Abstract
Rationale
Heavy drinking smokers constitute a sizeable and hard-to-treat subgroup of smokers, for whom tailored smoking cessation therapies are not yet available.
Objective
The present study used a double-blind, randomized, 2×2 medication design, testing varenicline alone (VAR; 1mg twice daily), naltrexone alone (NTX; 25mg once daily), varenicline plus naltrexone, and placebo for effects on neural activation to cigarette cues in a sample (n=40) of heavy drinking daily smokers (≥10 cigarettes/day).
Methods
All participants were tested after a 10–12 day titration period designed to reach steady state on the target medication. Participants underwent functional neuroimaging (fMRI) for examination of brain responses to visual smoking-related (vs. neutral) cues.
Results
Region of interest (ROI) analyses of brain responses to cigarette vs. neutral cues indicated that the combination of VAR+NTX was associated with reduced activation of the bilateral anterior cingulate cortex as compared to placebo and to NTX alone. Exploratory whole-brain analyses also indicated significant differences in brain activation during cigarette cues in the active medications versus placebo condition. All medications suppressed left nucleus accumbens activation relative to placebo, suggesting the possibility that both medications, either alone or in combination, reduce neural signals associated with appetitive behavior.
Conclusions
Although preliminary, these neuroimaging findings indicate that clinical studies of the combination of VAR+NTX for heavy drinkers trying to quit smoking may be warranted.
Keywords: Naltrexone, varenicline, heavy drinker, smoker, craving, fMRI
INTRODUCTION
Approximately 20–25% of all regular smokers are also heavy drinkers (1, 2). Heavy-drinking smokers experience more negative health consequences, such as deficits in brain morphology and functional impairments (3) and greater risk for various cancers (4) than individuals who are either smokers only or drinkers only. Alcohol use is thought to trigger lapses in smoking cessation, as greater alcohol intake is associated with lower odds of quitting smoking (2, 5, 6), and it is estimated that abstinent smokers are five times more likely to experience a smoking lapse during drinking episodes than at other times (6). Although heavy-drinking smokers constitute a distinct sub-population with a unique clinical profile and treatment needs (7, 8), there are no available treatments tailored to heavy-drinking smokers.
It may be possible to optimize smoking cessation treatments for heavy drinking smokers through the combination of effective pharmacotherapies for smoking and drinking. Varenicline, an α4β2 nicotinic acetylcholine receptor (nAChRs) partial agonist and α7 nAChR full agonist, has been found to be an effective smoking cessation aid (9). Its mechanisms of action stem from the stimulation of dopamine release while competing for nAChR binding, thereby preventing the full agonist action of nicotine (10). In the human laboratory, varenicline reduces craving and withdrawal and attenuates the rewarding effects of smoking (11–15). Functional neuroimaging studies found that varenicline increases activation of the dorsal anterior cingulate/medial frontal cortex, and dorsolateral prefrontal cortex during a working memory task (16) and an emotional processing task (17). Moreover, varenicline diminished brain activation in response to smoking cues in the ventral striatum and medial orbitofrontal cortex (18). Clinically, varenicline was found to be more effective as a smoking cessation agent than bupropion (9), nicotine replacement therapy (19), and placebo (9, 20–23).
Naltrexone is an opioid receptor antagonist with efficacy for the treatment of alcoholism (24). In combination with counseling and nicotine patches, adjunctive naltrexone increased cigarette smoking quit rates relative to placebo but only among participants with higher levels of depressive symptoms (25) and among women (26, 27). Two other studies found support for the use of naltrexone as an adjunct to treatment with bupropion and nicotine patch (28, 29), while one study did not support combining naltrexone with bupropion (30). Recent studies of naltrexone as a stand-alone smoking-cessation treatment found that it improves smoking quit rates (31), and may preferentially decrease both smoking and drinking in heavy-drinking smokers (32). A neuroimaging study of naltrexone found that it reduces ventral striatum activation during alcohol cues in alcohol-dependent individuals (33). More recently, a functional neuroimaging (fMRI) study of naltrexone found a medication by genotype interaction on neural responses to alcohol cues (34). No studies to date have examined the effects of naltrexone on neural responsivity to cigarette cues, and more broadly, no neuroimaging or clinical studies have tested combination of varenicline and naltrexone.
In summary, given the co-occurrence of smoking and drinking it has been convincingly argued that heavy drinking smokers constitute a distinct sub-population of smokers with a unique clinical profile and treatment needs (7, 8). Currently, there are no available pharmacological treatments tailored to heavy drinking smokers. Despite the mature status of smoking cessation research, this subpopulation is significant and efforts to develop novel treatment approaches for this group are warranted. One of the ways in which treatments can be optimized is through the combination of effective pharmacotherapies for smoking and drinking. Varenicline is an effective smoking cessation aid (9) that also reduces alcohol self-administration in the lab (35), alcohol craving (36), and alcohol consumption (36, 37) in smoking cessation trials. Naltrexone is a moderately effective treatment for alcohol problems (24) and has been found to improve smoking quit rates (31), particularly in heavy drinkers (32). These studies underscore the reciprocal effects of varenicline and naltrexone on both smoking and drinking outcomes and suggest that combining these medications for smoking cessation in heavy drinking smokers may be a viable and promising alternative. From a neuroimaging view point, since both medications have been found to attenuate neural activation to cues in the brain’s reward circuitry individually, the combination of varenicline and naltrexone is hypothesized to have additive effects on neural cue reactivity.
To advance medication development for heavy drinking smokers, the present study used a neuroimaging paradigm to test whether these medications, alone and in combination, would attenuate neural activation to cigarette cues. This study consisted of double-blind, randomized, 2×2 medication design, testing varenicline alone (1mg twice daily), naltrexone alone (25mg once daily), varenicline plus naltrexone (1mg twice daily and 25mg once daily, respectively), and placebo for their effects on neural response to cigarette versus control cues in a sample (n=40) of heavy drinking daily smokers (≥10 cigarettes/day). All participants underwent an fMRI task of neural responses to visual smoking cues after a 10–12-day titration period designed to reach a steady state on the target medication dosage. The objective of this study was to evaluate neural markers of response to the combination of varenicline plus naltrexone. It was hypothesized that combination pharmacotherapy would be more effective in attenuating neural activation to cigarette cues in brain regions previously implicated in smoking and alcohol cue processing (i.e., nucleus accumbens, insula, precuneus, anterior cingulate cortex, and the superior frontal gyrus) (38, 39). Region of interest (ROI) analyses were conducted to address the study goals, followed by exploratory whole brain comparisons.
METHODS
Participants
A community-based sample of daily smokers was recruited via online and print advertisements in the Los Angeles area. Inclusion criteria were: (1) age between 21 and 55 years; (2) endorsement of smoking 10 or more cigarettes per day; (3) current status of heavy drinking according to the National Institute on Alcohol Abuse and Alcoholism (NIAAA) guidelines (40): for men, >14 drinks per week or ≥5 drinks per occasion at least once per month over the past 12 months; for women, >7 drinks per week or ≥4 drinks per occasion at least once per month. Exclusion criteria were: (1) more than 3 months of smoking abstinence in the past year; (2) self-reported use of cocaine, methamphetamine, heroin or other illicit drugs (other than marijuana) in the previous 60 days or positive urine toxicology screen at assessment visit; (3) lifetime history of psychotic disorders, bipolar disorders, or major depression with suicidal ideation; (4) current symptoms of moderate depression (or higher), indexed by a score ≥20 on the Beck Depression Inventory-II (BDI-II) (41); (5) current use of psychiatric medications; (6) ineligibility on physical exam and laboratory tests; and (7) MRI contraindications/constraints, including left-handedness.
Demographic information, including age, sex, ethnicity, and years of education, was collected from all participants. Also obtained were self-reports of cigarette and alcohol use, and indices of nicotine and alcohol dependence (Table 1).
Table 1.
Demographic information, smoking behavior, and alcohol use for the 39 participants included in the analyses.
| Variable | All Subjects | Medication Condition – Mean (SD) or Frequency | |||
|---|---|---|---|---|---|
| VAR | NTX | VAR+NTX | PLAC | ||
| Age | 31.41 (8.81) | 33.70 (9.26) | 28.90 (8.42) | 27.40 (4.50) | 36.11 (10.48) |
| Sex – Male/Female | 24/15 | 7/3 | 8/2 | 4/6 | 5/4 |
| Ethnicity | |||||
| - Caucasian | 25 | 4 | 6 | 7 | 8 |
| - African Am. | 7 | 3 | 2 | 1 | 1 |
| - Asian | 3 | 0 | 2 | 1 | 0 |
| - Latino | 4 | 3 | 0 | 1 | 0 |
| Education (years) | 14.61 (3.76) | 13.90 (5.13) | 14.00 (4.45) | 15.50 (2.92) | 15.11 (1.83) |
| FTND* | 5.15 (1.66) | 5.20 (1.62) | 6.30 (1.16) | 4.80 (1.75) | 4.22 (1.56) |
| Cigarettes Per Day | 14.55 (7.45) | 12.77 (4.41) | 12.85 (5.99) | 16.05 (9.65) | 16.78 (8.98) |
| Smoking Days per Month | 29.49 (1.14) | 28.80 (1.75) | 30.00 (0.00) | 29.40 (1.08) | 29.78 (0.67) |
| Alcohol Dependence Scale | 12.59 (6.43) | 13.20 (5.45) | 10.10 (6.12) | 15.60 (8.53) | 11.33 (4.25) |
| Drinking Days per Month | 20.54 (7.79) | 22.30 (8.62) | 19.50 (7.75) | 19.50 (6.87) | 20.89 (8.81) |
| Alcohol Drinks per Drinking Day | 6.16 (3.22) | 5.22 (3.05) | 6.08 (3.27) | 7.72 (3.70) | 5.55 (2.60) |
Significant difference observed between NTX and PLAC groups on FTND (p = .006; p = .034, Bonferroni corrected); and between NTX and NTX+VAR (p = .034; p = .204, Bonferroni corrected)
Two of the 40 subjects (both in NTX group) reported using marijuana monthly (on average) and the remaining 38 subjects reported no current use of marijuana.
Procedures
Interested individuals called the laboratory and completed a telephone-screening interview. Individuals who were deemed eligible came to the laboratory for in-person screening. After receiving a full explanation of study procedures and providing written, informed consent, participants completed the screening visit. Carbon monoxide (CO) levels and a urine cotinine test were used to verify self-reported smoking patterns. Participants were required to have a Breath Alcohol Concentration (BrAC) of 0.00g/dl at the beginning of each visit. Participants deemed eligible following the in-person screening completed a physical exam and clinical labs. The physical examination included (inspection, auscultation, percussion and palpation), a medical and psychiatric history, a review of systems, inquiry about substance use, medication use and allergies. Vital signs and an EKG were also obtained and reviewed. Laboratories included: Sodium, potassium, chloride, carbon dioxide (CO2), serum calcium, serum total protein (TP), serum albumin, liver function assessment, bilirubin, alkaline phosphatase (ALP), aspartate amino transferase (AST or SGOT), alanine amino transferase (ALT or SGPT).
Individuals who passed the physical exam were then randomized to one of the four medication conditions. Participants then took the study medication for 9 days and completed a laboratory-based assessment of craving for cigarettes after 12-hrs of abstinence and after consuming alcohol; results of the laboratory component of the study are reported elsewhere (42). Participants from the laboratory study were randomly selected from each medication cell group for screening into the neuroimaging component of the study. Individuals who were interested and met eligibility criteria for scanning were provided with an extra 3 days of the study medication (at target dose) after the laboratory visit and completed the scan on medication days 10–12. Participants were not required to abstain from smoking prior to the neuroimaging session; however, breath alcohol concentration (BrAC) was required to be 0.00g/dl. At the time of scanning, a urine sample was collected to test for riboflavin fluorescence indicating medication compliance, and female participants completed a pregnancy test.
The participants were titrated on varenicline as follows: days 1–2, 0.5mg per day, days 3–5, 0.5mg twice per day, and days 6–12, 1mg twice per day. Naltrexone was administered at 25mg per day for a period of 12 days. The 25mg dose was selected based on study by O'Malley et al. (2009) comparing three doses of naltrexone (25, 50, and 100mg/day) as an adjunct to smoking cessation. This study found medication effects on drinking at 25mg/day, which has a more favorable side-effect profile than the usual 50mg dose, particularly for combination pharmacotherapy (43). Placebo pills were matched to the active medications in number of pills and packaging. Study medications were packed into opaque capsules with 50mg of riboflavin, and compliance with taking the medication was monitored by testing a urine sample for riboflavin content at each testing session by examining it under an ultraviolet light (44). Participants were given a 24-hour telephone number to call the physician to discuss side-effects, and physician office hours were available as needed. All procedures were approved by the Institutional Review Board of the University of California, Los Angeles.
Measures
The following individual differences measures were administered during the screening visit: (a) a demographics questionnaire used to collect information on age, sex, marital status, SES, ethnicity, income, and education; (b) the Beck Depression Inventory-II (BDI-II) administered at screening to identify and exclude individuals with current feelings of active suicidality and those with moderate to severe symptoms of depression; (c) frequency and quantity of alcohol/drug use; (d) a Smoking History Questionnaire to collect detailed history of nicotine use and quit attempts; (e) the Fagerstrom Test of Nicotine Dependence (FTND) to assess nicotine dependence (45); and (f) the Time Line Follow Back (TLFB) to assess smoking and alcohol use over the past 30 days (46).
Cigarette Cues Task
While in the scanner, participants underwent a cigarette cues task which consisted of viewing videotaped cues, developed on the basis of work by Brody et al (47). Similar tasks have been widely used in neuroimaging studies of tobacco users (48–52). Stimuli consisted of first-person perspective color videos with smoking content (e.g., person smoking a cigarette) or control content (e.g., person writing in a journal), each lasting 45 seconds. The paradigm consisted of 12 video cue trials (6 cigarette and 6 neutral) pseudorandomly presented across participants (first video always neutral), followed by an urge-rating period for a maximum of 10 seconds or until key press, 1 second of response feedback, and a 10-second rest period before initiation of the next trial. During the urge-rating period, participants were instructed to rate their current urge to smoke using a scale of 1 (no urge at all) to 4 (very high urge) using a 4-button response box placed in their right hand. The presentation of all stimuli and response collection were programmed using MATLAB (Mathworks, Natick, MA) and Psychtoolbox (www.psychtoolbox.org) on an Apple MacBook running Mac OSX (Apple Computers, Cupertino, CA). Visual and auditory stimuli were presented using MRI-compatible goggles and headphones (Resonance Technologies, Van Nuys, CA). The task averaged approximately 12 minutes in length with a 1 minute break at the halfway point.
MRI Data Acquisition, Preprocessing, and Registration
Neuroimaging was conducted using a 3 Tesla Siemens Trio MRI scanner. The protocol began with initial structural scans followed by a series of three functional paradigms, including the cigarette cues task. A T2-weighted, high resolution, matched-bandwidth, anatomical scan (MBW) (TR, 5s; TE, 34ms; FOV, 192mm; matrix, 128×128; sagittal plane; slice thickness, 4mm; 34 slices) and a magnetization-prepared rapid-acquisition gradient echo (MPRAGE) were acquired for each subject to enable registration (TR, 1.9s; TE, 2.26ms; FOV, 250mm; matrix, 256×256; sagittal plane; slice thickness, 1mm; 176 slices). The orientation for MBW and echoplanar image (EPI) scans was oblique axial to maximize brain coverage. The cigarette-cues scan included 100 functional T2*-weighted EPIs (slice thickness, 4mm; 34 slices; TR, 2s; TE, 30 ms; flip angle, 90°; matrix, 64 × 64; FOV, 192 mm; voxel size, 3×3×4mm3). The first six volumes collected were discarded to allow for T1 equilibrium effects.
FSL 4.1 (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl) was used for the imaging analyses. EPI images were motion corrected using the Motion Correction Linear Image Registration Tool (McFLIRT, Version 5.0) with the estimated motion parameters entered as covariates in the general linear model. The images were high-pass filtered (100-s cutoff) in the temporal domain using a Gaussian-weighted straight line with the FMRI Expert Analysis Tool (FEAT, Version 5.63). Non-brain tissue/skull removal was conducted for both structural and functional images with the Brain Extraction Tool (BET). The EPI images were first registered to the MBW, then to the MPRAGE using affine linear transformations, and finally into standard (Montreal Neurological Institute, MNI avg152 template) space for between subject analyses. Linear registrations using FSL’s FLIRT were refined with FNIRT nonlinear registration (53). Data from one subject (in the placebo group) was excluded from further analyses due to excessive motion (exceeding 3 mm of translation) resulting in a final sample of 39 individuals.
Regions of interest (ROIs) were chosen based on the findings of two large meta-analyses examining brain activation during smoking and alcohol cue exposure tasks (38, 39). ROIs identified for analyses were the left nucleus accumbens, ventral anterior insula, precuneus, bilateral rostral anterior cingulate cortex, and the right superior frontal gyrus. The left NAcc, the subcortical region of interest, was defined anatomically on an individual basis using automated segmentation procedures implemented in FSL's FMRIB's Integrated Registration and Segmentation Tool (FIRST) (54). FIRST was applied to each participant’s high resolution structural image (MPRAGE). The resultant NAcc ROIs were registered from native space to standard space and used to extract data from each participant’s Cigarette Cues vs. Neutral Cues contrast image.
To examine unbiased regions of interest for the cortical areas we used a "leave-one-out" procedure that allows definition of functional ROIs that are independent of the data extracted from them (55). Thirty-nine whole-brain one-sample t-tests were run for the Cigarette Cues vs. Neutral Cues contrast images. For each t-test, one participant was left out, and a spherical ROI (8mm radius) was defined around the peak voxel for each region. The left-out participant's data was extracted from this spherical ROI. Anatomically defined masks from the Harvard-Oxford Probabilistic Brain Atlas were used to constrain the search space for finding the peak voxel within the a priori determined anatomical regions (e.g., superior frontal gyrus). The mean peak voxels from the precuneus were located at x=2.4, y=−65.6, z=29.2, for superior frontal gyrus at x=−1.7, y=56.0, and z=29.7, for anterior cingulate cortex at x=−0.3, y=28, and z=12.4, and for the right anterior insula at x=42.6, y=10, and z=−10.3 (MNI coordinates).
Statistical Analyses
Statistical analyses on the fMRI data were performed using a multi-stage approach to implement a mixed-effects model treating participants as a random-effects variable. Explanatory variables for the cigarette-cues task were created by convolving stick functions representing the onset of the cigarette cue period for each trial type with a double-gamma hemodynamic response function (HRF) in FEAT. The events modeled included: Cigarette Cue and Neutral Cue exposure. The onset for each event was set at the initiation of the video cue with duration of 45 seconds. Temporal derivatives were included as covariates of no interest to improve statistical sensitivity. The Cigarette Cues vs. Neutral Cues contrast was computed, and the resulting Z-statistic images were thresholded with cluster-based corrections for multiple comparisons according to the theory of Gaussian Random Fields with a cluster-forming threshold (height threshold) of Z>2.30 and a probability threshold of p<0.05 (56). The following two sets of analyses were conducted: (1) ROI Analyses: Planned comparisons between medication groups on ROI data were conducted using independent t-tests on medication group means adjusted for FTND score; and (2) Whole Brain Analyses: Exploratory analyses of medication effects in the whole-brain were conducted by entering medication group (i.e., VAR, NTX, VAR+NTX, and PLAC) as a second-level predictor variable on the contrast map. Anatomical localization within each cluster (maximum Z statistics and MNI coordinates) was verified using the FSL Harvard-Oxford probabilistic atlas.
RESULTS
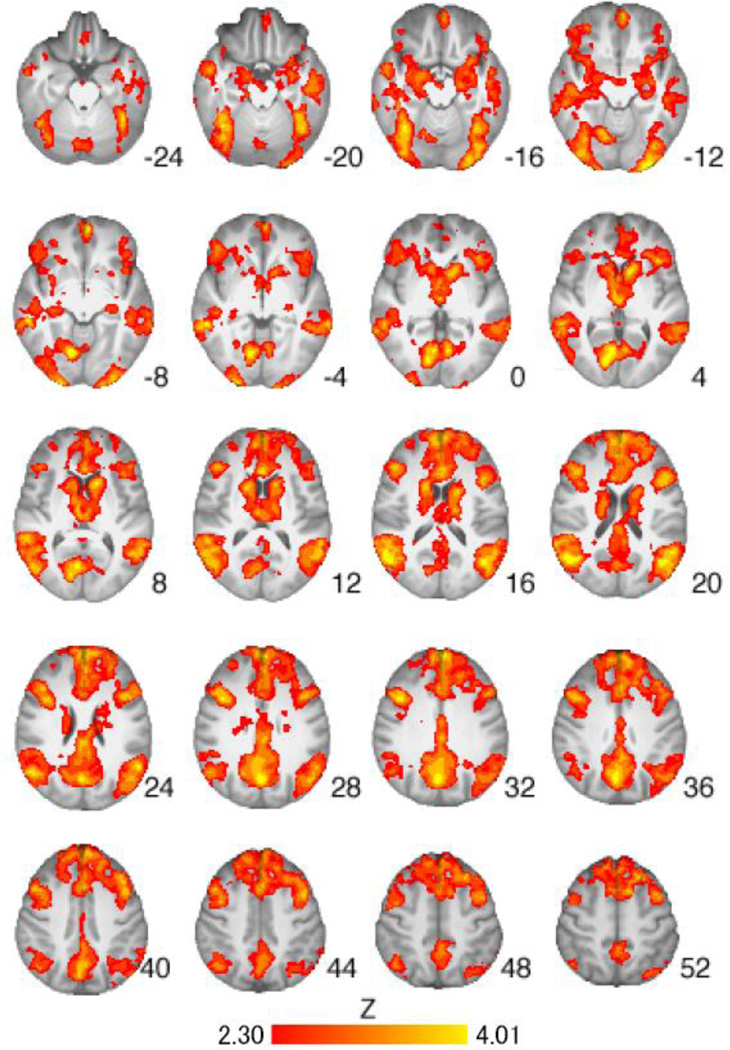
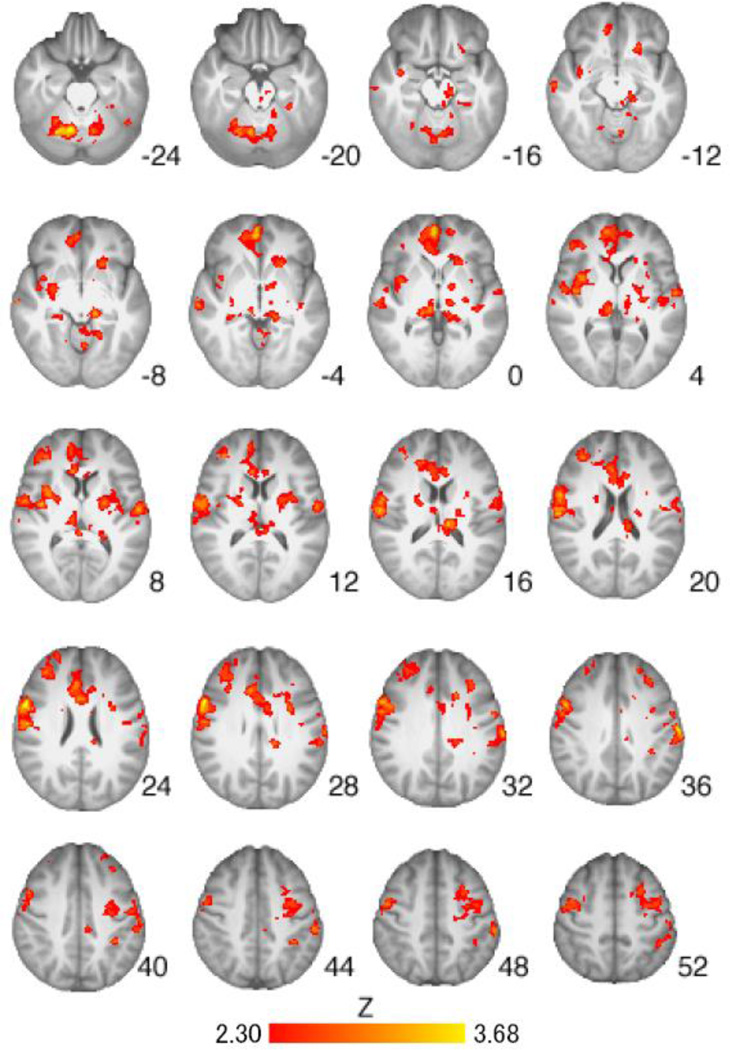
All urine samples tested positive for riboflavin and all participants had a BrAC of 0.00g/dl at each visit. There were no serious adverse events during the study. The medication groups did not differ on average subjective craving ratings to cigarette or control cues during the scan (ps>.05). The medication groups did not differ significantly on withdrawal (57), CO level pre-scan, CO level post-scan, or hours since last cigarette prior to scanning; nor did they differ on days since last alcohol intake (ps > .05). A total of 57.5% of the sample drank alcohol the day before the scan, 32.5% drank two days before, and 10% drank three days before. In addition, there were no significant correlations between cigarettes per day, drinks per drinking day, or total drinks during the medication titration period and ROI activation from the Cigarette vs Control Cues contrast (described in detail below). Whole-brain analysis of the Cigarette Cues versus Neutral Cues contrast across all participants revealed significant activation in brain regions implicated in cue reactivity, including the left nucleus accumbens, and bilateral precuneus, thalamus, and caudate nucleus (Table 2, Figure 1).
Table 2.
Locations of significant activation for the Cigarette cues versus Neutral cues contrast across all participants (n=39) (whole-brain cluster-corrected at Z>2.3, p<0.05)
| Brain Region | Hemisphere | Cluster Voxels |
Max Z | X | Y | Z |
|---|---|---|---|---|---|---|
| Precuneus/Thalamus/Putamen/ Accumbens (L)/ Caudate/ Anterior Cingulate Cortex/ Superior Frontal Gyrus/Middle Frontal Gyrus/Inferior Frontal Gyrus | R | 57355 | 4.01 | 2 | −70 | 28 |
| - Lingual Gyrus | R | 4.00 | 12 | −70 | −6 | |
| - Occipital Pole | L | 3.98 | −26 | −100 | −12 | |
| - Intracalcarine Cortex | R | 3.96 | 14 | −78 | 4 | |
| - Middle Temporal Gyrus | R | 3.96 | 58 | −40 | −6 | |
Note: X, Y, and Z MNI coordinates indicate the location of peak voxel activation (or local maxima for subregions) within each cluster. R, right, L, left.
Figure 1.
Brain activation for Cigarette Cues versus Neutral Cues contrast across all participants (n=39). Areas of activation included the left nucleus accumbens, and bilateral precuneus, thalamus, and caudate (see Table 2 for full list of regions). Z-statistic maps are whole-brain cluster-corrected, Z>2.30, p=0.05. Coordinates are in MNI space, and the brain is displayed in radiological convention (left = right).
A-Priori ROI Analyses
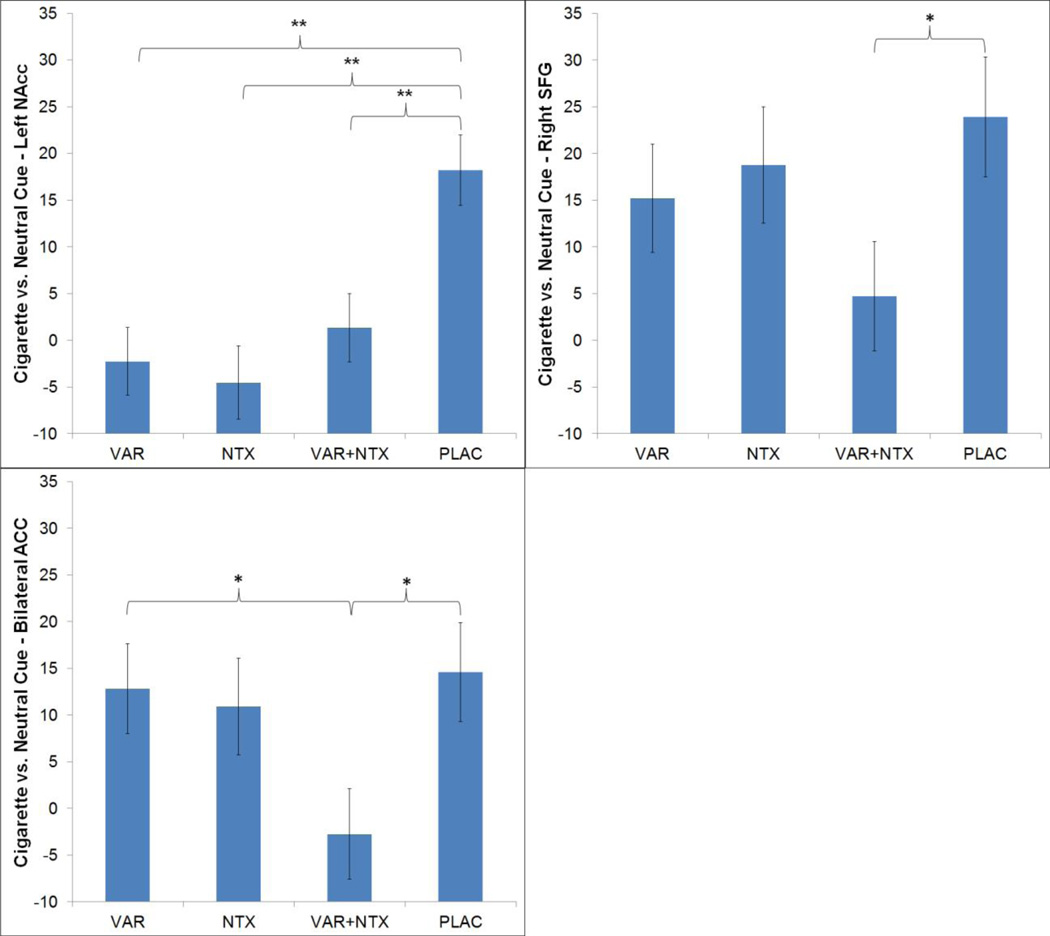
A priori defined ROI analyses of the Cigarette versus Neutral Cues contrast revealed significant medication effects, controlling for FTND score, in the precuneus, left NAcc, bilateral rostral ACC, and right SFG (Table 3, Figure 2). No medication effects were observed for the ventral anterior insula. Exploratory hierarchical linear regression analyses predicting ROI activation from the cigarette versus control cues contrast from in-scanner participant ratings of craving during the task revealed a significant relationship between craving and insula activation across all participants, while controlling for medication group and FTND score (β=−.347, t =−2.10, R2 change=.108, p=.04).
Table 3.
Comparisons of parameter estimates from the Cigarette versus Neutral Cues contrast between active medication groups versus placebo group within each region of interest. Listed are mean parameter estimates and 95% confidence intervals (CI)
| Variable | Medication Condition | ||
|---|---|---|---|
| VAR | NTX | VAR + NTX | |
| Left Nucleus Accumbens | −20.47 (−31.13; −9.81)** | −22.75 (−34.34; −11.15)** | −16.88 (−27.37; −6.40)** |
| Ventral Anterior Insula | −10.69 (−29.65; 8.27) | −8.78 (−29.39; 11.83) | −9.49 (−28.13; 9.15) |
| Precuneus | −12.57 (−30.81; 5.67)B | 13.75 (−6.07; 33.58)B | −2.08 (−20.01; 15.84) |
| Bilateral Rostral Anterior Cingulate Cortex | −1.78 (−16.36; 12.79)A | −3.69 (−19.54; 12.16) | −17.32 (−31.65; −2.99)*A |
| Right Superior Frontal Gyrus | −8.75 (−26.34; 8.84) | −5.18 (−24.30; 13.95) | −19.21 (−36.50; −1.92)* |
Note: All results are for ANCOVAs controlling for FTND score
p < .05;
p < .01
Significant difference between VAR + NTX vs. VAR alone
Significant difference between NTX alone vs. VAR alone
Please note that if a Bonferroni correction was implemented using a p-value threshold of p < .01 then the results for the Anterior Cingulate Cortex and Precuneus would remain statistically significant.
Figure 2.
Adjusted means and standard error of the mean for the nucleus accumbens (NAcc; a), right superior frontal gyrus (SFG; b), and bilateral rostral anterior cingulate cortex (ACC; c) ROI analyses of the Cigarette versus Neutral Cues contrast (controlling for FTND score). Significant group differences are indicated by * for p < .05 and ** for p < .01.
Exploratory Whole Brain Analyses
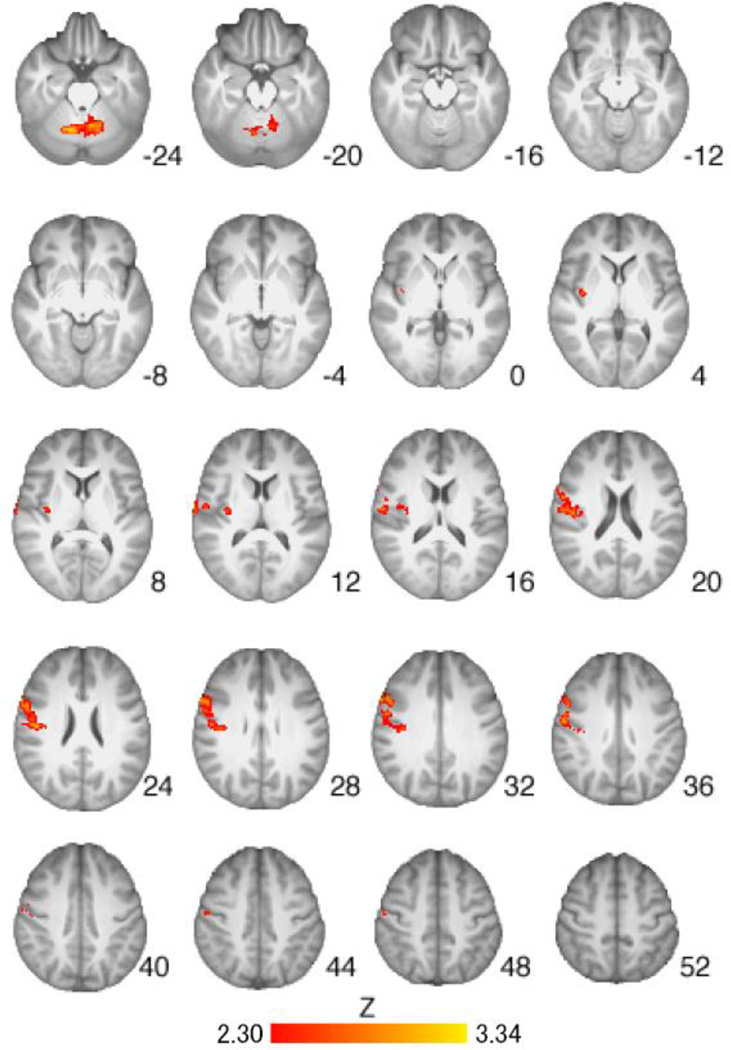
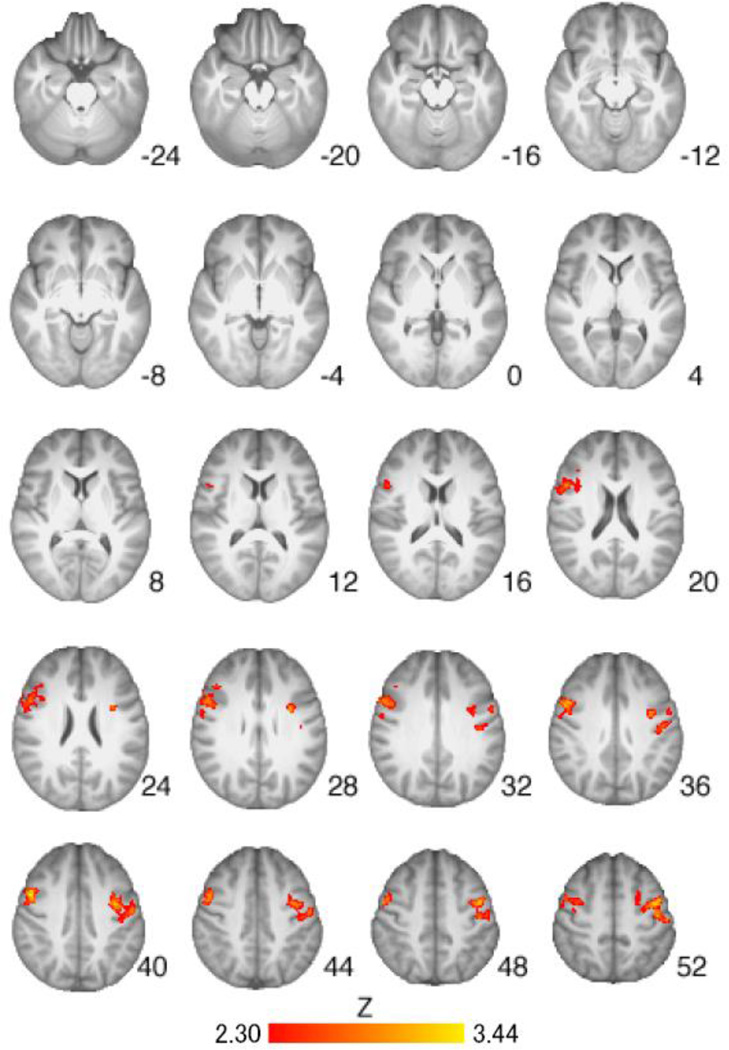
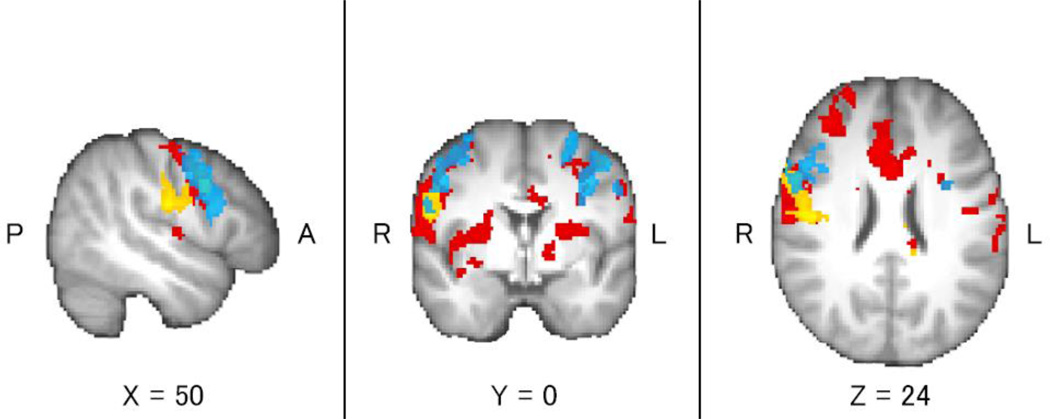
Regions of activation from the Cigarette Cues vs. Neutral Cues contrast were found to differ when comparing the placebo group to the medication groups. VAR alone was associated with less activation in the precentral gyrus, right insular cortex, left thalamus, and right caudate as compared to placebo (Table 4, Figure 3). NTX alone was associated with less activation in the right insular cortex, right putamen, right caudate, bilateral precentral gyrus, and right inferior frontal gyrus as compared to placebo (Table 4, Figure 4). The combination of VAR + NTX was associated with less activation to cigarette versus control cues in the bilateral orbitofrontal cortex, insular cortex, thalamus, caudate, and cerebellum, as compared to placebo (Table 4, Figure 5). Areas of overlap across medication group comparisons (for visualization purposes) are presented in Figure 6.
Table 4.
Locations of significant activation for the Cigarette cues versus Neutral cues contrast for all significant medication group comparisons: Placebo (PLAC) vs. Varenicline alone (VAR) (A), Placebo vs. Naltrexone alone (NTX) (B), Placebo versus combined Varenicline and Naltrexone (VAR+NTX). No other significant medication group comparisons were observed. All analyses were whole-brain cluster-corrected at Z>2.3, p<0.05.
| (A) Cigarette Cues vs. Neutral Cues – PLAC vs. VAR | ||||||
| Brain Region | Hemisphere |
Cluster Voxels |
Max Z | X | Y | Z |
| Cerebellum | R | 1849 | 3.34 | 18 | −60 | −30 |
| - Brain Stem | R | 3.04 | 14 | −36 | −40 | |
| Precentral Gyrus/Insula | R | 1115 | 3.16 | 60 | 14 | 30 |
| - Inferior Frontal Gyrus | R | 3.14 | 54 | 16 | 32 | |
| - Central Opercular Cortex | R | 3.12 | 48 | −14 | 22 | |
| - Postcentral Gyrus | R | 2.94 | 56 | −10 | 36 | |
| (B) Cigarette Cues vs. Neutral Cues – PLAC vs. NTX | ||||||
| Brain Region | Hemisphere |
Cluster Voxels |
Max Z | X | Y | Z |
| Precentral Gyrus/Postcentral Gyrus/Superior Frontal Gyrus | L | 1562 | 3.44 | −34 | −6 | 58 |
| Precentral Gyrus/Middle Frontal Gyrus | R | 1196 | 3.26 | 50 | 6 | 40 |
| - Inferior Frontal Gyrus | R | 2.91 | 52 | 14 | 30 | |
| (C) Cigarette Cues vs. Neutral Cues – PLAC vs. VAR+NTX | ||||||
| Brain Region | Hemisphere |
Cluster Voxels |
Max Z | X | Y | Z |
| Frontal Pole | R | 3281 | 3.42 | 4 | 56 | 0 |
| - Paracingulate gyrus | R | 3.37 | 4 | 50 | −4 | |
| - Anterior Cingulate Gyrus | R | 3.1 | 10 | 22 | 26 | |
| - Frontal Pole | R | 3.04 | 36 | 42 | 12 | |
| - Postcentral Gyrus/Middle Frontal Gyrus/Superior Frontal Gyrus/ Insula/ Putamen/Caudate/ Accumbens | L | 3191 | 3.42 | −62 | −18 | 36 |
| - Supramarginal Gyrus | L | 3.23 | −58 | −30 | 46 | |
| - Central Opercular Cortex | L | 3.18 | −58 | −12 | 8 | |
| - Superior Parietal Lobule | L | 3.14 | −38 | −48 | 58 | |
| Precentral Gyrus | R | 2983 | 3.62 | 56 | 10 | 26 |
| - Postcentral Gyrus | R | 3.36 | 54 | −12 | 24 | |
| - Insula | R | 3.24 | 34 | 6 | 6 | |
| Cerebellum | R | 2756 | 3.68 | 12 | −62 | −24 |
| Thalamus | L | 1247 | 3.17 | −10 | −26 | 16 |
| - Thalamus/Hippocampus | R | 3.1 | 8 | −26 | 0 | |
Note: X, Y, and Z MNI coordinates indicate the location of peak voxel activation (or local maxima for subregions) within each cluster. R, right, L, left.
Figure 3.
Brain activation for the Placebo versus Varenicline groups from the Cigarette Cues versus Neutral Cues contrast. Areas of activation included the precentral gyrus, right insular cortex, left thalamus, and right caudate (see Table 4 for full list of regions). Z-statistic maps are whole-brain cluster-corrected, Z>2.30, p=0.05. Coordinates are in MNI space, and the brain is displayed in radiological convention (left = right).
Figure 4.
Brain activation for Placebo versus Naltrexone groups from the Cigarette Cues versus Neutral Cues contrast. Areas of activation included right insular cortex, right putamen, right caudate, bilateral precentral gyrus, and right inferior frontal gyrus (see Table 4 for full list of regions). Z-statistic maps are whole-brain cluster-corrected, Z>2.30, p=0.05. Coordinates are in MNI space, and the brain is displayed in radiological convention (left = right).
Figure 5.
Brain activation for Placebo versus Varenicline + Naltrexone groups from the Cigarette Cues versus Neutral Cues contrast. Areas of activation included bilateral orbitofrontal cortex, insular cortex, thalamus, caudate, and cerebellum (see Table 4 for full list of regions). Z-statistic maps are whole-brain cluster-corrected, Z>2.30, p=0.05. Coordinates are in MNI space, and the brain is displayed in radiological convention (left = right).
Figure 6.
Brain activation from the Cigarette Cues versus Neutral Cues contrast (whole-brain cluster-corrected, Z>2.30, p=0.05) for the Placebo versus Varenicline groups (yellow), Placebo versus Naltrexone groups (blue), and Placebo versus Varenicline + Naltrexone groups (red), for visualization purposes.
DISCUSSION
The present study used a cue-exposure functional neuroimaging paradigm to elucidate whether a combination of effective medications for smoking cessation (VAR) and for alcohol misuse (NTX) would be superior to monotherapy and placebo at reducing neural response to cigarette cues among heavy drinking smokers. The greatest separation between the combination group (VAR + NTX) and placebo was found for the right superior frontal gyrus and the bilateral anterior cingulate cortex. Specifically, the combination group showed significant attenuation of right superior frontal gyrus activation relative to placebo but did not differ from VAR alone and NTX alone. Regarding the bilateral anterior cingulate ROI, however, the combination group differed significantly from placebo and from VAR alone, showing lower activation to cigarette versus neutral cues. These differences are intriguing as anterior cingulate activation was found to increase when smokers were instructed to suppress their craving (58, 59). Thus it is plausible to hypothesize that the greater attenuation of anterior cingulate activation by the combination of VAR+NTX may have clinical benefits by attenuating craving for cigarettes. Importantly, ROI analyses indicated that all medications suppressed left nucleus accumbens activation relative to placebo, suggesting the possibility that both medications, alone and in combination, reduce neural signals associated with appetitive behavior.
Exploratory whole brain analyses indicated that VAR was associated with less activation than placebo in the precentral gyrus, right insular cortex, left thalamus, and right caudate; a pattern of results that is consistent with recent fMRI studies of VAR (16–18). Naltrexone, in turn, reduced activation in the right insular cortex, right putamen, right caudate, bilateral precentral gyrus, and right inferior frontal gyrus compared to placebo, which was in line with studies of naltrexone’s neural effects in alcohol dependent individuals (33, 34). At the whole brain level, the combination of VAR+NTX was associated with reduced neural activation in the bilateral orbitofrontal cortex, insular cortex, thalamus, caudate, and cerebellum, compared to placebo. Together, these findings provide converging evidence, albeit preliminary, that the combination of VAR+NTX may be associated with attenuation of activation to cigarette versus neutral cues in brain regions that play a critical role in addiction broadly, and cigarette cue-reactivity in particular (38, 39).
These results must be interpreted in the context of the study strengths and limitations. Strengths include the randomized, double-blind, placebo-controlled design and the combination of experimental pharmacology with neuroimaging. The well phenotyped sample of community heavy drinkers, who smoke daily, is also a strength. Limitations include the reliance on non-treatment seeking smokers and the relatively small sample size for an fMRI study. In addition, we selected a 25mg/day dose of naltrexone on the basis of literature indicating this was a promising adjunctive dosage (43); however, since this study began, multiple reports have favored a 50 mg/day dose of naltrexone over the low-dose of 25 mg/day used in this study. In particular, a clinical trial by Toll et al. (2010) found that naltrexone at 25 mg/day was not significantly different from placebo (60). Further, recent smoking cessation trials of naltrexone at 50 mg/day have shown a benefit of naltrexone over placebo on quit rates as well as post-cessation weight gain (31, 61). Thus future studies of naltrexone in combination with varenicline should consider the standard dose of 50 mg/day as opposed to the low-dose naltrexone implemented in this study. Nevertheless, the combination of experimental psychopharmacology with neuroimaging to guide clinical studies of addiction represents a promising approach to screening novel medications, and combinations thereof (62–64). In particular, neuroimaging studies of pharmacotherapies for addiction are useful in demonstrating that these medications reach critical brain targets known for their involvement in the pathophysiology of the disorder, which in turn provides a proof-of-concept of their plausibility.
On balance, these preliminary results advance medication development for heavy drinking smokers by suggesting that the combination of VAR+NTX may be superior to placebo, and at times superior to monotherapy, in attenuating neural responsivity to cigarette versus control cues. Clinical studies of this combination for heavy drinkers trying to quit smoking appear warranted and may ultimately improve clinical care for a sizeable and hard-to-treat subgroup of smokers.
Acknowledgments
Sources of Funding
This research was supported grants from the California Tobacco Related Disease Research Program (TRDRP 18KT-0020) and from the National Institute on Drug Abuse (DA030898) to LAR. Support for this study was also provided by a grant from the UCLA Clinical and Translational Science Institute (CTSI), grants UL1RR033176 and UL1TR000124. KEC was supported by the UCLA Training Program in Translational Neuroscience of Drug Abuse (T32 DA024635). LAR is a paid consultant for GSK.
Footnotes
Disclosures/Conflicts of Interest
None of the authors have any other conflicts of interest to disclose.
REFERENCES
- 1.Dawson DA. Drinking as a risk factor for sustained smoking. Drug Alcohol Depend. 2000;59(3):235–249. doi: 10.1016/s0376-8716(99)00130-1. [DOI] [PubMed] [Google Scholar]
- 2.Toll BA, Cummings KM, O'Malley SS, Carlin-Menter S, McKee SA, Hyland A, Wu R, Hopkins J, Celestino P. Tobacco quitlines need to assess and intervene with callers' hazardous drinking. Alcohol Clin Exp Res. 2012;36(9):1653–1658. doi: 10.1111/j.1530-0277.2012.01767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durazzo TC, Cardenas VA, Studholme C, Weiner MW, Meyerhoff DJ. Non-treatment-seeking heavy drinkers: effects of chronic cigarette smoking on brain structure. Drug Alcohol Depend. 2007;87(1):76–82. doi: 10.1016/j.drugalcdep.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebbert JO, Janney CA, Sellers TA, Folsom AR, Cerhan JR. The association of alcohol consumption with coronary heart disease mortality and cancer incidence varies by smoking history. J Gen Intern Med. 2005;20(1):14–20. doi: 10.1111/j.1525-1497.2005.40129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hymowitz N, Cummings KM, Hyland A, Lynn WR, Pechacek TF, Hartwell TD. Predictors of smoking cessation in a cohort of adult smokers followed for five years. Tob Control. 1997;6(Suppl 2):S57–S62. doi: 10.1136/tc.6.suppl_2.s57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahler CW, Spillane NS, Metrik J. Alcohol use and initial smoking lapses among heavy drinkers in smoking cessation treatment. Nicotine Tob Res. 2010;12(7):781–785. doi: 10.1093/ntr/ntq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nat Neurosci. 2005;8(11):1465–1470. doi: 10.1038/nn1580. [DOI] [PubMed] [Google Scholar]
- 8.Littleton J, Barron S, Prendergast M, Nixon SJ. Smoking kills (alcoholics)! shouldn't we do something about it? Alcohol Alcohol. 2007;42(3):167–173. doi: 10.1093/alcalc/agm019. [DOI] [PubMed] [Google Scholar]
- 9.Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 10.Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD, 3rd, O'Neill BT. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48(10):3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- 11.Glover ED, Rath JM. Varenicline: progress in smoking cessation treatment. Expert Opin Pharmacother. 2007;8(11):1757–1767. doi: 10.1517/14656566.8.11.1757. [DOI] [PubMed] [Google Scholar]
- 12.Tonstad S. Smoking cessation efficacy and safety of varenicline, an alpha4beta2 nicotinic receptor partial agonist. J Cardiovasc Nurs. 2006;21(6):433–436. doi: 10.1097/00005082-200611000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Zierler-Brown SL, Kyle JA. Oral varenicline for smoking cessation. Ann Pharmacother. 2007;41(1):95–99. doi: 10.1345/aph.1H310. [DOI] [PubMed] [Google Scholar]
- 14.Brandon TH, Drobes DJ, Unrod M, Heckman BW, Oliver JA, Roetzheim RC, Karver SB, Small BJ. Varenicline effects on craving, cue reactivity, and smoking reward. Psychopharmacology (Berl) 2012;218(2):391–403. doi: 10.1007/s00213-011-2327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashare RL, Tang KZ, Mesaros AC, Blair IA, Leone F, Strasser AA. Effects of 21 days of varenicline versus placebo on smoking behaviors and urges among non-treatment seeking smokers. J Psychopharmacol. 2012;26(10):1383–1390. doi: 10.1177/0269881112449397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loughead J, Ray R, Wileyto EP, Ruparel K, Sanborn P, Siegel S, Gur RC, Lerman C. Effects of the alpha4beta2 partial agonist varenicline on brain activity and working memory in abstinent smokers. Biol Psychiatry. 2010;67(8):715–721. doi: 10.1016/j.biopsych.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Loughead J, Ray R, Wileyto EP, Ruparel K, O'Donnell GP, Senecal N, Siegel S, Gur RC, Lerman C. Brain activity and emotional processing in smokers treated with varenicline. Addict Biol. doi: 10.1111/j.1369-1600.2011.00324.x. in press. [DOI] [PubMed] [Google Scholar]
- 18.Franklin T, Wang Z, Suh JJ, Hazan R, Cruz J, Li Y, Goldman M, Detre JA, O'Brien CP, Childress AR. Effects of varenicline on smoking cue-triggered neural and craving responses. Arch Gen Psychiatry. 2011;68(5):516–526. doi: 10.1001/archgenpsychiatry.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mills EJ, Wu P, Lockhart I, Thorlund K, Puhan M, Ebbert JO. Comparisons of high-dose and combination nicotine replacement therapy, varenicline, and bupropion for smoking cessation: A systematic review and multiple treatment meta-analysis. Ann Med. 2012;44(6):588–597. doi: 10.3109/07853890.2012.705016. [DOI] [PubMed] [Google Scholar]
- 20.Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 21.Nides M, Oncken C, Gonzales D, Rennard S, Watsky EJ, Anziano R, Reeves KR. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch Intern Med. 2006;166(15):1561–1568. doi: 10.1001/archinte.166.15.1561. [DOI] [PubMed] [Google Scholar]
- 22.Oncken C, Gonzales D, Nides M, Rennard S, Watsky E, Billing CB, Anziano R, Reeves K. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Arch Intern Med. 2006;166(15):1571–1577. doi: 10.1001/archinte.166.15.1571. [DOI] [PubMed] [Google Scholar]
- 23.Williams KE, Reeves KR, Billing CB, Jr, Pennington AM, Gong J. A double-blind study evaluating the long-term safety of varenicline for smoking cessation. Curr Med Res Opin. 2007;23(4):793–801. doi: 10.1185/030079907x182185. [DOI] [PubMed] [Google Scholar]
- 24.Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 25.Walsh Z, Epstein A, Munisamy G, King A. The impact of depressive symptoms on the efficacy of naltrexone in smoking cessation. J Addict Dis. 2008;27(1):65–72. doi: 10.1300/J069v27n01_07. [DOI] [PubMed] [Google Scholar]
- 26.King A, de Wit H, Riley RC, Cao D, Niaura R, Hatsukami D. Efficacy of naltrexone in smoking cessation: a preliminary study and an examination of sex differences. Nicotine Tob Res. 2006;8(5):671–682. doi: 10.1080/14622200600789767. [DOI] [PubMed] [Google Scholar]
- 27.Byars JA, Frost-Pineda K, Jacobs WS, Gold MS. Naltrexone augments the effects of nicotine replacement therapy in female smokers. J Addict Dis. 2005;24(2):49–60. doi: 10.1300/J069v24n02_05. [DOI] [PubMed] [Google Scholar]
- 28.O'Malley SS, Cooney JL, Krishnan-Sarin S, Dubin JA, McKee SA, Cooney NL, Blakeslee A, Meandzija B, Romano-Dahlgard D, Wu R, Makuch R, Jatlow P. A controlled trial of naltrexone augmentation of nicotine replacement therapy for smoking cessation. Arch Intern Med. 2006;166(6):667–674. doi: 10.1001/archinte.166.6.667. [DOI] [PubMed] [Google Scholar]
- 29.Krishnan-Sarin S, Meandzija B, O'Malley S. Naltrexone and nicotine patch smoking cessation: a preliminary study. Nicotine Tob Res. 2003;5(6):851–857. doi: 10.1080/14622200310001614601. [DOI] [PubMed] [Google Scholar]
- 30.Toll BA, Leary V, Wu R, Salovey P, Meandzija B, O'Malley SS. A preliminary investigation of naltrexone augmentation of bupropion to stop smoking with less weight gain. Addict Behav. 2008;33(1):173–179. doi: 10.1016/j.addbeh.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King AC, Cao D, O'Malley SS, Kranzler HR, Cai X, deWit H, Matthews AK, Stachoviak RJ. Effects of naltrexone on smoking cessation outcomes and weight gain in nicotine-dependent men and women. J Clin Psychopharmacol. 2012;32(5):630–636. doi: 10.1097/JCP.0b013e3182676956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King A, Cao D, Vanier C, Wilcox T. Naltrexone decreases heavy drinking rates in smoking cessation treatment: an exploratory study. Alcohol Clin Exp Res. 2009;33(6):1044–1050. doi: 10.1111/j.1530-0277.2009.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K. Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry. 2008;65(4):466–475. doi: 10.1001/archpsyc.65.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schacht JP, Anton RF, Voronin KE, Randall PK, Li X, Henderson S, Myrick H. Interacting Effects of Naltrexone and OPRM1 and DAT1 Variation on the Neural Response to Alcohol Cues. Neuropsychopharmacology. doi: 10.1038/npp.2012.195. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKee SA, Harrison EL, O'Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry. 2009;66(2):185–190. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fucito LM, Toll BA, Wu R, Romano DM, Tek E, O'Malley SS. A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology (Berl) 2011;215(4):655–663. doi: 10.1007/s00213-010-2160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchell JM, Teague CH, Kayser AS, Bartlett SE, Fields HL. Varenicline decreases alcohol consumption in heavy-drinking smokers. Psychopharmacology (Berl) 2012;223(3):299–306. doi: 10.1007/s00213-012-2717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol. 2013;18(1):121–133. doi: 10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, Brown VL, Cinciripini PM. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. Neuroimage. 2012;60(1):252–262. doi: 10.1016/j.neuroimage.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.NIAAA. The physicians' guide to helping patietns with alcohol problems. Bathesda, MD: National Institutes of Health; 1995. [Google Scholar]
- 41.Beck AT, Steer RA, Brown G. Manual for the Beck Depression Inventory-II. San Antonio, TX: 1996. [Google Scholar]
- 42.Ray LA, Courtney KE, Ghahremani DG, Miotto K, Brody A, London ED. Varenicline, low dose naltrexone, and their combination for heavy-drinking smokers: human laboratory findings. Psychopharmacology (Berl) 2014 doi: 10.1007/s00213-014-3519-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Malley SS, Krishnan-Sarin S, McKee SA, Leeman RF, Cooney NL, Meandzija B, Wu R, Makuch RW. Dose-dependent reduction of hazardous alcohol use in a placebo-controlled trial of naltrexone for smoking cessation. Int J Neuropsychopharmacol. 2009;12(5):589–597. doi: 10.1017/S146114570800936X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Del Boca FK, Kranzler HR, Brown J, Korner PF. Assessment of medication compliance in alcoholics through UV light detection of a riboflavin tracer. Alcohol Clin Exp Res. 1996;20(8):1412–1417. doi: 10.1111/j.1530-0277.1996.tb01142.x. [DOI] [PubMed] [Google Scholar]
- 45.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 46.Sobell MB, Sobell LC, Klajner F, Pavan D, Basian E. The reliability of a timeline method for assessing normal drinker college students' recent drinking history: utility for alcohol research. Addict Behav. 1986;11(2):149–161. doi: 10.1016/0306-4603(86)90040-7. [DOI] [PubMed] [Google Scholar]
- 47.Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, Ho ML, Saxena S, Baxter LR, Jr, Madsen D, Jarvik ME. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59(12):1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- 48.Brody AL. Functional brain imaging of tobacco use and dependence. J Psychiatr Res. 2006;40(5):404–418. doi: 10.1016/j.jpsychires.2005.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McClernon FJ, Gilbert DG. Human functional neuroimaging in nicotine and tobacco research: basics, background, and beyond. Nicotine Tob Res. 2004;6(6):941–959. doi: 10.1080/14622200412331337394. [DOI] [PubMed] [Google Scholar]
- 50.McClernon FJ, Hiott FB, Huettel SA, Rose JE. Abstinence-induced changes in self-report craving correlate with event-related FMRI responses to smoking cues. Neuropsychopharmacology. 2005;30(10):1940–1947. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology. 2008;33(9):2148–2157. doi: 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- 52.King A, McNamara P, Angstadt M, Phan KL. Neural Substrates of Alcohol-Induced Smoking Urge in Heavy Drinking Nondaily Smokers. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andersson J, Jenkinson M, Smith S. Non-linear registration. FMRIB. 2007 Technical report TR07JA2. [Google Scholar]
- 54.Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56(3):907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Esterman M, Tamber-Rosenau BJ, Chiu YC, Yantis S. Avoiding non-independence in fMRI data analysis: leave one subject out. Neuroimage. 2010;50(2):572–576. doi: 10.1016/j.neuroimage.2009.10.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Worsley KJ. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: An Introduction to Methods. Oxford University Press; 2001. [Google Scholar]
- 57.Hughes J, Hatsukami DK. Errors in using tobacco withdrawal scale. Tob Control. 1998;7(1):92–93. doi: 10.1136/tc.7.1.92a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brody AL, Mandelkern MA, Olmstead RE, Jou J, Tiongson E, Allen V, Scheibal D, London ED, Monterosso JR, Tiffany ST, Korb A, Gan JJ, Cohen MS. Neural substrates of resisting craving during cigarette cue exposure. Biol Psychiatry. 2007;62(6):642–651. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Culbertson CS, Bramen J, Cohen MS, London ED, Olmstead RE, Gan JJ, Costello MR, Shulenberger S, Mandelkern MA, Brody AL. Effect of bupropion treatment on brain activation induced by cigarette-related cues in smokers. Arch Gen Psychiatry. 2011;68(5):505–515. doi: 10.1001/archgenpsychiatry.2010.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Toll BA, White M, Wu R, Meandzija B, Jatlow P, Makuch R, O'Malley SS. Low-dose naltrexone augmentation of nicotine replacement for smoking cessation with reduced weight gain: a randomized trial. Drug Alcohol Depend. 2010;111(3):200–206. doi: 10.1016/j.drugalcdep.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.King AC, Cao D, Zhang L, O'Malley SS. Naltrexone reduction of long-term smoking cessation weight gain in women but not men: a randomized controlled trial. Biol Psychiatry. 2013;73(9):924–930. doi: 10.1016/j.biopsych.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McKee SA, Weinberger AH, Shi J, Tetrault J, Coppola S. Developing and Validating a Human Laboratory Model to Screen Medications for Smoking Cessation. Nicotine Tob Res. doi: 10.1093/ntr/nts090. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ray LA, Hutchison KE, Tartter M. Application of human laboratory models to pharmacotherapy development for alcohol dependence. Curr Pharm Des. 2010;16(19):2149–2158. doi: 10.2174/138161210791516422. [DOI] [PubMed] [Google Scholar]
- 64.Litten RZ, Egli M, Heilig M, Cui C, Fertig JB, Ryan ML, Falk DE, Moss H, Huebner R, Noronha A. Medications development to treat alcohol dependence: a vision for the next decade. Addict Biol. 2012;17(3):513–527. doi: 10.1111/j.1369-1600.2012.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]