functions (i.e., measurements for the different sizes) generally superimpose, indicating that CFD accounts well for the relative sustained response. The functions superimpose at low CFD because there was no relative sustained response at low luminances or small sizes. Similarly, there was minimal or no relative sustained response for any long-wavelength stimuli, which accounts for the superimposed functions when plotted in CFD.
Light-adapted (cone-mediated) PLR
Figure 6a shows the mean PLRs (relative pupil response) for the five subjects elicited by 90°, short-wavelength stimuli of different luminance (i.e., different stimulus luminance at a constant size). Figure 6b shows similar data obtained with the long-wavelength stimulus. For both stimuli, the PLRs were characterized by a transient constriction followed by a rapid return to baseline. The recovery to baseline was slower for PLRs elicited by short-wavelength, high-luminance stimuli (≥1 log cd/m2; Figure 6a) compared to the PLRs elicited by photopically matched long-wavelength stimuli. The slower recovery can be attributed to a weak melanopsin contribution to the pupil response for the highest luminance stimuli. In general, the relative transient response increased as stimulus luminance increased. Figure 6c and d shows PLRs driven by 2.6 log cd/m2 short- and long-wavelength stimuli of different sizes, respectively (i.e., constant stimulus luminance at different sizes). The relative pupil size was independent of stimulus size for short-wavelength stimuli and minimally dependent on stimulus size for long-wavelength stimuli. In fact, only the smallest (4°) long-wavelength stimulus differed in the relative transient response. This minimal spatial summation is unlike that observed under the rod- and melanopsin-mediated conditions.
Figure 6.
Mean light-adapted PLRs for the five subjects for a series of stimulus luminance levels (a, b; top row) and size (c, d; bottom row). PLRs elicited by short- and long-wavelength stimuli are shown in the left and right columns, respectively. Stimulus luminance (a, b) and size (c, d) are given in the keys.
The relative transient responses for the different stimulus sizes and luminances shown in Figure 6 are quantified in Figure 7. Figure 7a and b shows the relative transient response as a function of log luminance (cd/m2) for short- and long-wavelength stimuli, respectively. Each function represents a series of responses obtained for stimuli of different luminance at a constant size (indicated in the key). For both short- and long-wavelength stimuli, the relative transient response depended on the stimulus luminance (all had positive slopes). The functions superimpose for the short-wavelength stimuli and for three of the four sizes for the long-wavelength stimuli. The exception was the long-wavelength function obtained for the 4° size as mentioned above (triangles; Figure 7b), which had a shallower slope compared to the other size functions.
Figure 7.
Mean (±1 SEM) light-adapted relative transient responses for the five subjects. Conventions as in Figure 4.
Figure 7c and d shows the relative transient response as a function of log stimulus area (deg2) for 2.6 log cd/m2 short- and long-wavelength stimuli, respectively. Each function represents a series of responses obtained for stimuli of different sizes at a constant luminance. Unlike the rod and melanopsin-mediated responses, there was essentially no size dependence for the short-wavelength stimulus at any luminance level (Figure 7c; all had slopes of approximately zero). Similarly, there was no size dependence for the long-wavelength stimulus for most of the stimulus sizes tested (Figure 7d) with the exception of a small increase in the relative transient response as size was increased from 4° to 16°.
The data of Figure 7a through d show that the light-adapted relative transient response is strongly dependent on stimulus luminance with minimal or no dependence on size. Given these findings, the data were replotted in terms of CFD in Figure 7e and f for the short- and long-wavelength stimuli, respectively. The functions (i.e., measurements for the different sizes) had positive slopes and were displaced horizontally for the short-wavelength stimuli, indicating that CFD cannot account for the cone-mediated relative transient response at any stimulus size (Figure 7e). A similar effect was observed for the long-wavelength stimuli (Figure 7f), but the parallel shift in the functions was less clear than for the short-wavelength stimuli. This is due to a weak dependence of the PLR on stimulus size as the stimulus was increased from 4° to 16°.
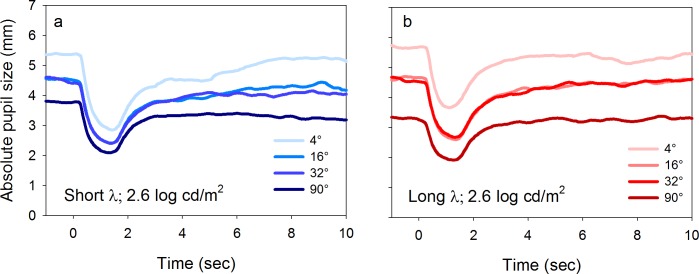
To ensure that the lack of spatial summation for the cone-mediated relative transient response was not due to a mechanical limitation of the pupil, the relative PLR elicited by the 2.6 log cd/m2 stimulus for each size (shown in Figure 6c, d) is replotted in absolute units (mm) in Figure 8a and b. The absolute transient constrictions for the 16° and 32° long-wavelength stimuli (2.59 and 2.67 mm, respectively) were larger than the absolute transient constriction elicited by the 90° stimulus (2.19 mm). Similar results were obtained for the short-wavelength stimulus (Figure 8a). Thus, if saturation of the cone-mediated relative transient response were due to a mechanical limitation of the pupil, then the absolute transient constrictions would be the same for all stimulus sizes, which was not observed. Rather, the stimulus luminance determined the relative transient response for all stimuli of 16° or larger, and the stimulus size had minimal effect. Note that the baseline pupil sizes of the functions shown in Figures 6 and 7 were different in absolute units (mm as shown in Figure 8). As the adaptation field size increased, the baseline pupil size decreased, consistent with previous reports (Watson & Yellott, 2012). However, the shifting baseline pupil size does not affect the cone-mediated pupil responses shown in Figures 6 and 7 because these measurements were normalized to the baseline pupil size.
Figure 8.
Mean light-adapted PLRs in response to 2.6 log cd/m2, short-wavelength (a) and long-wavelength (b) stimuli for four different sizes (given in the key). Data are shown in absolute units (mm).
Discussion
This study examined the spatial summation characteristics of the PLR using briefly presented stimuli under rod-, cone-, and melanopsin-mediated conditions. The spatial summation characteristics of the rod- and melanopsin-mediated PLRs were similar in that the product of stimulus luminance and size (CFD) accounted well for these pupil responses. That is, doubling the stimulus luminance had the same effect on the PLR as doubling the stimulus area. This indicates that under the rod- and melanopsin-mediated conditions, the pupil response is largely determined by the energy in the stimulus. In contrast to these results, the cone-mediated PLR was not well accounted for by CFD. This is because the cone-mediated PLR was highly dependent on stimulus luminance and minimally dependent on stimulus size. For example, a 2.6 log cd/m2 short-wavelength stimulus that subtended 4° of visual angle produced a PLR that was equivalent to that elicited by a stimulus of the same luminance that subtended 16° to 90° (Figure 6c). These findings highlight important differences in how stimulus luminance and size combine to govern the PLR under rod- and melanopsin-mediated conditions compared to cone-mediated conditions.
Previous studies have examined the joint effect of adapting field size and luminance on pupil size (Atchison et al., 2011; Stanley & Davies, 1995; Watson & Yellott, 2012), but these previous studies did not attempt to bias processing toward a specific receptor type, and importantly, only the steady-state pupil response was measured under conditions of constant retinal illumination. For example, a recent study by Watson and Yellott (2012) provided a formula based on earlier studies (Stanley & Davies, 1995; ten Doesschate & Alpern, 1967; Winn et al., 1994) that incorporated the effects of adapting field size and luminance, as well as the effects of age and binocular summation, to predict the size of the pupil. This study is important in that it allows the steady-state pupil size to be estimated for a given set of conditions when actual measurements are unavailable. However, this model is not intended to predict transient PLRs due to changes in stimulus luminance or size. Nevertheless, the formula proposed by Watson and Yellott (2012) is based primarily on the product of adapting field luminance and size (i.e., CFD) as the determinant for pupil size, which is consistent with our results obtained for the rod-mediated transient response and for the melanopsin-mediated sustained response.
The cone-mediated PLR could not be accounted for by CFD in the present study, which is in contrast to the steady-state, rod-mediated, and melanopsin-mediated pupil responses. The failure of CFD to account for the PLR is because the cone-mediated PLR is largely independent of stimulus size, at least for stimuli larger than 4°. Rather, the cone-mediated PLR is almost entirely dependent on stimulus luminance. Although the explanation for the lack of spatial summation is not clear, the spatial distribution of cone photoreceptors may play a role. That is, cone receptor density falls steeply with increasing eccentricity and is approximately an order of magnitude lower at an eccentricity of 3.5° compared to the density at the fovea (Curcio, Sloan, Kalina, & Hendrickson, 1990). Consequently, increasing the stimulus size beyond the central 7° may be expected to have relatively minimal effects on the cone-mediated PLR. However, future studies that are capable of delivering small stimuli while accounting for fixation instability are needed to provide further insight into the spatial summation characteristics of the PLR under cone-mediated conditions.
The present study used stimuli of relatively brief duration (1 s) and defined angular subtense to elicit rod-, cone-, and melanopsin-mediated PLRs. Stimuli that are of relatively short duration have the advantage of being more tolerable to the subjects, which helps to minimize eye blinks. Furthermore, previous work has showed no clear advantage of using long-duration stimuli for eliciting the melanopsin-mediated PLR (Lei et al., 2014; Park et al., 2011). An advantage of using small stimulus sizes is that deficits in retinal function may be spatially mapped. Indeed, multifocal chromatic PLRs have recently been recorded in normally sighted individuals and in patients with photoreceptor dysfunction (Carle, James, & Maddess, 2013; Skaat et al., 2013). Thus, stimuli of limited duration and spatial extent may be important clinically, and the present study may be useful for relating PLRs obtained with stimuli of small spatial extent to those obtained with the more typical large-field stimulation.
The results of the present study may also have clinical implications. For example, patients with visual field loss due to retinitis pigmentosa would be expected to have an abnormal rod-mediated PLR, but the cone-mediated PLR may be normal, provided the patient has at least 4° of central field remaining. This is expected because of the differences in spatial summation between the rod- and cone-mediated PLRs (Figures 3 and 6) and is consistent with a previous study that showed that five patients with late-stage retinitis pigmentosa had measurable cone-mediated PLRs but no recordable rod-mediated PLRs when using large-field (90°) stimuli (Park et al., 2011).
Conclusion
The PLR under rod-, cone-, and melanopsin-mediated conditions is dependent on stimulus luminance, consistent with a previous study that used large-field stimulation (Park et al., 2011). The present study extends this finding to show that the PLR under all three conditions is dependent on stimulus luminance for stimuli of limited angular subtense presented in the central visual field. Importantly, the PLR recorded under rod- and melanopsin-mediated conditions is strongly size-dependent, but this is not the case for the PLR recorded under cone-mediated conditions. Consequently, CFD accounts well for rod- and melanopsin-mediated PLRs but not the cone-mediated PLR.
Acknowledgments
This research was supported by NIH grants R00EY019510 (JM) and P30EY001792 and an unrestricted departmental grant from Research to Prevent Blindness.
Commercial relationships: none.
Corresponding author: J. Jason McAnany.
Email: jmcana1@uic.edu.
Address: University of Illinois at Chicago, Chicago, IL, USA
Contributor Information
Jason C. Park, Email: korsakoff@gmail.com.
J. Jason McAnany, Email: jmcana1@uic.edu.
References
- Atchison D. A., Girgenti C. C., Campbell G. M., Dodds J. P., Byrnes T. M., Zele A. J. (2011). Influence of field size on pupil diameter under photopic and mesopic light levels. Clinical and Experimental Optometry , 94 (6), 545–548. [DOI] [PubMed] [Google Scholar]
- Barrionuevo P. A., Nicandro N., McAnany J. J., Zele A. J., Gamlin P., Cao D. (2014). Assessing rod, cone, and melanopsin contributions to human pupil flicker responses. Investigative Ophthalmology & Visual Science , 55 (2), 719–727, http://www.iovs.org/content/55/2/719. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouma H. (1962). Size of the static pupil as a function of wavelength and luminosity of the light incident on the human eye. Nature , 193, 690–691. [DOI] [PubMed] [Google Scholar]
- Bradley M. M., Miccoli L., Escrig M. A., Lang P. J. (2008). The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology , 45 (4), 602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W. (1957). The depth of field of the human eye. Optica Acta: International Journal of Optics , 4 (4), 157–164. [Google Scholar]
- Carle C. F., James A. C., Maddess T. (2013). The pupillary response to color and luminance variant multifocal stimuli. Investigative Ophthalmology & Visual Science , 54 (1), 467–475, http://www.iovs.org/content/54/1/467. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Crawford B. H. (1936). The dependence of pupil size upon external light stimulus under static and variable conditions. Proceedings of the Royal Society of London. Series B - Biological Sciences , 121 (823), 376–395. [Google Scholar]
- Curcio C. A., Sloan K. R., Kalina R. E., Hendrickson A. E. (1990). Human photoreceptor topography. Journal of Comparative Neurology , 292 (4), 497–523. [DOI] [PubMed] [Google Scholar]
- Dacey D. M., Liao H. W., Peterson B. B., Robinson F. R., Smith V. C., Pokorny J., Gamlin P. D. (2005). Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature , 433 (7027), 749–754. [DOI] [PubMed] [Google Scholar]
- Feigl B., Zele A. J., Fader S. M., Howes A. N., Hughes C. E., Jones K. A., Jones R. (2012). The post-illumination pupil response of melanopsin-expressing intrinsically photosensitive retinal ganglion cells in diabetes. Acta Ophthalmologica , 90 (3), e230–e234. [DOI] [PubMed] [Google Scholar]
- Gamlin P. D., McDougal D. H., Pokorny J., Smith V. C., Yau K. W., Dacey D. M. (2007). Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Research , 47 (7), 946–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler A. D., Ecker J. L., Lall G. S., Haq S., Altimus C. M., Liao H. W., Hattar S. (2008). Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature , 453 (7191), 102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess E. H., Polt J. M. (1960). Pupil size as related to interest value of visual stimuli. Science , 132 (3423), 349–350. [DOI] [PubMed] [Google Scholar]
- Kankipati L., Girkin C. A., Gamlin P. D. (2010). Post-illumination pupil response in subjects without ocular disease. Investigative Ophthalmology & Visual Science , 51 (5), 2764–2769, http://www.iovs.org/content/51/5/2764. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardon R., Anderson S. C., Damarjian T. G., Grace E. M., Stone E., Kawasaki A. (2009). Chromatic pupil responses: Preferential activation of the melanopsin-mediated versus outer photoreceptor-mediated pupil light reflex. Ophthalmology , 116 (8), 1564–1573. [DOI] [PubMed] [Google Scholar]
- Kawasaki A., Collomb S., Leon L., Munch M. (2014). Pupil responses derived from outer and inner retinal photoreception are normal in patients with hereditary optic neuropathy. Experimental Eye Research , 120, 161–166. [DOI] [PubMed] [Google Scholar]
- Kawasaki A., Crippa S. V., Kardon R., Leon L., Hamel C. (2012). Characterization of pupil responses to blue and red light stimuli in autosomal dominant retinitis pigmentosa due to NR2E3 mutation. Investigative Ophthalmology & Visual Science , 53 (9), 5562–5569, http://www.iovs.org/content/53/9/5562. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Kawasaki A., Munier F. L., Leon L., Kardon R. H. (2012). Pupillometric quantification of residual rod and cone activity in leber congenital amaurosis. Archives of Ophthalmology , 130 (6), 798–800. [DOI] [PubMed] [Google Scholar]
- Lei S., Goltz H. C., Chandrakumar M., Wong A. M. (2014). Full-field chromatic pupillometry for the assessment of the postillumination pupil response driven by melanopsin-containing retinal ganglion cells. Investigative Ophthalmology & Visual Science , 55 (7), 4496–4503, http://www.iovs.org/content/55/7/4496. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Marg E., Morgan M. W. Jr. (1949). The pupillary near reflex: The relation of pupillary diameter to accommodation and the various components of convergence. American Journal of Optometry & Archives of American Academy of Optometry , 26 (5), 183–198. [PubMed] [Google Scholar]
- McCulloch D. L., Marmor M. F., Brigell M. G., Hamilton R., Holder G. E., Tzekov R., Bach M. (2015). ISCEV standard for full-field clinical electroretinography (2015 update). Documenta Ophthalmologica , 130 (1), 1–12. [DOI] [PubMed] [Google Scholar]
- McDougal D. H., Gamlin P. D. (2010). The influence of intrinsically-photosensitive retinal ganglion cells on the spectral sensitivity and response dynamics. Vision Research , 50 (1), 72–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura A. L., Nagy B. V., La Morgia C., Barboni P., Oliveira A. G., Salomao S. R., Ventura D. F. (2013). The pupil light reflex in Leber's hereditary optic neuropathy: Evidence for preservation of melanopsin-expressing retinal ganglion cells. Investigative Ophthalmology & Visual Science , 54 (7), 4471–4477, http://www.iovs.org/content/54/7/4471. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. C., Moura A. L., Raza A. S., Rhee D. W., Kardon R. H., Hood D. C. (2011). Toward a clinical protocol for assessing rod, cone, and melanopsin contributions to the human pupil response. Investigative Ophthalmology & Visual Science , 52 (9), 6624–6635, http://www.iovs.org/content/52/9/6624. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaat A., Sher I., Kolker A., Elyasiv S., Rosenfeld E., Mhajna M.… (2013). Pupillometer-based objective chromatic perimetry in normal eyes and patients with retinal photoreceptor dystrophies. Investigative Ophthalmology & Visual Science , 54 (4), 2761–2770, http://iovs.org/content/54/4/2761. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Stanley P. A., Davies A. K. (1995). The effect of field of view size on steady-state pupil diameter. Ophthalmic & Physiological Optics , 15 (6), 601–603. [PubMed] [Google Scholar]
- ten Doesschate J., Alpern M. (1967). Effect of photoexcitation of the two retinas on pupil size. Journal of Neurophysiology , 30 (3), 562–576. [DOI] [PubMed] [Google Scholar]
- Watson A. B., Yellott J. I. (2012). A unified formula for light-adapted pupil size. Journal of Vision , 12 (10): 13 1–16, http://www.journalofvision.org/content/12/10/12, doi:10.1167/12.10.12. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Winn B., Whitaker D., Elliott D. B., Phillips N. J. (1994). Factors affecting light-adapted pupil size in normal human subjects. Investigative Ophthalmology & Visual Science , 35 (3), 1132–1137, http://www.iovs.org/content/35/3/1132. [PubMed] [Article] [PubMed] [Google Scholar]