Abstract
Objective
Hydrocephalus, a complex condition characterized by progressive accumulation of cerebrospinal fluid within the ventricular system of the brain, affects ~6 in 10,000 infants and is heterogeneous in nature. Previous investigations of risk factors have not considered etiologic heterogeneity.
Methods
We conducted a case-control study of 1,748 children with hydrocephalus identified through birth certificate check boxes and ICD-9 codes of linked hospital discharge records through the first year of life. Control infants were identified from birth records (n=19,700), frequency-matched to cases by year of birth. Three mutually exclusive, non-exhaustive subgroups were identified: hydrocephalus associated with a neural tube defect (NTD-H; n=332); prenatal-onset hydrocephalus (PO-H; n=402); and hydrocephalus associated with intracranial hemorrhage (ICH-H; n=446). Within each group, we examined associations with maternal age, race/ethnicity, parity, diabetes and hypertension; and infant sex and gestational age. We used logistic regression to calculate odds ratios (OR) and 95% confidence intervals (CI).
Results
Asian ethnicity was independently associated with an inverse risk of all subtypes of hydrocephalus (NTD-H: OR: 0.44; 95% CI: 0.23–0.84; PO-H: OR: 0.47; 95% CI: 0.27–0.83; ICH-H: OR: 0.59; 95% CI: 0.33, 1.07), compared to whites. Pre-existing diabetes was associated to varying degrees with all three subtypes (NTD-H: OR: 1.94; 95% CI: 0.61–6.17; PO-H: OR: 5.20; 95% CI: 2.60–10.40; ICH-H: OR: 5.26; 95% CI: 2.85–9.69). Hypertension had a positive association with ICH-H (OR: 1.91; 95% CI: 1.46–2.52) but an inverse association with NTD-H (OR: 0.59; 95%CI: 0.36, 0.98). Gestational age ≤ 30 weeks was associated with all three subgroups, most notably ICH-H (OR: 443.56; 95% CI: 326.34–602.87); nearly two-thirds (64%) of ICH-H infants were born ≤ 30 weeks. Male sex was independently associated only with ICH-H (OR: 1.82; 95% CI: 1.40–2.39). No associations were observed with advanced or young maternal age or parity.
Conclusions
The different risk profiles seen among these three subgroups support the biologically heterogeneous nature of infantile hydrocephalus. Future research should take specific etiologic sub-types into account.
Keywords: hydrocephalus, epidemiology, myelomenigocele, intraventricular hemorrhage
INTRODUCTION
Hydrocephalus is a common but complex condition characterized by progressive accumulation of cerebrospinal fluid (CSF) within the ventricular system of the brain. Hydrocephalus can develop at any age, including during the prenatal period. Congenital hydrocephalus, which has been defined variably as hydrocephalus that is present at birth or that develops during the first year of life, was recently estimated to affect 5.9 in 10,000 infants during their initial birth hospitalization 1.
Hydrocephalus that develops during infancy is heterogeneous in nature, and can accompany a neural tube defect (NTD) or other central nervous system malformation 2, in which case it is usually develops in the second or third trimester and is present at birth. Infantile hydrocephalus can also be the result of extrinsic causes such as intracranial hemorrhage (ICH) or infection 3. In those situations, it is usually not present at birth, but develops later in the first year of life. Previous investigations of the risks for infancy-onset hydrocephalus have assessed both maternal and infant risk factors such as ethnicity, parity and infant gender, but analyses were limited by broad case definitions that did not take etiologic heterogeneity into account 1, 4, 5. As a result, risk factors have not been defined for subtypes of hydrocephalus, nor have they been compared across subtypes.
In the current analysis, we evaluated selected maternal and infant factors associated with hydrocephalus diagnosed in Washington State infants during their first year of life, compared to control infants without hydrocephalus. Because infantile hydrocephalus is heterogeneous, we hypothesized that risk factors would depend on the etiology; we chose to evaluate risk factors within three discrete, biologically related subgroups: hydrocephalus associated with an underlying neural tube defect (NTD); hydrocephalus present at birth but unrelated to NTD or ICH; and hydrocephalus associated with ICH.
METHODS
The Human Subjects Protection Review Boards at the University of Washington and the Washington State Department of Health approved the procedures used in the conduct of this study and determined that it was exempt from review.
Data sources
We conducted a population-based case-control study using the Birth Event Records Database (BERD), which contains linked hospital discharge-birth certificate data from Washington State from 1987 to 2012. Additional information was obtained from the Comprehensive Hospital Abstract Report System (CHARS), a statewide longitudinal inpatient hospital discharge database.
Selection of cases and controls
Cases were ascertained on the basis of ICD-9 codes for hydrocephalusrelated diagnoses and procedures captured in inpatient hospital billing records from the first year of life, as well as data reported on the birth certificate. Infants with hydrocephalus were identified using ICD-9 codes for hydrocephalus-related diagnoses recorded upon hospital discharge, including 331.3 and 331.4 (communicating hydrocephalus, obstructive hydrocephalus), 741.0 (spina bifida [NTD] with hydrocephalus) and 742.3 (congenital hydrocephalus), and several hydrocephalus-related procedure codes, including 002.2 (ventriculostomy), 002.3 (ventricular shunt placement) and 002.4 (ventricular shunt revision). In addition to identifying cases on the basis of diagnosis and procedure codes from hospital discharge data, potential cases were identified through birth certificates. From 1980–2002, Washington State birth certificates contained a checkbox for hydrocephalus. Infants with a birth certificate diagnosis of hydrocephalus but without one of the diagnosis or procedure codes that had been used for primary ascertainment were also included if they had an ICD9 diagnosis code of 741 (spina bifida) or 742 (other congenital anomalies of the nervous system). Those who had a birth certificate report of hydrocephalus but no accompanying procedure or diagnosis code consistent with this diagnosis were excluded. We initially identified 1,970 infants with hydrocephalus that had been born in Washington State between 1985 and 2002. We subsequently excluded 222 patients because they had a birth certificate checkbox but no compatible diagnosis or procedure code, which left 1,748 cases for analysis.
For comparison, controls (infants with no record of hydrocephalus) were randomly selected from the WA State birth certificate database at a control: case ratio of 10:1, and frequency matched to cases by birth year.
Delineation of subgroups
Using information from hospital discharge records, including ICD-9 diagnosis and procedure codes as well as length of stay, we defined three mutually exclusive but non-exhaustive subgroups as follows:
NTD-associated hydrocephalus (NTD-H) subgroup: all patients had an NTD-related ICD9 diagnosis code (741: spina bifida) or underwent an NTD-related procedure (03.51: repair of spinal meningocele, or 03.52: repair of spinal myelomenigocele). Because of the specificity of these diagnosis and procedure codes, we assume that this group consists entirely of patients with spinal myelomeningocele; however, these codes do not allow us to distinguish whether the NTD occurred in isolation or in conjunction with other physical anomalies or an underlying syndrome.
Prenatal-onset hydrocephalus (PO-H) subgroup: all patients had hydrocephalus designated on their birth certificates, had a hydrocephalus-related diagnosis code given within the first seven days of life, or underwent a hydrocephalus-related surgical procedure within the first 14 days of life, and did not meet criteria for inclusion in one of the other two subgroups. We assume that most patients within this group have developmental brain malformations such as aqueductal stenosis or obstructive intracranial cysts, though a small proportion could have had an unrecognized intraunterine infection or hemorrhage that led to hydrocephalus6.
Intracranial hemorrhage-associated hydrocephalus (ICH-H) subgroup: all patients had a hemorrhage-related ICD9 diagnosis code (772.1: intraventricular hemorrhage, or 767.0: subdural or cerebral hemorrhage, newborn only). We presume that most of the intracranial hemorrhage identified in this group was peri- or postanatal in onset, though some may have been prenatal in onset.
Using these criteria, we identified 332 patients with NTD-H, 402 patients with PO-H (not associated with NTD or ICH and 446 patients with ICH-H. Of these patients with ICH, 387 (87%) had been assigned diagnosis codes indicating intraventricular hemorrhage (IVH); the other 59 had been assigned codes indicating intracranial hemorrhage (which may also include IVH).
The remaining 578 infants did not fall into one of these three categories and were therefore not included in the subgroup analyses. Our presumption is that most uncategorized patients had other forms of acquired hydrocephalus (especially post-infectious and post-traumatic), later-onset developmental forms, and hydrocephalus that truly belonged in one of the three defined subgroups, but which had not been assigned the specific diagnosis or prodecure codes to enable it to be categorized as such.
Demographic information and risk factors
Maternal age (<20, 20–34, 35+ years), number of prior births (0, 1, 2+), and maternal race/ethnicity (White, Black, Hispanic, Asian, Native American, Native Hawaiian/Pacific Islander) were derived from birth certificate data, as was marital status and maternal participation in the Special Supplemental Nutrition Program for Women, Infants and Children (WIC), for which there was a checkbox present on birth certificates issued from 1992 through 2006. Maternal enrollment in Medicaid at the birth hospitalization was obtained from the hospital discharge records database. The presence of maternal hypertension was determined on the basis of a checkbox that was present on birth certificates issued from 1980–2002, and was supplemented with hypertension-related diagnosis codes (642: hypertension complicating childbirth, pregnancy and the puerperium, which includes several subcodes that allow distinction between pre-existing hypertension, gestational hypertension, pre-eclampsia and eclampsia.)
Maternal diabetes was defined on the basis of a checkbox that was present on birth certificates issued from 1980–2006, and was also supplemented with diabetes-related diagnosis codes (648.0: diabetes mellitus complicating childbirth, pregnancy and the puerperium; 648.83, gestational diabetes, antepartum). Infant sex was obtained from birth certificate data. Gestational age at birth (22–30, 31–36, 37–42, 43+ weeks) was obtained from birth certificates supplemented by discharge data.
Statistical analysis
We used logistic regression to calculate odds ratios (ORs) and 95% confidence intervals (CIs) to estimate the risk of infantile hydrocephalus for each subgroup associated with maternal age, race/ethnicity, parity, diabetes and hypertension, as well as the infant’s sex and gestational age. To further investigate the association of hydrocephalus with Asian ethnicity and male sex above and beyond what might be explained by other risk factors, we conducted an additional analysis controlling for gestational age, diabetes, hypertension, sex (for the analysis of effect of Asian ethnicity), and ethnicity (for analyses of effect of sex).
The possible confounding effects of marital status, parity, or WIC/Medicaid status were evaluated by comparing crude and adjusted ORs for each of our main exposures: maternal age, maternal race/ethnicity, diabetes, hypertension, and child’s sex and gestational age at birth. We performed these analyses for all hydrocephalus cases combined, and also for each of our three subgroups. A factor was considered a confounder after consideration of its relationship to the exposure and outcome, and if its adjusted and crude odds ratio differed by more than 10%.
Since the effect of the exposures on the risk for hydrocephalus did not differ within strata defined by maternal age and race, and since no potential confounder met our confounding criterion (above); we present risk estimates for each of our risk factors adjusted only by the matching variable, year of birth.
All analyses were performed using Stata12 software (Stata Statistical Software: Release 12. College Station, TX: StataCorp LP).
RESULTS
Women who gave birth to children with hydrocephalus were generally similar to the mothers of the control group with respect to maternal age and race/ethnicity (Table 1), although they were less likely to be Asian (4% of cases vs 8% controls). They were also less likely to have completed a level of education beyond high school (50% vs 55%). Mothers of infants with hydrocephalus were more likely to be unmarried (32% of cases vs 28% of controls), obese (27% vs 23%), and have a history of pre-existing diabetes (1.7% vs 0.5%). Compared to the control group, mothers of infants with hydrocephalus were more likely to qualify for WIC or Medicaid (48% vs 45%), and more commonly had a history of cigarette smoking during their pregnancy (15% vs 14%). Mothers of children with intracranial hemorrhage-associated hydrocephalus were more likely to have pre-existing hypertension (3.8%) as well as pre-eclampsia or eclampsia (11.1%) compared to controls (1.3% and 6.8% respectively). A greater proportion of infants with hydrocephalus were male (56% vs 51%) (Table 2). The ICH-H subgroup had the largest percentage of males (64.8%). Infants with hydrocephalus were also more likely to be of <37 weeks gestation compared to controls (44% vs 8%) and die within their first year of life (13% vs 1%). Among the three subgroups, the greatest proportions of deaths were seen in infants in the PO-H subgroup (26.4%), despite the fact that infants within the ICH-H subgroup were considerably more likely to be born <30 weeks gestation (64.2%; n=281).
Table 1.
Maternal characteristics of infants with and without hydrocephalus born in Washington State, 1987–2012
| CONTROLS | CASES | Case subgroup2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Infants without Hydrocephalus (N=19,700) | Infants with Hydrocephalus (N=1,748) | Neural tube defect (NTD-H) N=3222 |
Prenatal (PO-H) N=4022 |
Intracranial hemorrhage (ICH-H) N=4462 |
||||||
|
| ||||||||||
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
| Mother’s age (years) | ||||||||||
| <20 | 1,848 | (9.4) | 179 | (10.3) | 44 | (13.3) | 53 | (13.2) | 38 | (8.5) |
| 20–34 | 15,086 | (76.7) | 1323 | (75.7) | 251 | (75.6) | 290 | (72.1) | 343 | (77.1) |
| 35+ | 2,736 | (13.9) | 245 | (14.0) | 37 | (11.1) | 59 | (14.7) | 64 | (14.4) |
|
| ||||||||||
| Number of prior births | ||||||||||
| 0 | 8,085 | (41.8) | 738 | (43.9) | 127 | (38.8) | 166 | (42.9) | 202 | (47.8) |
| 1 | 6,232 | (32.2) | 452 | (26.9) | 87 | (26.6) | 105 | (27.1) | 110 | (26.0) |
| 2+ | 5,019 | (26.0) | 491 | (29.2) | 113 | (34.6) | 116 | (30.0) | 111 | (26.2) |
|
| ||||||||||
| Race/Ethnicity | ||||||||||
| White | 14,397 | (75.0) | 1,311 | (77.4) | 245 | (77.3) | 301 | (78.1) | 337 | (77.5) |
| Black | 856 | (4.5) | 83 | (4.9) | 11 | (3.5) | 22 | (5.5) | 23 | (5.3) |
| Hispanic | 1,941 | (10.1) | 166 | (9.8) | 40 | (12.6) | 39 | (9.8) | 35 | (8.1) |
| Asian | 1,468 | (7.6) | 74 | (4.4) | 10 | (3.2) | 16 | (4.0) | 23 | (5.3) |
| Native American | 443 | (2.3) | 48 | (2.8) | 11 | (3.5) | 8 | (2.0) | 12 | (2.8) |
|
| ||||||||||
| Education level 3 | ||||||||||
| Some HS or less | 2,749 | (17.6) | 275 | (19.8) | 61 | (25.0) | 68 | (23.3) | 68 | (17.6) |
| HS graduate | 4,363 | (27.9) | 414 | (29.8) | 69 | (28.3) | 79 | (27.1) | 122 | (31.5) |
| At least some college | 8,530 | (54.3) | 700 | (50.4) | 114 | (46.7) | 145 | (49.7) | 197 | (50.1) |
|
| ||||||||||
| WIC 4 or Medicaid participant | 8,157 | (45.1) | 809 | (48.2) | 158 | (49.1) | 194 | (49.4) | 199 | (47.3) |
|
| ||||||||||
| Unmarried | 5,577 | (28.4) | 564 | (32.4) | 99 | (30.0) | 125 | (31.3) | 158 | (35.5) |
|
| ||||||||||
| Smoked during pregnancy | 2,601 | (14.3) | 236 | (15.0) | 40 | (14.0) | 51 | (14.5) | 65 | (16.0) |
| CONTROLS | CASES | Case subgroup1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Infants without hydrocephalus (N=19,70012) | Infants with hydrocephalus (N=1,7482) | Neural tube defect (NTD-H) N=3222 |
Prenatal (PO-H) N=4022 |
Intracranial hemorrhage (ICH-H) N=4462 |
||||||
|
| ||||||||||
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
| BMI (kg/m2) | ||||||||||
| Underweight (<18.5) | 241 | (3.4) | 28 | (4.5) | 3 | (3.1) | 8 | (6.4) | 9 | (5.3) |
| Normal (18.5–24.9) | 3,422 | (47.7) | 273 | (44.3) | 45 | (46.4) | 45 | (36.0) | 86 | (50.3) |
| Overweight (25.0–29.9) | 1,874 | (26.1) | 151 | (24.5) | 22 | (22.7) | 38 | (30.4) | 37 | (21.6) |
| Obese (30.0+) | 1,645 | (22.9) | 165 | (26.7) | 27 | (27.8) | 34 | (27.2) | 39 | (22.8) |
|
| ||||||||||
| Diabetes | ||||||||||
| Pre-existing | 95 | (0.5) | 28 | (1.7) | 3 | (1.0) | 9 | (2.5) | 12 | (2.9) |
| Gestational | 705 | (3.8) | 65 | (4.0) | 12 | (3.8) | 17 | (4.7) | 16 | (3.9) |
| None | 17,851 | (95.7) | 1,530 | (94.3) | 302 | (95.3) | 337 | (92.8) | 383 | (93.2) |
|
| ||||||||||
| Hypertension | ||||||||||
| Pre-existing | 246 | (1.3) | 33 | (2.0) | 3 | (0.9) | 5 | (1.3) | 16 | (3.8) |
| Pre-eclampsia + eclampsia | 1,282 | (6.8) | 147 | (8.7) | 13 | (4.0) | 27 | (6.8) | 47 | (11.1) |
| None | 17,389 | (91.9) | 1,511 | (89.4) | 311 | (95.1) | 364 | (91.9) | 362 | (85.2) |
Case subgroups are mutually exclusive but not exhaustive.
Smaller totals within some categories reflect missing data.
Ascertained for years 1992–2012.
Special supplemental nutrition program for Women, Infants and Children, a federally funded program for low-income families.
Table 2.
Infant characteristics of infants with and without hydrocephalus born in Washington State, 1987–2012
| CONTROLS | CASES | Case subgroup1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Infants without hydrocephalus (N=19,70012) | Infants with hydrocephalus (N=1,7482) | Neural tube defect (NTD-H) N=3222 | Prenatal (PO-H) N=4022 | Intracranial hemorrhage (ICH-H) N=4462 | |||||||
|
| |||||||||||
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | ||
| Sex | Male | 10,071 | (51.1) | 985 | (56.4) | 162 | (48.8) | 206 | (51.2) | 289 | (64.8) |
|
| |||||||||||
| Gestational age (weeks) | 22–30 | 153 | (0.8) | 378 | (22.1) | 12 | (3.7) | 16 | (4.2) | 281 | (64.2) |
| 31–36 | 1,284 | (6.7) | 366 | (21.4) | 60 | (18.6) | 79 | (20.5) | 85 | (19.4) | |
| 37–41 | 17,146 | (89.4) | 929 | (54.4) | 243 | (75.5) | 277 | (72.0) | 70 | (16.0) | |
| 42+ | 595 | (3.1) | 35 | (2.1) | 7 | (2.2) | 13 | (3.4) | 2 | (0.5) | |
|
| |||||||||||
| Deceased | 125 | (0.6) | 231 | (13.2) | 26 | (7.8) | 106 | (26.4) | 44 | (9.9) | |
Case subgroups are mutually exclusive but not exhaustive
Smaller totals within some categories reflect missing data.
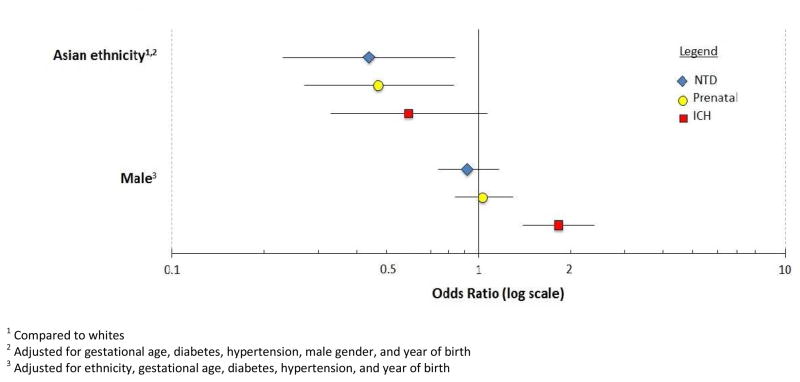
Maternal age and parity were not associated with any subtype of hydrocephalus (Table 3). Asian race/ethnicity was associated with an inverse risk of all three hydrocephalus subtypes even when controlling for other risk factors (Table 4, Figure 1); however, the risk of ICH-H among Asians may have been due to chance (NTD-H: OR: 0.44; 95% CI: 0.23–0.84; PO-H: OR: 0.47; 95% CI: 0.27–0.83; and ICH-H: OR: 0.59; 95% CI: 0.33, 1.07). Pre-existing diabetes was associated with a five-fold increased risk of P-OH and ICH-H (OR: 5.20; 95% CI: 2.60–10.40 and OR: 5.26; 95% CI: 2.85–9.69, respectively), but only atwo-fold higher risk for NTD-H (OR: 1.94; 95% CI: 0.61–6.17). Pre-existing hypertension was associated only with ICH-H, conveying an approximately three-fold increased risk (OR: 2.76; 95% CI: 1.65–4.63). Hypertension of any kind was inversely associated with NTD-H (pre-existing: OR: 0.71; 95%CI: 0.23, 2.24; all types: OR: 0.59; 95%CI: 0.36, 0.98). When controlling for other risk factors, male sex was independently associated only with ICH-H (OR: 1.82; 95% CI: 1.40–2.39) (Figure 1).
Table 3.
Adjusted1 risk estimates for infantile hydrocephalus, by subgroup.
| Case subgroup
|
||||||
|---|---|---|---|---|---|---|
| Neural tube defect (NTD-H) N=322 |
Prenatal (PO-H) N=402 |
Intracranial hemorrhage (ICH-H) N=446 |
||||
|
| ||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Maternal risk factors | ||||||
|
| ||||||
| Age > 35 years | 0.79 | (0.56, 1.11) | 1.08 | (0.82, 1.43) | 1.00 | (0.77, 1.31) |
|
| ||||||
| Parity: ≥1 prior birth | 1.12 | (0.90, 1.40) | 0.99 | (0.81, 1.20) | 0.85 | (0.70, 1.03) |
|
| ||||||
| Race/ethnicity | ||||||
| White | 1.00 | (ref) | 1.00 | (ref) | 1.00 | (ref) |
| Black | 0.77 | (0.42, 1.41) | 1.21 | (0.78, 1.88) | 1.10 | (0.72, 1.69) |
| Hispanic | 1.23 | (0.88, 1.72) | 0.95 | (0.68, 1.33) | 0.74 | (0.52, 1.05) |
| Asian | 0.41 | (0.22, 0.77) | 0.52 | (0.31, 0.87) | 0.63 | (0.41, 0.96) |
| Native American | - | - | 0.94 | (0.23, 3.84) | 1.93 | (0.78, 4.78) |
| Native Hawaiian/Pac Islander | 1.46 | (0.79, 2.69) | 0.84 | (0.41, 1.71) | 1.15 | (0.64, 2.06) |
|
| ||||||
| Diabetes | ||||||
| Pre-existing | 1.94 | (0.61, 6.17) | 5.20 | (2.60, 10.40) | 5.26 | (2.85, 9.69) |
| All types | 1.14 | (0.68, 1.93) | 1.78 | (1.18, 2.67) | 1.47 | (0.99, 2.18) |
|
| ||||||
| Hypertension | ||||||
| Pre-existing | 0.71 | (0.23, 2.24) | 0.99 | (0.41, 2.42) | 2.76 | (1.65, 4.63) |
| All types | 0.59 | (0.36, 0.98) | 1.01 | (0.70, 1.46) | 1.91 | (1.46, 2.52) |
|
| ||||||
| Infant risk factors | ||||||
|
| ||||||
| Male | 0.91 | (0.73, 1.13) | 1.01 | (0.83, 1.23) | 1.75 | (1.44, 2.13) |
|
| ||||||
| Gestational age (weeks) | ||||||
| 22–30 | 5.59 | (3.06, 10.20) | 6.54 | (3.85, 11.09) | 443.56 | (326.34, 602.87) |
| 31–36 | 3.32 | (2.49, 4.43) | 3.83 | (2.97, 4.95) | 16.14 | (11.70, 22.25) |
| 37–41 | 1.00 | (ref) | 1.00 | (ref) | 1.00 | (ref) |
| 42+ | 0.75 | (0.35, 1.61) | 1.21 | (0.69, 2.14) | 0.90 | (0.22, 3.69) |
Adjusted by year of birth
Table 4.
Adjusted odds ratios for Asian ethnicity and male gender, by subgroup.
| Case subgroup | ||||||
|---|---|---|---|---|---|---|
| Neural tube defect (NTD-H) N=322 |
Prenatal (PO-H) N=402 |
Intracranial hemorrhage (ICH-H) N=446 |
||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Asian ethnicity 1,2 | 0.44 | (0.23, 0.84) | 0.47 | (0.27, 0.83) | 0.59 | (0.33, 1.07) |
| Male gender 3 | 0.92 | (0.74, 1.17) | 1.03 | (0.84, 1.29) | 1.82 | (1.40, 2.39) |
Compared to whites
Adjusted for gestational age, diabetes, hypertension, male gender, and year of birth
Adjusted for ethnicity, gestational age, diabetes, hypertension, and year of birth
Figure 1.
Adjusted odds ratios for Asian ethnicity and male gender, by subgroup
Gestational age ≤ 30 weeks was associated with all three subtypes of hydrocephalus, but the risk was substantially higher in the ICH-H subgroup (OR: 467.8; 95% CI: 347.8–637.7); among these infants, nearly two thirds (n=281) were born at 30 weeks gestation or earlier.
DISCUSSION
Here, we assessed ORs for several maternal and child factors associated with infantile hydrocephalus. Since hydrocephalus has diverse clinical associations, we delineated three mutually exclusive subtypes: NTD-H, associated with neural tube defects; PO-H, prenatal in onset, and which we presume contains a high proportion of congenital malformations; and ICH-H, associated with intracranial hemorrhage. Our goal was to determine whether differences in etiology would be evidenced by risk profiles that varied by subgroup.
Among maternal risk factors, neither age nor parity was associated with any subtype of hydrocephalus; however, we noted a decreased risk associated with Asian ethnicity. After adjusting for multiple other risk factors, this association persisted in all three subtypes, though results were statistically significant only for NTD-H and PO-H, possibly due to small numbers of affected Asian infants. A previous study assessing risk factors for hydrocephalus in California noted a similarly reduced risk associated with Asian ethnicity; however, specific subgroups were not evaluated 1. The reduced risk for NTD-H and PO-H could arise from differential termination of pregnancy, since both NTD-H and PO-H may be diagnosed on prenatal ultrasound. However, it would not explain the reduced risk seen in ICH-H as well. Thus, there may be genetic factors possibly related to anatomy of the brain and skull that influence risk within this ethnic group.
Diabetes, particularly pre-existing diabetes, was associated with all three subtypes of hydrocephalus, though the result was not statistically significant in the NTD-H group, possibly because of small numbers. Two possible explanations exist for these associations. First, hyperglycemia is known to cause impaired expression of genes that are critical to central nervous system development 7, presumably leading to obstructive CNS malformations in NTD-H and PO-H. Second, diabetes has a strong association with premature delivery 8, which is strongly associated with ICH-H.
Hypertension (both pre-existing and pregnancy-related) was associated with an increased risk only for ICH-H. This is unsurprising, since hypertension is not a known risk factor for birth defects, but is strongly associated with preterm delivery 9. Interestingly, we noted an inverse association between NTD-H and hypertension. Hypertension seems unlikely to exert a protective effect for neural tube defects, so the nature of this association is unclear.
Among infant risk factors, prematurity was highly associated with all three subtypes of hydrocephalus, with similar risk increases seen in NTD-H and PO-H. Since the onset of hydrocephalus in both these subtypes is prior to delivery, we assume that premature delivery is either a consequence of the hydrocephalus or reflects other factors that led to – or are associated with – both hydrocephalus and premature delivery. ICH-H, by contrast, is post-natal in onset. Hemorrhage is a well-recognized complication of premature birth 10, 11, and post-hemorrhagic hydrocephalus is a known consequence of intracranial hemorrhage 12, 13. Therefore, the remarkably high OR seen here likely reflects the causal role of prematurity in this subtype.
Male sex was associated only with ICH-H. While the findings presented here are based on relatively few cases and are therefore imprecise, it is noteworthy that this finding is consistent with reports from multiple previous studies 14, 15. We believe this primarily reflects the increased risk of intraventricular hemorrhage seen in male preterm infants, which has been well described16–18. Though we anticipated a modestly increased male predominance in the PO-H group due to X-linked forms of hydrocephalus, this was not seen.
This study was limited by our reliance on hospital discharge data to classify infants into subgroups. Even within a relatively homogeneous diagnostic entity such as NTD or ICH, there may be key clinical information not captured within diagnosis codes, which may render these subgroups more biologically diverse than their names imply. The PO-H subgroup was delineated exclusively on the basis of hydrocephalus time of onset. Although we expect that most patients in this group have obstructive central nervous system malformations, prenatal factors such as infection could introduce additional heterogeneity into this group that we were unable to account for in our analyses.
The differing risk profiles seen across subtypes supports the notion of hydrocephalus as a heterogeneous entity, with risk factors that depend upon underlying biologic mechanism. The apparence inverse association seen with Asian ethnicity and all three subtypes of hydrocephalus deserves further investigation. Future research should take etiologic subtype into account.
Acknowledgments
The authors would like to thank the Washington State Department of Health for maintaining and allowing access to the databases used in this study. The authors also gratefully acknowledge the assistance of Mr. Bill O’Brien for data management, as well as Dr. Steven E. Hawes and Dr. Alyson Littman for guidance. The authors wish to thank Dr. Beth A. Mueller, for her careful review of this manuscript. This project was made possible by the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) under Award Number KL2TR000421, which provides training support for Drs. Tully and Capote.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official view of NCATS or NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jeng S, Gupta N, Wrensch M, Zhao S, Wu YW. Prevalence of congenital hydrocephalus in California, 1991–2000. Pediatr Neurol. 2011;45:67–71. doi: 10.1016/j.pediatrneurol.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Schrander-Stumpel C, Fryns JP. Congenital hydrocephalus: nosology and guidelines for clinical approach and genetic counselling. Eur J Pediatr. 1998;157:355–362. doi: 10.1007/s004310050830. [DOI] [PubMed] [Google Scholar]
- 3.Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Landingham M, Nguyen TV, Roberts A, Parent AD, Zhang J. Risk factors of congenital hydrocephalus: a 10 year retrospective study. Journal of neurology, neurosurgery, and psychiatry. 2009;80:213–217. doi: 10.1136/jnnp.2008.148932. [DOI] [PubMed] [Google Scholar]
- 5.Munch TN, Rasmussen ML, Wohlfahrt J, Juhler M, Melbye M. Risk factors for congenital hydrocephalus: a nationwide, register-based, cohort study. Journal of neurology, neurosurgery, and psychiatry. 2014 doi: 10.1136/jnnp-2013-306941. [DOI] [PubMed] [Google Scholar]
- 6.Tully HM, Dobyns WB. Infantile hydrocephalus: A review of epidemiology, classification and causes. Eur J Med Genet. 2014 doi: 10.1016/j.ejmg.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei D, Loeken MR. Increased DNA Methyltransferase 3b (Dnmt3b) -mediated CpG Island Methylation Stimulated by Oxidative Stress Inhibits Expression of a Gene Required for Neural Tube and Neural Crest Development in Diabetic Pregnancy. Diabetes. 2014 doi: 10.2337/db14-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knight KM, Thornburg LL, Pressman EK. Pregnancy outcomes in type 2 diabetic patients as compared with type 1 diabetic patients and nondiabetic controls. The Journal of reproductive medicine. 2012;57:397–404. [PubMed] [Google Scholar]
- 9.Morisaki N, Togoobaatar G, Vogel JP, et al. Risk factors for spontaneous and provider-initiated preterm delivery in high and low Human Development Index countries: a secondary analysis of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG: an international journal of obstetrics and gynaecology. 2014;121 (Suppl 1):101–109. doi: 10.1111/1471-0528.12631. [DOI] [PubMed] [Google Scholar]
- 10.Shalak L, Perlman JM. Hemorrhagic-ischemic cerebral injury in the preterm infant: current concepts. Clinics in perinatology. 2002;29:745–763. doi: 10.1016/s0095-5108(02)00048-9. [DOI] [PubMed] [Google Scholar]
- 11.Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatric research. 2010;67:1–8. doi: 10.1203/PDR.0b013e3181c1b176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitelaw A. Intraventricular haemorrhage and posthaemorrhagic hydrocephalus: pathogenesis, prevention and future interventions. Seminars in neonatology: SN. 2001;6:135–146. doi: 10.1053/siny.2001.0047. [DOI] [PubMed] [Google Scholar]
- 13.Strahle J, Garton HJ, Maher CO, Muraszko KM, Keep RF, Xi G. Mechanisms of hydrocephalus after neonatal and adult intraventricular hemorrhage. Translational stroke research. 2012;3:25–38. doi: 10.1007/s12975-012-0182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deak KL, Siegel DG, George TM, Gregory S, Ashley-Koch A, Speer MC. Further evidence for a maternal genetic effect and a sex-influenced effect contributing to risk for human neural tube defects. Birth Defects Res A Clin Mol Teratol. 2008;82:662–669. doi: 10.1002/bdra.20511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juriloff DM, Harris MJ. Hypothesis: the female excess in cranial neural tube defects reflects an epigenetic drag of the inactivating X chromosome on the molecular mechanisms of neural fold elevation. Birth Defects Res A Clin Mol Teratol. 2012;94:849–855. doi: 10.1002/bdra.23036. [DOI] [PubMed] [Google Scholar]
- 16.Cuestas E, Bas J, Pautasso J. Sex differences in intraventricular hemorrhage rates among very low birth weight newborns. Gender medicine. 2009;6:376–382. doi: 10.1016/j.genm.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Stevenson DK, Verter J, Fanaroff AA, et al. Sex differences in outcomes of very low birthweight infants: the newborn male disadvantage. Archives of disease in childhood Fetal and neonatal edition. 2000;83:F182–185. doi: 10.1136/fn.83.3.F182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tioseco JA, Aly H, Essers J, Patel K, El-Mohandes AA. Male sex and intraventricular hemorrhage. Pediatric critical care medicine: a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2006;7:40–44. doi: 10.1097/01.pcc.0000192341.67078.61. [DOI] [PubMed] [Google Scholar]