Summary
Endocytic transport plays a vital role in the establishment and maintenance of cell polarity. Many studies have demonstrated that endosome-dependent protein targeting is required for polarization of epithelial cells and neurons. Endocytic transport regulates several highly polarized cellular events, such as cell motility and division. Rab11 GTPase has been shown to be a master regulator of protein transport via recycling endosomes, and many recent studies have focused on the molecular machinery that mediates Rab11-dependent endocytic protein transport in polarized cells. This mini-review describes the recent advances in identifying and characterizing the role of Rab11 and its effector proteins that play important roles in polarized endocytic sorting and transport.
Keywords: Rab11, GTPase, Rab11-FIPs, Endosomes, Cytokinesis, Epithelial cells
Introduction
Eukaryotic cells compartmentalize biological functions in a series of organelles. In the exocytotic and endocytotic pathways, the unique composition of each compartment is maintained despite the continuous movement of proteins and lipids within the cell. This is accomplished by multiple sorting and recycling mechanisms during the formation, transport, tethering and fusion of transport vesicles.
Endosomes are the central hubs for sorting and transport of newly synthesized, as well as, endocytosed proteins. Traditionally, endosomes were shown to sort proteins to two main pathways: degradative and recycling. The sorting of proteins for degradation or recycling occurs in late endosomes, also known as sorting endosomes. It is generally accepted that early endosomes slowly mature to become late endosomes or multivesicular bodies. As part of this maturation, proteins destined for recycling back to plasma membrane or trans-Golgi network are removed via the formation of endocytic tubular extensions that involves several distinct sorting complexes, such as the retromer, AP-1 and AP-3 (Bonifacino and Lippincott-Schwartz, 2003). A large portion of these recycling proteins are transported via recycling endosomes, the endocytic compartment that has traditionally been morphologically defined by the presence of extensive tubulo-vesicular endocytic network containing transferrin receptor and Rab11 GTPase.
In addition to its role in constitutive recycling, recycling endosomes have emerged as an important pathway that mediates sorting and targeting of proteins during polarization of cells. For example, endocytic transport was shown to be required for establishment and maintenance of epithelial polarity (Apodaca et al., 1994; Casanova et al., 1999). Endosomes also were implicated in regulating cell polarization during cell division and motility (Jones et al., 2006; Prekeris and Gould, 2008). As a result it has become apparent that either recycling endosomes are comprised from several functionally distinct endocytic compartments or that an additional cargo sorting occurs at recycling endosomes that allows targeted delivery of specific proteins to a distinct cellular compartments during cell polarization. The first part of this mini-review will discuss the role of recycling endosomes in polarized protein transport in three well-understood polarized protein transport models: epithelial cell polarization, cell division and cell motility.
Rab11 GTPase has emerged as an important regulator of protein transport via recycling endosomes. One of the more intriguing aspects of Rab11 GTPase, is its involvement in the regulation of many distinct polarized endocytic transport steps. It has been suggested that the multi-functional properties of Rab11 GTPase may be explained by its ability to interact with multiple effector proteins, in particular with the members of Rab11 family-interacting proteins, Rab11-FIPs (Meyers and Prekeris, 2002). Consistent with this hypothesis, it was shown that various Rab11-FIPs form mutually exclusive complexes with Rab11 GTPase, forming a “targeting complexes” that appear to be involved in regulating different sorting/transport pathways via recycling endosomes (Meyers and Prekeris, 2002). The second part of this mini-review will discuss the role of distinct Rab11/Rab11-FIP “targeting complexes” in regulating polarized endocytic sorting and transport.
Endocytic transport and cell polarity
The role of endosomes in establishment and maintenance of epithelial cell polarity
Epithelial cells are structurally and functionally polarized to transport specific molecules selectively and unidirectionaly while maintaining trans-epithelial barrier. The selective transport is achieved by the partitioning of the plasma membrane into distinct domains: apical and basolateral. Both of these plasma membrane domains have distinct lipid compositions and contain specific sets of proteins that are maintained by a junctional complex. The apical plasma membrane is enriched in cholesterol, sphingolipids, glycolipids and various specific highly glycosylated proteins. In contrast, the basolateral membrane is enriched in E-cadherin, integrin molecules, as well as, growth factor receptors (Mostov et al., 2003; Nelson, 2003; Rodriguez-Boulan et al., 2004). Tight junction separate apical and basolateral domains and provide a diffusion barrier that prevents mixing of the apical and basolateral membrane components (Fig. 1A).
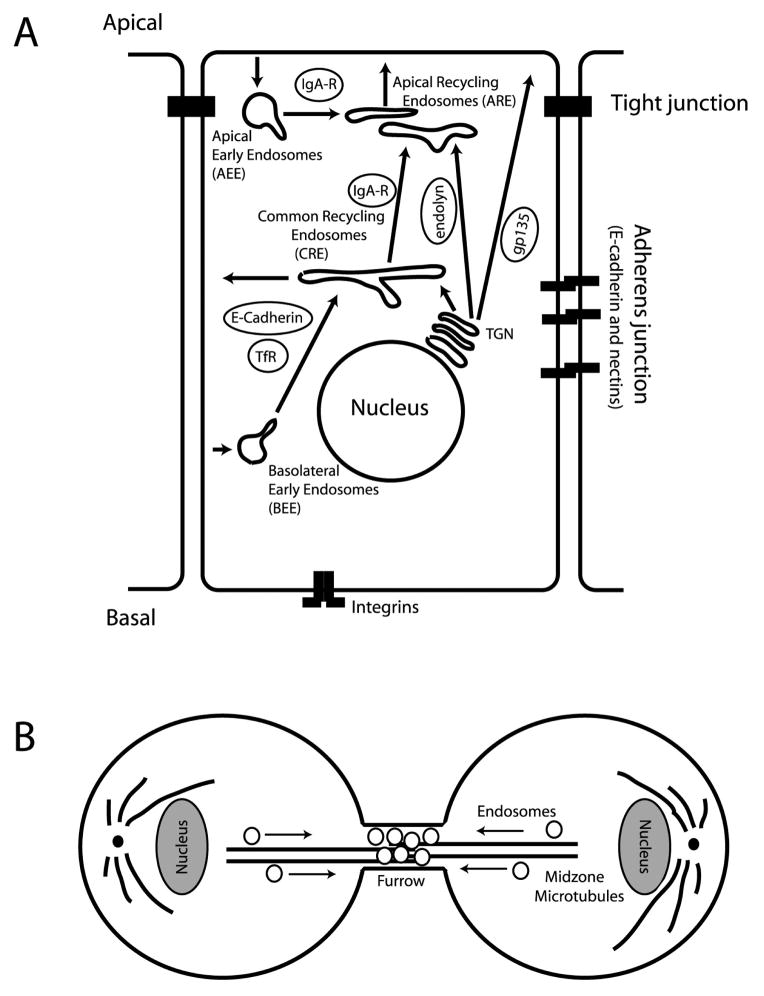
Fig. 1.
The models of polarized endocytic transport. A. Schematic representation of apical and basolateral endocytic protein transport in polarized epithelial cells. IgA-R, endolyn, gp135, TfR and E-Cadherin represent cargo proteins that are targeted to different plasma membrane domains of polarized cell. B. Schematic representation of recycling endosome transport during late telophase of mitotic cells.
Polarized endocytic transport also is an important regulator of epithelial cell polarity. Polarized epithelial cells have several domain specific endosomes: apical (AEE) and basolateral (BEE) early endosomes, as well as, a common recycling endosomes (CRE) (Fig. 1A). In addition, most polarized epithelial cells have specialized compartment of apical recycling endosomes (ARE) that are involved in regulated recycling of specialized apical proteins (Fig. 1A). For example, regulated insertion of H+/K+-ATPase into the apical plasma membrane of gastric parietal cells is responsible for selective secretion of hydrochloric acid into the stomach lumen and is mediated by ARE (Calhoun et al., 1998; Duman et al., 1999). A majority of apically or basolaterally internalized proteins go to AEE or BEE from which they are transferred to CRE. CRE is the site where plasma membrane-destined proteins are selectively segregated and sorted to their final destinations (Fig. 1A). For example, basolateral proteins such as E-cadherin, transferrin receptor (TfR) or LDL receptor (LDL-R) are targeted from CRE to basolateral plasma membrane via pathway that involves AP1B coat protein, as well as, the exocyst complex (Gan et al., 2002; Folsch et al., 2003; Yeaman et al., 2004; Gravotta et al., 2007). In contrast, polymeric IgA receptor (pIgA-R) that is involved in basolateral-to-apical transcytosis moves from CRE to ARE compartments before reaching apical plasma membrane (Apodaca et al., 1994).
In addition to sorting of endocytosed proteins, newly synthesized proteins exiting trans-Golgi Network (TGN) are sorted and delivered to either apical or basolateral plasma membrane. Interestingly, recent data suggest that from TGN many proteins are directed to endosomes before they are sorted to the apical or basolateral surfaces (Ang et al., 2004). For example, in Madin-Darby canine kidney (MDCK) cells E-cadherin is delivered from trans-Golgi Network (TGN) to CRE before delivery to basolateral plasma membrane (Lock et al., 2005; Lock and Stow, 2005). In contrast, apical proteins such as sialomucin endolyn appear to be targeted to ARE before they reach apical plasma membrane (Potter et al., 2006; Cresawn et al., 2007). These data suggest that apical and basolateral proteins are delivered to the plasma membrane via different classes of endosomes (Fig. 1A) (Cresawn et al., 2007). In addition, some apical proteins also may be delivered directly to plasma membrane in distinct population of apical transport carriers (Jacob and Naim, 2001; Ang et al., 2004), suggesting the existence of several biosynthetic pathways from TGN to apical plasma membrane. Consistent with that prediction, several studies have demonstrated that lipid-raft associated proteins (such as gp135) may be delivered from TGN directly to apical plasma membrane (Fig. 1A) (Guerriero et al., 2006; Delacour et al., 2007).
Endocytic transport and cytokinesis
Cytokinesis is the final stage of the cell cycle resulting in the physical separation of daughter cells. After replication of the genetic material, the mother cell divides by the formation of a furrow that constricts the cytoplasm, leaving two daughter cells connected by a thin cytoplasmic bridge. The resolution of this bridge, abscission, results in separation of two daughter cells. At least two distinct processes are required for successful cytokinesis: formation and contraction of an actomyosin contractile ring and delivery of membranes to the cleavage furrow (Fig. 1B) (O’Halloran, 2000; Scholey et al., 2003). Both these steps are tightly controlled and crucial for cell abscission. While the function of the actomyosin contractile ring in cell division is well understood, we are only beginning to understand the mechanisms and function of endocytic membrane transport during cytokinesis. The requirement of membrane transport to the site of division has been demonstrated in many different organisms including Caenorhabditis elegans and Drosophila melanogaster embryos (Skop et al., 2001; Hickson et al., 2003; Pelissier et al., 2003; Riggs et al., 2007).
Recycling endosomes have been identified as the organelles that are targeted to the cleavage furrow and play a key role in cytokinesis (Skop et al., 2001; Hickson et al., 2003; Pelissier et al., 2003; Wilson et al., 2004; Riggs et al., 2007). A number of reports have demonstrated the pronounced changes in endocytic recycling during mitosis (Pypaert et al., 1991; Raucher and Sheetz, 1999; Boucrot and Kirchhausen, 2007). For example, during metaphase and anaphase, the protein and membrane recycling back to plasma membrane is dramatically inhibited, resulting in the accumulation of endocytic organelles, as well as, decrease in PM surface area. In contrast, the initiation of the furrow formation and ingression during telophase stimulates a rapid increase in membrane and protein recycling back to the PM, in particular in the area of cleavage furrow. Importantly, these dynamic changes in the rates of endocytic recycling and the surface area of the PM seem to be required for the successful completion of cytokinesis (Boucrot and Kirchhausen, 2007). Consistent with that, the inhibition of various endocytic recycling regulating proteins, such as Rab11 GTPase, dynamin, α-adaptin, syntaxin 1 and VAMP8 result in cytokinesis block (Hickson et al., 2003; Low et al., 2003; Wilson et al., 2004).
The delivery of endocytic membranes to the furrow mediate several processes required for cytokinesis. First, the delivery and fusion of membranes at the tips of the furrow have been shown to be required to provide for the expanding plasma membrane surface. Second, the compound fusion of endosomes accumulated at the cleavage furrow is thought to mediate cell-cell separation (Fig. 1B) (Gromley et al., 2005). Third, it has been suggested that membrane transport to the furrow mediates the delivery of proteins and/or lipids that are required for abscission (for review see (Barr and Gruneberg, 2007; Prekeris and Gould, 2008)). What proteins and/or lipids are delivered to the furrow by these endosomes, remains largely unknown, and may include proteins that affect sphingomyelin, phosphatidylinositol 4,5-biphosphate (PI4,5P2) and phosphatidylethanolamine (PE) distribution at the furrow (Stock et al., 1999; Weber et al., 1999; Emoto and Umeda, 2000; Field et al., 2005), as well as, proteins regulating RhoA activation and actin cytoskeleton dynamics (Albertson et al., 2008).
The role of endosomes in cell motility
Cell adhesion and motility are dynamic processes requiring both temporal and spatial coordination of many cellular structures. While actin cytoskeleton dynamics plays a major role during cell movement, polarized membrane transport also is an important player in mediating cell motility. Vectorial cell migration necessitates the extension of lamellipodia or filopodia in the direction of movement. The generation of lamellipodia or filopodia requires massive directional influx of membrane (Nabi, 1999). This membrane influx may be achieved via targeted delivery of the membranes to the leading edge from the Golgi apparatus and/or endosomes. Interestingly, endosomes are involved in several other processes that require massive influx of plasma membrane, such as phagocytosis and cytokinesis (Cox et al., 2000; Skop et al., 2001; Wilson et al., 2004; Riggs et al., 2007).
Recent studies have shown that recycling endosomes regulate polarized recycling of various integrins (Roberts et al., 2001; Powelka et al., 2004). It is generally believed that endocytosis of the integrins at the retracting end of the moving cell and transport to the plasma membrane of the leading edge is part of the cellular machinery that regulates cell movement. Consistent with this hypothesis, it has been shown that endocytic recycling of α5β1 and 6β4 integrins contributes to the hypoxia-induced invasive migration of breast cancer cells, as well as, motility of fibroblasts (Yoon et al., 2005). Furthermore, some of endocytic proteins were shown to contribute to the invasiveness of breast and ovarian cancers (Cheng et al., 2004; Caswell and Norman, 2006).
The role of Rab11 GTPase in endocytic sorting and transport
Recently, Rab GTPases have emerged as master regulators of membrane transport. The first Rab GTPase, Sec4p, was cloned more then two decades ago. Since then, more than 60 Rab GTPases have been identified and shown to regulate distinct membrane transport pathways. Several Rabs were shown to regulate transport via recycling endosomes, including Rab11-family GTPases. The Rab11 family is composed of three closely related proteins: Rab11a, Rab11b and Rab25. All members of Rab11 family were shown to regulate endocytic recycling. Multiple studies have implicated Rab11 family GTPases in regulation of polarized endocytic transport. Over-expression of Rab11-dominant negative mutants affect apical and basolateral transport in epithelial cells, as well as, integrin transport to the leading edge of motile cells (Casanova et al., 1999; Powelka et al., 2004). Furthermore, Rab11a and Rab11b were shown to mediate recycling endosome transport to the cleavage furrow during cytokinesis, the step that is required for abscission of dividing cells (Wilson et al., 2004; Riggs et al., 2007).
As all Rab GTPases, in its GTP-bound state Rab11/25 interact with several proteins, known as Rab11/25 effectors. Several Rab11/25 effectors have been identified to date. Rab11a was shown to interact with Myosin Vb molecular motor and regulate endocytic recycling (Lapierre et al., 2001; Hales et al., 2002; Wilson et al., 2004; Riggs et al., 2007). Rab11a also was shown to bind to Sec15, a subunit of the exocyst complex (Prigent et al., 2003; Wu et al., 2005). The exocyst complex is known to regulate the polarized vesicle targeting and tethering in epithelial cells, neurons and dividing cells (Murthy et al., 2003; Yeaman et al., 2004; Fielding et al., 2005; Gromley et al., 2005). Thus, it was proposed that Sec15 binding to Rab11a mediates the recruitment of the exocyst complex to the endocytic carriers that mediate polarized protein recycling. Consistent with this hypothesis, Rab11 was shown to be required for the exocytic-dependent basolateral transport of E-cadherin in polarized epithelial cells (Lock et al., 2005; Lock and Stow, 2005). In addition to Myosin Vb and Sec15, Rab11/25 GTPases also were shown to bind to Rab11-family interacting proteins, known as Rab11-FIPs (Prekeris et al., 2000, 2001; Hales et al., 2001). Rab11-FIPs are a family of scaffolding proteins that play major roles in mediating endocytic recycling in a variety of cells. Since Rab11-FIPs form mutually exclusive complexes with Rab11, it was proposed that binding of Rab11 to different Rab11-FIPs work as a “targeting complexes” that regulate distinct endocytic transport pathways in polarized and non-polarized cells. The roles of Rab11-FIPs in regulating endocytic sorting and transport are the main focus of this mini-review.
The role of Rab11-FIPs in endocytic sorting and transport
Work from several laboratories have identified Rab11-FIPs as Rab11/25 effector proteins, which serve as “target complexes” in specific endocytic transport pathways. Rab11-FIP family consists from five members, which bind with high affinity (~50–100 nM) to Rab11a, Rab11b and Rab25 GTPases (Junutula et al., 2004; Peden et al., 2004; Tarbutton et al., 2005; Eathiraj et al., 2006) (Fig. 2). All Rab11-FIPs contain a highly conserved 20-amino acid motif at C-terminus of protein (Prekeris et al., 2001). This motif is known as Rab11-binding domain (RBD), since it mediates Rab11-FIP interaction with GTP-bound form of Rab11/25. The crystal structures of Rab11a complexes with Rab11-FIP2 and Rab11-FIP3 recently have been solved and show that Rab11/Rab11-FIPs form hetero-tetrameric complex with a parallel coiled-coil homo-dimer of α-helical RBD and two symmetric Rab11a molecules on both sides (Eathiraj et al., 2006; Jagoe et al., 2006; Shiba et al., 2006). Interestingly, Rab11-FIP binding induces an additional conformational change in GTP-bound Rab11a (Eathiraj et al., 2006), perhaps explaining high affinity interaction between Rab11-FIPs and Rab11/25 GTPases. Based on these data, it has been suggested that Rab11-FIPs are recruited to endocytic membrane through binding to active GTP-bound Rab11 and the cytosol exposed coiled-coil domains of FIPs may serve as a scaffolding platform for the recruitment of other membrane transport regulating proteins. Consistent with this hypothesis, several known endocytic transport regulators have been shown to specifically interact with various Rab11-FIPs (see below).
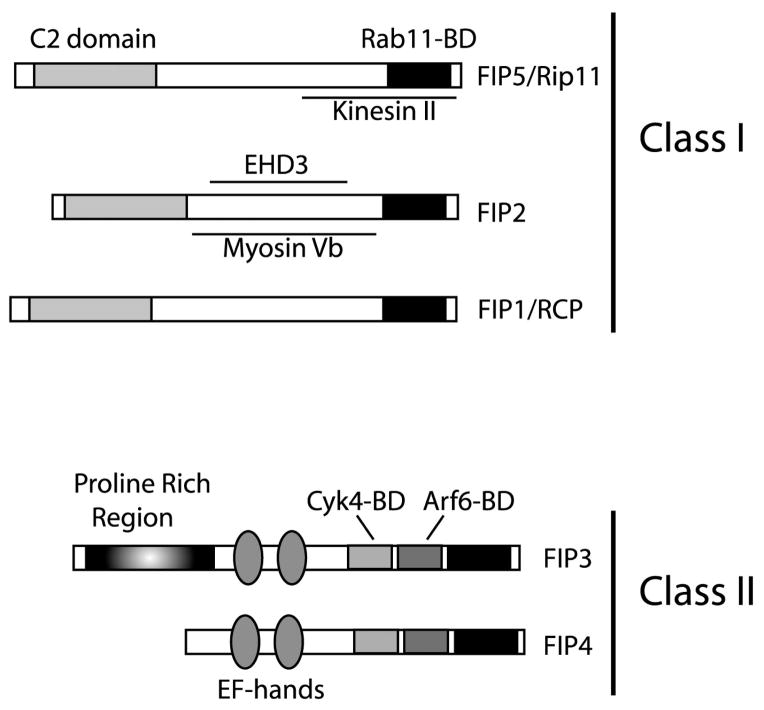
Fig. 2.
Rab11 family interacting proteins. Schematic representation of proteins belonging to Rab11 family interacting proteins (Rab11-FIPs). Rab11-BD stands for Rab11-binding domain. Arf6-BD stands for Arf6-binding domain. Cyk4-BD stands for Cyk4-binding domain.
Based on sequence homology all Rab11-FIPs are divided into two classes (Tarbutton et al., 2005) (Fig. 2). Class I FIPs (Rab11-FIP1/RCP, Rab11-FIP2 and Rab11-FIP5/Rip11) contain a C2 domain near N-terminus of protein (Prekeris et al., 2001). Recent work suggests that these C2 domains may interact with anionic phospholipids, specifically with PtdIn(3,4,5)P3 and phosphatidic acid (Lindsay and McCaffrey, 2004), although the functional significance of these interactions remains to be elucidated. All class I Rab11-FIPs have been shown to localize to recycling endosomes, where they regulate recycling of various plasma membrane receptors, such as epidermal growth factor receptor (EGFR), α5β1 integrins, transferrin receptor (TfR), chemokine receptors, GLUT4, FAT/36 and LDLR (Hales et al., 2001; Cullis et al., 2002; Fan et al., 2004; Lindsay and McCaffrey, 2004; Peden et al., 2004). In polarized epithelial cells class I FIPs are enriched at the apical recycling endosomes and were shown to regulate apical endocytic transport of polymeric IgA receptor (pIgAR) and H+K+-ATPase (Prekeris et al., 2000; Hales et al., 2001).
Class II FIPs (Rab11-FIP3 and Rab11-FIP4) do not have C2 domain, but are characterized by the presence of two EF-hands and proline rich region at the N-terminus (Prekeris et al., 2001). While class II Rab11-FIPs also are localized to recycling endosomes, they appear to play a role in regulating more specialized cell processes. Consistent with this, Rab11-FIP3 knock-down had little effect on recycling kinetics of TfR (Inoue et al., 2008). Instead, Rab11-FIP3 was shown to regulate recycling endosome targeting to the cleavage furrow during mitotic cell division, the step that is required for the successful completion of cytokinesis (Hickson et al., 2003; Horgan et al., 2004; Wilson et al., 2004; Fielding et al., 2005; Simon et al., 2008). In the following sections, we will discuss the recent research progress in understanding the roles and mechanisms of each member of Rab11-FIP protein complex.
Rab11-FIP1/RCP
Rab11-FIP1/RCP originally was isolated from yeast two-hybrid screen as a putative dual Rab4 and Rab11-interacting protein and named Rab coupling protein (RCP) (Lindsay et al., 2002). Later it was shown that RCP is a splice-isoform of Rab11-FIP1, which was earlier identified as Rab11-binding protein belonging to Rab11-FIPs (Hales et al., 2001). Importantly, binding analysis using several in vitro binding assays demonstrated that Rab11-FIP1/RCP binds to Rab4 with very low affinity (>30 μM) as compared to Rab11 binding (~50 nM) (Peden et al., 2004). Rab4 and Rab11 seem to bind to an overlapping site, since Rab11 very effectively competed with Rab4 for binding to Rab11-FIP1/RCP (Peden et al., 2004). Finally, while in vivo studies have confirmed that Rab11-FIP1/RCP binds to Rab11 and is required for Rab11-dependent endocytic recycling (Peden et al., 2004; Caswell et al., 2008), no in vivo evidence is consistent with Rab11-FIP1/RCP binding to Rab4 (Peden et al., 2004). Thus, it is not likely that Rab11-FIP1/RCP function as Rab11 and Rab4 coupling proteins. Instead, similarly to other Rab11-FIPs, Rab11-FIP1/RCP probably functions as a scaffolding protein that is recruited to recycling endosomes by binding to Rab11 GTPase.
Rab11-FIP1/RCP is known to mediate the recycling of several plasma membrane receptors. It was shown that the knock-down of Rab11-FIP1/RCP inhibits the sorting of TfR, as well as, β1 integrin to recycling pathways, resulting in increased degradation of these proteins (Peden et al., 2004) and unpublished data). Consistent with that, recent studies have demonstrated that Rab11-FIP1/RCP is required for α5β1 integrin recycling (Caswell et al., 2008). As a result, the inhibition of Rab11-FIP1/RCP by over-expression of dominant-negative mutant decreased the motility of cells on two-dimensional substrates, as well as, fibronectin-dependent migration of cells into three-dimensional matrices (Caswell et al., 2008). Rab11-FIP1/RCP also was shown to mediate protein recycling from phagosomes (Damiani et al., 2004). Finally, Rab11-FIP1/RCP was implicated endocytic transport of Langerin, thus regulating biogenesis of its characteristic organelles, known as Birbeck granules (Uzan-Gafsou et al., 2007). As other Rab11-FIPs, Rab11-FIP1/RCP probably acts as scaffolding factor that recruits other regulatory proteins to recycling endosomes. These factors are assumed to regulate the Rab11-FIP1/RCP-dependent sorting and/or transport of various proteins within endocytic recycling pathway. However, the proteins that bind directly to Rab11-FIP1/RCP and are required for endocytic recycling have not been identified.
Rab11-FIP2
Rab11-FIP2 is probably the best-studied member of class I Rab11-FIPs. Rab11-FIP2 was originally identified as Rab11 and Myosin Vb-binding protein (Hales et al., 2001, 2002; Prekeris et al., 2001). Importantly, Rab11 also directly binds to Myosin Vb (Lapierre et al., 2001). While the biochemical properties of Rab11-FIP2 and Myosin Vb interactions remain to be fully characterized, multiple studies have shown that the formation of Rab11/Rab11-FIP2/Myoins Vb complex plays an important role in mediating endocytic recycling. Over-expression of Myosin Vb or Rab11-FIP2 dominant-negative mutant results in a collapse of recycling endosomes around microtubule organizing center, resulting in the inhibition of the recycling of several plasma membrane receptors, such as transferrin receptor, epidermal growth factor receptor and chemokine receptor CXCR2 (Cullis et al., 2002; Hales et al., 2002; Fan et al., 2003, 2004; Naslavsky et al., 2006).
Rab11-FIP2 and Myosin Vb also were shown to regulate polarized endocytic transport. Rab11-FIP2 is highly enriched at the apical recycling endosomes and is required for transcytosis of polymeric IgA receptor, as well as, apical transport of aquaporin 2 water channels (Ducharme et al., 2007; Nedvetsky et al., 2007). The involvement of Rab11-FIP2 in apical transport appears to depend on its ability to interact with myosin Vb, since over-expression of myosin Vb tail domain, that acts as dominant-negative mutant, also inhibits polymeric IgA receptor and aquaporin 2 transport and affects the localization of apical recycling endosomes (Ducharme et al., 2007; Nedvetsky et al., 2007). Interestingly, Rab11-FIP2 and Myosin Vb protein complex also regulates AMPA receptor transport and targeting to the dendritic spines in neurons (Wang et al., 2008), suggesting that Rab11-FIP2 role is not limited to polarized transport in epithelial cells.
In addition to Myosin Vb, Rab11-FIP2 also was shown to interact with several Eps15 homology domain (EHD)-containing proteins, such as Reps1, EHD1 and EHD3 (Cullis et al., 2002; Naslavsky et al., 2006). EHD protein binding has been mapped to three NPF motifs situated at the middle region of Rab11-FIP2 (Cullis et al., 2002; Naslavsky et al., 2006). All mammalian EHD proteins are characterized by an N-terminal nucleotide binding domain and C-terminal EHD domain (Pohl et al., 2000). Studies from several laboratories have implicated EHD1 and EHD3 in the endocytic sorting of and recycling of plasma membrane proteins (Caplan et al., 2002; Rapaport et al., 2006; Gokool et al., 2007; Jovic et al., 2007; Naslavsky et al., 2007). While the mechanism of EHD1 and EHD3 action remains unclear, it was suggested that EHD1/3 can oligomerize, thus creating the “sorting subdomains” in early and recycling endosomes. The binding of EHD1/3 to Rab11-FIP2 may facilitate Rab11-FIP2 recruitment to these “sorting subdomains” during the generation of endocytic carriers.
Rab11-FIP5/Rip11
Rab11-FIP5/Rip11 is a founding member of Rab11-FIPs (Prekeris et al., 2000). Rab11-FIP5/Rip11 was first identified as putative SS-A/Ro auto-antigen interacting 75KD phosphoprotein (pp75) in the sera of systemic lupus erythematosus patients (Wang et al., 1999). Later, however, it was shown that Rab11-FIP5/Rip11 is actually a Rab11-interacting protein that is localized to apical recycling endosomes (ARE) in polarized epithelial cells (Prekeris et al., 2000). Over-expression of Rab11-FIP5/Rip11 dominant-negative mutant inhibits apical transcytosis of polymeric IgA receptor while having no effect on basolateral TfR recycling (Prekeris et al., 2000), suggesting that Rab11-FIP5/Rip11 regulates Rab11-dependent apical endocytic protein transport. In non-polarized cells Rab11-FIP5/Rip11 also was shown to regulate protein sorting and transport from “fast/constitutive” recycling pathway to “slow/regulated” recycling pathway (Schonteich et al., 2008). Consistent with that data, Rab11-FIP5/Rip11 was shown to regulate insulin-stimulated GLUT4 transport to plasma membrane in adipocytes (Welsh et al., 2007).
The mechanisms of Rab11-FIP5/Rip11-dependent protein sorting and transport remain to be fully understood. Like other Rab11-FIPs, Rab11-FIP5/Rip11 appears to act as a scaffolding factor, which mediates the recruitment of other membrane transport regulating proteins to recycling endosomes. Recent studies have shown that Rab11-FIP5/Rip11 directly binds to Kif3A/B subunits of Kinesin II molecular motor (Schonteich et al., 2008). Interestingly, Kinesin II is known to be required for growth and maintenance of apical primary cilia in epithelial cells. Thus, it was suggested that Kinesin II and Rab11-FIP5/Rip11 binding may be required for endocytic apical transport (Schonteich et al., 2008). Rab11-FIP5/Rip11 also was shown to interact with Rab GTPase-activating protein-AS160 (Welsh et al., 2007). Since AS160 is required for insulin-stimulated glucose uptake in adipocytes, Rab11-FIP5/Rip11 may be required for targeting AS160 to GLUT4 vesicles (Welsh et al., 2007).
Rab11-FIP3 and Rab11-FIP4
Rab11-FIP3 and Rab11-FIP4 belong to a class II Rab11-FIP family of proteins that are characterized by the presence of EF-hands and Rab11-Binding Motif (RBD) at the C-terminus of protein (Prekeris et al., 2001). While class I Rab11-FIPs have been implicated in regulation of endocytic protein sorting and recycling, class II Rab11-FIPs appear to have a more specialized roles. In mammalian cells Rab11-FIP3 was shown to regulate the targeting of recycling endosomes to the cleavage furrow, the process that is required for successful completion of cytokinesis (Hickson et al., 2003; Horgan et al., 2004; Wilson et al., 2004; Fielding et al., 2005; Simon et al., 2008). Indeed, the knock-down of Rab11-FIP3 by siRNA inhibits late cytokinesis, thus resulting in number of bi-nucleate and multi-nucleate cells (Wilson et al., 2004). Similarly, nuclear fallout protein (Nuf), a Drosophila homologue of Rab11-FIP3, was shown to regulate endosomal transport and actin polymerization during cellularization of Drosophila embryos (Hickson et al., 2003).
Recent studies have shown that the targeting of Rab11-FIP3-containing recycling endosomes to the cleavage furrow is dependent on Rab11-FIP3 binding to Cyk-4 protein (Simon et al., 2008). Cyk-4 is a member of a centralspindlin complex that accumulates at the midzone of dividing cells (Yuce et al., 2005). Cyk-4 works by recruiting RhoA GEF (guanine-nucleotide-exchange factor) ECT2 to the midzone (Yuce et al., 2005). Interestingly, both, ECT2 and Rab11-FIP3 compete for binding to Cyk-4, suggesting that ECT2 may regulate Rab11-FIP3 association with centralspindlin complex (Simon et al., 2008). It was proposed that during anaphase and early telophase Rab11-FIP3-containing endosomes cannot accumulate at the midzone because Cyk-4 is bound to ECT2. At late telophase, ECT2 dissociates from Cyk-4, the step that at least in part is controlled by the sequestration of ECT2 at the newly formed nucleus (Chalamalasetty et al., 2006). Removal of ECT2 allows the binding of Rab11-FIP3 to the centralspindlin complex, leading to accumulation of endosomes at the midbody (Simon et al., 2008). The tethering of Rab11-FIP3-endosomes to the midbody is further enhanced by Rab11-FIP3 interactions with Exocyst complex (via binding to Arf6 and Rab11 GTPases) (Prigent et al., 2003; Zhang et al., 2004; Fielding et al., 2005; Schonteich et al., 2007).
The role of Rab11-FIP3 during interphase remains to be determined. Several studies have shown that Rab11-FIP3 associates with perinuclear recycling endosomes (Wilson et al., 2004; Horgan et al., 2007; Inoue et al., 2008). Furthermore, the knock-down of Rab11-FIP3 was shown to affect the localization of recycling endosomes by causing their dispersion to the periphery of the cell (Horgan et al., 2007; Inoue et al., 2008). Since the knock-down of ASAP1 was shown to cause the dispersion of recycling endosomes, it was suggested that Rab11-FIP3 binding to ASAP1, and stimulation of ASAP1 Arf1 GAP activity may regulate the perinuclear location of recycling endosomes (Inoue et al., 2008). Surprisingly, while FIP3 knock-down affected the localization of recycling endosomes, it had no effect on the kinetics of the protein recycling via recycling endosomes (Horgan et al., 2007; Inoue et al., 2008). Thus, further studies will be needed to understand the role of Rab11-FIP3 in regulating the function of recycling endosomes during interphase.
While recent studies have identified several cellular pathways that are regulated by Rab11-FIP3, the role of Rab11-FIP4 remains to be determined. Similarly to Rab11-FIP3, Rab11-FIP4 binds to Arf6 and Rab11 GTPases, as well as, Cyk-4 (Fielding et al., 2005; Simon et al., 2008). Since Rab11-FIP4 is highly enriched in neurons and testes, it is possible that it may play a role in regulation of specialized forms of cell divisions or neuronal polarization.
Outlook and future perspectives
Rab11-FIPs have emerged as key regulators of polarized and non-polarized enocytic sorting and transport. Since Rab11-FIPs form mutually exclusive complexes with Rab11/25 GTPases it is likely that binding between Rab11/25 and various Rab11-FIPs form a pathway-specific “targeting complex” that mediate distinct endocytic transport steps. What remains unclear is what mechanisms regulate interaction between Rab11/25 and Rab11-FIPs. Most cells express several Rab11-FIP isoforms, thus one would expect that the ability of Rab11/25 to interact with distinct Rab11-FIPs is tightly regulated. Consistent with that, Rab11-FIP2 and Rab-FIP5/Rip11 were shown to be phosphorylated, although the functional significance of their phosphorylation remains unclear (Ducharme et al., 2007). Alternatively, secondary messengers, such as calcium could also play role in regulating Rab11-FIP function, especially since class II Rab11-FIPs are known to have EF-hand motifs (Prekeris et al., 2001). Thus, identification of the machinery that regulates Rab11-FIP binding to Rab11/25 and other interacting proteins will be important in understanding the dynamics and regulation of various endocytic transport pathways.
Multiple studies have indicated that Rab11-FIPs act as scaffolding proteins that are recruited to the endocytic membranes by high affinity binding to Rab11/25 GTPases. Recent work has identified several membrane transport proteins that interact with Rab11-FIP2, Rab11-FIP5/Rip11 and Rab11-FIP3. However, it remains to be determined what factors are recruited by Rab11-FIP1/RCP and Rab11-FIP4. Thus, the identification of the proteins that interact with distinct Rab11 and Rab11-FIP complexes will be fundamental to deciphering the mechanisms that regulate Rab11-dependent endocytic transport.
Acknowledgments
We thank Drs. Kathryn Howell and James Goldenring for critically reading this manuscript. RP is funded by NIH-NIDDK (DK064380) and Susan G. Komens Breast Cancer Research Foundation.
References
- Albertson R, Cao J, Hsieh TS, Sullivan W. Vesicles and actin are targeted to the cleavage furrow via furrow microtubules and the central spindle. J Cell Biol. 2008;181:777–90. doi: 10.1083/jcb.200803096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang AL, Taguchi T, Francis S, Folsch H, Murrells LJ, Pypaert M, Warren G, Mellman I. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J Cell Biol. 2004;167:531–43. doi: 10.1083/jcb.200408165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apodaca G, Katz LA, Mostov KE. Receptor-mediated transcytosis of IgA in MDCK cells is via apical recycling endosomes. J Cell Biol. 1994;125:67–86. doi: 10.1083/jcb.125.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr FA, Gruneberg U. Cytokinesis: placing and making the final cut. Cell. 2007;131:847–60. doi: 10.1016/j.cell.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Lippincott-Schwartz J. Coat proteins: shaping membrane transport. Nat Rev Mol Cell Biol. 2003;4:409–414. doi: 10.1038/nrm1099. [DOI] [PubMed] [Google Scholar]
- Boucrot E, Kirchhausen T. Endosomal recycling controls plasma membrane area during mitosis. Proc Natl Acad Sci USA. 2007;104:7939–7944. doi: 10.1073/pnas.0702511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun BC, Lapierre LA, Chew SC, Goldenring JR. Rab11a redistributes to apical secretory canaliculus during stimulation of gastric parietal cells. Am J Physiol. 1998;275:C163–70. doi: 10.1152/ajpcell.1998.275.1.C163. [DOI] [PubMed] [Google Scholar]
- Caplan S, Naslavsky N, Hartnell LM, Lodge R, Polishchuk RS, Donaldson JG, Bonifacino JS. A tubular EHD1-containing compartment involved in the recycling of major histocompatibility complex class I molecules to the plasma membrane. EMBO J. 2002;21:2557–67. doi: 10.1093/emboj/21.11.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova JE, Wang X, Kumar R, Bhartur SG, Navarre J, Woodrum JE, Altschuler Y, Ray GS, Goldenring JR. Association of Rab25 and Rab11a with the apical recycling system of polarized Madin-Darby canine kidney cells. Mol Biol Cell. 1999;10:47–61. doi: 10.1091/mbc.10.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell PT, Norman JC. Integrin trafficking and the control of cell migration. Traffic. 2006;7:14–21. doi: 10.1111/j.1600-0854.2005.00362.x. [DOI] [PubMed] [Google Scholar]
- Caswell PT, Chan M, Lindsay AJ, McCaffrey MW, Boettiger D, Norman JC. Rab-coupling protein coordinates recycling of alpha5beta1 integrin and EGFR1 to promote cell migration in 3D microenvironments. J Cell Biol. 2008;183:143–55. doi: 10.1083/jcb.200804140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalamalasetty RB, Hummer S, Nigg EA, Sillje HH. Influence of human Ect2 depletion and overexpression on cleavage furrow formation and abscission. J Cell Sci. 2006;119:3008–19. doi: 10.1242/jcs.03032. [DOI] [PubMed] [Google Scholar]
- Cheng KW, Lahad JP, Kuo WL, Lapuk A, Yamada K, Auersperg N, Liu J, Smith-McCune K, Lu KH, Fishman D, Gray JW, Mills GB. The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nat Med. 2004;10:1251–1256. doi: 10.1038/nm1125. [DOI] [PubMed] [Google Scholar]
- Cox D, Lee DJ, Dale BM, Calafat J, Greenberg S. A Rab11-containing rapidly recycling compartment in macrophages that promotes phagocytosis. Proc Natl Acad Sci USA. 2000;97:680–685. doi: 10.1073/pnas.97.2.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresawn KO, Potter BS, Oztan A, Guerriero CJ, Ihrke G, Goldenring JR, Apodaca G, Weisz OA. Differential involvement of endocytic compartments in the biosynthetic traffic of apical proteins. EMBO J. 2007;26:3737–3748. doi: 10.1038/sj.emboj.7601813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullis DN, Philip B, Baleja JD, Feig LA. Rab11-FIP2, an adaptor protein connecting cellular components involved in internalization and recycling of epidermal growth factor receptors. J Biol Chem. 2002;277:49158–49166. doi: 10.1074/jbc.M206316200. [DOI] [PubMed] [Google Scholar]
- Damiani MT, Pavarotti M, Leiva N, Lindsay AJ, McCaffrey MW, Colombo MI. Rab coupling protein associates with phagosomes and regulates recycling from the phagosomal compartment. Traffic. 2004;5:785–797. doi: 10.1111/j.1600-0854.2004.00220.x. [DOI] [PubMed] [Google Scholar]
- Delacour D, Greb C, Koch A, Salomonsson E, Leffler H, Le Bivic A, Jacob R. Apical sorting by galectin-3-dependent glycoprotein clustering. Traffic. 2007;8:379–88. doi: 10.1111/j.1600-0854.2007.00539.x. [DOI] [PubMed] [Google Scholar]
- Ducharme NA, Williams JA, Oztan A, Apodaca G, Lapierre LA, Goldenring JR. Rab11-FIP2 regulates differentiable steps in transcytosis. Am J Physiol Cell Physiol. 2007;293:C1059–C1072. doi: 10.1152/ajpcell.00078.2007. [DOI] [PubMed] [Google Scholar]
- Duman JG, Tyagarajan K, Kolsi MS, Moore HP, Forte JG. Expression of rab11a N124I in gastric parietal cells inhibits stimulatory recruitment of the H+-K+-ATPase. Am J Physiol. 1999;277:C361–C372. doi: 10.1152/ajpcell.1999.277.3.C361. [DOI] [PubMed] [Google Scholar]
- Eathiraj S, Mishra A, Prekeris R, Lambright DG. Structural Basis for Rab11-mediated Recruitment of FIP3 to Recycling Endosomes. J Mol Biol. 2006;364:121–135. doi: 10.1016/j.jmb.2006.08.064. [DOI] [PubMed] [Google Scholar]
- Emoto K, Umeda M. An essential role for a membrane lipid in cytokinesis. Regulation of contractile ring disassembly by redistribution of phosphatidylethanolamine. J Cell Biol. 2000;149:1215–1224. doi: 10.1083/jcb.149.6.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan GH, Lapierre LA, Goldenring JR, Richmond A. Differential regulation of CXCR2 trafficking by Rab GTPases. Blood. 2003;101:2115–24. doi: 10.1182/blood-2002-07-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan GH, Lapierre LA, Goldenring JR, Sai J, Richmond A. Rab11-family interacting protein 2 and myosin Vb are required for CXCR2 recycling and receptor-mediated chemotaxis. Mol Biol Cell. 2004;15:2456–2469. doi: 10.1091/mbc.E03-09-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field SJ, Madson N, Kerr ML, Galbraith KA, Kennedy CE, Tahiliani M, Wilkins A, Cantley LC. PtdIns(4,5)P2 functions at the cleavage furrow during cytokinesis. Curr Biol. 2005;15:1407–1412. doi: 10.1016/j.cub.2005.06.059. [DOI] [PubMed] [Google Scholar]
- Fielding AB, Schonteich E, Matheson J, Wilson G, Yu X, Hickson GR, Srivastava S, Baldwin SA, Prekeris R, Gould GW. Rab11-FIP3 and FIP4 interact with Arf6 and the exocyst to control membrane traffic in cytokinesis. EMBO J. 2005;24:3389–3399. doi: 10.1038/sj.emboj.7600803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folsch H, Pypaert M, Maday S, Pelletier L, Mellman I. The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains. J Cell Biol. 2003;163:351–362. doi: 10.1083/jcb.200309020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Y, McGraw TE, Rodriguez-Boulan E. The epithelial-specific adaptor AP1B mediates post-endocytic recycling to the basolateral membrane. Nat Cell Biol. 2002;4:605–609. doi: 10.1038/ncb827. [DOI] [PubMed] [Google Scholar]
- Gokool S, Tattersall D, Seaman MN. EHD1 interacts with retromer to stabilize SNX1 tubules and facilitate endosome-to-Golgi retrieval. Traffic. 2007;8:1873–1886. doi: 10.1111/j.1600-0854.2007.00652.x. [DOI] [PubMed] [Google Scholar]
- Gravotta D, Deora A, Perret E, Oyanadel C, Soza A, Schreiner R, Gonzalez A, Rodriguez-Boulan E. AP1B sorts basolateral proteins in recycling and biosynthetic routes of MDCK cells. Proc Natl Acad Sci USA. 2007;104:1564–1569. doi: 10.1073/pnas.0610700104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromley A, Yeaman C, Rosa J, Redick S, Chen CT, Mirabelle S, Guha M, Sillibourne J, Doxsey SJ. Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell. 2005;123:75–87. doi: 10.1016/j.cell.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Guerriero CJ, Weixel KM, Bruns JR, Weisz OA. Phosphatidylinositol 5-kinase stimulates apical biosynthetic delivery via an Arp2/3-dependent mechanism. J Biol Chem. 2006;281:15376–15384. doi: 10.1074/jbc.M601239200. [DOI] [PubMed] [Google Scholar]
- Hales CM, Griner R, Hobdy-Henderson KC, Dorn MC, Hardy D, Kumar R, Navarre J, Chan EK, Lapierre LA, Goldenring JR. Identification and characterization of a family of Rab11-interacting proteins. J Biol Chem. 2001;276:39067–39075. doi: 10.1074/jbc.M104831200. [DOI] [PubMed] [Google Scholar]
- Hales CM, Vaerman JP, Goldenring JR. Rab11 family interacting protein 2 associates with Myosin Vb and regulates plasma membrane recycling. J Biol Chem. 2002;277:50415–50421. doi: 10.1074/jbc.M209270200. [DOI] [PubMed] [Google Scholar]
- Hickson GRX, Matheson J, Riggs B, Maier VH, Fielding AB, Prekeris R, Sullivan W, Barr FA, Gould GW. Arfophilins are dual Arf/Rab11 binding proteins that regulate recycling endosome distribution and are related to Drosophila nuclear fallout. Mol Biol Cell. 2003 doi: 10.1091/mbc.E03-03-0160. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horgan CP, Oleksy A, Zhdanov AV, Lall PY, White IJ, Khan AR, Futter CE, McCaffrey JG, McCaffrey MW. Rab11-FIP3 is critical for the structural integrity of the endosomal recycling compartment. Traffic. 2007;8:414–430. doi: 10.1111/j.1600-0854.2007.00543.x. [DOI] [PubMed] [Google Scholar]
- Horgan CP, Walsh M, Zurawski TH, McCaffrey MW. Rab11-FIP3 localises to a Rab11-positive pericentrosomal compartment during interphase and to the cleavage furrow during cytokinesis. Biochem Biophys Res Commun. 2004;319:83–94. doi: 10.1016/j.bbrc.2004.04.157. [DOI] [PubMed] [Google Scholar]
- Inoue H, Ha VL, Prekeris R, Randazzo PA. Arf GAP ASAP1 interacts with Rab11 effector FIP3 and regulates pericentrosomal localization of transferrin receptor-positive recycling endosome. Mol Biol Cell. 2008;19:4224–4237. doi: 10.1091/mbc.E08-03-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R, Naim HY. Apical membrane proteins are transported in distinct vesicular carriers. Curr Biol. 2001;11:1444–1450. doi: 10.1016/s0960-9822(01)00446-8. [DOI] [PubMed] [Google Scholar]
- Jagoe WN, Lindsay AJ, Read RJ, McCoy AJ, McCaffrey MW, Khan AR. Crystal structure of rab11 in complex with rab11 family interacting protein 2. Structure. 2006;14:1273–1283. doi: 10.1016/j.str.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Jones MC, Caswell PT, Norman JC. Endocytic recycling pathways: emerging regulators of cell migration. Curr Opin Cell Biol. 2006;18:549–557. doi: 10.1016/j.ceb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Jovic M, Naslavsky N, Rapaport D, Horowitz M, Caplan S. EHD1 regulates beta1 integrin endosomal transport: effects on focal adhesions, cell spreading and migration. J Cell Sci. 2007;120:802–814. doi: 10.1242/jcs.03383. [DOI] [PubMed] [Google Scholar]
- Junutula JR, Schonteich E, Wilson GM, Peden AA, Scheller RH, Prekeris R. Molecular characterization of Rab11 interactions with members of the family of Rab11-interacting proteins. J Biol Chem. 2004;279:33430–33437. doi: 10.1074/jbc.M404633200. [DOI] [PubMed] [Google Scholar]
- Lapierre LA, Kumar R, Hales CM, Navarre J, Bhartur SG, Burnette JO, Provance DW, Mercer JA, Bahler M, Goldenring JR. Myosin vb is associated with plasma membrane recycling systems. Mol Biol Cell. 2001;12:1843–1857. doi: 10.1091/mbc.12.6.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay AJ, McCaffrey MW. The C2 domains of the class I Rab11 family of interacting proteins target recycling vesicles to the plasma membrane. J Cell Sci. 2004;117:4365–4375. doi: 10.1242/jcs.01280. [DOI] [PubMed] [Google Scholar]
- Lindsay AJ, Hendrick AJ, Cantalupo G, Senic-Matuglia F, Goud B, Bucci C, McCaffrey MW. Rab coupling protein (RCP), a novel Rab4 and Rab11 effector protein. J Biol Chem. 2002;277:12190–12199. doi: 10.1074/jbc.M108665200. [DOI] [PubMed] [Google Scholar]
- Lock JG, Stow JL. Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Mol Biol Cell. 2005;16:1744–55. doi: 10.1091/mbc.E04-10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock JG, Hammond LA, Houghton F, Gleeson PA, Stow JL. E-cadherin transport from the trans-Golgi network in tubulovesicular carriers is selectively regulated by golgin-97. Traffic. 2005;6:1142–1156. doi: 10.1111/j.1600-0854.2005.00349.x. [DOI] [PubMed] [Google Scholar]
- Low SH, Li X, Miura M, Kudo N, Quinones B, Weimbs T. Syntaxin 2 and endobrevin are required for the terminal step of cytokinesis in mammalian cells. Dev Cell. 2003;4:753–759. doi: 10.1016/s1534-5807(03)00122-9. [DOI] [PubMed] [Google Scholar]
- Meyers JM, Prekeris R. Formation of mutually exclusive Rab11 complexes with members of the family of Rab11-interacting proteins regulates Rab11 endocytic targeting and function. J Biol Chem. 2002;277:49003–49010. doi: 10.1074/jbc.M205728200. [DOI] [PubMed] [Google Scholar]
- Mostov K, Su T, ter Beest M. Polarized epithelial membrane traffic: conservation and plasticity. Nat Cell Biol. 2003;5:287–293. doi: 10.1038/ncb0403-287. [DOI] [PubMed] [Google Scholar]
- Murthy M, Garza D, Scheller RH, Schwarz TL. Mutations in the exocyst component Sec5 disrupt neuronal membrane traffic, but neurotransmitter release persists. Neuron. 2003;37:433–447. doi: 10.1016/s0896-6273(03)00031-x. [DOI] [PubMed] [Google Scholar]
- Nabi IR. The polarization of the motile cell. J Cell Sci. 1999;112 (Pt 12):1803–1811. doi: 10.1242/jcs.112.12.1803. [DOI] [PubMed] [Google Scholar]
- Naslavsky N, Rahajeng J, Chenavas S, Sorgen PL, Caplan S. EHD1 and Eps15 interact with phosphatidylinositols via their Eps15 homology domains. J Biol Chem. 2007;282:16612–16622. doi: 10.1074/jbc.M609493200. [DOI] [PubMed] [Google Scholar]
- Naslavsky N, Rahajeng J, Sharma M, Jovic M, Caplan S. Interactions between EHD proteins and Rab11-FIP2: a role for EHD3 in early endosomal transport. Mol Biol Cell. 2006;17:163–177. doi: 10.1091/mbc.E05-05-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedvetsky PI, Stefan E, Frische S, Santamaria K, Wiesner B, Valenti G, Hammer JA, Nielsen S, Goldenring JR, Rosenthal W, Klussmann E. A Role of Myosin Vb and Rab11-FIP2 in the Aquaporin-2 Shuttle. Traffic. 2007;8:110–123. doi: 10.1111/j.1600-0854.2006.00508.x. [DOI] [PubMed] [Google Scholar]
- Nelson WJ. Adaptation of core mechanisms to generate cell polarity. Nature. 2003;422:766–774. doi: 10.1038/nature01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Halloran TJ. Membrane traffic and cytokinesis. Traffic. 2000;1:921–826. [PubMed] [Google Scholar]
- Peden AA, Schonteich E, Chun J, Junutula JR, Scheller RH, Prekeris R. The RCP-Rab11 complex regulates endocytic protein sorting. Mol Biol Cell. 2004;15:3530–3541. doi: 10.1091/mbc.E03-12-0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelissier A, Chauvin JP, Lecuit T. Trafficking through Rab11 endosomes is required for cellularization during Drosophila embryogenesis. Curr Biol. 2003;13:1848–1857. doi: 10.1016/j.cub.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Pohl U, Smith JS, Tachibana I, Ueki K, Lee HK, Ramaswamy S, Wu Q, Mohrenweiser HW, Jenkins RB, Louis DN. EHD2, EHD3, and EHD4 encode novel members of a highly conserved family of EH domain-containing proteins. Genomics. 2000;63:255–262. doi: 10.1006/geno.1999.6087. [DOI] [PubMed] [Google Scholar]
- Potter BA, Weixel KM, Bruns JR, Ihrke G, Weisz OA. N-glycans mediate apical recycling of the sialomucin endolyn in polarized MDCK cells. Traffic. 2006;7:146–154. doi: 10.1111/j.1600-0854.2005.00371.x. [DOI] [PubMed] [Google Scholar]
- Powelka AM, Sun J, Li J, Gao M, Shaw LM, Sonnenberg A, Hsu VW. Stimulation-dependent recycling of integrin beta1 regulated by ARF6 and Rab11. Traffic. 2004;5:20–36. doi: 10.1111/j.1600-0854.2004.00150.x. [DOI] [PubMed] [Google Scholar]
- Prekeris R, Gould GW. Breaking up is hard to do -membrane traffic in cytokinesis. J Cell Sci. 2008;121:1569–1576. doi: 10.1242/jcs.018770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prekeris R, Davies JM, Scheller RH. Identification of a novel Rab11/25 binding domain present in Eferin and Rip proteins. J Biol Chem. 2001;276:38966–38970. doi: 10.1074/jbc.M106133200. [DOI] [PubMed] [Google Scholar]
- Prekeris R, Klumperman J, Scheller RH. A Rab11/Rip11 protein complex regulates apical membrane trafficking via recycling endosomes. Mol Cell. 2000;6:1437–1448. doi: 10.1016/s1097-2765(00)00140-4. [DOI] [PubMed] [Google Scholar]
- Prigent M, Dubois T, Raposo G, Derrien V, Tenza D, Rosse C, Camonis J, Chavrier P. ARF6 controls post-endocytic recycling through its downstream exocyst complex effector. J Cell Biol. 2003;163:1111–1121. doi: 10.1083/jcb.200305029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pypaert M, Mundy D, Souter E, Labbe JC, Warren G. Mitotic cytosol inhibits invagination of coated pits in broken mitotic cells. J Cell Biol. 1991;114:1159–1166. doi: 10.1083/jcb.114.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport D, Auerbach W, Naslavsky N, Pasmanik-Chor M, Galperin E, Fein A, Caplan S, Joyner AL, Horowitz M. Recycling to the plasma membrane is delayed in EHD1 knockout mice. Traffic. 2006;7:52–60. doi: 10.1111/j.1600-0854.2005.00359.x. [DOI] [PubMed] [Google Scholar]
- Raucher D, Sheetz MP. Membrane expansion increases endocytosis rate during mitosis. J Cell Biol. 1999;144:497–506. doi: 10.1083/jcb.144.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs B, Fasulo B, Royou A, Mische S, Cao J, Hays TS, Sullivan W. The concentration of Nuf, a Rab11 effector, at the microtubule-organizing center is cell cycle regulated, dynein-dependent, and coincides with furrow formation. Mol Biol Cell. 2007;18:3313–3322. doi: 10.1091/mbc.E07-02-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M, Barry S, Woods A, van der Sluijs P, Norman J. PDGF-regulated rab4-dependent recycling of alphavbeta3 integrin from early endosomes is necessary for cell adhesion and spreading. Curr Biol. 2001;11:1392–1402. doi: 10.1016/s0960-9822(01)00442-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Musch A, Le Bivic A. Epithelial trafficking: new routes to familiar places. Curr Opin Cell Biol. 2004;16:436–442. doi: 10.1016/j.ceb.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Scholey JM, Brust-Mascher I, Mogilner A. Cell division. Nature. 2003;422:746–52. doi: 10.1038/nature01599. [DOI] [PubMed] [Google Scholar]
- Schonteich E, Pilli M, Simon GC, Matern HT, Junutula JR, Sentz D, Holmes RK, Prekeris R. Molecular characterization of Rab11-FIP3 binding to ARF GTPases. Eur J Cell Biol. 2007;86:417–431. doi: 10.1016/j.ejcb.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Schonteich E, Wilson GM, Burden J, Hopkins CR, Anderson K, Goldenring JR, Prekeris R. The Rip11/Rab11-FIP5 and kinesin II complex regulates endocytic protein recycling. J Cell Sci. 2008;121:3824–3833. doi: 10.1242/jcs.032441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba T, Koga H, Shin HW, Kawasaki M, Kato R, Nakayama K, Wakatsuki S. Structural basis for Rab11-dependent membrane recruitment of a family of Rab11-interacting protein 3 (FIP3)/Arfophilin-1. Proc Natl Acad Sci USA. 2006;103:15416–15421. doi: 10.1073/pnas.0605357103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon GC, Schonteich E, Wu CC, Piekny A, Ekiert D, Yu X, Gould GW, Glotzer M, Prekeris R. Sequential Cyk-4 binding to ECT2 and FIP3 regulates cleavage furrow ingression and abscission during cytokinesis. EMBO J. 2008;27:1791–1803. doi: 10.1038/emboj.2008.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skop AR, Bergmann D, Mohler WA, White JG. Completion of cytokinesis in C. elegans requires a brefeldin A-sensitive membrane accumulation at the cleavage furrow apex. Curr Biol. 2001;11:735–46. doi: 10.1016/s0960-9822(01)00231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock A, Steinmetz MO, Janmey PA, Aebi U, Gerisch G, Kammerer RA, Weber I, Faix J. Domain analysis of cortexillin I: actin-bundling, PIP(2)-binding and the rescue of cytokinesis. EMBO J. 1999;18:5274–5284. doi: 10.1093/emboj/18.19.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarbutton E, Peden AA, Junutula JR, Prekeris R. Class I FIPs, Rab11-binding proteins that regulate endocytic sorting and recycling. Methods Enzymol. 2005;403:512–525. doi: 10.1016/S0076-6879(05)03045-4. [DOI] [PubMed] [Google Scholar]
- Uzan-Gafsou S, Bausinger H, Proamer F, Monier S, Lipsker D, Cazenave JP, Goud B, de la Salle H, Hanau D, Salamero J. Rab11A controls the biogenesis of Birbeck granules by regulating Langerin recycling and stability. Mol Biol Cell. 2007;18:3169–3179. doi: 10.1091/mbc.E06-09-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Buyon JP, Zhu W, Chan EK. Defining a novel 75-kDa phosphoprotein associated with SS-A/Ro and identification of distinct human autoantibodies. J Clin Invest. 1999;104:1265–1275. doi: 10.1172/JCI8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Edwards JG, Riley N, Provance DW, Karcher R, Li XD, Davison IG, Ikebe M, Mercer JA, Kauer JA, Ehlers MD. Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell. 2008;135:535–548. doi: 10.1016/j.cell.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber I, Gerisch G, Heizer C, Murphy J, Badelt K, Stock A, Schwartz JM, Faix J. Cytokinesis mediated through the recruitment of cortexillins into the cleavage furrow. EMBO J. 1999;18:586–594. doi: 10.1093/emboj/18.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh GI, Leney SE, Lloyd-Lewis B, Wherlock M, Lindsay AJ, McCaffrey MW, Tavare JM. Rip11 is a Rab11- and AS160-RabGAP-binding protein required for insulin-stimulated glucose uptake in adipocytes. J Cell Sci. 2007;120:4197–4208. doi: 10.1242/jcs.007310. [DOI] [PubMed] [Google Scholar]
- Wilson GM, Fielding AB, Simon GC, Yu X, Andrews PD, Peden AA, Gould GW, Prekeris R. The FIP3-Rab11 protein complex regulates recycling endosome targeting to the cleavage furrow during late cytokinesis. Mol Biol Cell. 2004;16:849–860. doi: 10.1091/mbc.E04-10-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Mehta SQ, Pichaud F, Bellen HJ, Quiocho FA. Sec15 interacts with Rab11 via a novel domain and affects Rab11 localization in vivo. Nat Struct Mol Biol. 2005;12:879–885. doi: 10.1038/nsmb987. [DOI] [PubMed] [Google Scholar]
- Yeaman C, Grindstaff KK, Nelson WJ. Mechanism of recruiting Sec6/8 (exocyst) complex to the apical junctional complex during polarization of epithelial cells. J Cell Sci. 2004;117:559–570. doi: 10.1242/jcs.00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SO, Shin S, Mercurio AM. Hypoxia stimulates carcinoma invasion by stabilizing microtubules and promoting the Rab11 trafficking of the alpha6beta4 integrin. Cancer Res. 2005;65:2761–2769. doi: 10.1158/0008-5472.CAN-04-4122. [DOI] [PubMed] [Google Scholar]
- Yuce O, Piekny A, Glotzer M. An ECT2-centralspindlin complex regulates the localization and function of RhoA. J Cell Biol. 2005;170:571–582. doi: 10.1083/jcb.200501097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XM, Ellis S, Sriratana A, Mitchell CA, Rowe T. Sec15 is an effector for the Rab11 GTPase in mammalian cells. J Biol Chem. 2004;279:43027–43034. doi: 10.1074/jbc.M402264200. [DOI] [PubMed] [Google Scholar]