Abstract
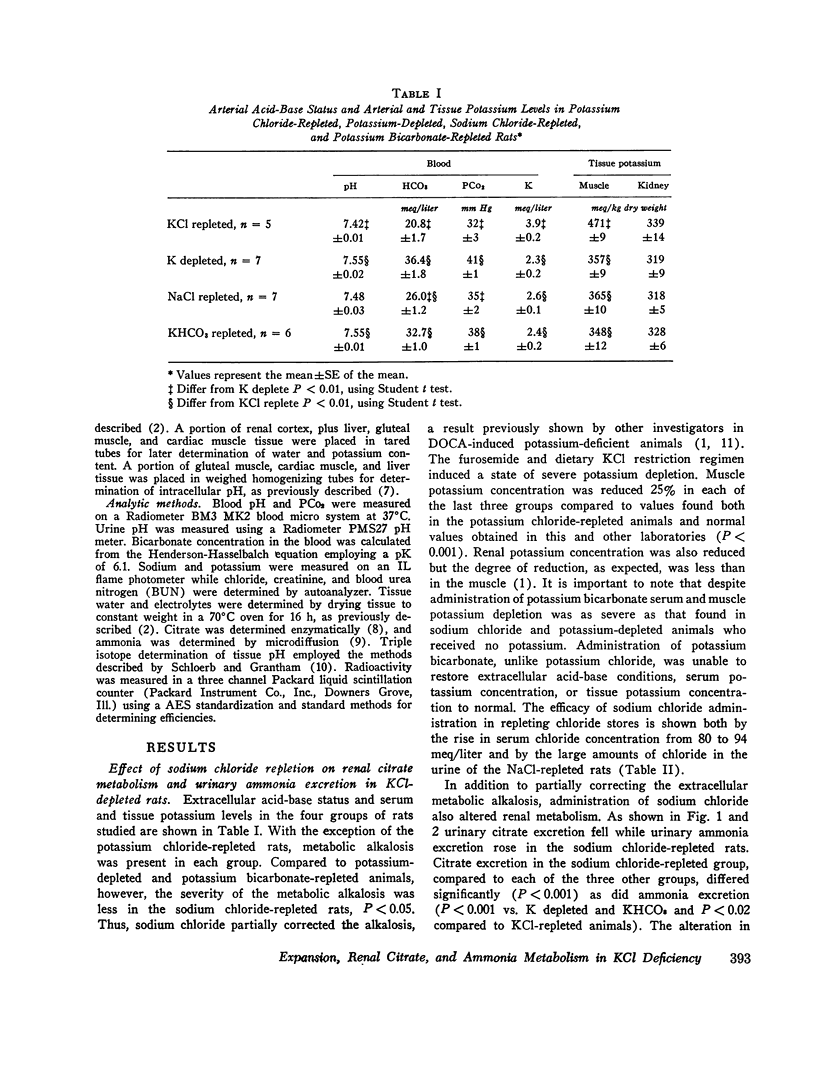
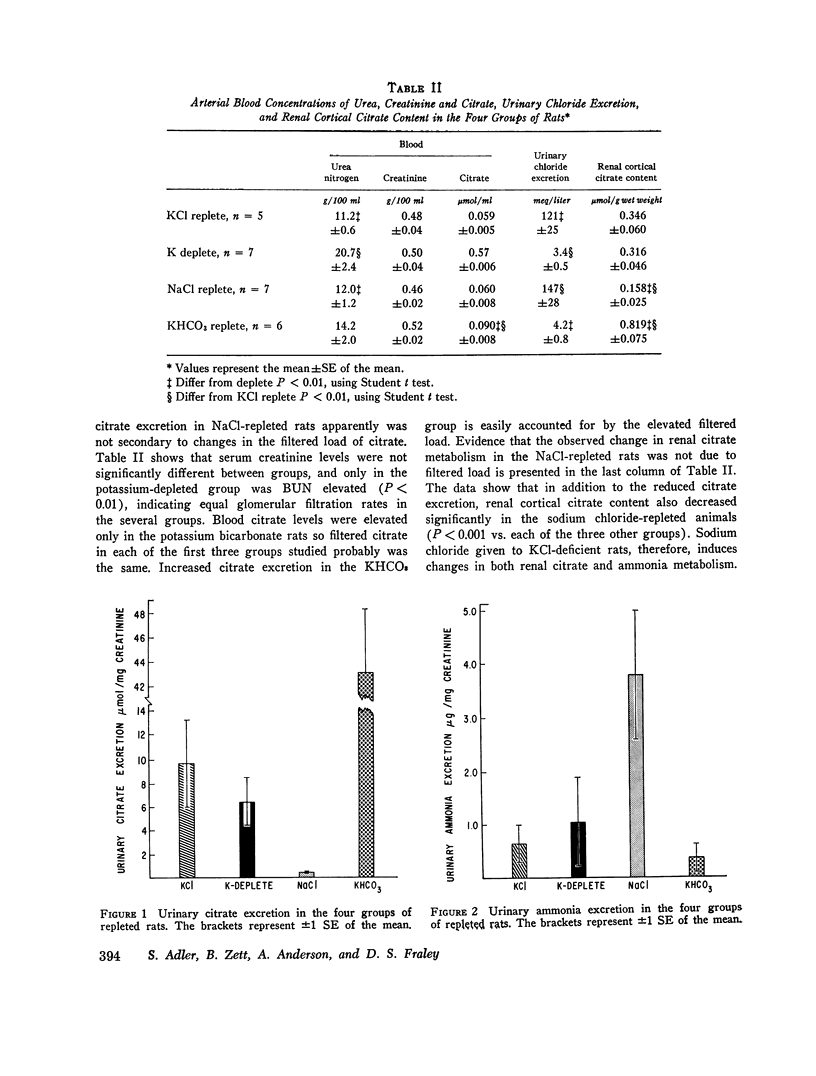
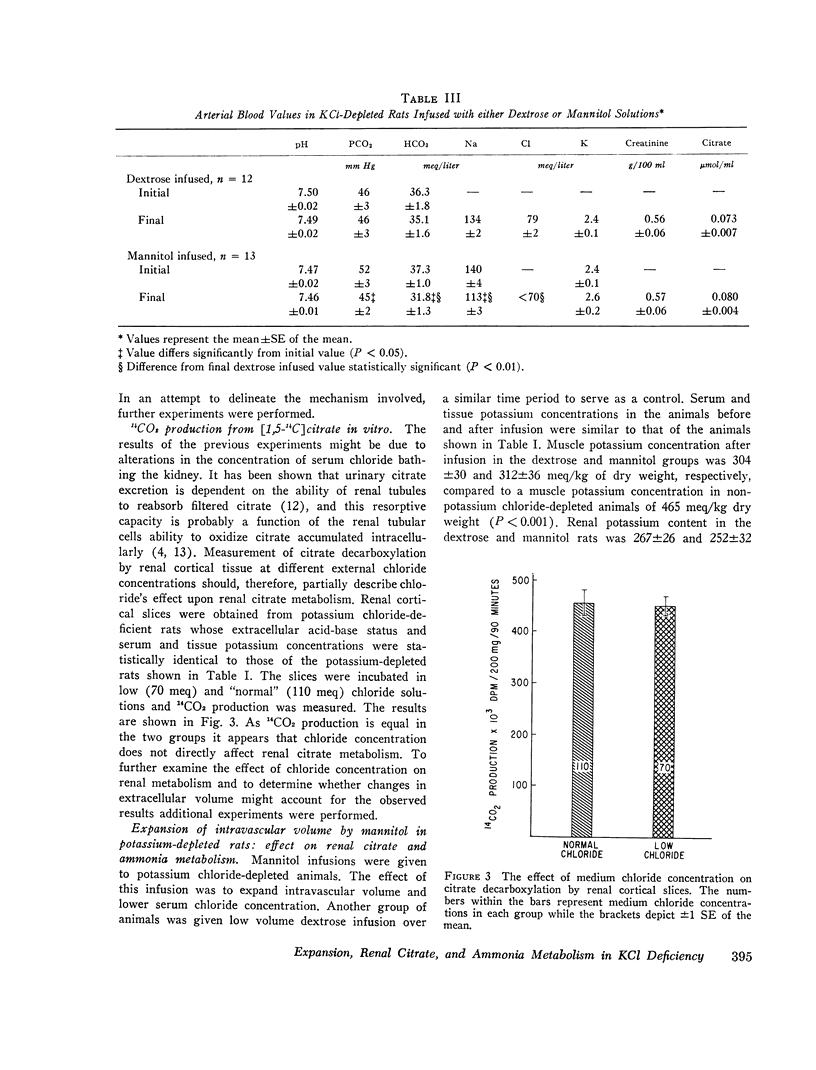
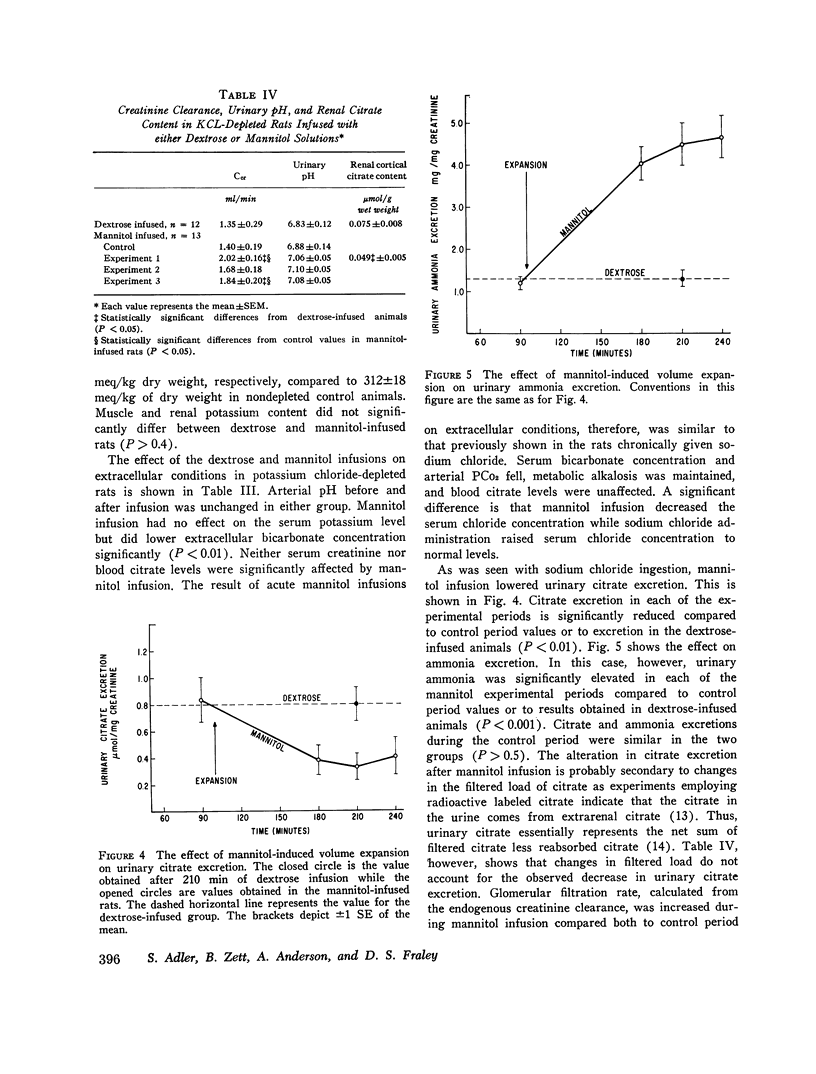
When rats with desoxycorticosterone acetate (DOCA)-induced potassium chloride deficiency are given sodium chloride there is simultaneously a partial correction of metabolic alkalosis and a marked reduction in urinary citrate excretion and renal citrate content. To examine DOCA's role in this phenomenon and to determine how sodium chloride alters renal metabolism, rats were made KC1 deficient using furosemide and a KC1-deficient diet. Renal citrate and ammonia metabolism were then studied after chronic oral sodium chloride administration or acute volume expansion with isotonic mannitol. Although both maneuvers partially corrected metabolic alkalosis, sodium chloride raised serum chloride concentration while mannitol significantly decreased it. Urinary citrate excretion decreased to 10% of control in rats given NaCl and to 50% of control in rats infused with mannitol. The filtered load of citrate was constant or increased indicating increased tubular citrate reabsorption. Renal cortical citrate content also decreased approximately 50%. Renal cortical slices from KCl-deficient rats incubated in low or normal chloride media produced equal amounts of 14CO2 from (1, 5-14C) citrate. In addition, urinary ammonia excretion increased by over 300% in both groups. This occurred in the mannitol group despite increased urinary pH and flow rate indicating a rise in renal ammonia production. It seems that neither DOCA nor an increase in serum chloride concentration explains the experimental results. Rather, it appears that volume expansion is responsible for increased renal tubular citrate reabsorption and renal ammonia production. As these renal metabolic responses ordinarily occur in response to acidosis, the data are consistent with the hypothesis that volume expansion reduces renal cell pH in 3KCl-deficient rats.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER S., ROY A., RELMAN A. S. INTRACELLULAR ACID-BASE REGULATION. I. THE RESPONSE OF MUSCLE CELLS TO CHANGES IN CO2 TENSION OR EXTRACELLULAR BICARBONATE CONCENTRATION. J Clin Invest. 1965 Jan;44:8–20. doi: 10.1172/JCI105129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADLER S., ROY A., RELMAN A. S. INTRACELLULAR ACID-BASE REGULATION. II. THE INTERACTION BETWEEN CO-2 TENSION AND EXTRACELLULAR BICARBONATE IN THE DETERMINATION OF MUSCLE CELL PH. J Clin Invest. 1965 Jan;44:21–30. doi: 10.1172/JCI105123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATKINS E. L., SCHWARTZ W. B. Factors governing correction of the alkalosis associated with potassium deficiency; the critical role of chloride in the recovery process. J Clin Invest. 1962 Feb;41:218–229. doi: 10.1172/JCI104473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler S., Anderson B., Zemotel L. Metabolic acid-base effects on tissue citrate content and metabolism in the rat. Am J Physiol. 1971 Apr;220(4):986–992. doi: 10.1152/ajplegacy.1971.220.4.986. [DOI] [PubMed] [Google Scholar]
- Adler S., Anderson B., Zett B. Regulation of citrate metabolism by cell pH in potassium-depleted rat diaphragm. Kidney Int. 1974 Aug;6(2):92–98. doi: 10.1038/ki.1974.84. [DOI] [PubMed] [Google Scholar]
- Adler S. The role of pH, PCO2, and bicarbonate in regulating rat diaphragm citrate content. J Clin Invest. 1970 Sep;49(9):1647–1655. doi: 10.1172/JCI106382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler S., Zett B., Anderson B. Renal citrate in the potassium-deficient rat: role of potassium and chloride ions. J Lab Clin Med. 1974 Sep;84(3):307–316. [PubMed] [Google Scholar]
- Adler S., Zett B., Anderson B. The effect of acute potassium depletion on muscle cell pH in vitro. Kidney Int. 1972 Sep;2(3):159–163. doi: 10.1038/ki.1972.86. [DOI] [PubMed] [Google Scholar]
- COOKE R. E., SEGAR W. E., CHEEK D. B., COVILLE F. E., DARROW D. C. The extrarenal correction of alkalosis associated with potassium deficiency. J Clin Invest. 1952 Aug;31(8):798–805. doi: 10.1172/JCI102665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway E. J., O'malley E. Microdiffusion methods. Ammonia and urea using buffered absorbents (revised methods for ranges greater than 10mug. N). Biochem J. 1942 Sep;36(7-9):655–661. doi: 10.1042/bj0360655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DENIS G., PREUSS H., PITTS R. THE PNH3 OF RENAL TUBULAR CELLS. J Clin Invest. 1964 Apr;43:571–582. doi: 10.1172/JCI104942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EARLEY L. E., FRIEDLER R. M. CHANGES IN RENAL BLOOD FLOW AND POSSIBLY THE INTRARENAL DISTRIBUTION OF BLOOD DURING THE NATRIURESIS ACCOMPANYING SALINE LOADING IN THE DOG. J Clin Invest. 1965 Jun;44:929–941. doi: 10.1172/JCI105210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON E. E. The metabolism of citrate-C-14 in normal and in fluoroinhibitor-poisoned rats. J Clin Invest. 1961 Sep;40:1719–1726. doi: 10.1172/JCI104394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROLLMAN A. P., HARRISON H. C., HARRISON H. E. The renal excretion of citrate. J Clin Invest. 1961 Jul;40:1290–1296. doi: 10.1172/JCI104358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman A. D., Fuisz R. E., Cahill G. F., Jr Renal gluconeogenesis in acidosis, alkalosis, and potassium deficiency: its possible role in regulation of renal ammonia production. J Clin Invest. 1966 Apr;45(4):612–619. doi: 10.1172/JCI105375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUDSON J. B., RELMAN A. S. Effects of potassium and rubidium on muscle cell bicarbonate. Am J Physiol. 1962 Jul;203:209–214. doi: 10.1152/ajplegacy.1962.203.1.209. [DOI] [PubMed] [Google Scholar]
- HUTH E. J., SQUIRES R. D., ELKINTON J. R. Experimental potassium depletion in normal human subjects. II. Renal and hormonal factors in the development of extracellular alkalosis during depletion. J Clin Invest. 1959 Jul;38(7):1149–1165. doi: 10.1172/JCI103891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZMAN R., VILLEE C. A., BEECHER H. K. Effect of increased carbon dioxide concentrations on fixed acid production in vitro. Am J Physiol. 1953 Feb;172(2):317–323. doi: 10.1152/ajplegacy.1953.172.2.317. [DOI] [PubMed] [Google Scholar]
- Kamm D. E., Fuisz R. E., Goodman A. D., Cahill G. F., Jr Acid-base alterations and renal gluconeogenesis: effect of pH, bicarbonate concentration, and PCO2. J Clin Invest. 1967 Jul;46(7):1172–1177. doi: 10.1172/JCI105610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LONGMORE W. J., HASTINGS A. B., MAHOWALD T. A. EFFECT OF ENVIRONMENTAL CO2 AND PH ON GLYCEROL METABOLISM BY RAT LIVER IN VITRO. J Biol Chem. 1964 Jun;239:1700–1704. [PubMed] [Google Scholar]
- Lowance D. C., Garfinkel H. B., Mattern W. D., Schwartz W. B. The effect of chronic hypotonic volume expansion on the renal regulation of acid-base equilibrium. J Clin Invest. 1972 Nov;51(11):2928–2940. doi: 10.1172/JCI107117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke R. G., Levitin H. Impaired renal conservation of chloride and the acid-base changes associated with potassium depletion in the rat. Clin Sci. 1967 Jun;32(3):511–526. [PubMed] [Google Scholar]
- MILNE M. D., SCRIBNER B. H., CRAWFORD M. A. Non-ionic diffusion and the excretion of weak acids and bases. Am J Med. 1958 May;24(5):709–729. doi: 10.1016/0002-9343(58)90376-0. [DOI] [PubMed] [Google Scholar]
- Moellering H., Gruber W. Determination of citrate with citrate lyase. Anal Biochem. 1966 Dec;17(3):369–376. doi: 10.1016/0003-2697(66)90172-2. [DOI] [PubMed] [Google Scholar]
- ORLOFF J., BERLINER R. W. The mechanism of the excretion of ammonia in the dog. J Clin Invest. 1956 Feb;35(2):223–235. doi: 10.1172/JCI103267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purkerson M. L., Lubowitz H., White R. W., Bricker N. S. On the influence of extracellular fluid volume expansion on bicarbonate reabsorption in the rat. J Clin Invest. 1969 Sep;48(9):1754–1760. doi: 10.1172/JCI106141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLOERB P. R., GRANTHAM J. J. INTRACELLULAR PH MEASUREMENT WITH TRITIATED WATER, CARBON-14 LABELED 5,5-DIMETHYL-2, 4-OXAZOLIDINEDIONE, AND CHLORIDE-36. J Lab Clin Med. 1965 Apr;65:669–676. [PubMed] [Google Scholar]
- SIMPSON D. P. INFLUENCE OF PLASMA BICARBONATE CONCENTRATION AND PH ON CITRATE EXCRETION. Am J Physiol. 1964 Apr;206:875–882. doi: 10.1152/ajplegacy.1964.206.4.875. [DOI] [PubMed] [Google Scholar]
- Simpson D. P., Angielski S. Regulation by bicarbonate ion of intramitochondrial citrate concentration in kidney mitochondria. Biochim Biophys Acta. 1973 Feb 27;298(1):115–123. doi: 10.1016/0005-2736(73)90016-3. [DOI] [PubMed] [Google Scholar]
- Simpson D. P. Regulation of renal citrate metabolism by bicarbonate ion and pH: observations in tissue slices and mitochondria. J Clin Invest. 1967 Feb;46(2):225–238. doi: 10.1172/JCI105525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson D. P., Sherrard D. J. Regulation of glutamine metabolism in vitro by bicarbonate ion and pH. J Clin Invest. 1969 Jun;48(6):1088–1096. doi: 10.1172/JCI106065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struyvenberg A., Morrison R. B., Relman A. S. Acid-base behavior of separated canine renal tubule cells. Am J Physiol. 1968 May;214(5):1155–1162. doi: 10.1152/ajplegacy.1968.214.5.1155. [DOI] [PubMed] [Google Scholar]
- WADDELL W. J., BUTLER T. C. Calculation of intracellular pH from the distribution of 5,5-dimethyl-2,4-oxazolidinedione (DMO); application to skeletal muscle of the dog. J Clin Invest. 1959 May;38(5):720–729. doi: 10.1172/JCI103852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell W. J., Bates R. G. Intracellular pH. Physiol Rev. 1969 Apr;49(2):285–329. doi: 10.1152/physrev.1969.49.2.285. [DOI] [PubMed] [Google Scholar]



