Abstract
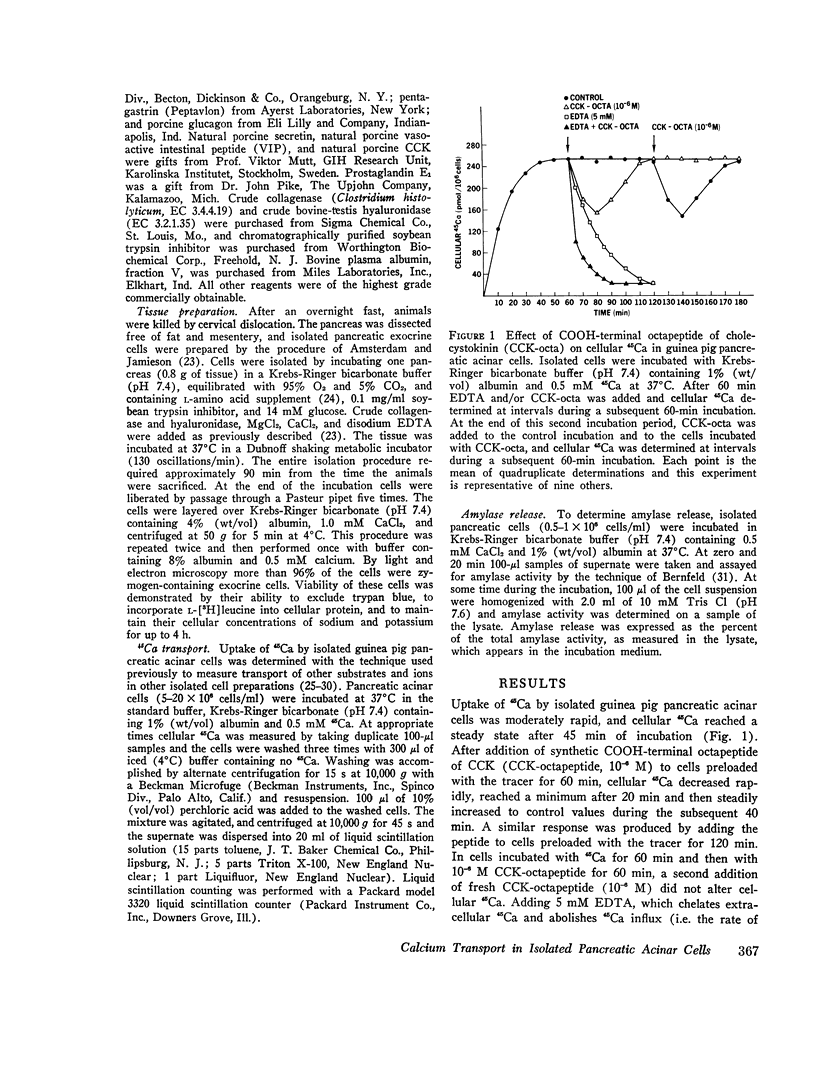
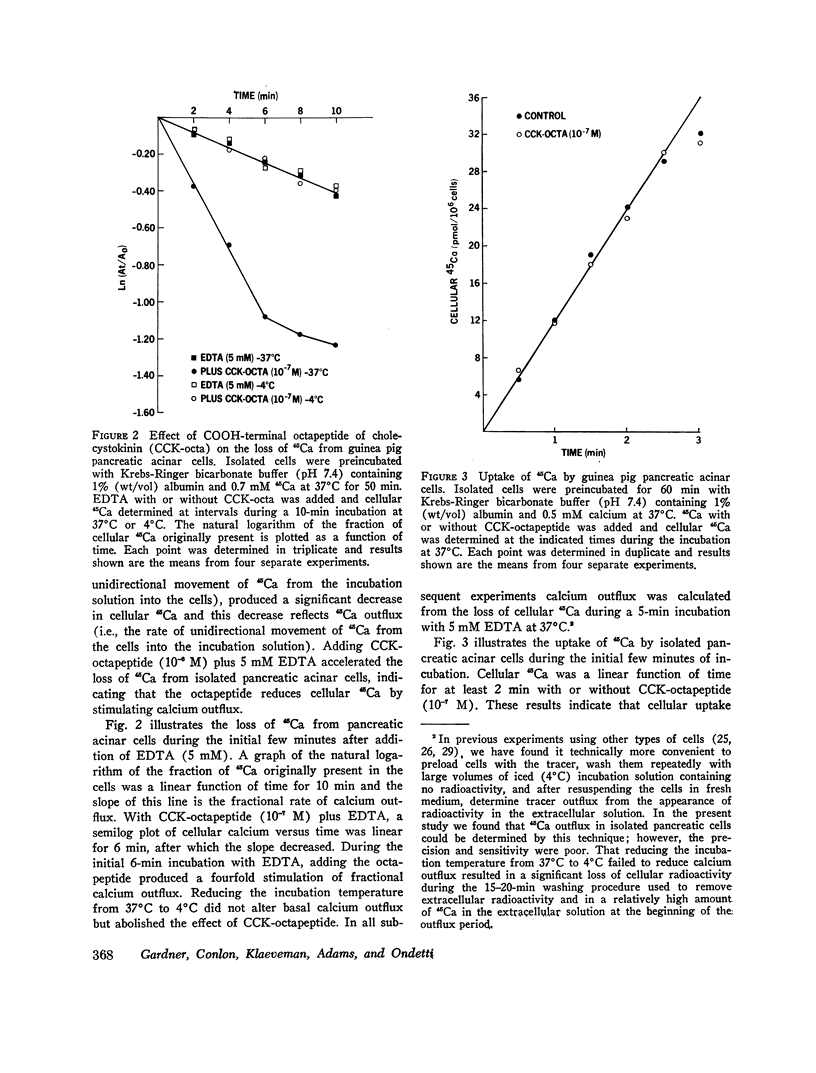
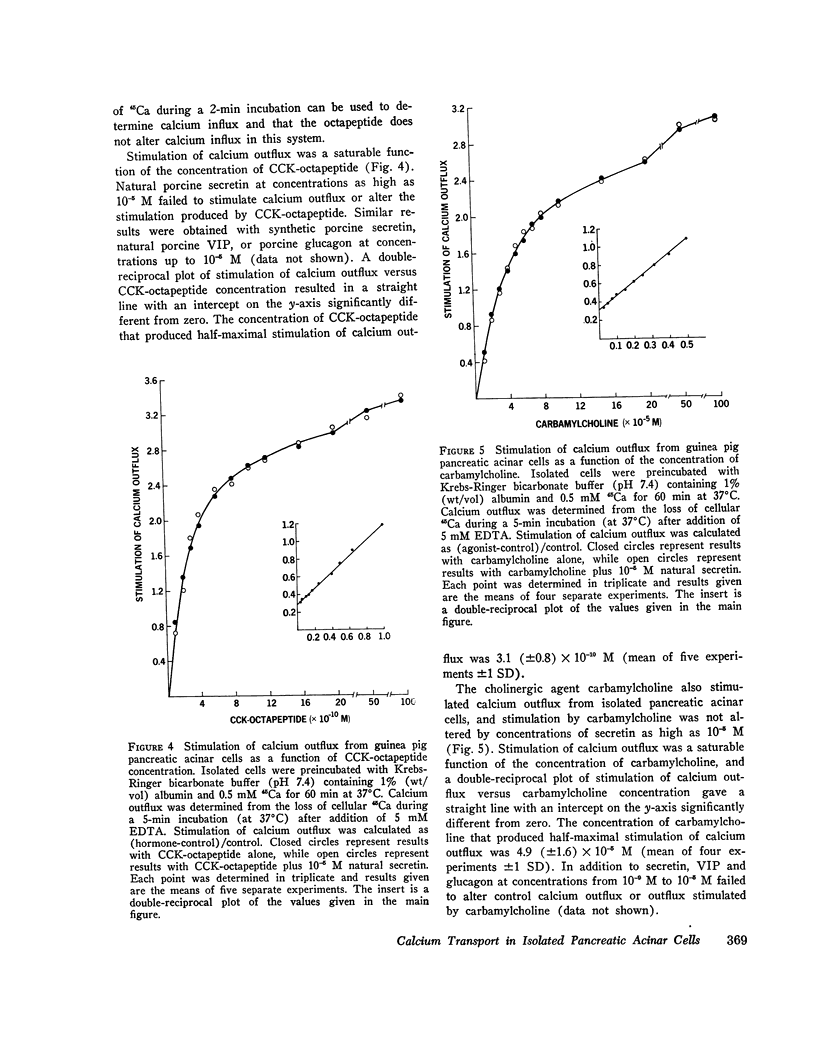
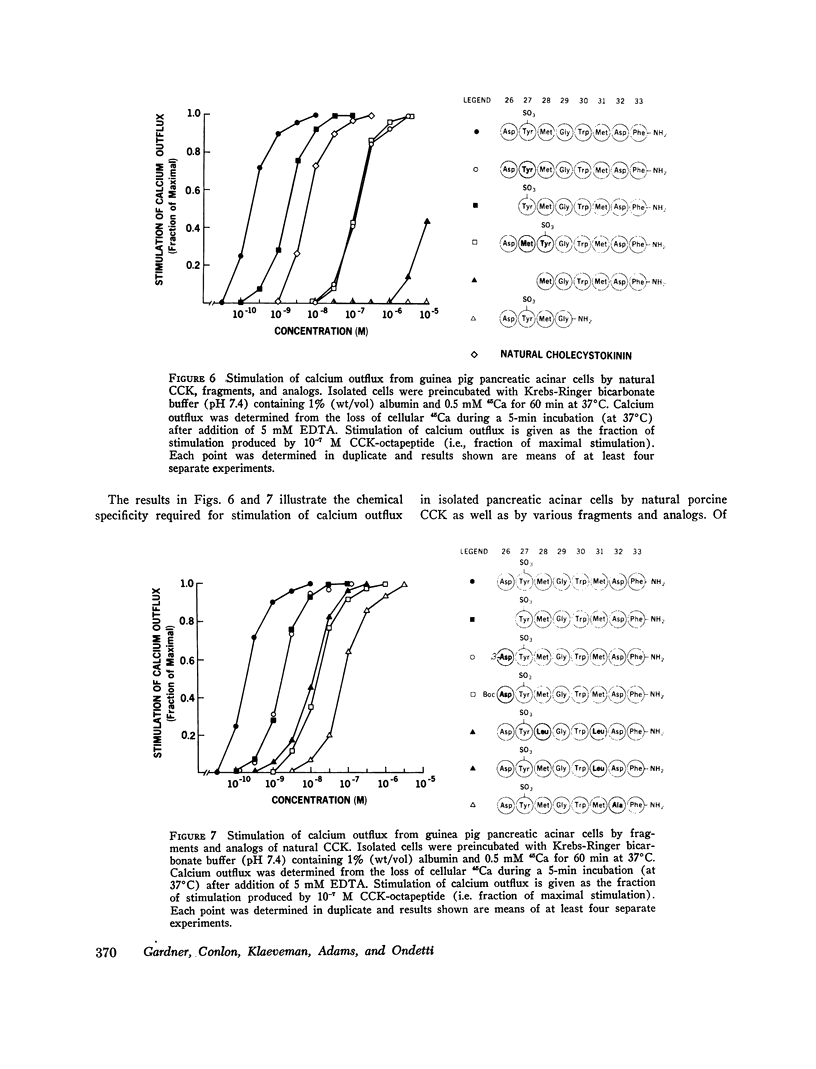
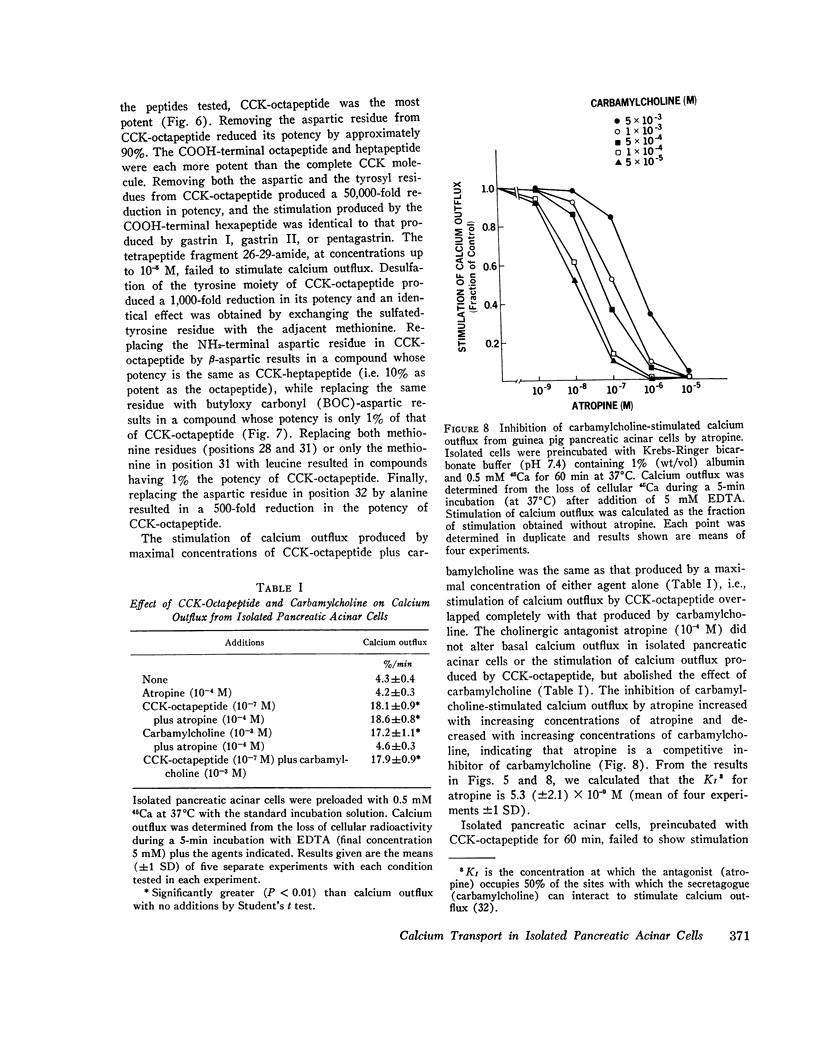
COOH-terminal octapeptide of cholecystokinin (CCK-octapeptide) and the cholinergic agent carbamylcholine each produced a fourfold stimulation of calcium outflux in guinea pig isolated pancreatic acinar cells. Neither agent altered calcium influx. Stimulation of calcium outflux was rapid and specific, was abolished by reducing the incubation temperature to 4 degrees C, and was a saturable function of the secretagogue concentration. The concentrations of CCK-octapeptide and carbamylcholine that produced half-maximal stimulation of calcium outflux were 3.1 x 10(-10) M and 4.9 x 10(-5) M, respectively. The cholinergic antagonist antropine competitively inhibited carbamylcholine stimulation of calcium outflux but did not alter stimulation produced by CCK-octapeptide. Stimulation of calcium outflux by maximal concentrations of carbamycholine plus CCK-octapeptide was the same as that produced by a maximal concentration of either agent alone.Calcium outflux became refractory to stimulation by secretagogues, and incubation with either CCK-ostapeptide or carbamylcholine produced a refractoriness to both agents. The relative potencies with CCK and its related fragments stimulated calcium outflux were CCK-octapeptide greater than heptapeptide greater than CCK greater than hexapeptide = gastrin. Secretin, glucagon, and vasoactive intestinal peptide, at concentrations as high as 10(-5) M, failed to alter calcium outflux and did not affect stimulation by CCK-octapeptide or by carbamycholine.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsterdam A., Jamieson J. D. Structural and functional characterization of isolated pancreatic exocrine cells. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3028–3032. doi: 10.1073/pnas.69.10.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argent B. E., Case R. M., Scratcherd T. Amylase secretion by the perfused cat pancreas in relation to the secretion of calcium and other electrolytes and as influenced by the external ionic environment. J Physiol. 1973 May;230(3):575–593. doi: 10.1113/jphysiol.1973.sp010205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauduin H., Rochus L., Vincent D., Dumont J. E. Role of cyclic 3',5'-amp in the action of physiological secretagogues on the metabolism of rat pancreas in vitro. Biochim Biophys Acta. 1971 Oct;252(1):171–183. doi: 10.1016/0304-4165(71)90106-1. [DOI] [PubMed] [Google Scholar]
- Benz L., Eckstein B., Matthews E. K., Williams J. A. Control of pancreatic amylase release in vitro: effects of ions, cyclic AMP, and colchicine. Br J Pharmacol. 1972 Sep;46(1):66–67. doi: 10.1111/j.1476-5381.1972.tb06849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. M., Gardner J. D. Sequence of events mediating the effect of cholera toxin on rat thymocytes. J Clin Invest. 1974 Apr;53(4):1149–1158. doi: 10.1172/JCI107653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Clausen T. The relationship between calcium exchange and enzyme secretion in the isolated rat pancreas. J Physiol. 1973 Nov;235(1):75–102. doi: 10.1113/jphysiol.1973.sp010379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. Pancreatic acinar cells: measurement of membrane potential and miniature depolarization potentials. J Physiol. 1972 Aug;225(1):1–13. doi: 10.1113/jphysiol.1972.sp009926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimerl S., Savion N., Heichal O., Selinger Z. Induction of enzyme secretion in rat pancreatic slices using the ionophore A-23187 and calcium. An experimental bypass of the hormone receptor pathway. J Biol Chem. 1974 Jun 25;249(12):3991–3993. [PubMed] [Google Scholar]
- Fölsch U. R., Wormsley K. G. Pancreatic enzyme response to secretin and cholecystokinin-pancreozymin in the rat. J Physiol. 1973 Oct;234(1):79–94. doi: 10.1113/jphysiol.1973.sp010335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner J. D., Conlon T. P. The effects of sodium and potassium on ouabain binding by human erythrocytes. J Gen Physiol. 1972 Nov;60(5):609–629. doi: 10.1085/jgp.60.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner J. D., Klaeveman H. L., Bilezikian J. P., Aurbach G. D. Effect of beta-adrenergic catecholamines on sodium transport in turkey erythrocytes. J Biol Chem. 1973 Aug 25;248(16):5590–5597. [PubMed] [Google Scholar]
- Gardner J. D., Simopoulos A. P., Lapey A., Shibolet S. Altered membrane sodium transport in Bartter's syndrome. J Clin Invest. 1972 Jun;51(6):1565–1571. doi: 10.1172/JCI106953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfine I. D., Gardner J. D., Neville D. M., Jr Insulin action in isolated rat thymocytes. I. Binding of 125 I-insulin and stimulation of -aminoisobutyric acid transport. J Biol Chem. 1972 Nov 10;247(21):6919–6926. [PubMed] [Google Scholar]
- Gregory R. A. The Bayliss-Starling lecture 1973. The gastrointestinal hormones: a review of recent advances. J Physiol. 1974 Aug;241(1):1–32. doi: 10.1113/jphysiol.1974.sp010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler S., Fast D., Tenenhouse A. Role of Ca 2+ and cyclic AMP in protein secretion from rat exocrine pancreas. Biochim Biophys Acta. 1972 Oct 25;279(3):561–572. doi: 10.1016/0304-4165(72)90178-x. [DOI] [PubMed] [Google Scholar]
- Hokin L. E. Effects of calcium omission on acetylcholine-stimulated amylase secretion and phospholipid synthesis in pigeon pancreas slices. Biochim Biophys Acta. 1966 Jan 25;115(1):219–221. doi: 10.1016/0304-4165(66)90066-3. [DOI] [PubMed] [Google Scholar]
- Kanno T. Calcium-dependent amylase release and electrophysiological measurements in cells of the pancreas. J Physiol. 1972 Oct;226(2):353–371. doi: 10.1113/jphysiol.1972.sp009988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knodell R. G., Toskes P. P., Reber H. A., Brooks F. P. Significance of cyclic AMP in the regulation of exocrine pancreas secretion. Experientia. 1970 May 15;26(5):515–517. doi: 10.1007/BF01898480. [DOI] [PubMed] [Google Scholar]
- Kulka R. G., Sternlicht E. Enzyme secretion in mouse pancreas mediated by adenosine-3'5'-cyclic phosphate and inhibited by adenosine-3'-phosphate. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1123–1128. doi: 10.1073/pnas.61.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhlouf G. M. The neuroendocrine design of the gut. The play of chemicals in a chemical playground. Gastroenterology. 1974 Jul;67(1):159–184. [PubMed] [Google Scholar]
- Matthews E. K., Petersen O. H. Pancreatic acinar cells: ionic dependence of the membrane potential and acetycholine-induced depolarization. J Physiol. 1973 Jun;231(2):283–295. doi: 10.1113/jphysiol.1973.sp010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews E. K., Petersen O. H., Williams J. A. Pancreatic acinar cells: acetylcholine-induced membrane depolarization, calcium efflux and amylase release. J Physiol. 1973 Nov;234(3):689–701. doi: 10.1113/jphysiol.1973.sp010367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J. H., Spingola J., Grossman M. I. Endogenous cholecystokinin potentiates exogenous secretin on pancreas of dog. Am J Physiol. 1971 Sep;221(3):742–747. doi: 10.1152/ajplegacy.1971.221.3.742. [DOI] [PubMed] [Google Scholar]
- Nishiyama A., Petersen O. H. Pancreatic acinar cells: membrane potential and resistance change evoked by acetylcholine. J Physiol. 1974 Apr;238(1):145–158. doi: 10.1113/jphysiol.1974.sp010515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondetti M. A., Rubin B., Engel S. L., Pluscec J., Sheehan J. T. Cholecystokinin-pancreozymin: recent developments. Am J Dig Dis. 1970 Feb;15(2):149–156. doi: 10.1007/BF02235646. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Matthews E. K. The effect of pancreozymin and acetylcholine on the membrane potential of the pancreatic acinar cells. Experientia. 1972 Sep 15;28(9):1037–1038. doi: 10.1007/BF01918657. [DOI] [PubMed] [Google Scholar]
- Ridderstap A. S., Bonting S. L. Cyclic AMP and enzyme secretion by the isolated rabbit pancreas. Pflugers Arch. 1969;313(1):62–70. doi: 10.1007/BF00586329. [DOI] [PubMed] [Google Scholar]
- Robberecht P., Christophe J. Secretion of hydrolases by perfused fragments of rat pancreas: effect of calcium. Am J Physiol. 1971 Apr;220(4):911–917. doi: 10.1152/ajplegacy.1971.220.4.911. [DOI] [PubMed] [Google Scholar]
- Rutten W. J., de Pont J. J., Bonting S. L. Adenylate cyclase in the rat pancreas properties and stimulation by hormones. Biochim Biophys Acta. 1972 Jul 3;274(1):201–213. doi: 10.1016/0005-2736(72)90294-5. [DOI] [PubMed] [Google Scholar]
- Schramm M. Secretion of enzymes and other macromolecules. Annu Rev Biochem. 1967;36:307–320. doi: 10.1146/annurev.bi.36.070167.001515. [DOI] [PubMed] [Google Scholar]
- Williams J. A., Lee M. Pancreatic acinar cells: use of Ca++ ionophore to separate enzyme release from the earlier steps in stimulus-secretion coupling. Biochem Biophys Res Commun. 1974 Sep 23;60(2):542–548. doi: 10.1016/0006-291x(74)90274-5. [DOI] [PubMed] [Google Scholar]