Abstract
Background
It remains unclear if patients with clinical stage T2 N0 (cT2 N0) esophageal cancer should be offered induction therapy vs surgical intervention alone.
Methods
This was a retrospective cohort study of cT2 N0 patients undergoing induction therapy, followed by surgical resection, or resection alone, at the Johns Hopkins Hospital from 1989 to 2009. Kaplan-Meier analysis was used to compare all-cause mortality in cT2 N0 patients who had resection alone vs those who had induction chemoradiation therapy, followed by resection.
Results
A study cohort of 69 patients was identified and divided into two groups: 55 patients (79.7%) received induction therapy and 14 (20.3%) did not. No statistically significant difference in 5-year survival rate was observed for the two groups: 49.5% for the resection-only group and 53.8% for the induction group. More than 50% of cT2 N0 patients were understaged.
Conclusions
For cT2 N0 esophageal cancer patients, the benefit of neoadjuvant therapy is still unclear. Induction therapy for cT2 N0 did not translate into a statistically significant improvement in survival. However, due to the significant understaging of T2 N0 patients, we recommend neoadjuvant therapy to all cT2N0 patients before operation.
Although there are generally accepted paradigms for the use of neoadjuvant chemoradiation therapy with subsequent operation for early and locally advanced stages of esophageal cancer, controversy persists about its use in patients who do not fit comfortably into these groups, namely patients with T2 N0 disease. Rohatgi and colleagues [1] found that 68% of T2 N0 esophageal cancers were understaged and recommended use of neoadjuvant therapy in this clinical group. However, Mariette and colleagues [2] did not find an improvement in overall survival, but rather, an increase in postoperative mortality rates in stage I and II patients treated with neoadjuvant chemoradiation therapy compared with operation alone. Hurmuzlu and colleagues [3] found no significant survival advantage in stage IIA or III patients treated with preoperative high-dose chemoradiation therapy vs operation alone, and Stiles and colleagues [4] similarly found no difference between these two treatment groups for stage IIA patients. In an observational study, Rice and colleagues [5] found a decreased 5-year survival rate in clinical T2 N0 patients undergoing induction therapy vs operation.
These conflicting results mostly stem from the fact that these were all observational cohort studies and not randomized clinical trials. Moreover, there is the difficulty of planning treatment based on clinical staging (c), which may not be an accurate reflection of the actual pathologic stage (p). Although some cT2 N0 patients are understaged, for example, the degree of understaging may not be significant enough for neoadjuvant therapy to be beneficial for these patients. In addition, the indiscriminate allocation of chemoradiation therapy to those who may not benefit may compound local and systemic toxicities, thereby increasing death. Also, there may be a significant portion of patients who are overstaged in which neoadjuvant therapy would produce more harm than good.
Many of the studies that have compared neoadjuvant therapy plus surgical resection vs resection alone include a wide range of clinical stages: T1 to T3, N0 to N1, M0 [3, 6]. There are very little data on the benefits of neoadjuvant therapy for cT2 N0 specifically. The purpose of this investigation was to examine whether there was any survival benefit to preoperative neoadjuvant chemotherapy for cT2 N0 patients.
Material and Methods
This study was approved by the Johns Hopkins Institutional Review Board, who exempted the need for patient consent, and abides by Health Insurance Portability and Accountability Act compliance standards.
Data Collection
A retrospective study was performed of a cohort with preoperative cT2 N0 esophageal cancer at Johns Hopkins diagnosed from March 1989 to May 2009. Patients were entered from case logs from several thoracic surgeons as well as from the Johns Hopkins Hospital Multidisciplinary Esophageal Cancer Database. Elements of the database were obtained from patient hospital records, as well as electronic and paper files. Since 2000, periodic data audits have been performed by archival and on-line record reviews for quality assurance. These reviews have consistently verified more than 90% accuracy of the database with source materials.
There was systematic follow-up of any patients with incomplete data through their referring physicians. In almost all cases, recurrence was documented radio-graphically, with pathologic assessment in a few cases. Deaths were determined from a combination of surgeons’ case logs and the Social Security Death Index.
Patient Population
Patient selection criteria included a biopsy-proven esophageal carcinoma and a documented assessment at the Johns Hopkins Hospital. Patients were only included if their preoperative staging was assessed as T2 N0 by the operative surgeon according to the Sixth Edition of the American Joint Committee on Cancer (AJCC) [7]. Preoperative clinical staging was done by compiling the results from endoscopic ultrasound (EUS), computed tomography (CT), positron emission tomography, and often, barium esophagrams. In this cohort, the first use of EUS was in 1995, whereas positron emission tomography was first used to stage esophageal cancer preoperatively in 2000.
The pathology record was used to derive a recorded pathologic stage for all patients. The American Society of Anesthesiologists 5-grade classification system was used as an index of preoperative comorbidity [8].
Esophagectomies were separated into transhiatal esophagectomy, three-incision esophagectomy, Ivor Lewis esophagectomy, and esophagogastrectomy. Preoperative chemotherapy regimens were categorized as fluorouracil (5-FU) and platinum only, non-5-FU and platinum (replacement of either or both with paclitaxe docetaxel capecitabine, and irinotecan), or unknown. Preoperative radiotherapy was categorized as less than 4,400 cGy, 4,400 cGy or more, or dose unknown.
Pathologic response to neoadjuvant chemoradiation was determined at the time of pathologic examination of the surgical specimen.
Statistical Analysis
Comparison of medians, dichotomous, and categoric variables was performed using the nonparametric Wilcoxon rank sum test and χ2 test for homogeneity, respectively. The Fisher exact test was applied to dichotomous and categoric variables with cells containing fewer than five observations. Time to all-cause mortality was estimated using the Kaplan-Meier method. Overall survival was defined as time from date of diagnosis to death or the last follow-up. Patients were censored if they were still alive at time of analysis or were lost to follow-up. Recurrence-free survival was defined as the time from the date of surgery to the event of recurrence. Patients in the recurrence-free survival analysis were censored if they died before the event of recurrence, were lost to follow-up, or were administratively censored if recurrence-free at time of analysis. Differences in overall survival and recurrence-free survival between the two groups were estimated using the log-rank test.
Univariate Cox proportional hazards regression models demonstrated associations between specific covariates and outcome, defined as recurrence-free survival. Multivariable models were built by including single covariates into the model with treatment group and assessed the degree to which the unadjusted hazard ratio was altered. Covariate adjustment in the final multivariable model was ultimately determined by differences by treatment group on observed characteristics within the entire cohort, prior belief, and clinical and biologic plausibility. Results of Cox regression are reported as hazard ratios with 95% confidence intervals. Statistical analysis was performed using STATA 10.0 software (StataCorp LP, College Station, TX).
Results
Patients
A study cohort of 69 patients with preoperative cT2 N0 esophageal cancer was identified and their data are summarized in Table 1. These patients were separated into two groups: group A consisted of 14 patients (20.3%) who were treated with an operation alone, and group B comprised 55 patients (79.7%) who received induction therapy and an operation. Both groups were comparable in sex, race, and smoking behavior.
Table 1.
Characteristics of cT2 N0 Patients by Treatment Modality (N = 69)
| Variablesa | Operation Only | Induction Therapy + Operation |
p Value |
|---|---|---|---|
| Patients | 14 (20.3) | 55 (79.7) | |
| Age at diagnosis, years | 69 (66–75) | 61 (53–66) | 0.014 |
| Sex | 0.980 | ||
| Male | 12 (85.7) | 47 (85.5) | |
| Female | 2 (14.3) | 8 (14.5) | |
| Race | 0.429 | ||
| White | 13 (92.9) | 49 (89.1) | |
| Black | 0 (0.0) | 4 (73) | |
| Other | 1 (7.1) | 2 (3.6) | |
| Smoked cigarettes | 0.734 | ||
| Never | 4 (28.5) | 13 (23.6) | |
| Ever | 10 (71.4) | 42 (76.4) | |
| Pack-years smoked | 40 (30–55) | 35 (20–50) | 0.727 |
| Histology | 0.030 | ||
| Adenocarcinoma | 14 (100) | 40 (72.7) | |
| Squamous cell | 0 (0.0) | 15 (27.3) | |
| Diagnostic Imaging | 0.025 | ||
| Computed tomography only | 1 (7.1) | 23 (41.8) | |
| Endoscopic ultrasound | 13 (92.9) | 32 (58.2) | |
| Neoadjuvant chemotherapy | |||
| 5-FU and platinum only | … | 37 (67.3) | |
| Non-5-FU and platinum | … | 12 (21.8) | |
| Unknown | … | 6 (10.9) | |
| Neoadjuvant radiotherapy dose |
|||
| <4,400 cGy | … | 4 (7.3) | |
| $ 4,400 cGy | … | 46 (83.6) | |
| Dose unknown | … | 5 (9.1) | |
| Surgical procedure | >0.99 | ||
| Transhiatal esophagectomy | 11 (78.6) | 39 (70.9) | |
| 3-Incision esophagectomy | 2 (14.3) | 7 (12.7) | |
| Ivor Lewis | 0 (0.0) | 3 (5.5) | |
| Esophagogastrectomy | 1 (7.1) | 6 (10.9) | |
| ASA classification | 0.762 | ||
| 1 | 0 (0.0) | 0 (0.0) | |
| 2 | 3 (21.4) | 9 (16.4) | |
| 3 | 11 (78.6) | 45 (81.8) | |
| 4 | 0 (0.0) | 1 (1.8) | |
| 5 | 0 (0.0) | 0 (0.0) | |
| Margin | 0.127 | ||
| R0 | 10 (71.4) | 50 (90.9) | |
| R1 | 3 (21.4) | 4 (7.3) | |
| Unknown | 1 (7.1) | 1 (1.8) | |
| Adjuvant chemotherapy | 0.865 | ||
| Received | 4 (28.6) | 17 (30.9) | |
| Did not receive | 10 (71.4) | 38 (69.1) | |
| Recurrent disease | 0.133 | ||
| Recurrence | 3 (21.4) | 21 (38.2) | |
| No recurrence | 11 (78.6) | 34 (61.8) | |
| Date of surgery, year | 2003 (2001–2004) | 1996 (1992–2002) | 0.001 |
| Time in days between | |||
| Diagnosis and operation | 28.5 (21–41) | 111 (93–129) | <0.001 |
| Diagnosis and treatment | 28.5 (21–41) | 32 (21–51) | 0.310 |
| Postop hospital LOS, days | 11 (9–18) | 10 (8–12) | 0.103 |
| Pathologic stage (T N M) | |||
| T0 N0 | 0 (0) | 22 (40) | |
| T1 N0 | 3 (21.4) | 6 (10.9) | |
| T2 N0 | 4 (28.6) | 5 (9.1) | |
| T3 N0 | 1 (7.1) | 5 (9.1) | |
| T1 N1 | 1 (7.1) | 2 (3.6) | |
| T2 N1 | 3 (21.4) | 10 (18.2) | |
| T3 N1 | 2 (14.3) | 5 (9.1) | |
| Pathologic stage (grouped) | |||
| <pT2 N0 | 3 (21.4) | 28 (50.9) | |
| >pT2 N0 | 11 (78.6) | 27 (49.1) |
Categoric data are presented as number (%); continuous data as median (interquartile range).
ASA = American Society of Anesthesiologists; LOS = length of stay; Postop = postoperative; T N M = tumor size, lymph nodes, metastasis.
All group A patients had adenocarcinoma. In group B, 40 patients (72.7%) had adenocarcinoma and 15 (27.2%) had squamous cell carcinoma. For adenocarcinomas, 26 (48.1%) were located in the lower third and 26 (48.1%) at the gastroesophageal junction. For squamous cell, 1 (6.7%) was in the upper third, 3 (20.0%) were in the middle third, 8 (53.3%) were in the lower third, and 2 (13.3%) were at the gastroesophageal junction.
Diagnostic imaging modalities differed significantly by groups. In group A, all but 1 patient was preoperatively staged by EUS, whereas in group B, 23 patients (41.8%) were staged by CT alone and 32 (58.2%) by EUS.
Treatment
Platinum and 5-FU and >4,400 cGy were the most commonly used neoadjuvant treatments (Table 1). Approximately one-third of the patients in each treatment group received adjuvant chemotherapy. Transhiatal esophagectomy was the most common procedure, comparable between the two groups. Operations were performed at a median year of 2003 (interquartile range [IQR], 2001 to 2004) in group A and at year 1996 (IQR, 1992 to 2002) in group B (p = 0.001).
Postoperative Staging
Postoperative pathologic staging (p) in group A showed that 3 patients (21.4%) were clinically overstaged (pT1 N0), 4 (28.6%) had a corresponding pathologic stage (pT2 N0), and 7 (50%) were clinically understaged (pT3 N0, pT1 N1, pT2 N1, and pT3 N1). In group B, 22 patients (40.0%) had a complete response to induction therapy (pT0 N0), 6 (10.9%) had a partial response (pT1 N0), and 27 (49.1%) had no response, with the same or worse pathologic staging postoperatively (pT2 N0, pT3 N0, pT0 N1, pT1 N1, pT2 N1, pT3 N1). Postoperative adjuvant chemotherapy was administered to 4 patients (28.6%) in group A and to 17 patients (30.9%) in group B. Margins were positive in 3 patients (21.4%) in group A and in 4 patients (7.3%) in group B.
Univariate and Multivariate Analysis
Univariate analysis using 5-year recurrence free survival as the outcome (Table 2) and treatment group, age, sex, race, histology, diagnostic procedure, diagnosis year, surgical procedure, American Society of Anesthesiologists grade, length of hospital stay, smoking status, and margins as the variables, failed to show any variable to be predictive of outcome. Multivariate analysis using the same variables showed EUS had a positive effect on outcome (hazard ratio, 0.02; 95% confidence interval, 0.00 to 0.26; p = 0.004).
Table 2.
Association Between Measured Variables and 5-Year Recurrence-Free Survival of T2 N0 Esophageal Patients Who Received Surgical Treatment (N = 60)a
| Variable | Crude HR (95% CI) | p Value | Adjusted HR (95% CI) | p Value |
|---|---|---|---|---|
| Treatment group | ||||
| Operation only | 1.00 | Referent | >0.99 | |
| Neoadjuvant + operation | 4.20 (0.56–31.68) | 0.164 | 7.21 (0.71–73.81) | 0.096 |
| Age | 0.98 (0.94–1.03) | 0.402 | 1.01 (0.95–1.08) | 0.737 |
| Sex | ||||
| Male | 1.00 | Referent | >0.99 | |
| Female | 0.66 (0.15–2.88) | 0.578 | 0.75 (0.14–3.91) | 0.735 |
| Race | ||||
| White | 1.00 | Referent | >0.99 | |
| African American/other | 0.44 (0.06–3.30) | 0.423 | 0.30 (0.03–3.24) | 0.323 |
| Histology | ||||
| Adenocarcinoma | 1.00 | Referent | >0.99 | |
| Squamous cell | 0.89 (0.29–2.75) | 0.846 | 1.50 (0.31–7.20) | 0.611 |
| Diagnostic procedure | ||||
| Computed tomography | 1.00 | Referent | >0.99 | |
| Endoscopic ultrasound | 0.39 (0.15–1.04) | 0.060 | 0.02 (0.00–0.26) | 0.004 |
| Diagnosis year | 0.99 (0.91–1.07) | 0.743 | 1.37 (1.12–1.69) | 0.002 |
| Surgical procedure | ||||
| Transhiatal | 1.00 | Referent | >0.99 | |
| Otherb | 0.43 (0.10–1.87) | 0.260 | 0.19 (0.03–1.13) | 0.068 |
| ASA classification | ||||
| 1–2 | 1.00 | Referent | >0.99 | |
| 3–4 | 0.58 (0.20–1.67) | 0.313 | 0.48 (0.12–1.97) | 0.308 |
| Hospital length of stay | 1.03 (0.98–1.09) | 0.232 | 1.06 (0.99–1.14) | 0.115 |
| Smoked cigarettes | ||||
| Never | 1.00 | Referent | >0.99 | |
| Ever | 0.82 (0.29–2.32) | 0.704 | 2.28 (0.57–9.10) | 0.570 |
The analysis excluded 7 patients who were margin positive and 2 patients with unknown margin status.
Other procedures: 3-rntision esophagectomy, Ivor Lewis, esophagogastrectomy.
ASA = American Society of Anesthesiologists; CI = confidence interval; HR = hazard ratio.
Complications
Complications within 30 days postoperatively and comorbidities between the two treatment groups were not significantly different (Table 3), except that group A had a higher rate of infectious postoperative complications, 6 (42.9%) vs 7 patients (12.7%) in group B. In group A, these included four wound infections, one urinary tract infection, and one septic complication. In group B, there were four wound infections and two urinary tract infections. Median length of stay after surgery did not differ significantly between the two groups.
Table 3.
Intraoperative and Postoperative Complications and Comorbidities by Treatment Modality (N = 69)
| Variable | Operation Only No. (%) |
Induction Therapy + Operation No. (%) |
p Value |
|---|---|---|---|
| Patients | 14 (203) | 55(79.7) | |
| Intra-op complications | |||
| Blood transfusion | 0 (0.0) | 5(9.1) | 0.575 |
| Post-op complications | |||
| Pulmonarya | 5 (35.7) | 15 (27.7) | 0.534 |
| Cardiovascularb | 3 (21.4) | 11 (20.0) | 0.906 |
| Infectionc | 6(42.9) | 7 (12.7) | 0.019 |
| Mental status change | 0 (0.0) | 1 (1.82) | 0.899 |
| Anastomotic leak | 4(28.6) | 6 (10.9) | 0.109 |
| Other complicationsd | 9(643) | 18 (32.7) | 0.063 |
| Any complication | 11 (78.6) | 33 (60.0) | 0.231 |
Pulmonary embolus, atelectasis, pleural effusion, pneumonia, acute respiratory distress syndrome, pneumothorax aspiration, and respiratory failure.
Myocardial infarction and dysrhythmia.
Sepsis, urinary tract infection, and wound infection.
Fever, weight loss, stricture, tracheal injury, and vocal cord paralysis.
Recurrence
The sites of recurrence include esophagus, mediastinum, lung/pleura, gastrointestinal tract, liver, bone, and adrenal gland. In group A, 3 patients (21.4%) recurred, including 1 distant recurrence (33.3%), whereas in group B, 21 patients (38.2%) had a recurrence (p = 0.133), including 15 distant recurrences (71.4%). All of the recurrences were within the first year, with the exception of 1 patient in group A and 3 patients in group B.
Survival
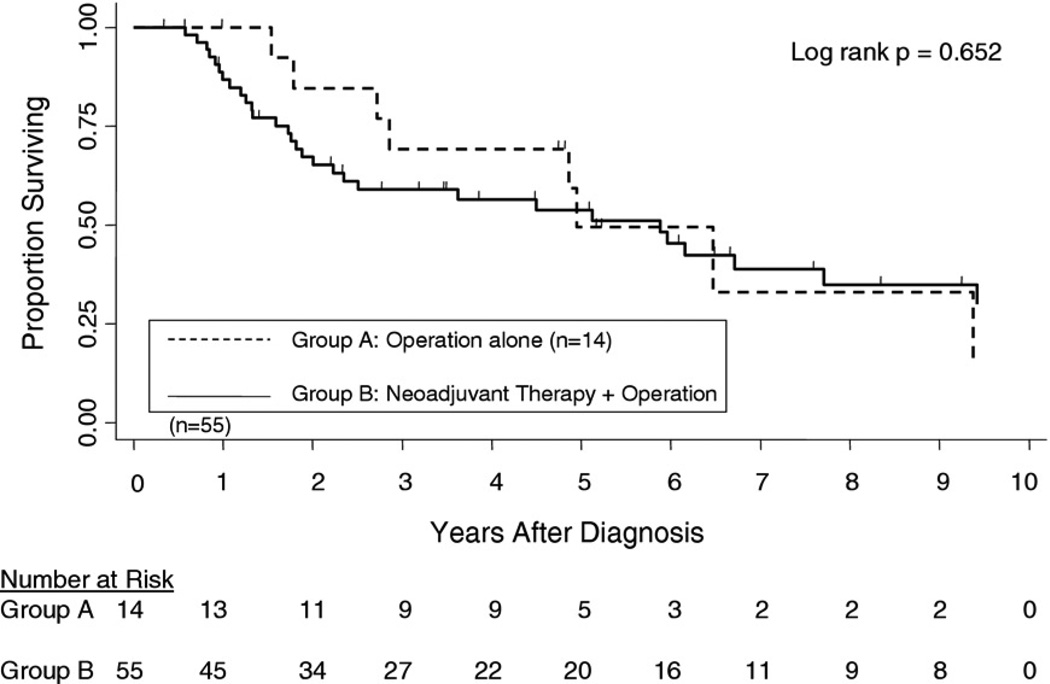
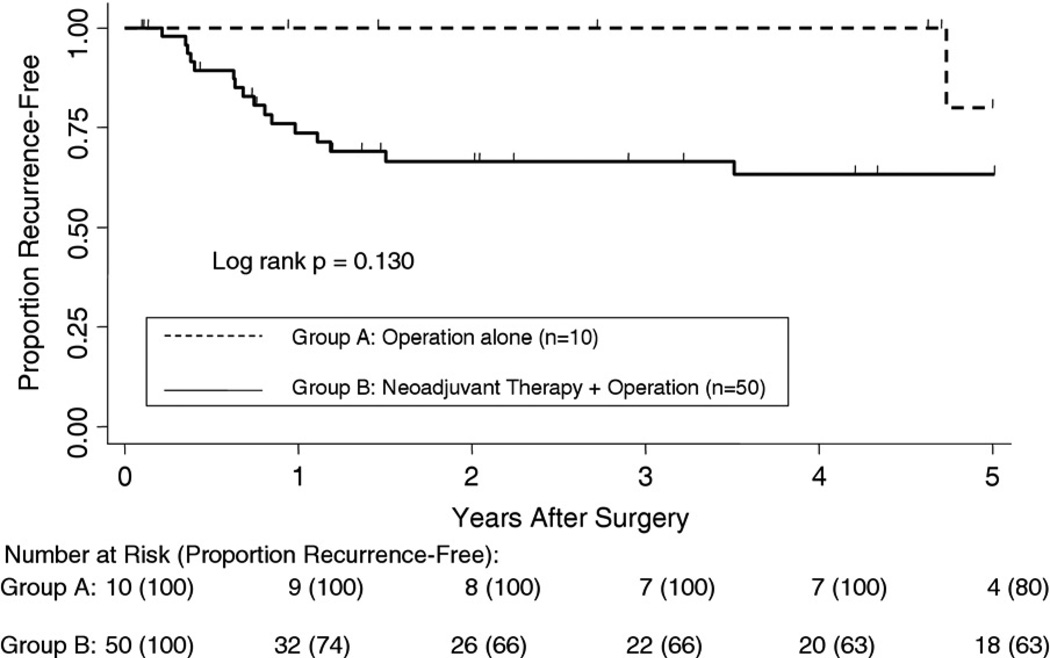
All-cause mortality and recurrence-free survival were measured as the primary outcomes. Kaplan-Meier analysis showed no statistical difference in all-cause mortality between the two groups starting from time of diagnosis (Fig 1). There were no 30- or 90-day deaths in either group. Although there was a trend for better survival for the operation-alone group from years 1 to 4, the curves converged and crossed at 5 years, with survival rate of 49.5% and 53.8% for groups A and B, respectively (p = 0.652). The median survival after diagnosis was 59.4 months for group A and 70.6 months for group B. The 5-year recurrence-free survival (Fig 2) was 80% for group A and 63% for group B (p = 0.130).
Fig 1.
Kaplan-Meier curves of overall survival rates for 14 esophageal cancer patients staged as cT2 N0 who underwent operations alone vs 55 cT2 N0 patients who received neoadjuvant chemoradiation therapy, followed by operation, with 5-year survival rates of 49.5% vs 53.8%, respectively.
Fig 2.
Kaplan-Meier curves of recurrence-free survival rates for 10 esophageal cancer patients staged as cT2 N0 who underwent operations alone vs 50 cT2 N0 patients who received neoadjuvant chemoradiation therapy, followed by an operation, with 5-year recurrence-free survival rates of 80% vs 63%, respectively. This analysis excluded 7 patients who were margin-positive and 2 patients with unknown margin status.
Comment
The optimal therapy for esophageal cancer continues to evolve. Treatment of cT2 N0 disease has raised much debate, with some institutions offering multimodality therapy and others not. Similar to Rohatgi and colleagues [1], about 50% of cT2 N0 patients in our operative group were clinically understaged. Rohatgi and colleagues [1] hypothesized that because most of their cT2 N0 patients were understaged and that neoadjuvant therapy was of known therapeutic benefit to patients with locally advanced pathologically staged esophageal cancer, neoadjuvant therapy offered to understaged cT2 N0 patients would lead to a survival advantage for cT2 N0 patients. The significant understaging seen in our cohort supports this hypothesis as well.
However, there was still no difference in 5-year survival or recurrence-free survival rates between the two groups. Why this did not translate into higher survival for the neoadjuvant group may be due to differences in the degree of understaging between the two groups. In group B, a greater proportion of patients were diagnosed with CT scan, a less accurate staging modality, which could have led to a greater degree of understaging in that group. It is important to note that in the operative group, all but 1 patient received EUS preoperatively. EUS is the current gold standard for esophageal cancer staging and a known variable in determining the T classification, especially for T2 cancers [9]; however, even with an EUS probe, we, as well as Rohatgi [1], Stiles [4], and Rice [5] and their colleagues, still had difficulty preoperatively in accurately determining the correct pathologic stage of cT2 N0 patients. In fact, only 28% of our cT2 N0 operation-alone patients had pT2 N0, which is in line with the 13% found by Rice and colleagues [5]. Orditura and colleagues [10] concluded that for all stages of esophageal cancer, complete responders have longer survival than partial responders or those with persistent disease. Given this well-known finding as well as the frequent understaging in cT2 N0 disease, it remains our current practice to offer all of our cT2 N0 patients neoadjuvant chemoradiation therapy before surgical intervention.
It is interesting that although not statistically significant, the rate of recurrence was higher in the neoadjuvant group. Similar to the finding by Meguid and colleagues [11], these recurrences were mostly distant, as opposed to mostly local recurrences in the operation-only group. Stiles and colleagues [4] concluded that there was no difference in nodal metastases with cT2 N0 disease with or without neoadjuvant therapy. The difference was only seen with cT3 N0 disease. These findings led to the idea that chemotherapy may be ineffective against micrometastatic disease in cT2 N0 esophageal cancer.
The lack of power of our study was due to the small number of cT2 N0 patients. Although this was one of the major limitations in our study, it should be appreciated that the presentation of cT2 N0 patients in esophageal cancer is not common. In fact, this study of 69 patients represents one of the larger cohorts of T2 N0 patients published in the literature to date.
Another limitation is that our two groups were not randomized and thus may not be equivalent in variables that are difficult to measure. For example, our analysis assumed that there was the same degree of clinical understaging in both groups of T2 N0 patients. This may not hold true, because there is a significant difference in the time interval and staging method between the two groups. To address the issue of heterogeneity between the two groups, we performed a multivariate analysis of the entire cohort to identify significant variables that might affect outcome. Only the use of EUS proved to have a positive effect on outcome.
Tumor location, an important factor in the prognosis of T2 to T3 N0 M0 squamous cell cancers [12], was rather atypical in the squamous cell cancer cohort, as lesions in 10 of 15 patients (66.6%) were located in the distal esophagus. However, Alexandrou [13], Gupta [14], and Salazar [15] an their colleagues compared survival outcomes for distal adenocarcinoma and squamous cell esophageal cancers and found they were similar. Our multivariate analysis also showed no survival rate differences for adenocarcinoma vs squamous cell carcinoma, which are histologically distinct tumors.
Most importantly, there is a physician selection bias that cannot be accounted for, because choices to recommend neoadjuvant therapy may be a physician’s intuitive sense about the progression of the disease and how well the patient would tolerate chemoradiation. This might have affected our outcomes, because even though a patient may be clinically staged T2 N0, other factors may lead a physician to believe the patient may actually be at a higher stage and subsequently recommend induction therapy.
T2 N0 esophageal carcinoma is difficult to diagnose accurately, even with the gold standard of EUS. A recent report by Stiles and colleagues [11], using all the latest diagnostic tools, showed 60% of cT2 N0 patients have node-positive disease. Despite many studies, including our own cohort, showing understaging of these patients, there are currently no consistent data demonstrating statistically significant survival advantages in giving induction chemoradiotherapy to these patients. Our results do suggest, however, that because such a large group of patients are clinically understaged, it may be of benefit to give patients neoadjuvant therapy since usually being able to downstage the patient significantly improves survival rates for that particular group of chemotherapy-responsive patients [11]. Although this is a small, retrospective study, the confirmation of understaging in the modernly defined cT2 N0 in yet another cohort raises the possibility that cT2 N0 patients might benefit more from treatment with neoadjuvant therapy and subsequent operation in frequency of responders and long-term survival rates if our current ability to assess real pathologic stage preoperatively remains poor. Thus, we recommend treating cT2 N0 patients with neoadjuvant therapy, followed by operation. A large controlled trial or multi-institutional pooling of data is needed to gain better insight into this issue.
Abbreviations and Acronyms
- ASA
American Society of Anesthesiologists
- CI
confidence interval
- CT
computed tomography
- cT2 N0
clinical stage T2 N0
- EUS
endoscopic ultrasound
- HR
hazard ratio
- IQR
interquartile range
- PET
positron emission tomography
- pT2 N0
pathologic stage T2 N0
- R0
margin negative
- R1
margin positive
DISCUSSION
DR DAVID R. JONES (Charlottesville, VA)
I enjoyed your talk very much. I was just sitting here trying to think about what I am going to do based on your talk, and I am not sure. So I guess my question to you is: Did you think about looking at other things besides just endoscopic ultrasound (EUS) or computed tomography (CT)? These just look at the T status and the N status only, and CT does it poorly. You said positron emission tomography (PET) scanning was performed, which is probably our best surrogate, noninvasive marker for the biology of the disease. So what would happen if you had a standardized uptake value (SUV) max of 8 or 10, for a T2 N0 by EUS, should that patient get induction therapy? So the question is, did you look at your PET data in addition to the other radiographic imaging?
MS ZHANG
For the PET data that we use from our institution, the radiologists at Hopkins don’t typically use SUV as a parameter. They rate the activity as mild, moderate, or intense. So the PET scan is mostly taken into consideration in detection of lymph node status.
DR JONES
But we know that PET is worse than EUS for lymph node status.
DR YANG
As Jennifer said, we don’t use SUV value, so I think the hotter the activity, sure, I think we would consider using induction therapy. Twenty-five percent of our PET scans actually come from the outside. So, again, it is hard to quantitate them all. So using the PET scan for T stage only for a T2, at least in our data, I think we are sort of going toward induction therapy for all the T2s, probably irrespective of what the PET showed.
DR MARK KRASNA (Towson, MD)
Jennifer, that was an excellent presentation. Just a comment and then a question. The comment, to follow-up on what Dr Jones said: Apparently we are seeing more of this disease, because at each meeting, we are hearing more and more about these alleged T2 N0s. So, again, I think that either it is because we are identifying more patients with Barrett’s and we are finding the cancers earlier, which is a good thing, or we were mistaken in years past when we thought that everybody that we identified with what we thought was a T2 was really a T3 and every T3 had N1 disease. So the one obvious question that comes from this, similar to the question we had yesterday in our discussion, is, of those T2s that were misstaged, how many remained T2 but were actually N1 and not N0 and how many of those were upstaged to T3, if you have that information?
The second question—and, again, Steve, I don’t mean to make you get up but this really is for your whole group—I know that during that time period there was also a transition in some of your staff, and I know that one of the surgeons who used to work with you was a big believer in transhiatal esophagectomy. Can you tell us if there was any difference in how you approached the T2 N0 patients? If they went to surgery alone, was that a transhiatal esophagectomy; if they got neoadjuvant with chemoradiation, was it an Ivor Lewis perhaps? Was there any difference in the surgical technique during that period? I enjoyed the paper.
MS ZHANG
Thank you. In terms of upstaging in the operation-only group, out of the 7 patients, or the 50% that were upstaged, we only had 3 patients that had T2 N1s, and the rest were a different T stage. Most of the surgical procedures were transhiatal. So about 80% of the patients in the operation-only group were transhiatal and about 53% or so in the chemotherapy group plus operation were transhiatal. But that is also given that about 20% of those patients were marked as unspecified esophagectomies as well.
DR KRASNA
I was just asking is it possible that you understaged the N stage at the time of the resection if the surgery-alone group actually had transhiatal surgery.
DR YANG
That is probably the likely cause, because there is a bias at our institution to do transhiatals, and I would bet, again, with the two groups, that most of them were transhiatals as opposed to Ivor Lewises.
DR STEPHEN CASSIVI (Rochester, MN)
In this day and age of our workforce problems and trying to recruit the best and the brightest and a more diverse profession, I am rising to congratulate once again Steve Yang for his work. I don’t know whether anyone realized it, but Jennifer is a medical student, and she got up and presented in front of a whole group of varied thoracic surgeons, and this is not the first time that Steve has brought a medical student and mentored them through that. So it is a real feather in both of your caps.
My question is with regards to this paper and the one that Dr Yacoub presented yesterday. I think what we are seeing right now is that the gray zone of esophageal staging—although we think we are much better than we used to be—is in the T2 N0 area. I know you talked about maybe implementing using the PET or whatever, but what can we do other than maybe taking this home? I mean, I am guilty of it, too. I send my EUS to an endoscopist of an ultrasonographer to do. But maybe when something is so operator-dependent, maybe we should bring these home to us in thoracic surgery.
MS ZHANG
I think that is definitely a good point and something that we can explore further in terms of, say, these patients are getting EUS outside with people that you don’t have any experience with. Does it change their later pathologic staging if you bring them to the Mayo Clinic, for example, with more experienced operators? That is a good question.
DR YANG
Continuing the discussion from yesterday, is this operator-dependent or is it dependent upon our technology? We use the 15 MHz, and, again, about 25% of our EUSs are done outside the institution, and even within our institution, we have 4 people who do EUSs, and they range from just having finished their fellowship to someone who has been there for 15 years. And so is the onus on us doing it? Again, for us who don’t do it routinely, maybe then we will question our own. Even though we are a little bull-headed in thoracic surgery, we will question our own reliability, and we don’t do EUS as much as the gastrointestinal folks. I am not sure we will ever answer this issue of quality of the EUS’s. I think that will evolve over time, as yesterday we talked about the 20 MHz and sampling the lymph nodes better, et cetera. So I think that will come in time.
DR WAYNE L. HOFSTETTER (Houston, TX)
Jennifer, I think you did a really nice job on the presentation. I just wanted to make a comment and then a question and then really to extend an invitation.
This is a group of patients that we don’t see that often, and similar to Tom Rice’s paper from the Cleveland Clinic, the presentation contains a small sample size, and therefore, it is difficult to reach conclusions. And so in the end, you had talked about performing either a multiinstitutional validation or a randomized trial. When you do the statistical numbers for this, the sample size required to perform a prospective study with adequate power is inaccessible. We just don’t have a frequent enough presentation of this group of patients. So I agree with you that this will really require multiinstitutional validation.
Like you, I have been searching for reliable evidence to guide therapy in this group of patients. We queried the Society of Thoracic Surgeons database for cT2 N0 patients, and unfortunately, the data that we are keeping at the Society of Thoracic Surgeons isn’t robust enough. It doesn’t have enough granularity to determine what their overall treatment was. The invitation that I am sending out to your group is to pool our data in attempts to validate whether treatment with chemoradiation or surgery alone is indicated in this group of patients.
In terms of inaccurate staging, our group participated in a retrospective study to try and delineate those patients who are at higher risk for pN1 disease, and we have published a multiinstitutional validated nomogram. Rather than relying on PET scan, which is somewhat insensitive for regional disease, we found that the most sensitive indicators of lymph node involvement have been EUS-determined tumor length and depth. So to summarize, in patients that we find a longer length T2 tumor that would translate to a higher risk on our nomogram of underdiagnosis, these are the patients we focus on giving neoadjuvant chemoradiation.
DR ROBERT J. CERFOLIO (Birmingham, AL)
Nice presentation. I think because the T2 N0s are equivocal, you should have one person do them. If someone is sent to me with a T2 N0, I order a repeat EUS fine-needle aspiration at our place. So that is one thing I would say I do a little differently. Second, age should be a big factor. If the patient is 75 and older and they are T2, I am going to change my protocol a little and probably just go ahead and resect them, but if they are younger, we treat most all of them with neoadjuvant therapy when our one observer, our one endosonographer that we use for all our T2s, corroborates that it is T2 N0. And, finally, every T2 N0 should either have an Ivor Lewis, in my opinion, a robotic lymph node, video-assisted thoracoscopic procedure, whatever it is, some way to get all the lymph nodes out of the chest, because you are going to understage them if you don’t do a complete thoracic lymphadenectomy.
MS ZHANG
Thank you. That is a great comment. Actually if you look at our cohort as well, we see that the surgery-only group tends to be older, almost 70, with the chemotherapy adjuvant group being around 60.
Footnotes
Presented at the Fifty-seventh Annual Meeting of the Southern Thoracic Surgical Association, Orlando, FL, Nov 3-6, 2010.
References
- 1.Rohatgi A, Naji S, Hamouda A, et al. Effect of understaging of early oesophageal cancers on treatment. J Clin Oncol. 2009;(27(suppl)) abstr el5572. [Google Scholar]
- 2.Mariette C, Seitz F, Maillard E, et al. Surgery alone versus chemoradiotherapy followed by surgery for localized esophageal cancer: analysis of a randomized controlled phase HI trial FFCD 9901. J Clin Oncol. 2010;28(15 suppl) doi: 10.1200/JCO.2013.53.6532. abstr 4005. [DOI] [PubMed] [Google Scholar]
- 3.Hurmuzlu M, Øvrebø K, Monge OR, Smaaland R, Wentzel-Larsen T, Viste A. High-dose chemoradiotherapy followed by surgery versus surgery alone in esophageal cancer: a retrospective cohort study. World J Surg Oncol. 2010;8:46. doi: 10.1186/1477-7819-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stiles B, Mirza F, Coppolino A, et al. Clinical T2-T3N0M0 esophageal cancer: the risk of node positive disease. Ann Thorac Surg. 2011;92:491–498. doi: 10.1016/j.athoracsur.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Rice T, Mason D, Murthy S, et al. T2N0M0 esophageal cancer. J Thorac Cardiovasc Surg. 2007;133:317–324. doi: 10.1016/j.jtcvs.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 6.Matsubara H. Neoadjuvant chemoradiation therapy for the treatment of esophageal carcinoma. Intl J Clin Oncol. 2008;13:474–478. doi: 10.1007/s10147-008-0853-4. [DOI] [PubMed] [Google Scholar]
- 7.Greene FPD, Fleming L, Fritz A, et al. AJCC cancer staging handbook. 6th ed. New York: Springer-Verlag; 2002. Esophagus; pp. 91–95. [Google Scholar]
- 8.Keats AS. The ASA classification of physical status—a recapitulation. Anesthesiology. 1978;49:233–236. doi: 10.1097/00000542-197810000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Rice T, Rusch V, Ishwaran H, Blackstone E. Cancer of the esophagus and esophagogastric junction. Cancer. 2010:3763–3773. doi: 10.1002/cncr.25146. [DOI] [PubMed] [Google Scholar]
- 10.Orditura M, Galizia G, Morgillo F, et al. Complete response to preoperative chemoradiation and survival in esophageal cancer: a pooled analysis of three single-institution phase II trials. Dis Esophagus. 2011:1442–2050. doi: 10.1111/j.1442-2050.2011.01220.x. [DOI] [PubMed] [Google Scholar]
- 11.Meguid RA, Hooker CM, Taylor JT, et al. Recurrence after neoadjuvant chemoradiation and surgery for esophageal cancer: Does the pattern of recurrence differ for patients with complete response and those with partial or no response? Gen Thorac Surg. 2009;138:1309–1316. doi: 10.1016/j.jtcvs.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rice T, Blackstone E, Rusch V. A cancer staging primer: esophagus and esophagogastric junction. J Thorac Cardiovasc Surg. 2010;139:527–529. doi: 10.1016/j.jtcvs.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Alexandrou A, Davis PA, Law S, et al. Squamous cell carcinoma and adenocarcinoma of the lower third of the esophagus and gastric cardia: similarities and differences. Dis Esophagus. 2002;15:290–295. doi: 10.1046/j.1442-2050.2002.00272.x. [DOI] [PubMed] [Google Scholar]
- 14.Gupta NM, Jindal R, Prakash O, et al. Comparison of the clinical profile and outcome for squamous cell carcinoma and adenocarcinoma of the distal esophagus and cardia in India. Surg Today. 2001;31:400–404. doi: 10.1007/s005950170129. [DOI] [PubMed] [Google Scholar]
- 15.Salazar JD, Doty JR, Lin JW, et al. Does cell type influence post-esophagectomy survival in patients with esophageal cancer? Dis Esophagus. 1998;11:168–171. doi: 10.1093/dote/11.3.168. [DOI] [PubMed] [Google Scholar]