Abstract
Reading instruction can direct attention to different unit sizes in print-to-speech mapping, ranging from grapheme-phoneme to whole-word relationships. Thus, attentional focus during learning might influence brain mechanisms recruited during reading, as indexed by the N170 response to visual words. To test this, two groups of adults were trained to read an artificial script under instructions directing attention to grapheme-phoneme versus whole-word associations. N170 responses were subsequently contrasted within an active reading task. Grapheme-phoneme focus drove a left-lateralized N170 response relative to the right-lateralized N170 under whole-word focus. These findings suggest a key role for attentional focus in early reading acquisition.
A central challenge in early reading acquisition is learning to link visual word forms (i.e., spelling) to spoken words (i.e., speech). Different unit sizes afford this mapping: attention can be focused on relating letters to sounds within words thereby concentrating on grapheme-phoneme associations, or on linking larger units such as letter clusters, onsets, rimes, and whole words to corresponding sounds. Directing learner’s attention to levels of representation that promote accurate and robust word knowledge can therefore serve an important role in education, given that reading ability is acquired specifically through instruction (McCandliss, Beck, Sandak, & Perfetti, 2003). Reading development theorists agree that focusing a student’s attention on individual letters and their relations to phonemes enhances the quality of word representations, especially for struggling readers who may have relatively low phonological awareness skills, and thus have difficulty focusing attention on such mappings (Ehri, 1991; Perfetti, 1991). Establishing stable grapheme-phoneme connections and specifying this information in the correct position in the word (e.g., Restricted Interactive Model, Perfetti, 1991) has been proposed to mediate successful reading acquisition. Furthermore, the ability to manipulate learned grapheme-phoneme associations is regarded as central to reading development as this skill not only contributes to the strengthening and refining of familiar word representations but also enables self-teaching of novel words (Share & Stanovich, 1995). In sum, phonological abilities (Bradley & Bryant, 1983; Goswami, 1993) and emerging decoding skills (Share & Stanovich, 1995) constitute the core prerequisites for normal reading development, and intentionally directing attention to mappings below the level of entire word units is a crucial component of these skills. Given the vital role of attention in beginning reading instruction, the neural processes engaged specifically in focusing attention on grapheme-phoneme versus whole-word relationships during and beyond training beckon a better understanding.
DEVELOPMENTAL DYNAMICS IN NEURAL NETWORKS FOR READING: PROGRESSIVE TUNING OF LEFT VENTRAL REGIONS
Fluent reading skill rests upon rapid, accurate word recognition abilities. In the skilled reader, as demonstrated by extensive neuroimaging work, these processes are sub-served by a cortical network including a domain-general anterior (inferior frontal) system and two consolidated posterior circuits: ventral (occipito-temporal) and dorsal (temporo-parietal) (Jobard, Crivello, & Tzourio-Mazoyer, 2003; Vigneau et al., 2006). Although the components of the reading network typically act in concert to integrate orthographic, phonological, and semantic word aspects, relative functional specializations have been proposed. Compared with the fast ventral system, the anterior and posterior dorsal circuits engage slower, computationally demanding processes (Breier, Simos, Zouridakis, & Papanicolaou, 1998; Tarkiainen, Helenius, Hansen, Cornelissen, & Salmelin, 1999). Throughout development the patterns of activation in the reading circuitry change substantially (for review, see Schlaggar & McCandliss, 2007). Initially, children recruit a widely distributed network, including left temporo-parietal, frontal and right posterior areas for word recognition (Booth et al., 2001; Turkeltaub, Gareau, Flowers, Zeffiro, & Eden, 2003). As reading skill accrues, beginners show enhanced engagement of left occipito-temporal ventral areas, which become increasingly tuned in responsiveness to the writing system being learned (Brem et al., 2006; Gaillard, Balsamo, Ibrahim, Sachs, & Xu, 2003; Pugh et al., 2001; Schlaggar et al., 2002; Turkeltaub, Gareau, Flowers, Zeffiro, & Eden, 2003).
Multiple studies have converged on the observation that the earliest region in the ventral visual stream that exhibits sensitivity to visual input resembling written words versus similar low-level control input is a left-lateralized region in mid-fusiform gyrus, termed the visual word form area (VWFA) (Cohen et al., 2002; McCandliss, Cohen, & Dehaene, 2003). Different lines of evidence have established that activity in the VWFA and nearby left ventral regions contributes to reading function (McCandliss, Cohen, & Dehaene, 2003). In literate adults the patterns of left ventral engagement can be linked to orthographic structure properties (e.g., letter position and bigram frequency), as well as to behavioral measures of word recognition (e.g., reaction times) (Binder, Medler, Westbury, Liebenthal, & Buchanan, 2006; Dehaene et al., 2004). Furthermore, lesions in left posterior regions in the vicinity of the VWFA are associated with reading deficits such as pure alexia (Cohen et al., 2003). Children’s reading abilities, across both normal and reading-impaired ranges, positively correlate with left occipito-temporal activations (Shaywitz et al., 2002). Notably, right ventral areas exhibit reduced performance-related involvement over the course of reading development (Turkeltaub, Gareau, Flowers, Zeffiro, & Eden, 2003) and skill improvement (Shaywitz et al., 2002). Collectively, these findings support the notion that fluent word recognition is associated with experience-driven functional refinement of perceptual regions that support reading skill, manifested as more focal, left-lateralized recruitment of ventral occipito-temporal regions.
PERCEPTUAL EXPERTISE FOR WORD FORMS: THE N170 VISUAL ERP RESPONSE
While successful in localizing reading circuitry, neuroimaging studies using low temporal resolution techniques (e.g., functional magnetic resonance imaging (fMRI) and positron emission tomography (PET)) provide limited insight into the reported effects with respect to the contribution of early perceptual versus post-perceptual processing. Electrophysiological recordings, on the other hand, due to their excellent temporal resolution, prove to be invaluable tools for investigating the impact of top-down attention to different levels of representation during perception of visual word forms (Posner & McCandliss, 1993). In the event-related potential (ERP), rapid processing of category-specific visual information is reliably indexed by the N170 component, which peaks between 150 and 200 msec following visual stimulus onset (Bentin, Mouchetant-Rostaing, Giard, Echallier, & Pernier, 1999; Rossion, Joyce, Cottrell, & Tarr, 2003; Schendan, Ganis, & Kutas, 1998), a time-range proposed by eye movement investigations to reflect initial word recognition processes (for review, see Rayner & Pollatsek, 1989).
The N170 response has been linked to perceptual expertise effects reflecting cumulative visual experience within domains that are common to most individuals (e.g., faces and words in literate adults, Bentin et al., 1999; Rossion et al., 2003) and also within domains that are specific to some individuals (e.g., experts for fingerprints [Busey & Vanderkolk, 2005] or cars [Gauthier, Curran, Curby, & Collins, 2003]). The characteristic N170 occipito-temporal negativity in adults tends to be right-lateralized or bilateral for faces and most objects of expertise (Bentin, Allison, Puce, Perez, &McCarthy, 1996; Rossion et al., 2003; Schendan et al., 1998; Tanaka & Curran, 2001).
N170 expertise effects for visual word forms, in contrast to N170 effects for other classes of perceptual expertise, are predominantly left-lateralized (Bentin et al., 1999; Brem et al., 2005; Maurer, Brandeis, & McCandliss, 2005; Maurer, Brem, Bucher, & Brandeis, 2005; Schendan et al., 1998; Maurer, Zevin, & McCandliss, 2008). They are generally characterized as reflecting pre-semantic sensitivity to properties of letter strings that distinguish word forms from closely visually matched symbol or shape strings (Bentin et al., 1999; Maurer et al., 2005; Rossion et al., 2003). Importantly, the sensitivity of the N170 response can be modulated by focusing attention on different linguistic representations associated with visual word forms. For instance, Bentin and colleagues (1999) reported that when task demands focused attention on lexical/phonological representations, N170 amplitudes elicited by non-words (unpronounceable consonant strings) differed from those elicited by words, yet when attention was focused on visual/orthographic representations, these same word/non-word stimuli elicited equivalent N170 responses (Bentin et al., 1999).
The left-lateralized N170 ERP response to visual words has been linked to neural activity in the VWFA region. The orthographic N170 response is generated in left occipito-temporal regions as demonstrated by intracranial recordings (Allison, McCarthy, Nobre, Puce, & Belger, 1994) and source localization estimates of scalp-recorded electroencephalography (Maurer et al., 2005; Rossion et al., 2003) and magnetoencephalography (Tarkiainen, Helenius, & Salmelin, 2003). Furthermore, individual differences in word-induced N170 amplitude have been shown to systematically correlate with metabolic activity in the VWFA in response to words (Brem et al., 2006). In dyslexia, early visual discrimination of letter strings is specifically compromised in both children (Maurer et al., 2007) and adults (Helenius, Tarkiainen, Cornelissen, Hansen, & Salmelin, 1999). Taken together these observations argue that the left-lateralized N170 perceptual expertise for word forms contributes to reading function and plausibly reading skill development.
LEFT LATERALIZATION OF THE VISUAL WORD FORM N170: THE PHONOLOGICAL MAPPING HYPOTHESIS
Developmental studies have revealed that the N170 expertise effect for visual word forms emerges with reading acquisition (Maurer et al., 2005; Maurer et al., 2006). The characteristic left lateralization of the effect shows a pattern of protracted development over the course of gaining reading proficiency (Maurer et al., 2007; Maurer et al., 2006; Parviainen, Helenius, Poskiparta, Niemi, & Salmelin, 2006). Behaviorally the rise of fluent reading skills involves progressive integration of orthographic with phonological and lexico-semantic word features (McCandliss et al., 2003), a process supported by increasing decoding abilities throughout learning (Share & Stanovich, 1995). Indeed, the phonological mapping hypothesis (Maurer & McCandliss, 2007) postulates that the grapheme-phoneme decoding of visual words, exercised consistently and repeatedly over the course of reading acquisition, drives the characteristic left lateralization of the N170 expertise effect for written words (given the predominant engagement of the left hemisphere in phonological processing, it accordingly induces left lateralization of the visual word form N170 response.) Here we extend this hypothesis to specifically regard the role of attention to grapheme-phoneme unit sizes in print-to-speech mapping as a factor that is important for the emergence of the left-lateralized N170 response to word forms. Such attentional focus proposition is in line with the proposed contribution of extensively trained patterns of selective attention to relevant attributes to the development of perceptual expertise for objects (for a discussion, see Palmeri, Wong, & Gauthier, 2004). Importantly, this attentional focus aspect of the phonological mapping hypothesis motivates particular predictions based on the specific reading instruction approach. If attention to grapheme-phoneme relationships is emphasized and reinforced during reading training, visual word forms should elicit a left-lateralized N170 response. Conversely, if grapheme-phoneme mappings are not highlighted, and thus not easily focused upon, as in the case of children with weak phonological skills or adults learning to read a script in which the grapheme-phoneme mappings have been obscured, a left-lateralized N170 topography should not emerge. It is worth noting that the choice to contrast whole-word versus grapheme-phoneme mapping levels in the present study is unrelated to debates in the literature regarding dual reading routes in the adult expert state (Coltheart, Rastle, Perry, Langdon, & Ziegler, 2001) but rather reflects the importance of explicitly and systematically directing attention to sub-lexical phonological units motivated by the literature on early reading acquisition (McCandliss et al., 2003; Schlaggar & McCandliss, 2007).
ARTIFICIAL ORTHOGRAPHY TRAINING IN ADULTS: ISOLATING THE IMPACT OF ATTENTIONAL FOCUS DURING READING ACQUISITION
Training literate adults to read a novel artificial writing system is an approach complementary to developmental studies that crucially allows an experimentally controlled manipulation of attentional focus during instruction in relative isolation from other influences. Artificial orthography training studies in skilled adult readers have demonstrated differences in behavioral performance on tasks during and following whole-word versus grapheme-phoneme training (Bishop, 1964; Bitan, Manor, Morocz, & Karni, 2005; McCandliss, Schneider, & Smith, 1997). fMRI results have been promising as well, reporting differential involvement of components of the reading circuitry depending on the artificial script training strategy (Bitan et al., 2005; Xue, Chen, Jin, & Dong, 2006). Importantly, focus on phonological features during learning of new words, as contrasted with visual or semantic features, has been shown to specifically alter activity in the left occipito-temporal ventral stream (Sandak et al., 2004; Xue et al., 2006). Whether such attentional focus effects are actual modulations of early perceptual processes applied to visual word forms, as opposed to later post-perceptual processes, remains an open question.
PRESENT STUDY: AIMS, DESIGN, AND HYPOTHESIS
The present study examined adult learning in a short-term training session with an artificial orthography and used ERP measures to investigate the impact of attending to different levels of representation in relating print to speech on subsequently tested N170 response to visual words. The experiment entailed teaching two groups to associate written words with corresponding spoken words, presented under identical conditions during learning and testing. The only manipulation was the instructional content of a single slide presented at the onset of training. This instructional manipulation was designed to bias one group of learners (the whole-word group) to focus attention on each visual character, as a whole, in the writing system and associate it with an entire spoken English word, and to bias a second group of learners (the grapheme-phoneme group) to focus attention on embedded letter-like figures within each visual character and associate them with phonemes in each spoken English word. Thus the design isolated the influence of attentional focus during training, while controlling for typically confounded factors, such as stimulus characteristics and individual differences among learners. A post-training reading verification task, identical for the two training groups, assessed learning and transfer, and provided ERP probes of whether differential neural circuitry was recruited based on instructional focus. The current experiment tested an aspect of the phonological mapping hypothesis that we consider to be central to issues of early literacy, namely that the left lateralization of the N170 response to recently trained visual words is linked to the degree to which students focus their attention on grapheme-phoneme relationships while acquiring new relationships between print and speech.
METHODS
Participants
Right-handed native English speakers with normal reading abilities (TOWRE, Torgesen, Wagner, & Rashotte, 1999) and normal or corrected-to-normal vision took part in the study. Additional inclusion criteria were based on the reading verification task: behavioral (accuracy >80% with trained characters) and ERP data quality (signal-to-noise ratio >1.75). The reported data are from two equally-sized experimental groups matched for age and sex (30 subjects in total: mean age = 25 years; 10 male; all right-handed). Participants provided informed consent in an experimental protocol approved by the Institutional Review Board Committee of the Weill Medical College of Cornell University.
Stimuli
We created a novel artificial script, which consisted of word characters containing embedded letter-like figures evident only when instruction draws attention to them. This feature of the characters made it possible to experimentally manipulate attentional focus by revealing the underlying grapheme-phoneme mapping to only half of the subjects, while withholding the appropriate segmentation cues from the other half. The embedded letter-like figures were stacked in a vertical manner, rendering them dissimilar to familiar alphabetic fonts and enabling whole character integration (Nelson, Liu, Fiez, & Perfetti, 2009). Eight consonants (b, d, m, n, k, r, s, t) and four vowels (a, i, e, u) were used to compose 32 simple consonant-vowel-consonant English words. The embedded letter-like figures were novel black line-drawings on white background, and each character subtended 2.4° horizontal and 2.6° vertical visual angle. Auditory words spanned 600 msec on average (SD = 55 msec) and were spoken by a female native English speaker.
Training in Artificial Orthography
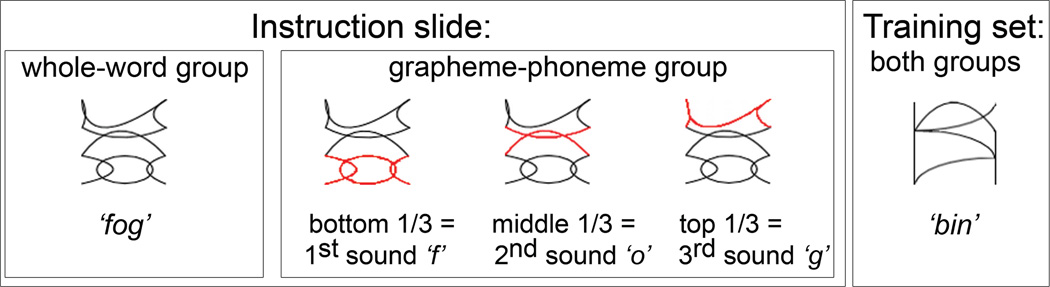
All subjects learned to associate an auditory word with each visual character. Participants were trained in either the whole-word condition or in the phoneme-grapheme condition. Training was identical except for the different instruction slide in the beginning of the training phase prescribing the use of one of the two strategies. The whole-word group (N = 15) was instructed to link whole characters with auditory words, while the phoneme-grapheme group (N = 15) was focused on associating embedded letter figures with sounds within words (Figure 1). Training lasted approximately 20 minutes, over the course of which participants were presented with 16 visual character-auditory word pairs, with 20 non-consecutive repetitions per pair. A trial began with the presentation of the visual character, which stayed on the screen for 2234 msec. 1334 msec following visual stimulus onset, the corresponding auditory word was played over the speakers. Presentation of an irrelevant face stimulus of a fearful or neutral expression for 300 msec preceded each trial. This facial expression manipulation (reported elsewhere: Blau, Maurer, Tottenham, & McCandliss, 2007) was counterbalanced across our training conditions and was not related to the present study.
FIGURE 1.
Manipulating attentional focus during training in artificial orthography. Participants were trained in either the whole-word condition or in the phoneme-grapheme condition. Training was identical for both groups (exactly the same visual characters and auditory words were presented), except for the instructional slide at the onset of training, which prescribed the use of one of two learning strategies. The grapheme-phoneme group was focused on linking the hidden letters with sounds within words, whereas the whole-word group was asked to associate whole visual characters with entire auditory words. (Figure is available in color online)
Reading Verification Task
The reading verification task was a two-alternative forced choice judgment of whether the presented visual word character matches with the auditory word. A trial commenced with fixation (mean duration 750 msec) followed by the presentation of a visual character (mean duration 2000 msec). Next, an auditory word (mean duration 600 msec) was presented, 667–1000 msec after the onset of the visual character. To assess alphabetic transfer, in addition to the trained characters, the task included word characters of the same script that were novel but decodable based on the grapheme-phoneme relationships. Trained and transfer character sets were counterbalanced across subjects and groups using three sets that were closely matched (100% overlap at the letter type level and 92% overlap on average at the token level). There was a trained character block (16 words, 16 repetitions) and a transfer character block (16 words, 8 repetitions). The overall task duration was approximately 13 minutes, and participants could take breaks between blocks, if desired. “Yes” and “no” trials were presented with equal probability. Electroencephalogram (EEG) was recorded and ERPs to the visual symbol were reported for the reading verification task. The task was identical for the two training groups.
EEG Recording and Preprocessing
EEG recording was acquired using a 128-channel Geodesic Sensor Net 200 (Electrical Geodesics Inc., Eugene, Oregon) referenced to the vertex electrode (Tucker, 1993). Data were sampled at 250 Hz/channel with calibrated technical zero baselines and filters set at 0.1–100 Hz. Electrode impedances were below 50 kΩ. Spline interpolation was applied to channels with excessive artifacts and eye blink correction followed in BESA 5.1 software. EEG data were then digitally band-pass filtered (1–30 Hz, 24 dB/oct), epoched from −150 msec pre-stimulus (visual character) to 750 msec post-stimulus. Artifacts exceeding ±100 µV in any channel were automatically rejected. Single-subject averaging was done separately for each condition (trained, transfer characters). In Brain Vision Analyzer, ERPs were re-referenced to average reference, then Global Field Power (GFP; spatial root mean squared of amplitude values at all electrodes) and grand averages were computed collapsed over training group and character condition, as well as separately for each character type for each group (Lehmann & Skrandies, 1980).
ERP Analyses
Given our a priori hypothesis that attentional focus during training modulates subsequent N170 lateralization, we employed a data-driven approach sensitive to topographic differences, including lateralization, to identify the time range over which the two training groups exhibited differential stimulus processing. Accordingly, we conducted topographic bootstrapping tests (topographic analysis of variance, TANOVA, Strik, Fallgatter, Brandeis, & Pascual-Marqui, 1998) using LORETA software package on normalized ERP maps (GFP = 1) between the two training groups for each time point in the range of early latency ERP components (0–400 msec) for each character condition. To account for multiple comparisons (over 100 time-frames) the criterion for statistical significance was set at three or more consecutive time-frames each significant at the p < .05 alpha level (such joint probability of p < .05 over three frames (i.e., 0.05*0.05*0.05) is lower than an equivalent Bonferroni-corrected p value (i.e., 0.05/100)). TANOVA on normalized maps detects systematic topographic differences between the two training groups (independent of overall amplitude variations) and was used to determine the time-window for further investigation. Over the interval 0–400 msec following visual character onset, significant differences (p < .05) between the grapheme-phoneme and the whole-word group were found only in the 186–198 msec interval. This was the case independently for both trained and transfer characters. Notably, no time-frames in the P100 range showed significant group differences. Next, we set out to confirm that this segment, obtained based on group differences, temporally corresponded to the N170 component in the robust ERP response associated with visual word processing across any condition. Thus, we performed adaptive segmentation based on minima in the GFP of the ERP response to the visual character (collapsed over training group and character type), which identified the N170 component as spanning from 170 to 218 msec after visual character presentation (Brandeis, Vitacco, & Steinhausen, 1994; Lehmann & Skrandies, 1980; Maurer et al., 2005). This supported expectations that the training effect, as revealed by the difference-based TANOVA, occurred during the N170 component. Therefore, samples at 186, 190, 194, and 198 msec were defined as the N170 response of interest in the present study, and statistical comparisons were performed between conditions over this averaged (186–198 msec) segment. The central findings were also tested over the extended 170–218 msec time window, and the 170–218 msec segment results corroborated the N170 results.
Two indices were computed for the N170 segment map at the individual level for each character condition: (1) GFP (strength of the electric field) aimed at attesting that observed differences are purely topographic, that is, in the absence of GFP difference; (2) topographic 3D centroids (center of gravity for positive and negative map regions; x-, y-, z-axis locations presented in Talairach space (Talairach & Tournoux, 1988)), which reduce topographic map complexity to six quantifiable parameters (Brandeis et al., 1994; Maurer, Blau, Yoncheva, & McCandliss, 2010/this issue; Maurer et al., 2005), aimed at testing lateralization effects. We consider an ERP component to be characterized by a stable topographic map (Lehmann & Skrandies, 1980) that, when average-referenced, consists of negativities and positivities, which can be quantified by a pair of corresponding topographic 3D centroids (a positive and a negative centroid).
To facilitate comparison with conventional ERP analysis approaches and to further characterize lateralization effects, selected waveforms at left and right occipito-temporal sites were also studied. Based on the N170 segment collapsed across group and condition, the homologous left and right hemisphere electrode pairs showing the (pair-wise) most negative values along with the six immediately adjacent electrodes within each hemisphere were identified. This resulted in a left hemisphere channel cluster (channels 51, 52, 58, 59, 60, 65, and 66) and a right hemisphere cluster (channels 85, 86, 91, 92, 93, 97, and 98). Relative to hallmarks of the 10–20 system, the left hemisphere cluster roughly encompassed P7, while the right hemisphere cluster roughly encompassed P8 (Luu & Ferree, 2000). N170 ERP values from each channel were averaged within a hemisphere group, for which mean N170 amplitudes (over the 186–198 msec range), as well as peak N170 amplitudes (in the 192 msec ± 10 time-frames range) were computed for each character type at the individual level. We focused on left and right occipito-temporal channel groups since these sites have been shown to be most sensitive to differences between objects of expertise and control stimuli (Maurer et al., 2005; Tanaka & Curran, 2001). Additionally, the time-course of the training effect was illustrated at selected waveforms. The sites that showed the maximal group differences for trained characters in the N170 segment were identified and potentials at these channels were averaged with the potentials of their neighboring channels within channel clusters chosen to reflect divisions within the 10–20 landmark system (Luu & Ferree, 2000). The grapheme-phoneme group had larger negative potentials compared to the whole-word group over occipito-temporal sites at the left mastoid (LM) cluster, which included channels 56, 63, 64, 57. Correspondingly, the grapheme-phoneme group also had larger positive potentials compared to the whole-word group over central sites at the right-hemisphere central cluster, which was centered approximately at C4 and included channels 88, 94, 104, 105, 106, 111, 112. For these “C4” and “LM” clusters timepoint-wise between-group t-tests were computed for trained and transfer characters. Again, to account for multiple comparisons over the 0–400 msec time-range, significant effects were defined as at least three consecutive p < .05 timeframes.
Analyses of GFP, centroid locations, and N170 amplitude values were conducted in SPSS. Multivariate analyses of variance (MANOVA) for repeated measures with within-subject factors “character type” (trained vs. transfer) and between-subject factor “group” (whole-word vs. grapheme-phoneme training condition) was performed as well as planned comparisons separately for “character type” and “group.” The centroid analyses included “polarity” (positive vs. negative centroid) as an additional factor, and the three location dimensions of the centroids (x-, y-, and z-axes) were treated as multivariate dependent measures. Polarity is only reported when it interacts significantly with other factors. Effects on the x-axis indicate lateralization effects. The waveform analyses also included “hemisphere” as a factor. Behavioral data were assessed using t-tests. Significance level was set at 0.05 for all tests.
RESULTS
Behavioral Data
Consistent with previous findings (McCandliss et al., 1997), the whole-word group showed an advantage in behavioral performance over the grapheme-phoneme group when tested in the reading verification task with trained characters. This was the case both in terms of accuracy (mean 95.1 % ± SD 3.9 versus 89.2 % ± 5.6: t(28) = 4.76, p < .001) and reaction times (895.5 msec ± 140.2 versus 1080.5 msec ± 155.4: t(28) = 3.24, p < .005). Notably, in the transfer condition of the reading verification test, the whole-word group performed at chance (t(14) = 0.48, ns) with accuracy significantly lower than the grapheme-phoneme group (58 % ± 8.9 vs. 78.5 % ± 7.6: t(28) = 2.56, p < .001). Reaction times for transfer characters were comparable between the two groups (whole-word 1077.0 msec ± 198.0 vs. 1148.9 msec ± 159.9: t(28) = 1.70, ns).
Differences Between Training Groups in Consecutive ERP Maps
Differential ERP responses between the two training conditions over time were examined using a topographic analysis of variance (TANOVA) on normalized maps conducted separately for trained and transfer characters. Processing of visual word characters differed (p < .05) between groups from 186 to 198 msec (independently for both character types). An adaptive GFP minima segmentation approach, which is not biased by group differences, but rather reflects the robust N170 ERP response associated with visual character processing for all conditions collectively, was used to confirm that the 186–198 msec belonged to the N170 response. Therefore, the N170 interval was defined as samples 186 to 198 msec, and ERPs averaged over this segment were used for all subsequent analyses.
N170 Time Interval
GFP analysis
Overall N170 map strength, as indexed by GFP, did not differ significantly between training groups for trained characters (t(28) = 1.033, p = .311, ns) and for transfer words (t(28) = 1.252, p = .221, ns). The similarity of GFP across the two groups was independent of character type (ANOVA with factors “character type” and “group” showed no significant main effect of character type: F(1, 28) = 0.39, p = .535, ns or interaction with group).
Topographic centroid effects
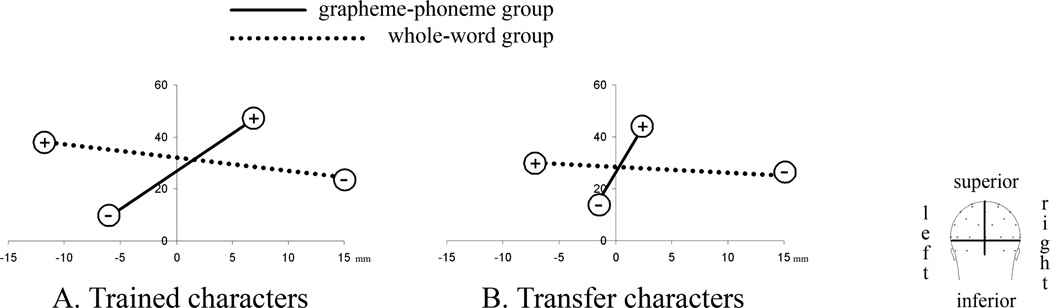
Assessment of topographic differences between training groups was performed based on centroid measures, which describe the distribution of positivity and negativity on the scalp surface. The 3D locations of the positive and negative centroids were tested using multivariate analyses of variance (MANOVA) for repeated measures with within-subject factor “polarity” (positive vs. negative centroid) and between-subject factor “group” (whole-word vs. grapheme-phoneme group). Significant contrast main effects and polarity interactions (p < .05) at the multivariate level were followed by univariate tests to identify the spatial direction (x-, y-, and z-axes) of the effect.
The positivity/negativity distribution differed significantly between the two training groups for trained characters (multivariate MANOVA: “polarity” by “group” F(3,26) = 3.067, p < .05; Figure 2a). In particular, the grapheme-phoneme group exhibited a more left-lateralized negativity relative to the whole-word group (significant univariate x-axis: F(1,28) = 5.506, p < .05).1 A similar group difference was observed for the transfer characters (multivariate MANOVA: “polarity” by “group” F(3,26) = 3.021, p < .05; univariate axes: x-axis F(1,28) = 3.143, p < .1, z-axis F(1,28) = 3.291, p < .1; Figure 2b).2 A comprehensive MANOVA corroborated that the pattern of differential lateralization between the two training conditions was not dependent on character type (MANOVA: “polarity” by “group” F(3,26) = 3.542, p < .05 (x-axis F(1,28) = 4.834, p < .05; z-axis F(1,28) = 3.208, p < .1); “character type” by “polarity” F(3,26) = 1.889, ns; “character type” by “polarity” by “group” F(3,26) = 0.528, ns). Since the occipito-temporal negativity is the hallmark of the N170 component we also zoomed in on the negative centroids in order to confirm that the two training conditions showed differentially lateralized ERPs in the reading test (“group”: F(3,26) = 3.013, p < .05 (significant x-axis F(1,28) = 4.225); main effect of “character type” and “character type” by “group” interactions are ns: F < 2).
FIGURE 2.
Centroid locations reflecting N170 topographies in response to (a) trained and (b) transfer characters in the reading verification task. The most prominent difference in centroid positions between the two training groups is in the coordinates along the x-axis (left-right). Note that for both character types the center of the N170 negativity of the grapheme-phoneme group is more left-lateralized than that of the whole-word group.
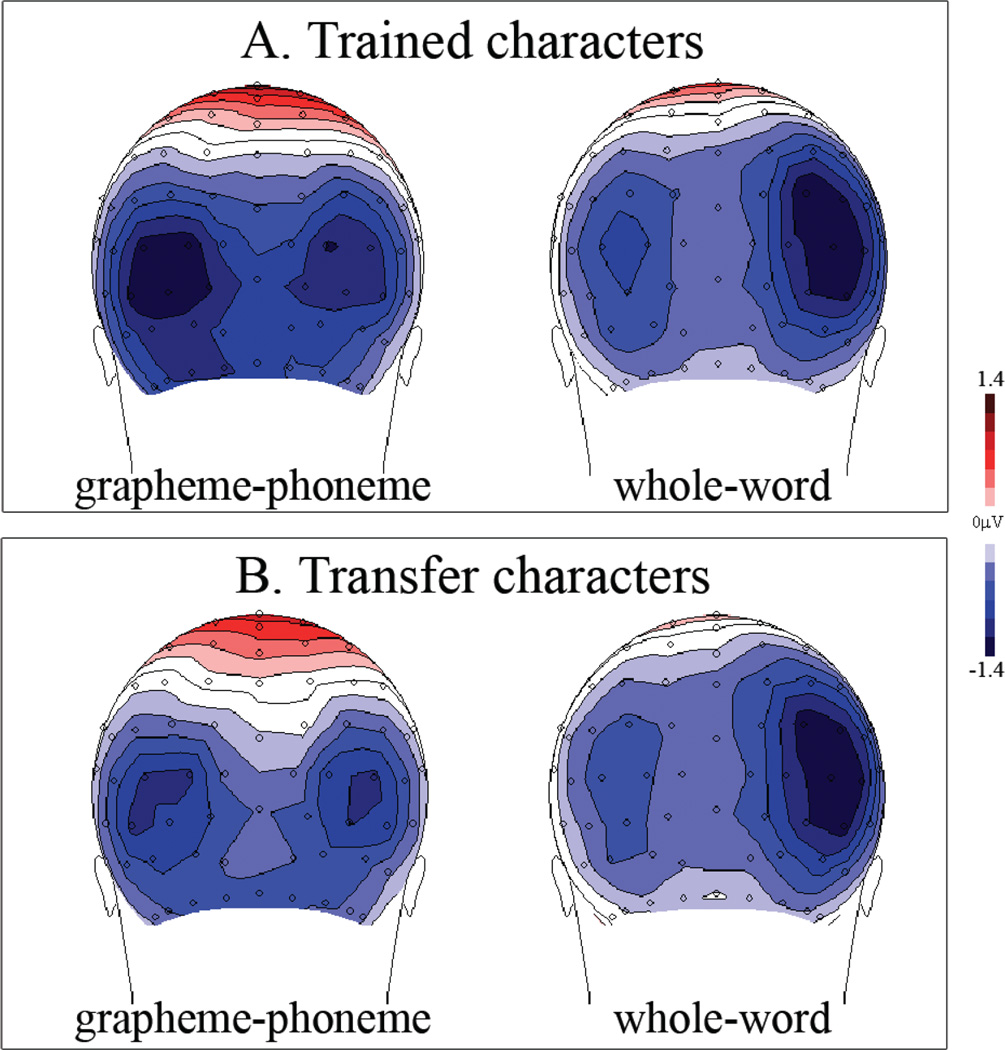
Overall, in the N170 window the grapheme-phoneme group, irrespective of character typecondition, exhibited a predominantly left-lateralized topography over occipito-temporal regions as compared to the more right-lateralized topography of the whole-word training condition (Figure 3).
FIGURE 3.
Topographic maps of the N170 ERP in response to (a) trained and (b) transfer characters in the reading verification task. The grapheme-phoneme group exhibits a predominantly left-lateralized topography over occipito-temporal regions relative to the right-lateralized topography of the whole-word group. (Figure is available in color online)
Selected waveform analyses
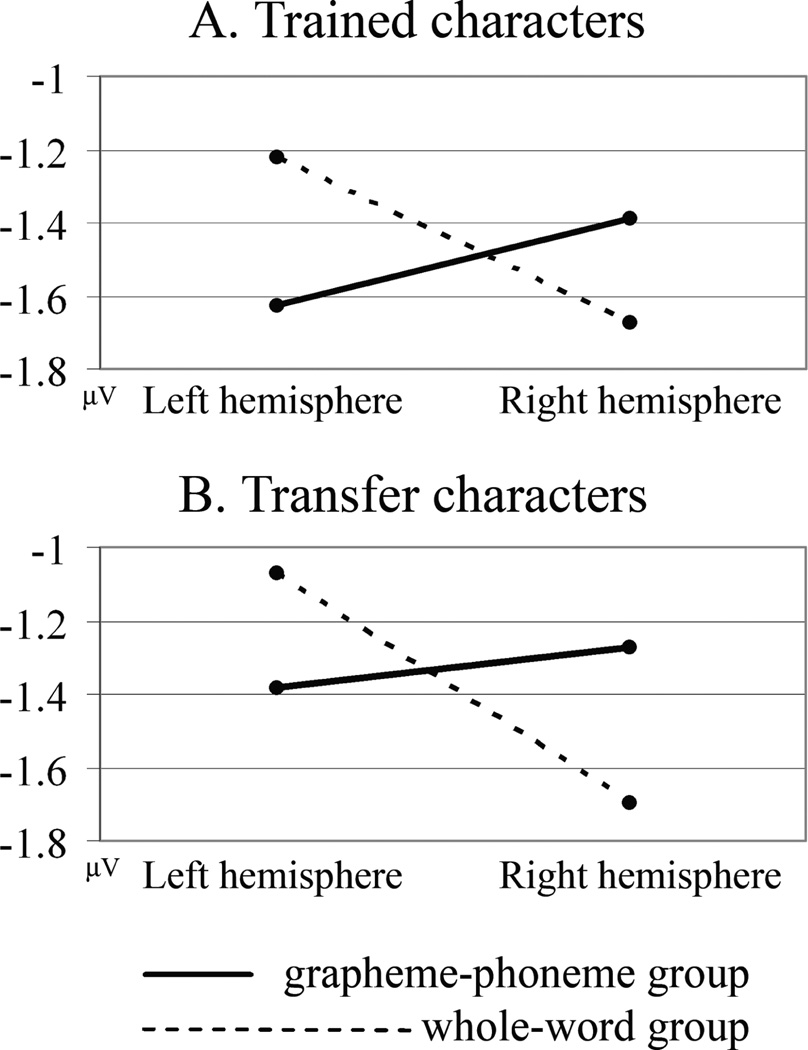
Lateralization training effects were also studied at the waveform level. Consistent with topographic centroid findings, peak N170 amplitude differences between training groups differed across left and right hemisphere locations. This was the case for both trained characters (“group” by “hemisphere” interaction, F(1,28) = 7.084, p < .05; Figure 4a) and transfer characters (“group” by “hemisphere” interaction, F(1,28) = 7.288, p < .05; Figure 4b). Again, the differential lateralization was comparable for both character types as indicated by ANOVA analysis (“group” by “hemisphere” interaction, F(1,28) = 7.188, p < .05; “character type” by “group” and “character type” by “group” by “hemisphere” interactions are all ns, F < 2.6). Mean N170 amplitudes showed a pattern similar to peak N170 amplitudes: a significantly more right-lateralized N170 response in the whole-word group compared to the grapheme-phoneme group irrespective of character type (ANOVA: “group” by “hemisphere” interaction, F(1,28) = 4.291, p < .05; “character type” by “group” and “character type” by “group” by “hemisphere” interactions are all ns, F < .933). The relative lateralization difference of the N170 ERP between the two training conditions was thus corroborated in the waveform analysis.
FIGURE 4.
N170 amplitudes at left (“P7”) and right (“P8”) occipito-temporal channel clusters in response to (a) trained and (b) transfer characters in the reading verification task. An interaction between hemisphere and training condition is evident for both trained and transfer symbols. The grapheme-phoneme group shows larger N170 amplitudes in the left than in the right hemisphere, while the whole-word group exhibits the reverse pattern with a stronger N170 response in the right hemisphere.
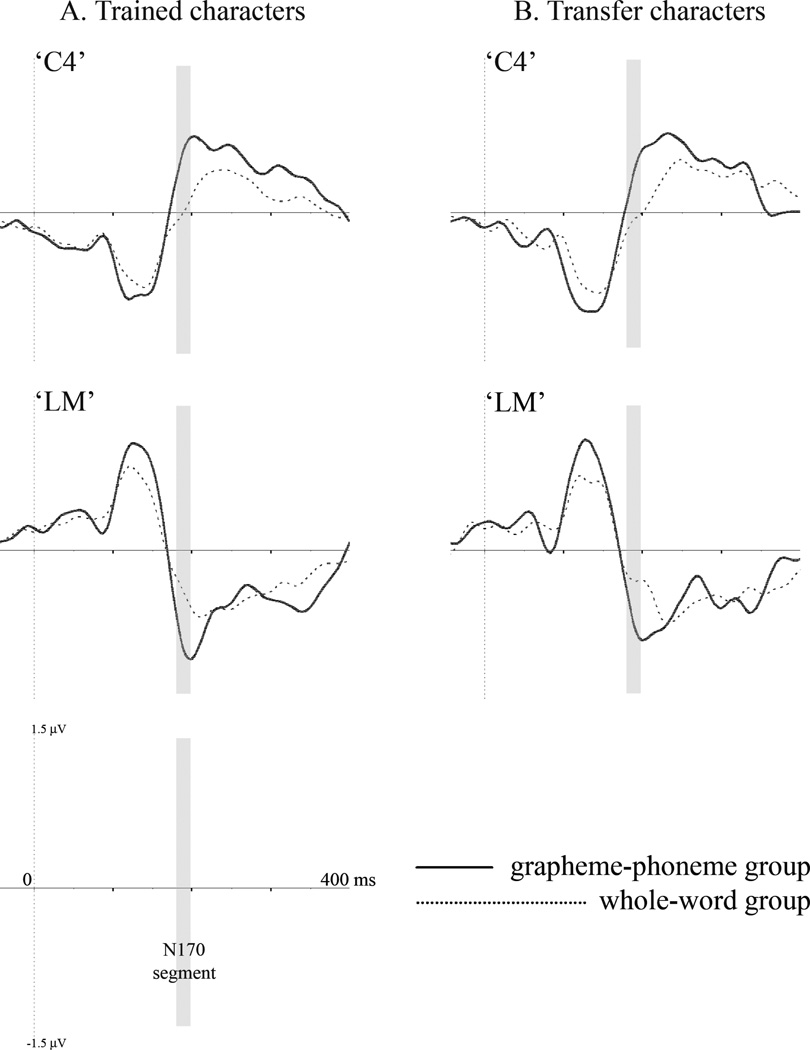
The time-course of the training group difference is illustrated in Figure 5. In the left inferior occipito-temporal “LM” cluster, for both trained and transfer characters, the only time-frames significant at the p < .05 level were confined to the N170 segment. For trained characters the significant group effect also passed the three consecutive time-point restriction (190–198 msec). A similar trend emerged for transfer characters, with two consecutive time-points significant at the p < .05 level (186–190 msec), but this effect did not surpass the three consecutive sample constraint. In the right central “C4” cluster, a significant training effect was also only observed in the range of the N170 response (consecutive p < .05 time-points for trained characters: 182–206 msec; for transfer characters: 186–218 msec). Consistent with the whole-map findings, the waveform-level effect illustrations also indicate that current training effects are specific to the time-range of the N170 response.
FIGURE 5.
Grand-average waveforms of (a) trained and (b) transfer character event-related potentials (ERPs) in the reading verification task at left inferior occipito-temporal channel cluster “LM” and right central cluster “C4.” For both character types, the gray bars indicate the boundaries of the N170 segment (186–198 msec).
DISCUSSION
The present results indicate that attentional focus on different unit sizes of representations that relate print to speech systematically impacted learning, transfer, and the left lateralization of the N170 response to the newly learned words. This effect was observed under between-group training conditions that maintained similar general training goals, equal learning time, identical visual stimuli, identical auditory stimuli, and identical mappings between visual and auditory stimuli. Post-training, visual characters elicited a left-lateralized N170 response in the grapheme-phoneme group relative to the right-lateralized N170 ERP of the whole-word group in the identical for the two groups reading verification task. This left-lateralized N170 effect in the grapheme-phoneme group was observed for trained as well as for transfer words, with a more robust lateralization bias for trained characters. Behavioral performance on trained items revealed that both groups successfully learned to associate visual characters with the corresponding spoken words. However, while the whole-word group exhibited a slight advantage for verifying exactly matching words versus close distracters (composed of different combinations of embedded letter-figures), the grapheme-phoneme group showed an advantage in the alphabetic transfer test. Finally, the whole-word group’s accuracy for transfer items was indistinguishable from chance, indicating that no detectable implicit learning took place in the absence of explicit instruction of grapheme-phoneme mappings.
The differential N170 lateralization between the groups is interpreted here in the light of the present experimental design, which sought to equate several aspects of the learning situation, commonly confounded in natural settings. First, the stimuli (visual and auditory) and the visual-auditory pairings were identical for the two groups, therefore ruling out the possibility that the group ERP lateralization effects were related to previous associations with the novel visual stimuli, the nature of particular visual-auditory pairings, or specific stimulus properties. Ensuring identical bottom-up stimulation is in line with laterality accounts focusing on hemispheric specialization for sensory information processing (e.g., high/low spatial frequency model (Sergent, 1983)). Second, participants viewed and listened to the stimuli for the same amount of time within the same general learning task context (i.e., both groups had the explicit goal of learning to associate visual with spoken word stimuli). Third, since the visual word characters and the embedded letter-figures were novel to both groups prior to training, confounds of previous experience, typically related to skill differences, were prevented. Fourth, both groups participated in identical post-training assessment, in which ERPs were collected to each visual stimulus, in advance of the auditory stimulus, and therefore in advance of the match/mismatch decision. Thus, the N170 response should not reflect processes tied to accuracy even though behavioral performance was not fully equated across groups. Moreover, the left-lateralized N170 effect for the grapheme-phoneme group relative to the whole-word group was similar across both trained and transfer items, indicating that group accuracy differences are unlikely to account for the between-group differences in the N170 effect. The lack of feedback during testing further reduces the likelihood that the group effect reflects learning during this phase of the experiment, although some degree of learning has been demonstrated to occur with and without feedback in similar adult language training studies (McClelland, Fiez, & McCandliss, 2002).
We focus discussion next on the nature of the experimental manipulation, which was restricted to initial instruction that directed attention to small versus large units of representations for mapping print to speech. Given that all other stimulus and task related factors were identical, the differential visual word form N170 responses based on training condition must be driven by a class of top-down processes, which we characterize as attentional focus. This account highlights the fact that all unit sizes were simultaneously present for both groups and that both groups carried out the same general goal of learning to associate novel print with familiar spoken words, yet top-down instructional biases led them to attend to different visual and phonological representations and their associations. This view fits with the notion of perceptual expertise effects as emergent properties of learned selective attention to relevant attributes (discussed in Palmeri et al., 2004). In the current study, the group-specific N170 lateralization patterns were not restricted to the specific letter combinations encountered during training (i.e., patterns for trained and transfer characters were largely equivalent). This indicates that instruction of the correspondence between visual and auditory words (irrespective of focus on specific unit size) led to a generalization of the N170 response to the artificial orthography as a stimulus class based on the trained individual instances. Crucially, explicit attentional focus on grapheme-phoneme mappings was necessary for transfer of alphabetic knowledge. Thus, the differential N170 response between the two groups on the post-training reading verification test is due to the bias toward a representational level (grapheme-phoneme vs. whole-word) acquired during training.
To further refine and clarify our interpretation of the present results as reflecting attentional focus on different unit sizes in mapping print to speech, it may be useful to differentiate this construct, from other, more general forms of attention known to influence early ERP responses (i.e., visuo-spatial attention, global-local attention, and the continuum from controlled to automatic processing). First, let us consider simple visuo-spatial attentional effects. These are typically characterized by reliable retinotopic organization and a latency corresponding to the P100 component of the visual ERP (Di Russo, Martinez, & Hillyard, 2003; Woldorff et al., 1997). In the present study, the visual characters contained vertically stacked letter-figures promoting bottom-to-top attentional shifts as opposed to left-right shifts; moreover, each character was presented centrally and contained differentiating features distributed equivalently over the left and right visual hemifields. Additionally, prior to the N170 time-range there were no statistically significant whole-map differences between the training groups for either trained or transfer characters. Thus, while visuo-spatial attentional effects cannot be ruled out based on the current experimental manipulation and results, there is little evidence to suggest that such processes underlie the observed N170 group difference. Another possible explanation for the present ERP results could be differences in visual attention to global versus local stimulus features. Hemispheric asymmetries in cortical activation when attending to global versus local features in a hierarchically organized stimulus have been previously demonstrated (Fink, Marshall, Halligan, & Dolan, 1999). However, reports of such global/local lateralization of the N170 visual ERP response, in particular, have been inconsistent (Evans, Shedden, Hevenor, & Hahn, 2000; Han, Liu, Yund, & Woods, 2000; Jiang & Han, 2005). Finally, another potential framework for the current ERP findings is to consider attention as it relates to the typical trajectory of learning and the associated transition from controlled, attention-demanding processing to automatic processing (e.g., Schneider & Shiffrin, 1977). Processing novel stimuli or performing novel tasks are typically thought to rely on controlled, voluntary processes, which require attention. With extensive learning, stimulus responses become automated, and attention is required less or not at all. Accuracy data, however, demonstrate that both training groups were in the early phases of learning, especially when considered within the time-scale of other perceptual expertise training studies, which typically involve many hours of practice over multiple sessions (Gauthier, Williams, Tarr, & Tanaka, 1998; Tanaka, Curran, & Sheinberg, 2005). Further, following the same amount of training, divergence in N170 lateralization between the two groups was not observed in a different task (Maurer et al., 2010/this issue), suggesting that levels of automaticity (or lack thereof) do not drastically differ between groups. Overall, although general attentional influences cannot be excluded, these forms of attentional processing fail to offer compelling explanations of the present ERP results given the lines of reasoning detailed earlier. Our favored interpretation of the observed N170 effects is that they are due to an additional, specific form of attention, which can be characterized as attentional focus on larger versus smaller unit sizes in relating print to speech.
The interpretation of the differential N170 lateralization based on training focus is in agreement with the phonological mapping hypothesis, which holds that left-lateralized N170 expertise effects for words are related to print-to-speech mapping at the level of grapheme-phoneme associations (Maurer & McCandliss, 2007). The present study extends this framework to highlight the crucial role of attention to such unit sizes during learning and practice, even when stimuli and learning intentions are held constant. A series of related investigations, examining these attentional phenomena in greater detail, provide an informative context for the current findings. A recent fMRI study revealed that left VWFA was differentially engaged when focusing attention on phonological as opposed to general acoustic features within complex auditory stimuli that combined speech and tones (Yoncheva, Zevin, Maurer, & McCandliss, 2010). This was the case when contrasting two equally difficult tasks performed on identical stimuli, thus isolating the impact of attentional focus on phonology, and demonstrating how such focus impacts regions associated with orthography, even in the absence of any visual stimulation. In a parallel paradigm, ERP responses during stimulus encoding were shown to be modulated by intentionally focusing on phonological distinctions within spoken words (Yoncheva, Maurer, Daruwalla, Zevin, & McCandliss, 2008). In both the fMRI and the ERP studies these top-down attentional effects were observed without visual word presentation, pointing to an attentional influence on early integration of grapheme and phoneme representations. Accordingly, the current results may reflect aspects of acquired print-to-speech associations, and not simply top-down biases on phonological processing.
Furthermore, the current ERP findings are consistent with several lines of fMRI evidence suggesting that skilled readers’ intentional engagement of phonological representations may substantially influence brain mechanisms shaped by the early stages of learning a novel script. For instance, adults who had learned to associate an artificial writing system with corresponding speech sounds exhibited a predominantly left-lateralized engagement of posterior extrastriate areas in response to newly learned characters (Xue et al., 2006). Such left-hemispheric dominance, importantly, was not observed following training with visual word forms alone, and failed to emerge even after an intensive, two-week visual-only training program. This reinforces the notion that phonological instruction is qualitatively distinct from purely visual instruction, and that engagement of the left-lateralized response to visual stimuli may be more linked to phonological-orthographic processing rather than to visual familiarity alone. Similarly, focusing learners’ attention on phonological, as opposed to visual, aspects of visually presented pseudowords were shown to modulate later fMRI responses in a left ventral occipito-temporal region, likely including the VWFA, during a test that presented the trained pseudowords under identical task conditions (Sandak et al., 2004). In addition, another artificial orthography training study reported that learning to link novel letter forms specifically with speech sounds, in contrast to the control condition linking with non-speech sounds, led to differential responses in left fusiform and left occipito-parietal regions (Hashimoto & Sakai, 2004). Taken together these fMRI findings suggest that attention to phonological associations with visual orthographic stimuli during learning modulates VWFA activity during later exposures to the visual stimuli.
As reviewed in the introduction, substantial evidence links cortical activity in the VWFA, implicated in the training effects detailed earlier, to the visual word form N170 response (Allison et al., 1994; Brem et al., 2006; Maurer et al., 2005; Tarkiainen et al., 2003). Importantly, relating the present ERP results to the reviewed fMRI training studies helps clarify the nature of the impact of attending to phonological and orthographic representations on early learning by providing critical information about the time-course of these influences. The current study demonstrates that differential attentional focus during training modulates early perceptual expertise for word forms, reflected in the N170 effects, which precede later post-perceptual and decision-making processes. Thus, the present artificial orthography training study extends insights into the key component in acquiring perceptual expertise for a novel script, namely explicit training of grapheme-phoneme associations, and the contribution of attentional focus in this process.
Findings based on skilled adult readers may hold implications for considering the course of reading acquisition throughout development. The rise of reading skills in children is associated with functional refinement of perceptual brain mechanisms supporting expertise, mainly characterized by the transition to increasingly more focal, left-lateralized ventral recruitment (Schlaggar & McCandliss, 2007). The protracted development of the word-specific N170 topography as it becomes more expert-like, specifically in terms of lateralization, likely parallels cognitive hallmarks in the reading acquisition trajectory. During the initial learning steps, an important facilitator of literacy acquisition might be the establishment of familiarity with the visual script. This process potentially draws on object recognition circuitry, given the predominantly right-lateralized word N170 in kindergarten children with high levels of letter knowledge (Maurer et al., 2005). The increasingly left-lateralized response in 2nd graders (Maurer et al., 2007; Maurer et al., 2006) might correspond to later reading instruction phases that involve active pursuit of specific learning goals, drawing attention to representations promoting phonological processing. Such parallels between the rise of reading skill and visual word form N170 are congruent with reading development accounts postulating the need for progressive disengagement of posterior right hemisphere visual representations over the course of successful reading acquisition (Bakker, 1990; Orton, 1937; Schlaggar & McCandliss, 2007). Further, these parallels are in line with the patterns of engagement of ventral regions in reading throughout development, exhibiting increasingly stronger left lateralization (Shaywitz et al., 2002; Turkeltaub et al., 2003). Finally, complementing this view is the neural signature of developmental dyslexia, in which posterior regions show reduced overall activation and notably a predominantly right-lateralized engagement, especially during decoding of words and pseudowords (Helenius et al., 1999; Shaywitz et al., 2002).
One challenge to directly relating the current work to the typical trajectory of reading acquisition is the time-scale on which learning takes place. Given the protracted development of the left-lateralized N170 expertise for visual word forms in children (Brem et al., 2006; Maurer et al., 2005; Maurer et al., 2007; Maurer et al., 2006), the rapid emergence of an expert-like electro-physiological response following a mere 20 minutes of training might seem puzzling. Indeed, adult training studies show that acquiring perceptual expertise in a new domain typically requires multiple hours of instruction and practice (Gauthier et al., 1998; Tanaka et al., 2005). We propose that the fast rise of the N170 group effects following the brief training period most likely reflects a neural assimilation phenomenon, whereby the newly learned visual characters are processed using a well-established native language reading circuitry (for discussion, see Nelson et al., 2009). This notion is congruent with findings of experience-based plasticity of extrastriate regions. For instance, following brief training in mirror-reversed script reading, learning effects in activation in left fusiform and left inferior temporal areas were demonstrated for novel visual stimuli (Poldrack & Gabrieli, 2001), suggesting that, at least under some conditions, short-term training with novel stimuli leads to recruitment of pre-established expertise networks associated with reading expertise.
Although N170 responses are typically linked to early perceptual responses, the findings of group differences in the current study do not necessarily reflect automatic, task-invariant responses to these newly trained stimuli. In a related study, ERP responses to the recently learned artificial script were probed using a task with very shallow encoding demands: a visual immediate-repetition detection task. Under these minimal decoding demands, the post-training N170 topography was predominantly right-lateralized irrespective of training condition (Maurer et al., 2010/this issue). This finding counters the notion that short-term training resulted in the novel stimuli being fully and automatically assimilated into subjects’ perceptual expertise circuitry associated with left-lateralized N170 responses to English visual word forms. Our interpretation of these potentially conflicting results obtained under the reading verification task versus the visual repetition detection task is that attention to grapheme-phoneme associations may be a necessary but not a sufficient condition for assimilation of N170 visual word form processing following short training. In fact, additional processing goals (potentially instantiated by explicit task demands to associate orthography and phonology) are needed to ensure assimilation into the neural circuitry specialized for reading in the native language. Accordingly, training led to a left-lateralized N170 response to the newly learned characters manifested only under test conditions of visual processing in the service of phonological analysis. Thus, artificial orthography processing did not automatically assimilate into the native language reading network (even after grapheme-phoneme mappings had been learned) but required intentionally relating visual orthographic to phonological representations.
Interestingly, the temporal extent of the post-test group N170 effect in the current study was quite different from the temporal extent of the pre–post N170 training effect in the visual repetition detection study. In the present study, the post-test group ERP difference was phasic and restricted to the N170 component. In the visual repetition detection study, however, the pre–post ERP training effect spanned beyond the N170 component and was sustained (the total duration of the effect was 220 msec), presumably reflecting more general processes related to training-induced visual familiarity (Maurer et al., 2010/this issue). Collectively the current findings point to the key role of attention to grapheme-phoneme representations during training, together with task demands that explicitly require “reading” newly trained stimuli in producing neural assimilation of the cortical circuitry supporting left-lateralized N170 responses associated with reading expertise.
Artificial orthography training of expert readers was used in the present study as a model system to investigate the role of factors at play during the initial stages of reading acquisition. Specifically, attentional focus to different unit sizes in relating print to speech was shown to critically bias learning outcome in terms of both behavior and training-induced changes in the N170 response. The present results demonstrated that left-lateralized N170 responses were associated with attention to grapheme-phoneme association units rather than attention to whole-word associations between print and speech. In children with reading difficulties, it is likely that this ability to attend to grapheme-phoneme associations is masked by difficulties on the phonological side, such as inabilities to focus attention on sub-syllabic phonological units (McCandliss & Noble, 2003; Noble, Wolmetz, Ochs et al., 2006). In the current study, literate adults’ tendency to attend to grapheme-phoneme associations was discouraged in the whole-word condition by the design of the visual characters, which masked letter segmentation. We propose here that in both the case of children with phonological deficits and adults learning visual word forms when letter segmentation is obscured, both groups similarly fail to focus attention on grapheme-phoneme mappings. Thus, even though learning is taking place at the level of whole-word associations, such training does not lead to the left-lateralized N170 visual word form response, characteristic of reading expertise.
CONCLUSION AND BROADER IMPLICATIONS
In sum, this artificial orthography training study demonstrates how specific attentional focus during learning impacts the neural bases of expertise recruited beyond training. In particular, attending to grapheme-phoneme associations during training eventually engages processes linked to perceptual expertise for reading, as indexed by the left-lateralized N170 ERP response. The present results suggest that such expertise effects can be observed after even short-term training, potentially reflecting a form of neural assimilation, in which pre-existing perceptual expertise circuitry associated with skilled reading is recruited in service of encoding the newly learned stimuli. These insights from well-controlled training studies in literate adults, isolating the impact of attentional focus during learning from typically confounded stimulus- and task-related factors, complement developmental investigations of the acquisition of visual expertise for reading.
This study’s emphasis on attentional focus to different unit sizes in relating print to speech constitutes an alternative to bottom-up perceptual accounts of the nature of deficits in developmental reading disabilities that interfere with the attainment of normal adult levels of perceptual expertise. Furthermore, isolating top-down attention focusing mechanisms from other factors allows specific manipulations of focus type (e.g., different unit sizes) to better characterize the role of these processes in building perceptual expertise. This idea fits well with recent demonstrations that the nature of the educational experiences through which children are first introduced to letters can directly impact the recruitment of left ventral visual regions when they later view letters (McCandliss, 2010; James, 2010). This may directly inform reading intervention efforts, suggesting that instructional cues that direct attention toward appropriate unit sizes (i.e., grapheme-phoneme representations in the alphabetic English orthography) might be a key ingredient to successful remediation. In keeping with this notion, McCandliss and colleagues (2003) examined the impact of reading instruction techniques designed to encourage children with poor decoding skills to focus attention on grapheme-phoneme relationships within visual word forms, and demonstrated significant gains in both word recognition ability and alphabetic transfer to novel words (McCandliss et al., 2003). Notably, the results of the grapheme-phoneme group in the current study, linking recruitment of perceptual expertise circuits in adult learning to attentional focus to appropriate aspects of phonology and orthography, parallel such intervention findings in children still learning to read. Thus, the present work provides a model context for investigating how such attentional effects might relate to the development of left-lateralized perceptual expertise responses in typically developing children, and furthermore might potentially suggest impaired top-down processing in developmental dyslexia that could be specifically targeted for remediation.
ACKNOWLEDGMENTS
The authors thank Dr. Michael Worden for sharing his expert technical knowledge of the EGI system, the reviewers for their critiques, and Dr. Eva Hulse for her help with editing.
This work was supported by grants to BDM from the National Science Foundation (REC-0337715) and National Institutes of Health (NIDCD-R01-DC007694) and the Swiss National Science Foundation (Fellowship for Prospective Researchers: UM).
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
The group difference in lateralization for trained characters was also significant in the extended N170 interval spanning 170–218 msec (2 × 2 ANOVA on the x-axis for trained characters with within-subject factor “polarity” and between-subject factor “group”: “polarity” by “group” F(1,28) = 4.764, p < .05).
The group lateralization difference for transfer characters in the 170–218 msec interval also exhibited a non-significant trend (2 × 2 ANOVA on the x-axis for transfer characters with within-subject factor “polarity” and between-subject factor “group”: “polarity” by “group” F(1,28) = 3.080, p = .09).
Contributor Information
Yuliya N. Yoncheva, Department of Psychology and Human Development, Vanderbilt University, Nashville, Tennessee
Vera C. Blau, Vanderbilt Brain Institute, Vanderbilt University, Nashville, Tennessee
Urs Maurer, Department of Child and Adolescent Psychiatry, University of Zurich, Zurich, Switzerland.
Bruce D. McCandliss, Department of Psychology and Human Development, Vanderbilt University, Nashville, Tennessee
REFERENCES
- Allison T, McCarthy G, Nobre A, Puce A, Belger A. Human extrastriate visual cortex and the perception of faces, words, numbers, and colors. Cerebral Cortex. 1994;4:544–554. doi: 10.1093/cercor/4.5.544. [DOI] [PubMed] [Google Scholar]
- Bakker DJ. Neuropsychological treatment of dyslexia. New York: Oxford University Press; 1990. [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. Journal of Cognitive Neuroscience. 1996;8:551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentin S, Mouchetant-Rostaing Y, Giard MH, Echallier JF, Pernier J. ERP manifestations of processing printed words at different psycholinguistic levels: Time course and scalp distribution. Journal of Cognitive Neuroscience. 1999;11:235–260. doi: 10.1162/089892999563373. [DOI] [PubMed] [Google Scholar]
- Binder JR, Medler DA, Westbury CF, Liebenthal E, Buchanan L. Tuning of the human left fusiform gyrus to sublexical orthographic structure. Neuroimage. 2006;33:739–748. doi: 10.1016/j.neuroimage.2006.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop C. Transfer effects of word and letter training in reading. Journal of Verbal Learning and Verbal Behavior. 1964;3:215–221. [Google Scholar]
- Bitan T, Manor D, Morocz IA, Karni A. Effects of alphabeticality, practice and type of instruction on reading an artificial script: An fMRI study. Cognitive Brain Research. 2005;25:90–106. doi: 10.1016/j.cogbrainres.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Blau VC, Maurer U, Tottenham N, McCandliss BD. The face-specific N170 component is modulated by emotional facial expression. Behavioral and Brain Functions. 2007;3:7. doi: 10.1186/1744-9081-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Van Santen FW, Harasaki Y, Gitelman DR, Parrish TB, et al. The development of specialized brain systems in reading and oral-language. Child Neuropsychology. 2001;7:119–141. doi: 10.1076/chin.7.3.119.8740. [DOI] [PubMed] [Google Scholar]
- Bradley L, Bryant PE. Categorizing sounds and learning to read - a causal connection. Nature. 1983;301:419–421. [Google Scholar]
- Brandeis D, Vitacco D, Steinhausen HC. Mapping brain electric micro-states in dyslexic children during reading. Acta Paedopsychiatrica. 1994;56:239–247. [PubMed] [Google Scholar]
- Breier JI, Simos PG, Zouridakis G, Papanicolaou AC. Relative timing of neuronal activity in distinct temporal lobe areas during a recognition memory task for words. Journal of Clinical and Experimental Neuropsychology. 1998;20:782–790. doi: 10.1076/jcen.20.6.782.1116. [DOI] [PubMed] [Google Scholar]
- Brem S, Bucher K, Halder P, Summers P, Dietrich T, Martin E, et al. Evidence for developmental changes in the visual word processing network beyond adolescence. Neuroimage. 2006;29:822–837. doi: 10.1016/j.neuroimage.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Brem S, Lang-Dullenkopf A, Maurer U, Halder P, Bucher K, Brandeis D. Neurophysiological signs of rapidly emerging visual expertise for symbol strings. Neuroreport. 2005;16:45–48. doi: 10.1097/00001756-200501190-00011. [DOI] [PubMed] [Google Scholar]
- Busey TA, Vanderkolk JR. Behavioral and electrophysiological evidence for configural processing in fingerprint experts. Vision Research. 2005;45:431–448. doi: 10.1016/j.visres.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain. 2002;125:1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Cohen L, Martinaud O, Lemer C, Lehericy S, Samson Y, Obadia M, et al. Visual word recognition in the left and right hemispheres: Anatomical and functional correlates of peripheral alexias. Cerebral Cortex. 2003;13:1313–1333. doi: 10.1093/cercor/bhg079. [DOI] [PubMed] [Google Scholar]
- Coltheart M, Rastle K, Perry C, Langdon R, Ziegler J. DRC: A dual route cascaded model of visual word recognition and reading aloud. Psychological Review. 2001;108(1):204–256. doi: 10.1037/0033-295x.108.1.204. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Jobert A, Naccache L, Ciuciu P, Poline JB, Le Bihan D, et al. Letter binding and invariant recognition of masked words: Behavioral and neuroimaging evidence. Psychological Science. 2004;15:307–313. doi: 10.1111/j.0956-7976.2004.00674.x. [DOI] [PubMed] [Google Scholar]
- Di Russo F, Martinez A, Hillyard SA. Source analysis of event-related cortical activity during visuo-spatial attention. Cerebral Cortex. 2003;13:486–499. doi: 10.1093/cercor/13.5.486. [DOI] [PubMed] [Google Scholar]
- Ehri LC. Learning to read and spell words. In: Rieben L, Perfetti CA, editors. Learning to read: Basic research and its implications. Hillsdale, NJ: L. Erlbaum Associates; 1991. pp. 57–73. [Google Scholar]
- Evans MA, Shedden JM, Hevenor SJ, Hahn MC. The effect of variability of unattended information on global and local processing: Evidence for lateralization at early stages of processing. Neuropsychologia. 2000;38(3):225–239. doi: 10.1016/s0028-3932(99)00080-9. [DOI] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Halligan PW, Dolan RJ. Hemispheric asymmetries in global/local processing are modulated by perceptual salience. Neuropsychologia. 1999;37:31–40. doi: 10.1016/s0028-3932(98)00047-5. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Balsamo LM, Ibrahim Z, Sachs BC, Xu B. fMRI identifies regional specialization of neural networks for reading in young children. Neurology. 2003;60(1):94–100. doi: 10.1212/wnl.60.1.94. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Curran T, Curby KM, Collins D. Perceptual interference supports a non-modular account of face processing. Nature Neuroscience. 2003;6:428–432. doi: 10.1038/nn1029. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Williams P, Tarr MJ, Tanaka J. Training ‘greeble’ experts: A framework for studying expert object recognition processes. Vision Research. 1998;38:2401–2428. doi: 10.1016/s0042-6989(97)00442-2. [DOI] [PubMed] [Google Scholar]
- Goswami U. Phonological skills and learning to read. Annals of the New York Academy of Sciences. 1993;682:296–311. doi: 10.1111/j.1749-6632.1993.tb22977.x. [DOI] [PubMed] [Google Scholar]
- Han S, Liu W, Yund EW, Woods DL. Interactions between spatial attention and global/local feature selection: An ERP study. Neuroreport. 2000;11:2753–2758. doi: 10.1097/00001756-200008210-00029. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Sakai KL. Learning letters in adulthood: Direct visualization of cortical plasticity for forming a new link between orthography and phonology. Neuron. 2004;42:311–322. doi: 10.1016/s0896-6273(04)00196-5. [DOI] [PubMed] [Google Scholar]
- Helenius P, Tarkiainen A, Cornelissen P, Hansen PC, Salmelin R. Dissociation of normal feature analysis and deficient processing of letter-strings in dyslexic adults. Cerebral Cortex. 1999;9:476–483. doi: 10.1093/cercor/9.5.476. [DOI] [PubMed] [Google Scholar]
- James KH. Sensori-motor experience leads to changes in visual processing in the developing brain. Developmental Science. 2010;13:279–288. doi: 10.1111/j.1467-7687.2009.00883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Han SH. Neural mechanisms of global/local processing of bilateral visual inputs: An ERP study. Clinical Neurophysiology. 2005;116:1444–1454. doi: 10.1016/j.clinph.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: A metanalysis of 35 neuroimaging studies. Neuroimage. 2003;20:693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Lehmann D, Skrandies W. Reference-free identification of components of checkerboard-evoked multichannel potential fields. Electroencephalography and Clinical Neurophysiology. 1980;48:609–621. doi: 10.1016/0013-4694(80)90419-8. [DOI] [PubMed] [Google Scholar]
- Luu P, Ferree T. Determination of the Geodesic Sensor Nets’ average electrode positions and their 10–10 international equivalents. Eugene, OR: Electrical Geodesics, Inc.; 2000. [Google Scholar]
- Maurer U, Blau VC, Yoncheva Y, McCandliss BD, et al. Development of visual expertise for reading: Rapid emergence of visual familiarity for an artificial script. Developmental Neuropsychology. 2010;35(4):404–422. doi: 10.1080/87565641.2010.480916. /this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer U, Brandeis D, McCandliss BD. Fast, visual specialization for reading in English revealed by the topography of the N170 ERP response. Behavioral and Brain Functions. 2005;1:13. doi: 10.1186/1744-9081-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer U, Brem S, Bucher K, Brandeis D. Emerging neurophysiological specialization for letter strings. Journal of Cognitive Neuroscience. 2005;17:1532–1552. doi: 10.1162/089892905774597218. [DOI] [PubMed] [Google Scholar]
- Maurer U, Brem S, Bucher K, Kranz F, Benz R, Steinhausen HC, et al. Impaired tuning of a fast occipito-temporal response for print in dyslexic children learning to read. Brain. 2007;130:3200–3210. doi: 10.1093/brain/awm193. [DOI] [PubMed] [Google Scholar]
- Maurer U, Brem S, Kranz F, Bucher K, Benz R, Halder P, et al. Coarse neural tuning for print peaks when children learn to read. Neuroimage. 2006;33:749–758. doi: 10.1016/j.neuroimage.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Maurer U, McCandliss BD. The development of visual expertise for words: The contribution of electrophysiology. In: Grigorenko EL, Naples AJ, editors. Single-word reading: Biological and behavioral perspectives. Mahwah, NJ: Lawrence Erlbaum Associates; 2007. pp. 43–64. [Google Scholar]
- Maurer U, Zevin JD, McCandliss BD. Left-lateralized N170 effects of visual expertise in reading: evidence from Japanese syllabic and logographic scripts. Journal of Cognitive Neuroscience. 2008;20(10):1878–1891. doi: 10.1162/jocn.2008.20125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCandliss BD. Educational neuroscience: The early years. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:8049–8050. doi: 10.1073/pnas.1003431107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCandliss BD, Beck IL, Sandak R, Perfetti CA. Focusing attention on decoding for children with poor reading skills: Design and preliminary tests of the word building intervention. Scientific Studies of Reading. 2003;7:75–104. [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. The visual word form area: Expertise for reading in the fusiform gyrus. Trends in Cognitive Sciences. 2003;7:293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Noble KG. The development of reading impairment: A cognitive neuroscience model. Mental Retardation & Developmental Disabilities Research Reviews. 2003;9:196–204. doi: 10.1002/mrdd.10080. [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Schneider W, Smith T. Learning to read new visual symbols as integrated wholes or component parts; Paper presented at the 38th Annual Meeting of the Psychonomic Society; 1997. Nov, [Google Scholar]
- McClelland JL, Fiez JA, McCandliss BD. Teaching the /r/ /l/ discrimination to Japanese adults: Behavioral and neural aspects. Physiology & Behavior. 2002;77:657–662. doi: 10.1016/s0031-9384(02)00916-2. [DOI] [PubMed] [Google Scholar]
- Nelson JR, Liu Y, Fiez J, Perfetti CA. Assimilation and accommodation patterns in ventral occipito-temporal cortex in learning a second writing system. Human Brain Mapping. 2009;30(3):810–820. doi: 10.1002/hbm.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Wolmetz ME, Ochs LG, Farah MJ, McCandliss BD. Brain-behavior relationships in reading acquisition are modulated by socioeconomic factors. Developmental Science. 2006;9:642–654. doi: 10.1111/j.1467-7687.2006.00542.x. [DOI] [PubMed] [Google Scholar]
- Orton ST. Reading, writing and speech problems in children. London, UK: Chapman & Hall; 1937. [Google Scholar]
- Palmeri TJ, Wong ACN, Gauthier I. Computational approaches to the development of perceptual expertise. Trends in Cognitive Sciences. 2004;8(8):378–386. doi: 10.1016/j.tics.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Parviainen T, Helenius P, Poskiparta E, Niemi P, Salmelin R. Cortical sequence of word perception in beginning readers. Journal of Neuroscience. 2006;26(22):6052–6061. doi: 10.1523/JNEUROSCI.0673-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfetti CA. Representations and awareness in the acquisiton of reading competence. In: Rieben L, Perfetti CA, editors. Learning to read: Basic research and its implications. Hillsdale, NJ: L. Erlbaum Associates; 1991. pp. 33–44. [Google Scholar]
- Poldrack RA, Gabrieli JD. Characterizing the neural mechanisms of skill learning and repetition priming: Evidence from mirror reading. Brain. 2001;124:67–82. doi: 10.1093/brain/124.1.67. [DOI] [PubMed] [Google Scholar]
- Posner MI, McCandilss BD. Converging methods for investigating lexical access. Psychological Science. 1993;4(5):305–309. [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, et al. Neurobiological studies of reading and reading disability. Journal of Communication Disorders. 2001;34:479–492. doi: 10.1016/s0021-9924(01)00060-0. [DOI] [PubMed] [Google Scholar]
- Rayner K, Pollatsek A. The psychology of reading. London, United Kingdom: Prentice-Hall International; 1989. [Google Scholar]
- Rossion B, Joyce CA, Cottrell GW, Tarr MJ. Early lateralization and orientation tuning for face, word, and object processing in the visual cortex. Neuroimage. 2003;20:1609–1624. doi: 10.1016/j.neuroimage.2003.07.010. [DOI] [PubMed] [Google Scholar]
- Sandak R, Mencl WE, Frost SJ, Rueckl JG, Katz L, Moore DL, et al. The neurobiology of adaptive learning in reading: A contrast of different training conditions. Cognitive, Affective & Behavioral Neuroscience. 2004;4:67–88. doi: 10.3758/cabn.4.1.67. [DOI] [PubMed] [Google Scholar]
- Schendan HE, Ganis G, Kutas M. Neurophysiological evidence for visual perceptual categorization of words and faces within 150 ms. Psychophysiology. 1998;35:240–251. [PubMed] [Google Scholar]
- Schlaggar BL, Brown TT, Lugar HM, Visscher KM, Miezin FM, Petersen SE. Functional neuro-anatomical differences between adults and school-age children in the processing of single words. Science. 2002;296:1476–1479. doi: 10.1126/science.1069464. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, McCandliss BD. Development of neural systems for reading. Annual Review of Neuroscience. 2007;30:475–503. doi: 10.1146/annurev.neuro.28.061604.135645. [DOI] [PubMed] [Google Scholar]
- Schneider W, Shiffrin RM. Controlled and automatic human information processing: I. Detection, search, and attention. Psychological Review. 1977;84:1–66. [Google Scholar]
- Sergent J. Role of the input in visual hemispheric asymmetries. Psychological Bulletin. 1983;93(3):481–512. [PubMed] [Google Scholar]
- Share DL, Stanovich KE. Cognitive processes in early reading development: Accommodating individual differences into a model of acquisition. Issues in education. 1995;1:1–57. [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, Skudlarski P, et al. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biological Psychiatry. 2002;52(2):101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Strik WK, Fallgatter AJ, Brandeis D, Pascual-Marqui RD. Three-dimensional tomography of event-related potentials during response inhibition: Evidence for phasic frontal lobe activation. Electroencephalography and Clinical Neurophysiology. 1998;108:406–413. doi: 10.1016/s0168-5597(98)00021-5. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimentional proportional system - an approach to cerebral imaging. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Tanaka J, Curran T. A neural basis for expert object recognition. Psychological Science. 2001;12:43–47. doi: 10.1111/1467-9280.00308. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Curran T, Sheinberg DL. The training and transfer of real-world perceptual expertise. Psychological Science. 2005;16:145–151. doi: 10.1111/j.0956-7976.2005.00795.x. [DOI] [PubMed] [Google Scholar]
- Tarkiainen A, Helenius P, Hansen PC, Cornelissen PL, Salmelin R. Dynamics of letter string perception in the human occipitotemporal cortex. Brain. 1999;122:2119–2132. doi: 10.1093/brain/122.11.2119. [DOI] [PubMed] [Google Scholar]
- Tarkiainen A, Helenius P, Salmelin R. Category-specific occipitotemporal activation during face perception in dyslexic individuals: An MEG study. Neuroimage. 2003;19:1194–1204. doi: 10.1016/s1053-8119(03)00161-7. [DOI] [PubMed] [Google Scholar]
- Torgesen J, Wagner R, Rashotte C. Test of Word Reading Efficiency (TOWRE) Austin, TX: PRO-ED; 1999. [Google Scholar]
- Tucker DM. Spatial sampling of head electrical fields: The geodesic sensor net. Electroencephalography and Clinical Neurophysiology. 1993;87:154–163. doi: 10.1016/0013-4694(93)90121-b. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nature Neuroscience. 2003;6:767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, et al. Meta-analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. Neuroimage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Woldorff MG, Fox PT, Matzke M, Lancaster JL, Veeraswamy S, Zamarripa F, et al. Retinotopic organization of early visual spatial attention effects as revealed by PET and ERPs. Human Brain Mapping. 1997;5:280–286. doi: 10.1002/(SICI)1097-0193(1997)5:4<280::AID-HBM13>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Xue G, Chen C, Jin Z, Dong Q. Language experience shapes fusiform activation when processing a logographic artificial language: An fMRI training study. Neuroimage. 2006;31:1315–1326. doi: 10.1016/j.neuroimage.2005.11.055. [DOI] [PubMed] [Google Scholar]
- Yoncheva YN, Maurer U, Daruwalla Z, Zevin JD, McCandliss BD. The temporal dynamics of listening to versus hearing words: Attention modulates both early stimulus encoding and preparatory activity. Journal of Cognitive Neuroscience, Supplement ISSN 1096–8857. 2008:268. [Google Scholar]
- Yoncheva YN, Zevin JD, Maurer U, McCandliss BD. Auditory selective attention to speech modulates activity in the visual word form area. Cerebral Cortex. 2010;20(3):622–632. doi: 10.1093/cercor/bhp129. [DOI] [PMC free article] [PubMed] [Google Scholar]