Abstract
Recently, recycling endosomes have emerged as a key components required for the successful completion of cytokinesis. Furthermore, FIP3 (family of Rab11-interacting protein 3), a Rab11 GTPase-binding protein, has been implicated in targeting the recycling endosomes to the midbody of dividing cells. Previously, we have shown that FIP3/Rab11-containing endosomes associate with centrosomes until anaphase, at which time they translocate to the cleavage furrow. At telophase, FIP3/Rab11-containing endosomes move from the furrow into the midbody, and this step is required for abscission. While several other proteins were implicated in regulating FIP3 targeting to the cleavage furrow, the mechanisms regulating the dynamics of FIP3-containing endosomes during mitosis have not been defined. To identify the factors regulating FIP3 targeting to the furrow, we used a combination of siRNA (small interfering RNA) screens and proteomic analysis to identify Cyk-4/MgcRacGAP (GTPase-activating protein) and kinesin I as FIP3-binding proteins. Furthermore, kinesin I mediates the transport of FIP3-containing endosomes to the cleavage furrow. Once in the furrow, FIP3 binds to Cyk-4 as part of centralspindlin complex and accumulates at the midbody. Finally, we demonstrated that ECT2 regulates FIP3 association with the centralspindlin complex. Thus we propose that kinesin I, in concert with centralspindlin complex, plays a role in temporal and spatial regulation of endosome transport to the cleavage furrow during cytokinesis.
Keywords: cytokinesis, exocyst, FIP3, kinesin I, membrane traffic, recycling endosome
Review
Regulation of cytokinesis involves many different cellular pathways. In animal cells at least two distinct processes play essential roles during cytokinesis: formation and constriction of an actomyosin contractile ring and the delivery of new membranes during the ingression and abscission steps of cytokinesis [1,2]. While we are only beginning to understand the role of membrane traffic during cytokinesis, it is evident that membrane delivery to the cleavage furrow is critical for cell division. The requirement of membrane transport to the site of division has been demonstrated in many different organisms including Xenopus laevis, Caenorhabditis elegans, as well as in Drosophila melanogaster syncitial embryos [2-5]. Membrane transport during mitosis plays several roles. First, membrane delivery to the cleavage furrow promotes localized enrichment of distinct proteins and lipids. Indeed, it was reported that the cleavage furrow is enriched in phosphatidylinositol 4,5-bisphosphate and gangliosides [6,7]. Furthermore, dynamic changes in asymmetric distribution of phosphatidylethanolamine at the furrow were also shown to be required for successful completion of cytokinesis [8]. Secondly, the division of one cell into two requires the delivery of new membrane, simply to provide the necessary surface area required for furrow ingression. Finally, the delivery and co-ordinated fusion of transport vesicles mediates abscission, the terminal step of cytokinesis that results in separation of the daughter cells.
Previous studies have identified recycling endosomes as organelles that are targeted to the cleavage furrow to provide extra membrane for cytokinesis [9]. A number of reports have demonstrated the pronounced changes in endocytic recycling during mitosis [10,11]. For example, during metaphase and anaphase, protein and membrane recycling back to the PM (plasma membrane) is dramatically inhibited, resulting in the accumulation of endocytic organelles as well as a decrease in PM surface area [12]. In contrast, the initiation of furrow formation and ingression during telophase stimulates a rapid increase in membrane and protein recycling back to the PM, especially in the area of the cleavage furrow [13]. Interestingly, these dynamic changes in the rates of endocytic recycling and in the surface area of the PM seem to be required for the successful completion of cytokinesis [13]. Consistent with this, the inhibition of specific endocytic recycling-regulating proteins, such as dynamin, α-adaptin, syntaxin 1, syntaxin 2 and VAMP8 (vesicle-associated membrane protein 8) result in cytokinesis block [4,14-16].
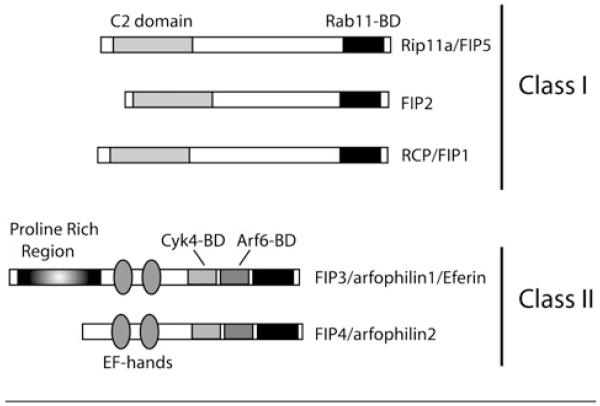
Since significant data supports that targeting of recycling endosomes to the cleavage furrow is required for cytokinesis, much effort has been invested in identifying the machinery that temporally and spatially regulates endocytic traffic during cell division. Previous studies have shown that endosomes containing Rab11, a small monomeric GTPase known to regulate recycling endosomes, accumulate around centrosomes during metaphase and anaphase and move to the furrow and the midbody at late telophase [9,17]. The inhibition of Rab11, which results in a block of cytokinesis in mammalian, C. elegans and D. melanogaster cells, produces a defect that appears to result from the inability of recycling endosomes to be targeted to the cleavage furrow [4,18]. Thus it was proposed that Rab11 GTPase regulates transport and targeting of recycling endosomes to the cleavage furrow [9,18]. As with all GTPases, Rab11 functions by cycling between GTP- and GDP-bound forms. GTP-bound (active) Rab11 then recruits various effector proteins to the endocytic membranes [17]. Several years ago, we and others identified novel FIPs (family of Rab11-interacting proteins), which all share a highly conserved, 20-amino-acid motif at the C-terminus of protein, known as a RBD (Rab11-binding domain) (Figure 1, [19-22]). Rab11-FIP3 (Rab11-binding FIP3, hereafter FIP3) belongs to the class II FIPs and was previously shown to be required for the abscission step of cytokinesis [17,23]. Consistent with this finding, the FIP3 homologue in D. melanogaster, known as Nuf (nuclear fallout), was also shown to regulate membrane addition to the cleavage furrow during cellularization. Syncytial D. melanogaster embryos, deficient in Nuf, failed to add sufficient membrane to the actively ingressing furrows to complete cellularization [5]. In addition to Rab11, FIP3 also binds to Arf6, another GTPase that is targeted to the cleavage furrow and is implicated in regulating cytokinesis [9]. Although the role of Arf6 in cell division remains unclear, it was suggested that Arf6 may regulate endosome targeting to the furrow [24,25]. Collectively, these and other studies provide compelling support for the role of FIP3 in regulating the targeting of recycling endosomes to the cleavage furrow.
Figure 1. Schematic representation of the FIP protein family.
Class I FIP proteins include FIP1, 2 and 5, whereas class II FIP proteins include FIP3 and 4. Both classes contain the highly conserved 20-amino-acid Rab11-binding domain. BD, binding domain.
Since the transport of recycling endosomes to the cleavage furrow is a tightly controlled event, it is expected that endosomal transport during mitosis is regulated by multiple transport and tethering proteins [9,17,24]. Our previous studies suggested that midzone microtubules are involved in transport of Rab11/FIP3-containing endosomes to the cleavage furrow [17,24]. To support these results, an siRNA (small interfering RNA) screen of kinesin molecular motors have identified kinesin I as the microtubule motor that mediates transport of recycling endosomes during mitosis (M. Pawlus and R. Prekeris, unpublished work). Consistent with this, we have shown that kinesin I co-imunoprecipitates with Rab11/FIP3 protein complex. Depletion of kinesin I blocks the delivery of FIP3 to the furrow, and as consequence results in the inhibition of cytokinesis (M. Pawlus and R. Prekeris, unpublished work).
While kinesin I mediates the transport of Rab11/FIP3-containing endosomes along midzone microtubules, other factors are expected to tether the endosomes to the midbody. Several reports have shown that recycling endosomes accumulate at the midbody at late telophase, the step that appears to be required for abscission [17,24]. Recently Cyk-4/MgcRacGAP (GTPase-activating protein) was identified as a putative FIP3-interacting protein (G.C. Simon and R. Prekeris, unpublished work). Cyk-4 is a member of a centralspindlin protein complex that accumulates at the midzone during cytokinesis and regulates the formation and initiation of actomyosin ring contraction [26-28]. Centralspindlin works by recruiting the RhoA GEF (guanine-nucleotide-exchange factor) ECT2 to the midzone at early anaphase. Recruitment of ECT2 to the midzone in early anaphase was shown to activate RhoA GTPase, thus regulating actomyosin ring formation and contraction [29-31]. At late telophase, ECT2 is removed from the centralspindlin complex and sequestered in the nucleus, the step that is required for the disassembly of the actomyosin ring and the final separation of daughter cells [32]. While the exact mechanism of ECT2 re-localization at late telophase is not fully understood, it seems to depend, at least in part, on reformation of the nucleus [32]. Consistent with this, previous work has shown that mutation of nuclear localization signal in ECT2 results in accumulation of ECT2 at the midbody during late telophase and consequently inhibiton of abscission [32]. This provides an important regulatory mechanism for complete nuclear segregation before the separation of daughter cells. In addition, we have shown that ECT2 and FIP3 compete for interaction with Cyk-4 (G.C. Simon and R. Prekeris, unpublished work). Thus we propose that the removal of ECT2 from the centralspindlin complex at late telophase is required for the recruitment of FIP3/Rab11-containing endosomes to the midbody. As a result, the sequential interaction of centralspindlin with ECT2 and FIP3 may serve as means of temporal regulation of actomyosin ring contraction and endosome-dependent abscission steps during cytokinesis.
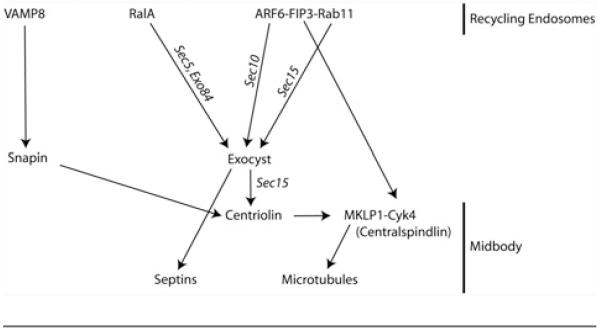
In summary, our data show that in addition to regulating actomyosin ring contraction, centralspindlin also appears to regulate the tethering of recycling endosomes to the midbody at late telophase. In addition to centralspindlin, other proteins are also implicated in regulating targeting of recycling endosomes to the cleavage furrow. Results from several labs have shown that the exocyst complex is required for the targeting of post-Golgi secretory vesicles as well as endosomes to the furrow [24,33]. The exocyst is a multiprotein complex that consists of Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70 and Exo84 subunits [34]. Interestingly, Sec15 binds to Rab11, while Sec10 was shown to interact with Arf6 [24]. Since Rab11 and Arf6 also bind to FIP3, it is tempting to speculate that the exocyst complex may also regulate FIP3 targeting to the furrow (Figure 2). Consistent with this hypothesis, the exocyst complex was shown to co-imunoprecipitate with FIP3 and depletion of the exocyst complex subunits by siRNA inhibits the abscission step of cytokinesis [24]. In addition to FIP3, several other proteins were also shown to regulate endocytic recruitment to the furrow. RalA, another small monomeric GTPase, has been implicated in endosomal targeting by binding to Sec5 and Exo84 subunits of exocyst complex [35,36]. Furthermore, the endocytic SNARE (soluble N-ethylmaleimide-sensitive fusion protein-attachment protein receptor) VAMP8 was shown to bind to snapin and the centriolin complex, which are also localized to the midbody at late telophase (Figure 2) [16]. What is the function of the multiple endocytic-tethering factors? While the contributions of all of these interactions in targeting the recycling endosomes to the cleavage furrow remain to be fully understood, it is likely that these multiple interactions work as ‘belt and braces’ to ensure the specificity of membrane targeting. Indeed, while exocyst and snapin are enriched at the cleavage furrow, they are also present at other subcellular compartments, thus they alone cannot ensure the specificity of endosome targeting to the midbody. Thus FIP3 and Cyk4 binding may be required to ensure the specificity of recycling endosome targeting to the midbody. In contrast, kinesin I, the exocyst complex and centriolin, in combination with ECT2, may ensure the timing of recycling endosome transport and accumulation at the furrow. As a result, combinatorial interactions between multiple protein complexes may be required for the temporal and spacial regulation of endocytic transport during cytokinesis.
Figure 2. Schematic representation of protein-protein interactions that mediate targeting of recycling endosomes to the midbody.
Proteins localized to the recycling endosomes, such as VAMP8, RalA and the complex ARF6-FIP3-Rab11, interact with snapin and the exocyst complex, which causes targeting of the endosomes to the midbody during cytokinesis.
Abbreviations used
- FIP
family of Rab11-interacting protein
- GAP
GTPase-activating protein
- Nuf
nuclear fallout
- PM
plasma membrane
- siRNA
small interfering RNA
- VAMP
vesicle-associated membrane protein
References
- 1.Rappaport R. Establishment and organization of the cleavage mechanism. Soc. Gen. Physiol. Ser. 1975;30:287–304. [PubMed] [Google Scholar]
- 2.Bluemink JG, de Laat SW. New membrane formation during cytokinesis in normal and cytochalasin B-treated eggs of Xenopus laevis. I: electron microscope observations. J. Cell Biol. 1973;59:89–108. doi: 10.1083/jcb.59.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danilchik MV, Funk WC, Brown EE, Larkin K. Requirement for microtubules in new membrane formation during cytokinesis of Xenopus embryos. Dev. Biol. 1998;194:47–60. doi: 10.1006/dbio.1997.8815. [DOI] [PubMed] [Google Scholar]
- 4.Jantsch-Plunger V, Glotzer M. Depletion of syntaxins in the early Caenorhabditis elegans embryo reveals a role for membrane fusion events in cytokinesis. Curr. Biol. 1999;9:738–745. doi: 10.1016/s0960-9822(99)80333-9. [DOI] [PubMed] [Google Scholar]
- 5.Rothwell WF, Zhang CX, Zelano C, Hsieh TS, Sullivan W. The Drosophila centrosomal protein Nuf is required for recruiting Dah, a membrane associated protein, to furrows in the early embryo. J. Cell Sci. 1999;112:2885–2893. doi: 10.1242/jcs.112.17.2885. [DOI] [PubMed] [Google Scholar]
- 6.Han JK. Oscillation of inositol polyphosphates in the embryonic cleavage cycle of the Xenopus laevis. Biochem. Biophys. Res. Commun. 1995;206:775–780. doi: 10.1006/bbrc.1995.1109. [DOI] [PubMed] [Google Scholar]
- 7.Janetopoulos C, Borleis J, Vazquez F, Iijima M, Devreotes P. Temporal and spatial regulation of phosphoinositide signaling mediates cytokinesis. Dev. Cell. 2005;8:467–477. doi: 10.1016/j.devcel.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Emoto K, Kobayashi T, Yamaji A, Aizawa H, Yahara I, Inoue K, Umeda M. Redistribution of phosphatidylethanolamine at the cleavage furrow of dividing cells during cytokinesis. Proc. Natl. Acad. Sci. U.S.A. 1996;93:12867–12872. doi: 10.1073/pnas.93.23.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hickson GR, Matheson J, Riggs B, Maier VH, Fielding AB, Prekeris R, Sullivan W, Barr FA, Gould GW. Arfophilins are dual Arf/Rab 11 binding proteins that regulate recycling endosome distribution and are related to Drosophila nuclear fallout. Mol. Biol. Cell. 2003;14:2908–2920. doi: 10.1091/mbc.E03-03-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreiner T, Moore HP. Membrane traffic between secretory compartments is differentially affected during mitosis. Cell Regul. 1990;1:415–424. doi: 10.1091/mbc.1.5.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warren G, Davoust J, Cockcroft A. Recycling of transferrin receptors in A431 cells is inhibited during mitosis. EMBO J. 1984;3:2217–2225. doi: 10.1002/j.1460-2075.1984.tb02119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raucher D, Sheetz MP. Membrane expansion increases endocytosis rate during mitosis. J. Cell Biol. 1999;144:497–506. doi: 10.1083/jcb.144.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boucrot E, Kirchhausen T. Endosomal recycling controls plasma membrane area during mitosis. Proc. Natl. Acad. Sci. U.S.A. 2007;104:7939–7944. doi: 10.1073/pnas.0702511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cayrol C, Cougoule C, Wright M. The β2-adaptin clathrin adaptor interacts with the mitotic checkpoint kinase BubR1. Biochem. Biophys. Res. Commun. 2002;298:720–730. doi: 10.1016/s0006-291x(02)02522-6. [DOI] [PubMed] [Google Scholar]
- 15.Chanez AL, Hehl AB, Engstler M, Schneider A. Ablation of the single dynamin of T. brucei blocks mitochondrial fission and endocytosis and leads to a precise cytokinesis arrest. J. Cell Sci. 2006;119:2968–2974. doi: 10.1242/jcs.03023. [DOI] [PubMed] [Google Scholar]
- 16.Low SH, Li X, Miura M, Kudo N, Quinones B, Weimbs T. Syntaxin 2 and endobrevin are required for the terminal step of cytokinesis in mammalian cells. Dev. Cell. 2003;4:753–759. doi: 10.1016/s1534-5807(03)00122-9. [DOI] [PubMed] [Google Scholar]
- 17.Wilson GM, Fielding AB, Simon GC, Yu X, Andrews PD, Hames RS, Frey AM, Peden AA, Gould GW, Prekeris R. The FIP3-Rab11 protein complex regulates recycling endosome targeting to the cleavage furrow during late cytokinesis. Mol. Biol. Cell. 2005;16:849–860. doi: 10.1091/mbc.E04-10-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riggs B, Rothwell W, Mische S, Hickson GR, Matheson J, Hays TS, Gould GW, Sullivan W. Actin cytoskeleton remodeling during early Drosophila furrow formation requires recycling endosomal components Nuclear-fallout and Rab11. J. Cell Biol. 2003;163:143–154. doi: 10.1083/jcb.200305115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin OH, Ross AH, Mihai I, Exton JH. Identification of arfophilin, a target protein for GTP-bound class II ADP-ribosylation factors. J. Biol. Chem. 1999;274:36609–36615. doi: 10.1074/jbc.274.51.36609. [DOI] [PubMed] [Google Scholar]
- 20.Prekeris R, Davies JM, Scheller RH. Identification of a novel Rab11/25 binding domain present in Eferin and Rip proteins. J. Biol. Chem. 2001;276:38966–38970. doi: 10.1074/jbc.M106133200. [DOI] [PubMed] [Google Scholar]
- 21.Lindsay AJ, Hendrick AG, Cantalupo G, Senic-Matuglia F, Goud B, Bucci C, McCaffrey MW. Rab coupling protein (RCP), a novel Rab4 and Rab11 effector protein. J. Biol. Chem. 2002;277:12190–12199. doi: 10.1074/jbc.M108665200. [DOI] [PubMed] [Google Scholar]
- 22.Meyers JM, Prekeris R. Formation of mutually exclusive Rab11 complexes with members of the family of Rab11-interacting proteins regulates Rab11 endocytic targeting and function. J. Biol. Chem. 2002;277:49003–49010. doi: 10.1074/jbc.M205728200. [DOI] [PubMed] [Google Scholar]
- 23.Horgan CP, Walsh M, Zurawski TH, McCaffrey MW. Rab11-FIP3 localises to a Rab11-positive pericentrosomal compartment during interphase and to the cleavage furrow during cytokinesis. Biochem. Biophys. Res. Commun. 2004;319:83–94. doi: 10.1016/j.bbrc.2004.04.157. [DOI] [PubMed] [Google Scholar]
- 24.Fielding AB, Schonteich E, Matheson J, Wilson G, Yu X, Hickson GR, Srivastava S, Baldwin SA, Prekeris R, Gould GW. Rab11-FIP3 and FIP4 interact with Arf6 and the exocyst to control membrane traffic in cytokinesis. EMBO J. 2005;24:3389–3399. doi: 10.1038/sj.emboj.7600803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schweitzer JK, D’Souza-Schorey C. Localization and activation of the ARF6 GTPase during cleavage furrow ingression and cytokinesis. J. Biol. Chem. 2002;277:27210–27216. doi: 10.1074/jbc.M201569200. [DOI] [PubMed] [Google Scholar]
- 26.Kitamura T, Kawashima T, Minoshima Y, Tonozuka Y, Hirose K, Nosaka T. Role of MgcRacGAP/Cyk4 as a regulator of the small GTPase Rho family in cytokinesis and cell differentiation. Cell. Struct. Funct. 2001;26:645–651. doi: 10.1247/csf.26.645. [DOI] [PubMed] [Google Scholar]
- 27.Hirose K, Kawashima T, Iwamoto I, Nosaka T, Kitamura T. MgcRacGAP is involved in cytokinesis through associating with mitotic spindle and midbody. J. Biol. Chem. 2001;276:5821–5828. doi: 10.1074/jbc.M007252200. [DOI] [PubMed] [Google Scholar]
- 28.Mishima M, Pavicic V, Gruneberg U, Nigg EA, Glotzer M. Cell cycle regulation of central spindle assembly. Nature. 2004;430:908–913. doi: 10.1038/nature02767. [DOI] [PubMed] [Google Scholar]
- 29.Chalamalasetty RB, Hummer S, Nigg EA, Sillje HH. Influence of human Ect2 depletion and overexpression on cleavage furrow formation and abscission. J. Cell Sci. 2006;119:3008–3019. doi: 10.1242/jcs.03032. [DOI] [PubMed] [Google Scholar]
- 30.Kimura K, Tsuji T, Takada Y, Miki T, Narumiya S. Accumulation of GTP-bound RhoA during cytokinesis and a critical role of ECT2 in this accumulation. J. Biol. Chem. 2000;275:17233–17236. doi: 10.1074/jbc.C000212200. [DOI] [PubMed] [Google Scholar]
- 31.Yuce O, Piekny A, Glotzer M. An ECT2-centralspindlin complex regulates the localization and function of RhoA. J. Cell Biol. 2005;170:571–582. doi: 10.1083/jcb.200501097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saito S, Liu XF, Kamijo K, Raziuddin R, Tatsumoto T, Okamoto I, Chen X, Lee CC, Lorenzi MV, Ohara N, Miki T. Deregulation and mislocalization of the cytokinesis regulator ECT2 activate the Rho signaling pathways leading to malignant transformation. J. Biol. Chem. 2004;279:7169–7179. doi: 10.1074/jbc.M306725200. [DOI] [PubMed] [Google Scholar]
- 33.Gromley A, Yeaman C, Rosa J, Redick S, Chen CT, Mirabelle S, Guha M, Sillibourne J, Doxsey SJ. Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell. 2005;123:75–87. doi: 10.1016/j.cell.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 34.TerBush DR, Maurice T, Roth D, Novick P(1996) The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- 35.Chen XW, Inoue M, Hsu SC, Saltiel AR. RalA-exocyst-dependent recycling endosome trafficking is required for the completion of cytokinesis. J. Biol. Chem. 2006;281:38609–38616. doi: 10.1074/jbc.M512847200. [DOI] [PubMed] [Google Scholar]
- 36.Jin R, Junutula JR, Matern HT, Ervin KE, Scheller RH, Brunger AT. Exo84 and Sec5 are competitive regulatory Sec6/8 effectors to the RalA GTPase. EMBO J. 2005;24:2064–2074. doi: 10.1038/sj.emboj.7600699. [DOI] [PMC free article] [PubMed] [Google Scholar]