Abstract
Purpose of review
Recurrent urinary tract infection (rUTI) is a serious clinical problem, yet effective therapeutic options are limited, especially against multidrug-resistant uropathogens. In this review, we explore the development of a clinically relevant model of rUTI in previously infected mice and review recent developments in bladder innate immunity that may affect susceptibility to rUTI.
Recent findings
Chronic bladder inflammation during prolonged bacterial cystitis in mice causes bladder mucosal remodelling that sensitizes the host to rUTI. Although constitutive defenses help prevent bacterial colonization of the urinary bladder, once infection occurs, induced cytokine and myeloid cell responses predominate and the balance of immune cell defense and bladder immunopathology is critical for determining disease outcome, in both naïve and experienced mice. In particular, the maintenance of the epithelial barrier appears to be essential for preventing severe infection.
Summary
The innate immune response plays a key role in determining susceptibility to rUTI. Future studies should be directed towards understanding how the innate immune response changes as a result of bladder mucosal remodelling in previously infected mice, and validating these findings in human clinical specimens. New therapeutics targeting the immune response should selectively target the induced innate responses that cause bladder immunopathology, while leaving protective defenses intact.
Keywords: cystitis, mucosal immunology, urinary tract infection, uropathogenic Escherichia coli
INTRODUCTION
Urinary tract infections (UTIs) are very common and highly recurrent: approximately 60% of all women will have a UTI during their lifetime [1,2], and 20–30% of women with an acute UTI will have a recurrence within 6 months [3,4], accounting for 10.5 million ambulatory care visits in 2007 in the United States alone [5]. Of particular concern is the fact that some women experience highly recurrent UTI and may have half a dozen or more recurrences in the year following an initial episode [6,7]. A recurrent UTI (rUTI) negatively impacts patient quality of life and contributes significantly to the extremely high financial burden of UTI, which, through direct medical costs and indirect costs such as lost work output, is estimated to be more than $5 billion in the United States alone [3,4,8]. Unfortunately, clinicians have few therapeutic options for preventing rUTI and often resort to daily antibiotic prophylaxis [9,10], though multidrug resistance among uropathogens is a rapidly increasing concern [11]. Therefore, novel therapeutic approaches are needed to combat rUTI. Although approaches targeting the bacteria, such as vaccines and antivirulence therapies, are being pursued, the diversity among uropathogens presents considerable difficulties. For example, although over 80% of uncomplicated UTI are caused by uropathogenic Escherichia coli (UPEC), this pathotype is actually extremely diverse in terms of virulence factor profiles and genomic content [12]. Furthermore, whether a patient is susceptible to symptomatic infection or an asymptomatic colonization seems to be dictated by host factors, in addition to or even rather than bacterial determinants [13,14]. Therefore, an alternative therapeutic approach is to target the host by employing strategies that enhance the ability of the host immune system to prevent or rapidly resolve UTI. However, the pathogenesis of rUTI is poorly understood and significant work is needed in order to develop effective immunomodulatory therapies against rUTI. In this review, we will discuss the development of a clinically relevant murine model of rUTI and review recent developments in bladder innate immunity that may affect susceptibility to rUTI.
THE BIOLOGY OF URINARY TRACT INFECTION
The vast majority of UTI affect the lower urinary tract, which includes the bladder (cystitis), and ascension to the kidneys is rare in the absence of anatomical abnormalities [15–17]. Upon introduction into the urinary bladder, UPEC and some other Gram-negative uropathogens can invade superficial cells of the bladder epithelium (urothelium) and replicate rapidly within the host cell cytosol, forming clonal, biofilm-like intracellular bacterial communities (IBCs) (Fig. 1). This acute pathogenic cascade allows UPEC to replicate in a protected intracellular niche, thereby avoiding professional phagocytic cells and antibiotics while dramatically increasing in number, with each IBC giving rise to 10 000–100 000 bacterial cells [18–20]. Although first observed in mice, IBCs have been found in urine sediments from women and children with UTI [21–23].
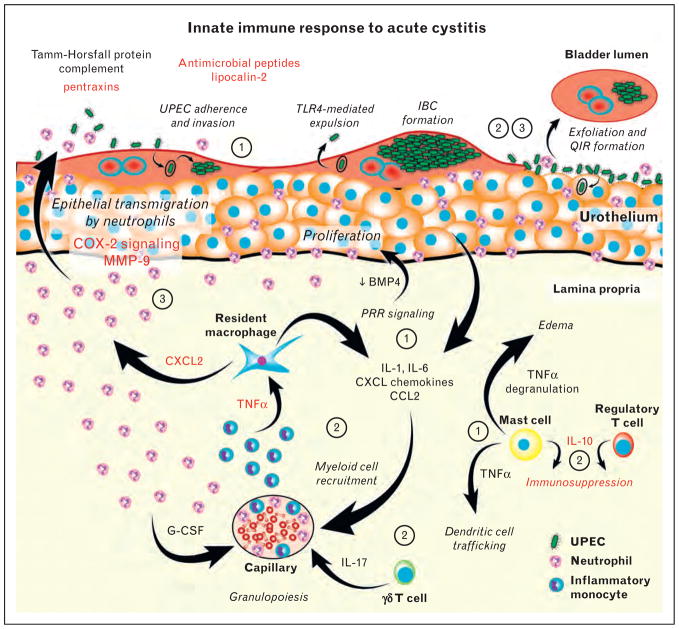
FIGURE 1.
The innate immune response to acute UPEC cystitis. During acute UPEC infection of the urinary bladder, a series of coordinated and sequential host – pathogen interactions determine disease outcome. The circled numbers indicate a sequence of initial events during acute cystitis: ‘1’ indicates events that happen within the first 1 – 2 h of experimental colonization; ‘2’ indicates events that occur within the first 4 – 6 h of colonization; and ‘3’ indicates events that occur from 6 to 24 h postinfection. Responses discussed in this literature review are highlighted in red. BMP4, bone morphogenetic protein 4; G-CSF, granulocyte colony stimulating factor; IBC, intracellular bacterial community; IL-1, interleukin 1; MMP-9, matrix metallopeptidase 9; QIR, quiescent intracellular reservoir; PRR, pattern recognition receptor; TLR4, Toll-like receptor 4; TNFα, tumour necrosis factor alpha [18 – 52,53▪▪,54▪,55▪▪,56,57,58▪,59▪,60,61].
The primary reservoir for uropathogenic bacteria has been assumed to be the gastrointestinal tract (GIT), which in turn can seed the vaginal and periurethral flora [62–66]. However, once the initial ascending UTI has occurred, subsequent rUTI may either be a re-ascension from the GIT, vaginal or periurethral reservoir or a reseeding from a persistent lower urinary tract nidus, for example urinary calculus or other persistent reservoir in the bladder. Bladder reservoirs were first observed in patients between rUTI episodes [67]. Subsequent studies in mice demonstrated that even after sterilization of the urine by antibiotics, UPEC could persist latently within urothelial cells, wherein they can potentially seed reinfections [68–71]. These bacterial quiescent intracellular reservoirs (QIRs) are small collections of UPEC (4–10 dormant bacteria) in Lamp1+ endosomes. They can emerge and cause recurrent infection either naturally [69,70] or when a urothelial exfoliation response is triggered by bladder damage [68,71]. QIRs are completely distinct from the process by which UPEC invade and replicate within urothelial cells during acute infection to form IBCs [18,19]. IBCs are transient, forming and maturing over a matter of hours with the cytosol, whereas QIRs are latent infections that can last for months confined within the endosomal compartment. QIRs may contribute to rUTI, though whether QIRs are found in humans is an open question.
The bladder mucosal defenses against bacterial infection include both constitutive and induced factors. The lower urinary tract is lined by a specialized stratified epithelium known as the urothelium, which acts not only as a permeability barrier but also as a barrier to bacterial adherence and invasion [24–27]. Continuous low shear force flow of filtrate and urine in the kidneys and ureters and intermittent high shear force urine flow in the bladder and urethra during urination act as formidable barriers to bacterial ascension and colonization. Furthermore, soluble factors in the urine, such as Tamm–Horsfall protein, complement and antimicrobial peptides, also play a role in defense against bacterial UTI [28–30]. Upon colonization of the bladder wall and invasion of the urothelium, pattern recognition receptor signalling in both the stromal and haematopoietic cell compartments plays a critical role in inducing a vigorous innate immune response, including activation of urothelial and bladder resident immune cells and coordinated cytokine and chemokine secretion, resulting in recruitment of myeloid cells, predominantly neutrophils and inflammatory monocytes, to the bladder [31–48,72] (Fig. 1). The outer layers of the urothelium respond to infection by undergoing cell death and shedding infected cells into the urine stream in a process termed exfoliation [73,74], and the underlying urothelial cells become hyperproliferative as long as infection ensues [39,75,76]. Adaptive immune responses are not well understood, but appear to contribute to immune defense against challenge [34,77,78], perhaps explaining why the majority of women who suffer an acute UTI do not develop recurrent infection.
MODELING RECURRENT URINARY TRACT INFECTION
Host factors affecting risk of recurrence in premenopausal women have been studied in detail and are described fully elsewhere [2,79–83]. For the purpose of this review, it is important to highlight that there are three main risk factors for symptomatic rUTI in premenopausal women (Table 1): bacterial exposure, a prior history of UTI and host genetics. Importantly, rUTI might be influenced by a combination of some or all of these host determinants, or other host determinants that have not yet been identified. Fully understanding the bacterial and host drivers of recurrence will require a tractable animal model of rUTI. It is imperative to note that the vast majority of our knowledge of the mechanisms of determinants of UTI pathogenesis, as well as the efficacy of novel therapeutics and vaccines, stems from experiments performed in naïve mice with pristine bladders. Although these studies have been tremendously informative, they do not take into account the effects of mucosal remodelling consequent to the chronic bladder inflammation that occurs in patients suffering from rUTI [84].
Table 1.
Risk factors for recurrent urinary tract infection in premenopausal women
| Exposure | Sexual activity |
|---|---|
| Spermicides | |
| Alteration of the vaginal microbiota | |
| Urine retention | |
| History | Childhood history of UTI |
| Prior UTI | |
| Genetics | Maternal history of rUTI |
| Secretor of non-ABO blood group antigens | |
| TLR polymorphisms |
TLR, Toll-like receptor; UTI, urinary tract infection.
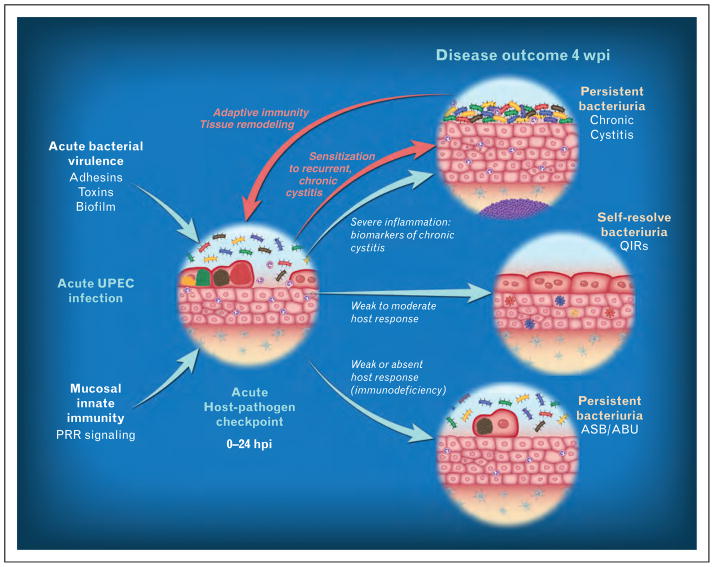
Inbred mice are powerful tools to understand biological processes because they are genetically identical and can be used to isolate a particular trait of interest in a way that cannot be done in an outbred population. Naïve C57BL/6 mice are relatively resistant to acute cystitis, resolving infection rapidly, and, when infected serially at 2-week intervals with UPEC, become progressively more resistant to bacterial rechallenge [78]. Although this model can provide useful information about the adaptive immune response to UTI, it does not shed light on the clinical problem of rUTI. In contrast, UPEC infection in C3H/HeN and closely related mouse strains results in two distinct outcomes: chronic bacterial cystitis, which is defined as persistent high-titre bacteriuria, high-titre bladder infection and chronic bladder inflammation lasting for the life of the animal; or spontaneous resolution of cystitis [76,85]. Placebo-controlled studies indicate that in the absence of effective antibiotic therapy, up to 60% of women experience bacteriuria lasting months after an initial infection, often despite improvement of symptoms [86,87]. In chronic cystitis in mice, the bladder mucosa develops lymphonodular hyperplasia in the bladder submucosa, and urothelial hyperplasia with a lack of uroplakin expression, similar to lesions seen in human patients with persistent bacteriuria, whether symptomatic or not [84,88]. The development of chronic infection in mice can be predicted as early as 24 hpi by elevation of serum and urine cytokine biomarkers associated primarily with the neutrophil response to infection [76]. Elevation of these bio-markers correlated with the presence of severe acute bladder inflammation. Treatment of mice with the immunosuppressive drug dexamethasone reduced the severity of acute inflammation and protected against chronic infection. Thus, an inappropriately severe bladder inflammatory response predisposes to chronic infection (Fig. 2).
FIGURE 2.
Model for susceptibility to acute, chronic and recurrent cystitis. We hypothesize that an acute host – pathogen checkpoint exists that determines disease outcome within the first 24 h of the acute infection. If the immune response is too limited, the host may fail to clear the infection and consequently will develop persistent bacteriuria and bladder infection that may be asymptomatic. In contrast, an immune response that is too severe may cause bladder immunopathology during acute cystitis that leads to the development of persistent bacteriuria and bladder infection that may be symptomatic. This is accompanied by bladder disease indicative of chronic cystitis, which, in mice, is accompanied by urothelial hyperplasia with a lack of terminal differentiation. Furthermore, evidence in mice suggests that a severe first-time infection that is left untreated for 2 weeks or more causes changes to the bladder mucosa that render it more sensitive to bacteria upon reinoculation. Figure adapted from [89].
Importantly, C3H/HeN mice that are allowed to be infected with UPEC for 2 or more weeks become ‘sensitized’ or predisposed to developing severe acute and chronic cystitis when challenged 4 or more weeks after the initial infection is cleared with oral antibiotics [76]. This model is to our knowledge the only clinically relevant model of rUTI in use, and together with the epidemiological data raises the following hypothesis: an episode of UTI that is severe, prolonged and early in life results in chronic bladder inflammation that in turn causes remodelling of the bladder and increases susceptibility to infection later in life when women become sexually active [8]. Lending further support to this hypothesis is the key finding that in the C3H model of chronic cystitis, the early innate response is critical for determining disease outcome [76]. Thus, in this review we will focus on recent advances in our understanding of innate immune defenses against UPEC infection of the bladder mucosa, in an attempt to understand how these responses may contribute to resistance or susceptibility to rUTI.
RECENT ADVANCES IN URINARY TRACT INFECTION INNATE IMMUNOLOGY
The urinary tract is subject to frequent bacterial exposures and has therefore developed an arsenal of innate defense mechanisms, both constitutive and induced. Understanding these innate defense mechanisms may reveal insight into whether and how these protections break down in the case of rUTI and provide new therapeutic targets for the treatment of rUTI.
Innate immune responses to uropathogenic Escherichia coli urinary tract infection
Antimicrobial peptides are important defenses against microorganisms. Cathelicidin (LL-37) is constitutively expressed in the urinary tract, and has been demonstrated to play a key role in protection from UTI [30]. A recent study found that uncomplicated UTI patients had significantly elevated levels of LL-37 during infection compared with postinfection. Interestingly, fecal E. coli isolates from healthy controls were more susceptible to LL-37 than fecal and UTI isolates from UTI patients were, suggesting a potential mechanism for UPEC persistence in the GIT [49]. Beta defensin-1 (BD1) is also constitutively expressed in the urinary tract and has been suggested to play a role in pyelonephritis [50] and defense against Gram-positive uropathogens [51]. However, lower UTI patients did not differ from healthy controls in their urine levels of BD1 [49]. Another recent study demonstrated that Defb1−/− mice did not differ from wild-type C57BL/6 mice in their acute bladder and kidney bacterial burden after experimental UPEC infection. BD3 and BD14 were found to be bactericidal against UPEC in vitro, but further studies are needed to examine their role in UTI [52].
Humoral innate immunity also plays a role in UTI. This arm of the innate immune system is composed of the complement cascade and soluble pattern recognition molecules such as pentraxins. PTX3 is a highly conserved, prototypic pentraxin known to be protective against bacterial and fungal infections. Recently, a role for PTX3 in UPEC UTI was reported. Compared with wild-type C57BL/6 mice, PTX3-deficient mice had impaired bladder and kidney bacterial clearance and significantly more inflammation of the urinary tract, likely due to defective phagocytosis by PTX3-deficient neutrophils. Importantly, polymorphisms in PTX3 were associated with increased susceptibility to UTI in humans [53▪▪].
It has long been known that neutrophils are an important innate cellular defense against UTI [36]. Although CXCL chemokines secreted by the infected mucosa certainly play a role in recruiting neutrophils to the urinary tract, how this response is coordinated has been poorly understood. A recent study [54▪] suggests that bladder resident macrophages recruit both neutrophils and Ly6C+ inflammatory monocytes to the urinary bladder during acute UTI, and that the inflammatory monocytes play a role in ‘licensing’ resident macrophages to induce bladder epithelial transmigration by neutrophils. The authors demonstrated in C57BL/6J mice that recruited monocytes express tumour necrosis factor-alpha (TNFα), which induces further CXCL2 (chemokine [C-X-C motif] ligand 2) expression by bladder resident macrophages, which in turn induces matrix metalloproteinase-9 expression by the neutrophils, enhancing their capacity for crossing the epithelium. However, disruption of this ‘licensing’ resulted in only very minor (~two-fold) differences in acute bladder bacterial burdens and did not affect disease outcome, bringing into question how critical this pathway is to bladder immune defense.
In C3H/HeN mice, severe acute immunopathology leads to the development of chronic cystitis [76]. Recently, the same group found evidence in both humans and mice that this acute immunopathology is a result of exacerbated bladder epithelial transmigration by neutrophils [55▪▪]. They identified serum cytokine and growth factor biomarkers of rUTI in adult women that implicated a magnified myeloid cell immune response during acute cystitis as a risk factor for future rUTI. In C3H/HeN mice, temperance, but not elimination, of the neutrophil response to the UPEC-infected urinary bladder significantly protected against severe acute and chronic infection. In contrast to the reported findings of Schiwon et al. [54▪], the exacerbated bladder epithelial transmigration in C3H/HeN mice was not dependent upon inflammatory monocyte recruitment, but was instead sensitive to inhibition of cyclooxygenase-2 (COX-2), the enzyme that catalyzes the rate-limiting step in prostanoid synthesis. Treatment of mice with NSAIDs that inhibit all isoforms of cyclooxygenase, or with COX-2 specific inhibitors, reduced epithelial transmigration by neutrophils and prevented mucosal wounding during acute cystitis and protected naïve mice against chronic infection. A history of chronic cystitis was previously shown to sensitize mice to developing recurrent chronic cystitis upon subsequent bacterial challenge (Fig. 2) [76]. Proteomics of bladder epithelial cells isolated from these ‘sensitized’ mice during the convalescent period after the initial infection and antibiotic treatment revealed changes in protein expression that would likely intensify their innate immune and exfoliation responses to infection, particularly under conditions of oxidative stress. Importantly, COX-2 inhibitors were also protective against recurrent cystitis upon bacterial challenge of these sensitized mice [55▪▪].
Noncanonical innate immune responses to urinary tract infection
Recent work has revealed new insight into host defense mechanisms that, while innate, lie outside the realm of traditional innate immunity. A key concept is the so-called ‘nutritional immunity’, or the careful control and sequestration of transition metals by the host in order to limit their availability to pathogens [56]. To circumvent nutritional immunity, many pathogenic and nonpathogenic bacteria possess an arsenal of siderophores, small molecules that scavenge and import free iron. Most clinical UPEC isolates produce multiple siderophores, and a large-scale metabolomics study revealed that urine isolates from patients with rUTI preferentially express salmochelin and yersiniabactin [90]. Recently, a novel role for yersiniabactin in UTI was identified. This siderophore was found to bind to copper in urine from both infected mice and women with acute cystitis, in order to protect UPEC from copper toxicity [91]. Cupric yersiniabactin was found to have superoxide dismutase like activity that prevented bacterial killing by copper-replete phagocytic cells [92]. To counter bacterial scavenging of transition metals, the host produces the antimicrobial protein lipocalin-2, which binds and inactivates siderophores. Although lipocalin-2 expression is induced in the bladder epithelium during cystitis [57,72], a recent study found that α-intercalated cells of the collecting duct of the kidney act as sentinels of the upper urinary tract during cystitis, sensing infection in the lower urinary tract and expressing and secreting lipocalin-2 into the urine filtrate in a Toll-like receptor 4 (TLR4)-dependent manner [58▪]. Mice lacking either lipocalin-2 or intercalated cells were susceptible to more severe and prolonged cystitis.
Although not classically considered a component of innate immunity, autophagy is known to play a protective role in the context of several infectious diseases, such as listeriosis and toxoplasmosis [93]. A recent study found that ATG16L1 deficiency protected mice from UTI. Atg16L1-hypomorphic mice had more rapid clearance of bacteriuria and retained fewer QIRs than wild-type mice did. Thus, the persistence of loss-of-function variants in autophagy proteins in humans may be due to a protective role against common bacterial infections such as UTI [94]. The pattern recognition receptor NOD2 (nucleotide-binding oligomerization domain-containing protein 2) is known to interact with ATG16L1, but NOD2 was not found to play a role in ATG16L1 deficiency mediated UTI resistance in mice or humans [95].
Uropathogenic Escherichia coli subversion of innate immunity and bladder tolerance
Why do prolonged UPEC exposures, either through persistent asymptomatic colonization of the bladder in the case of asymptomatic bacteriuria (ASB/ABU) or through frequent recurrent infections in the case of rUTI, not result in a protective adaptive response in many patients? New evidence suggests that UPEC infection can directly or indirectly promote a tolerogenic bladder environment by suppressing host inflammatory responses. Although it has been known that virulent UPEC have the capacity to suppress host inflammation compared with K12 strains [96–98], Lutay et al. [99▪▪] elegantly demonstrated that at least some ASB strains have the capacity to actively influence host gene expression on a broad scale. This study, notable for its use of both model cell lines and therapeutic ASB inoculations of humans, revealed that the model ASB strain E. coli 83972 caused significant suppression of innate immune response gene expression by suppressing the phosphorylation of host RNA polymerase II. Acute pyelonephritis strains were less able to suppress Pol II phosphorylation. Similarly, UPEC infection can result in the expression of the broadly anti-inflammatory cytokine IL-10 by monocytes and urothelial cells in vitro [100], as well as mast cells, regulatory T cells and macrophages in vivo [59▪]. In particular, mast cell derived IL-10 was found to promote a tolerogenic bladder environment that suppressed the adaptive immune response to a subsequent challenge bladder infection [59▪]. However, bladder mast cell derived TNF has been shown to recruit large numbers of dendritic cells to infected bladders [60], suggesting a pro-inflammatory role for bladder mast cells as well. Thus, bladder exposure to UPEC may elicit not only pro-inflammatory but also anti-inflammatory host responses, which could prevent the development of an adaptive response.
CONCLUSION
UTI is a complex syndrome, and bladder colonization ranges from asymptomatic bacteriuria to chronic and recurrent cystitis (Fig. 2). Naïve mice with pristine bladders are extremely useful for modeling an initial, uncomplicated UTI, but likely do not accurately model the significant clinical problem of highly recurrent UTI. Thus, recent studies of innate immune responses to UTI must be evaluated in the context of the animal model used and may not reflect general innate responses in the context of rUTI. Recent work in mice that have experienced a prior UTI has identified bladder mucosal remodeling that differs depending upon the length of the initial infection [55▪▪]. These changes, determined by the outcome of the initial infection, likely alter the mucosal response to subsequent bacterial challenge. Indeed, some of the innate immune factors identified in naïve mice have been suggested to play a role in mucosal remodelling at other sites [61,101]. To further our understanding of the host factors underlying rUTI, we must include the known risk factors of bacterial exposure, history and genetics in any mouse model. In addition, we should incorporate relevant data from patients suffering from rUTI whenever possible. By refining the way in which we model rUTI, we can ensure that future studies more accurately increase our understanding of disease pathogenesis and facilitate the development of new, more effective therapeutic strategies to combat this important clinical problem.
KEY POINTS.
Urinary tract infections (UTIs) are very common and highly recurrent, and rising antibiotic resistance to uropathogens necessitates the development of alternative therapeutic approaches.
Sensitization to recurrent UTI can occur in mice as a consequence of chronic bladder inflammation during prolonged bacterial cystitis, likely due to mucosal remodelling that alters the propensity for protective and damaging mucosal responses.
The innate mucosal immune defenses of the urinary bladder are numerous and formidable and include both constitutive and induced components.
If bacterial colonization is established, soluble and cellular mediators of inflammation are induced and the balance of immune cell defense and bladder immunopathology is critical for determining disease outcome.
Future studies of recurrent UTI should be directed towards understanding how the innate immune response changes as a result of bladder mucosal remodelling in previously infected mice and validating these findings in human clinical specimens.
Acknowledgments
We thank Karen Dodson for critical reading of this manuscript and helpful discussion.
Financial support and sponsorship
This work was supported by the National Institutes of Health and Office of Research on Women’s Health Specialized Center of Research (DK64540, DK51406, AI48689, AI29549, AI49950 and AI95542 to S.J.H., and a Mucosal Immunology Studies Team consortium U01AI095776 pilot grant and a Mentored Clinical Scientist Research Career Development Award K08 AI083746 to T.J.H.), and by the National Science Foundation (Graduate Research Fellowship to V.P.O.).
Footnotes
Conflicts of interest
The authors declare that there are no conflicts of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Dis Mon. 2003;49:53–70. doi: 10.1067/mda.2003.7. [DOI] [PubMed] [Google Scholar]
- 2.Foxman B, Barlow R, D’Arcy H, et al. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol. 2000;10:509–515. doi: 10.1016/s1047-2797(00)00072-7. [DOI] [PubMed] [Google Scholar]
- 3.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113 (Suppl 1A):5S–13S. doi: 10.1016/s0002-9343(02)01054-9. [DOI] [PubMed] [Google Scholar]
- 4.Foxman B. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect Dis Clin North Am. 2014;28:1–13. doi: 10.1016/j.idc.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Schappert SM, Rechtsteiner EA. Ambulatory medical care utilization estimates for. Vital Health Stat 13. 2007;2011:1–38. [PubMed] [Google Scholar]
- 6.Ikaheimo R, Siitonen A, Heiskanen T, et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis. 1996;22:91–99. doi: 10.1093/clinids/22.1.91. [DOI] [PubMed] [Google Scholar]
- 7.Hopkins WJ, Elkahwaji J, Beierle LM, et al. Vaginal mucosal vaccine for recurrent urinary tract infections in women: results of a phase 2 clinical trial. J Urol. 2007;177:1349–1353. doi: 10.1016/j.juro.2006.11.093. [DOI] [PubMed] [Google Scholar]
- 8.Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol. 2010;7:653–660. doi: 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- 9.Al-Badr A, Al-Shaikh G. Recurrent urinary tract infections management in women: a review. Sultan Qaboos Univ Med J. 2013;13:359–367. doi: 10.12816/0003256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams G, Craig JC. Long-term antibiotics for preventing recurrent urinary tract infection in children. Cochrane Database Syst Rev. 2011:CD001534. doi: 10.1002/14651858.CD001534.pub3. [DOI] [PubMed] [Google Scholar]
- 11.Taneja N, Rao P, Arora J, Dogra A. Occurrence of ESBL & Amp-C beta-lactamases & susceptibility to newer antimicrobial agents in complicated UTI. Indian J Med Res. 2008;127:85–88. [PubMed] [Google Scholar]
- 12.Marrs CF, Zhang L, Foxman B. Escherichia coli mediated urinary tract infections: are there distinct uropathogenic E. coli (UPEC) pathotypes? FEMS Microbiol Lett. 2005;252:183–190. doi: 10.1016/j.femsle.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 13.Ragnarsdottir B, Samuelsson M, Gustafsson MC, et al. Reduced toll-like receptor 4 expression in children with asymptomatic bacteriuria. J Infect Dis. 2007;196:475–484. doi: 10.1086/518893. [DOI] [PubMed] [Google Scholar]
- 14.Ragnarsdottir B, Lutay N, Gronberg-Hernandez J, et al. Genetics of innate immunity and UTI susceptibility. Nat Rev Urol. 2011;8:449–468. doi: 10.1038/nrurol.2011.100. [DOI] [PubMed] [Google Scholar]
- 15.Hooton TM, Stamm WE. Diagnosis and treatment of uncomplicated urinary tract infection. Infect Dis Clin North Am. 1997;11:551–581. doi: 10.1016/s0891-5520(05)70373-1. [DOI] [PubMed] [Google Scholar]
- 16.Scholes D, Hooton TM, Roberts PL, et al. Risk factors associated with acute pyelonephritis in healthy women. Ann Intern Med. 2005;142:20–27. doi: 10.7326/0003-4819-142-1-200501040-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melekos MD, Naber KG. Complicated urinary tract infections. Int J Antimicrob Agents. 2000;15:247–256. doi: 10.1016/s0924-8579(00)00168-0. [DOI] [PubMed] [Google Scholar]
- 18.Anderson GG, Palermo JJ, Schilling JD, et al. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301:105–107. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 19.Justice SS, Hung C, Theriot JA, et al. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc Natl Acad Sci U S A. 2004;101:1333–1338. doi: 10.1073/pnas.0308125100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosen DA, Pinkner JS, Jones JM, et al. Utilization of an intracellular bacterial community pathway in Klebsiella pneumoniae urinary tract infection and the effects of FimK on type 1 pilus expression. Infect Immun. 2008;76:3337–3345. doi: 10.1128/IAI.00090-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosen DA, Hooton TM, Stamm WE, et al. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 2007;4:e329. doi: 10.1371/journal.pmed.0040329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robino L, Scavone P, Araujo L, et al. Detection of intracellular bacterial communities in a child with Escherichia coli recurrent urinary tract infections. Pathogens Dis. 2013;68:78–81. doi: 10.1111/2049-632X.12047. [DOI] [PubMed] [Google Scholar]
- 23.Robino L, Scavone P, Araujo L, et al. Intracellular bacteria in the pathogenesis of Escherichia coli urinary tract infection in children. Clin Infect Dis. 2014;59:e158–e164. doi: 10.1093/cid/ciu634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hurst RE, Zebrowski R. Identification of proteoglycans present at high density on bovine and human bladder luminal surface. J Urol. 1994;152 (5 Pt 1):1641–1645. doi: 10.1016/s0022-5347(17)32495-3. [DOI] [PubMed] [Google Scholar]
- 25.Hu P, Meyers S, Liang FX, et al. Role of membrane proteins in permeability barrier function: uroplakin ablation elevates urothelial permeability. Am J Physiol Renal Physiol. 2002;283:F1200–F1207. doi: 10.1152/ajprenal.00043.2002. [DOI] [PubMed] [Google Scholar]
- 26.Bishop BL, Duncan MJ, Song J, et al. Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat Med. 2007;13:625–630. doi: 10.1038/nm1572. [DOI] [PubMed] [Google Scholar]
- 27.Wu XR, Kong XP, Pellicer A, et al. Uroplakins in urothelial biology, function, and disease. Kidney Int. 2009;75:1153–1165. doi: 10.1038/ki.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Springall T, Sheerin NS, Abe K, et al. Epithelial secretion of C3 promotes colonization of the upper urinary tract by Escherichia coli. Nat Med. 2001;7:801–806. doi: 10.1038/89923. [DOI] [PubMed] [Google Scholar]
- 29.Bates JM, Raffi HM, Prasadan K, et al. Tamm-Horsfall protein knockout mice are more prone to urinary tract infection: rapid communication. Kidney Int. 2004;65:791–797. doi: 10.1111/j.1523-1755.2004.00452.x. [DOI] [PubMed] [Google Scholar]
- 30.Chromek M, Slamova Z, Bergman P, et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med. 2006;12:636–641. doi: 10.1038/nm1407. [DOI] [PubMed] [Google Scholar]
- 31.de Man P, van Kooten C, Aarden L, et al. Interleukin-6 induced at mucosal surfaces by gram-negative bacterial infection. Infect Immun. 1989;57:3383–3388. doi: 10.1128/iai.57.11.3383-3388.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hedges S, Anderson P, Lidin-Janson G, et al. Interleukin-6 response to deliberate colonization of the human urinary tract with gram-negative bacteria. Infect Immun. 1991;59:421–427. doi: 10.1128/iai.59.1.421-427.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirose T, Kumamoto Y, Matsukawa M, et al. Study on local immune response in Escherichia coli-induced experimental urinary tract infection in mice –infiltration of Ia-positive cells, macrophages, neutrophils, T cells and B cells. Kansenshogaku Zasshi. 1992;66:964–973. doi: 10.11150/kansenshogakuzasshi1970.66.964. [DOI] [PubMed] [Google Scholar]
- 34.Hopkins WJ, James LJ, Balish E, Uehling DT. Congenital immunodeficiencies in mice increase susceptibility to urinary tract infection. J Urol. 1993;149:922–925. doi: 10.1016/s0022-5347(17)36260-2. [DOI] [PubMed] [Google Scholar]
- 35.Ko YC, Mukaida N, Ishiyama S, et al. Elevated interleukin-8 levels in the urine of patients with urinary tract infections. Infect Immun. 1993;61:1307–1314. doi: 10.1128/iai.61.4.1307-1314.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haraoka M, Hang L, Frendeus B, et al. Neutrophil recruitment and resistance to urinary tract infection. J Infect Dis. 1999;180:1220–1229. doi: 10.1086/315006. [DOI] [PubMed] [Google Scholar]
- 37.Jones-Carson J, Balish E, Uehling DT. Susceptibility of immunodeficient gene-knockout mice to urinary tract infection. J Urol. 1999;161:338–341. [PubMed] [Google Scholar]
- 38.Godaly G, Hang L, Frendeus B, Svanborg C. Transepithelial neutrophil migration is CXCR1 dependent in vitro and is defective in IL-8 receptor knockout mice. J Immunol. 2000;165:5287–5294. doi: 10.4049/jimmunol.165.9.5287. [DOI] [PubMed] [Google Scholar]
- 39.Mysorekar IU, Mulvey MA, Hultgren SJ, Gordon JI. Molecular regulation of urothelial renewal and host defenses during infection with uropathogenic Escherichia coli. J Biol Chem. 2002;277:7412–7419. doi: 10.1074/jbc.M110560200. [DOI] [PubMed] [Google Scholar]
- 40.Schilling JD, Martin SM, Hung CS, et al. Toll-like receptor 4 on stromal and hematopoietic cells mediates innate resistance to uropathogenic Escherichia coli. Proc Natl Acad Sci U S A. 2003;100:4203–4208. doi: 10.1073/pnas.0736473100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malaviya R, Ikeda T, Abraham SN, Malaviya R. Contribution of mast cells to bacterial clearance and their proliferation during experimental cystitis induced by type 1 fimbriated E. coli. Immunol Lett. 2004;91:103–111. doi: 10.1016/j.imlet.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 42.Engel D, Dobrindt U, Tittel A, et al. Tumor necrosis factor alpha- and inducible nitric oxide synthase-producing dendritic cells are rapidly recruited to the bladder in urinary tract infection but are dispensable for bacterial clearance. Infect Immun. 2006;74:6100–6107. doi: 10.1128/IAI.00881-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andersen-Nissen E, Hawn TR, Smith KD, et al. Cutting edge: Tlr5−/− mice are more susceptible to Escherichia coli urinary tract infection. J Immunol. 2007;178:4717–4720. doi: 10.4049/jimmunol.178.8.4717. [DOI] [PubMed] [Google Scholar]
- 44.Song J, Bishop BL, Li G, et al. TLR4-initiated and cAMP-mediated abrogation of bacterial invasion of the bladder. Cell Host Microbe. 2007;1:287–298. doi: 10.1016/j.chom.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song J, Abraham SN. TLR-mediated immune responses in the urinary tract. Curr Opin Microbiol. 2008;11:66–73. doi: 10.1016/j.mib.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ingersoll MA, Kline KA, Nielsen HV, Hultgren SJ. G-CSF induction early in uropathogenic Escherichia coli infection of the urinary tract modulates host immunity. Cell Microbiol. 2008;10:2568–2578. doi: 10.1111/j.1462-5822.2008.01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sivick KE, Schaller MA, Smith SN, Mobley HL. The innate immune response to uropathogenic Escherichia coli involves IL-17A in a murine model of urinary tract infection. J Immunol. 2010;184:2065–2075. doi: 10.4049/jimmunol.0902386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith NJ, Varley CL, Eardley I, et al. Toll-like receptor responses of normal human urothelial cells to bacterial flagellin and lipopolysaccharide. J Urol. 2011;186:1084–1092. doi: 10.1016/j.juro.2011.04.112. [DOI] [PubMed] [Google Scholar]
- 49.Nielsen KL, Dynesen P, Larsen P, et al. Role of urinary cathelicidin LL-37 and human beta-defensin 1 in uncomplicated Escherichia coli urinary tract infections. Infect Immun. 2014;82:1572–1578. doi: 10.1128/IAI.01393-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hiratsuka T, Nakazato M, Ihi T, et al. Structural analysis of human beta-defensin-1 and its significance in urinary tract infection. Nephron. 2000;85:34–40. doi: 10.1159/000045627. [DOI] [PubMed] [Google Scholar]
- 51.Morrison G, Kilanowski F, Davidson D, Dorin J. Characterization of the mouse beta defensin 1, Defb1, mutant mouse model. Infect Immun. 2002;70:3053–3060. doi: 10.1128/IAI.70.6.3053-3060.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Becknell B, Spencer JD, Carpenter AR, et al. Expression and antimicrobial function of beta-defensin 1 in the lower urinary tract. PLoS One. 2013;8:e77714. doi: 10.1371/journal.pone.0077714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53▪▪.Jaillon S, Moalli F, Ragnarsdottir B, et al. The humoral pattern recognition molecule PTX3 is a key component of innate immunity against urinary tract infection. Immunity. 2014;40:621–632. doi: 10.1016/j.immuni.2014.02.015. The soluble pattern recognition molecule PTX3 protected against acute UTI in mice and polymorphisms in this gene were associated with susceptibility to UTI humans. [DOI] [PubMed] [Google Scholar]
- 54▪.Schiwon M, Weisheit C, Franken L, et al. Crosstalk between sentinel and helper macrophages permits neutrophil migration into infected uroepithelium. Cell. 2014;156:456–468. doi: 10.1016/j.cell.2014.01.006. The authors present data suggesting that inflammatory monocytes ‘license’ bladder resident macrophages to induce bladder epithelial transmigration by neutrophils. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55▪▪.Hannan TJ, Roberts PL, Riehl TE, et al. Inhibition of cyclooxygenase-2 prevents chronic and recurrent cystitis. EBioMedicine. 2014;1:46–57. doi: 10.1016/j.ebiom.2014.10.011. The authors present evidence from women and mice that an exacerbated neutrophilic inflammatory response during an initial UTI causes bladder immunopathology and prolonged infection that predisposes to bladder mucosal remodelling and recurrent UTI and demonstrate that COX-2 inhibitors may have a clinical benefit by preventing bladder epithelial wounding. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol. 2012;10:525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reigstad CS, Hultgren SJ, Gordon JI. Functional genomic studies of uropathogenic Escherichia coli and host urothelial cells when intracellular bacterial communities are assembled. J Biol Chem. 2007;282:21259–21267. doi: 10.1074/jbc.M611502200. [DOI] [PubMed] [Google Scholar]
- 58▪.Paragas N, Kulkarni R, Werth M, et al. alpha-Intercalated cells defend the urinary system from bacterial infection. J Clin Invest. 2014;124:2963–2976. doi: 10.1172/JCI71630. This interesting study utilized inbred mouse strains that differed in their susceptibility to pyelonephritis to discover that α-intercalated cells of the kidney collecting duct respond to UTI by expressing cytokines and lipocalin-2 and acidifying the urine, regardless of whether kidney infection was present or not. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59▪.Chan CY, St John AL, Abraham SN. Mast cell interleukin-10 drives localized tolerance in chronic bladder infection. Immunity. 2013;38:349–359. doi: 10.1016/j.immuni.2012.10.019. UPEC infection in mice resulted in a bladder mast cell derived IL-10 response that promoted an anti-inflammatory, tolerogenic bladder environment and prevented the development of an adaptive response to infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shelburne CP, Nakano H, St John AL, et al. Mast cells augment adaptive immunity by orchestrating dendritic cell trafficking through infected tissues. Cell Host Microbe. 2009;6:331–342. doi: 10.1016/j.chom.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Savchenko AS, Inoue A, Ohashi R, et al. Long pentraxin 3 (PTX3) expression and release by neutrophils in vitro and in ulcerative colitis. Pathol Int. 2011;61:290–297. doi: 10.1111/j.1440-1827.2011.02651.x. [DOI] [PubMed] [Google Scholar]
- 62.Navas-Nacher EL, Dardick F, Venegas MF, et al. Relatedness of Escherichia coli colonizing women longitudinally. Mol Urol. 2001;5:31–36. doi: 10.1089/109153601750124285. [DOI] [PubMed] [Google Scholar]
- 63.Foxman B, Manning SD, Tallman P, et al. Uropathogenic Escherichia coli are more likely than commensal E. coli to be shared between heterosexual sex partners. Am J Epidemiol. 2002;156:1133–1140. doi: 10.1093/aje/kwf159. [DOI] [PubMed] [Google Scholar]
- 64.Chen SL, Wu M, Henderson JP, et al. Genomic diversity and fitness of E. coli strains recovered from the intestinal and urinary tracts of women with recurrent urinary tract infection. Sci Transl Med. 2013;5:184ra60. doi: 10.1126/scitranslmed.3005497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamamoto S, Tsukamoto T, Terai A, et al. Genetic evidence supporting the fecal-perineal-urethral hypothesis in cystitis caused by Escherichia coli. J Urol. 1997;157:1127–1129. [PubMed] [Google Scholar]
- 66.Moreno E, Andreu A, Pigrau C, et al. Relationship between Escherichia coli strains causing acute cystitis in women and the fecal E. coli population of the host. J Clin Microbiol. 2008;46:2529–2534. doi: 10.1128/JCM.00813-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elliott TS, Reed L, Slack RC, Bishop MC. Bacteriology and ultrastructure of the bladder in patients with urinary tract infections. J Infect. 1985;11:191–199. doi: 10.1016/s0163-4453(85)92997-4. [DOI] [PubMed] [Google Scholar]
- 68.Mysorekar IU, Hultgren SJ. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc Natl Acad Sci U S A. 2006;103:14170–14175. doi: 10.1073/pnas.0602136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mulvey MA, Schilling JD, Hultgren SJ. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun. 2001;69:4572–4579. doi: 10.1128/IAI.69.7.4572-4579.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schilling JD, Lorenz RG, Hultgren SJ. Effect of trimethoprim-sulfamethoxazole on recurrent bacteriuria and bacterial persistence in mice infected with uropathogenic Escherichia coli. Infect Immun. 2002;70:7042–7049. doi: 10.1128/IAI.70.12.7042-7049.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blango MG, Ott EM, Erman A, et al. Forced resurgence and targeting of intracellular uropathogenic Escherichia coli reservoirs. PLoS One. 2014;9:e93327. doi: 10.1371/journal.pone.0093327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Duell BL, Carey AJ, Tan CK, et al. Innate transcriptional networks activated in bladder in response to uropathogenic Escherichia coli drive diverse biological pathways and rapid synthesis of IL-10 for defense against bacterial urinary tract infection. J Immunol. 2012;188:781–792. doi: 10.4049/jimmunol.1101231. [DOI] [PubMed] [Google Scholar]
- 73.Mulvey MA, Lopez-Boado YS, Wilson CL, et al. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science. 1998;282:1494–1497. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- 74.Mulvey MA, Schilling JD, Martinez JJ, Hultgren SJ. Bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. Proc Natl Acad Sci U S A. 2000;97:8829–8835. doi: 10.1073/pnas.97.16.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mysorekar IU, Isaacson-Schmid M, Walker JN, et al. Bone morphogenetic protein 4 signaling regulates epithelial renewal in the urinary tract in response to uropathogenic infection. Cell Host Microbe. 2009;5:463–475. doi: 10.1016/j.chom.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hannan TJ, Mysorekar IU, Hung CS, et al. Early severe inflammatory responses to uropathogenic E. coli predispose to chronic and recurrent urinary tract infection. PLoS Pathog. 2010;6:e1001042. doi: 10.1371/journal.ppat.1001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Langermann S, Palaszynski S, Barnhart M, et al. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science. 1997;276:607–611. doi: 10.1126/science.276.5312.607. [DOI] [PubMed] [Google Scholar]
- 78.Thumbikat P, Waltenbaugh C, Schaeffer AJ, Klumpp DJ. Antigen-specific responses accelerate bacterial clearance in the bladder. J Immunol. 2006;176:3080–3086. doi: 10.4049/jimmunol.176.5.3080. [DOI] [PubMed] [Google Scholar]
- 79.Hooton TM, Scholes D, Hughes JP, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. N Engl J Med. 1996;335:468–474. doi: 10.1056/NEJM199608153350703. [DOI] [PubMed] [Google Scholar]
- 80.Scholes D, Hooton TM, Roberts PL, et al. Risk factors for recurrent urinary tract infection in young women. J Infect Dis. 2000;182:1177–1182. doi: 10.1086/315827. [DOI] [PubMed] [Google Scholar]
- 81.Hawn TR, Scholes D, Li SS, et al. Toll-like receptor polymorphisms and susceptibility to urinary tract infections in adult women. PLoS One. 2009;4:e5990. doi: 10.1371/journal.pone.0005990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tabel Y, Berdeli A, Mir S. Association of TLR2 gene Arg753Gln polymorphism with urinary tract infection in children. Int J Immunogenet. 2007;34:399–405. doi: 10.1111/j.1744-313X.2007.00709.x. [DOI] [PubMed] [Google Scholar]
- 83.Karoly E, Fekete A, Banki NF, et al. Heat shock protein 72 (HSPA1B) gene polymorphism and Toll-like receptor (TLR) 4 mutation are associated with increased risk of urinary tract infection in children. Pediatr Res. 2007;61:371–374. doi: 10.1203/pdr.0b013e318030d1f4. [DOI] [PubMed] [Google Scholar]
- 84.Schlager TA, LeGallo R, Innes D, et al. B cell infiltration and lymphonodular hyperplasia in bladder submucosa of patients with persistent bacteriuria and recurrent urinary tract infections. J Urol. 2011;186:2359–2364. doi: 10.1016/j.juro.2011.07.114. [DOI] [PubMed] [Google Scholar]
- 85.Hopkins WJ, Gendron-Fitzpatrick A, Balish E, Uehling DT. Time course and host responses to Escherichia coli urinary tract infection in genetically distinct mouse strains. Infect Immun. 1998;66:2798–2802. doi: 10.1128/iai.66.6.2798-2802.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mabeck CE. Treatment of uncomplicated urinary tract infection in nonpregnant women. Postgrad Med J. 1972;48:69–75. [PMC free article] [PubMed] [Google Scholar]
- 87.Ferry SA, Holm SE, Stenlund H, et al. The natural course of uncomplicated lower urinary tract infection in women illustrated by a randomized placebo controlled study. Scand J Infect Dis. 2004;36:296–301. doi: 10.1080/00365540410019642. [DOI] [PubMed] [Google Scholar]
- 88.Hansson S, Hanson E, Hjalmas K, et al. Follicular cystitis in girls with untreated asymptomatic or covert bacteriuria. J Urol. 1990;143:330–332. doi: 10.1016/s0022-5347(17)39950-0. [DOI] [PubMed] [Google Scholar]
- 89.Hannan TJ, Totsika M, Mansfield KJ, et al. Host-pathogen checkpoints and population bottlenecks in persistent and intracellular uropathogenic Escherichia coli bladder infection. FEMS Microbiol Rev. 2012;36:616–648. doi: 10.1111/j.1574-6976.2012.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Henderson JP, Crowley JR, Pinkner JS, et al. Quantitative metabolomics reveals an epigenetic blueprint for iron acquisition in uropathogenic Escherichia coli. PLoS Pathog. 2009;5:e1000305. doi: 10.1371/journal.ppat.1000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chaturvedi KS, Hung CS, Crowley JR, et al. The siderophore yersiniabactin binds copper to protect pathogens during infection. Nat Chem Biol. 2012;8:731–736. doi: 10.1038/nchembio.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chaturvedi KS, Hung CS, Giblin DE, et al. Cupric yersiniabactin is a virulence-associated superoxide dismutase mimic. ACS Chem Biol. 2014;9:551–561. doi: 10.1021/cb400658k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao Z, Fux B, Goodwin M, et al. Autophagosome-independent essential function for the autophagy protein Atg5 in cellular immunity to intracellular pathogens. Cell Host Microbe. 2008;4:458–469. doi: 10.1016/j.chom.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang C, Mendonsa GR, Symington JW, et al. Atg16L1 deficiency confers protection from uropathogenic Escherichia coli infection in vivo. Proc Natl Acad Sci U S A. 2012;109:11008–11013. doi: 10.1073/pnas.1203952109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang C, Yuan X, Ma E, et al. NOD2 is dispensable for ATG16L1 deficiency-mediated resistance to urinary tract infection. Autophagy. 2014;10:331–338. doi: 10.4161/auto.27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hunstad DA, Justice SS, Hung CS, et al. Suppression of bladder epithelial cytokine responses by uropathogenic Escherichia coli. Infect Immun. 2005;73:3999–4006. doi: 10.1128/IAI.73.7.3999-4006.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Loughman JA, Hunstad DA. Attenuation of human neutrophil migration and function by uropathogenic bacteria. Microbes Infect. 2011;13:555–565. doi: 10.1016/j.micinf.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lau ME, Loughman JA, Hunstad DA. YbcL of uropathogenic Escherichia coli suppresses transepithelial neutrophil migration. Infect Immun. 2012;80:4123–4132. doi: 10.1128/IAI.00801-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99▪▪.Lutay N, Ambite I, Gronberg Hernandez J, et al. Bacterial control of host gene expression through RNA polymerase II. J Clin Invest. 2013;123:2366–2379. doi: 10.1172/JCI66451. The ASB strain E. coli 83972 was found to broadly suppress host gene expression by suppressing phosphorylation of RNA polymerase II at serine 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Duell BL, Carey AJ, Dando SJ, et al. Human bladder uroepithelial cells synergize with monocytes to promote IL-10 synthesis and other cytokine responses to uropathogenic Escherichia coli. PLoS One. 2013;8:e78013. doi: 10.1371/journal.pone.0078013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pouwels SD, Heijink IH, ten Hacken NH, et al. DAMPs activating innate and adaptive immune responses in COPD. Mucosal Immunol. 2014;7:215–226. doi: 10.1038/mi.2013.77. [DOI] [PubMed] [Google Scholar]