Synopsis
Segregation of the apical and basolateral plasma membrane domains is the key distinguishing feature of epithelial cells. A series of interrelated cues and processes follow this primary polarization event, resulting in the morphogenesis of the mammalian epithelium. This review focuses on the role of the interactions between the extracellular matrix and neighbouring cells during the initiation and establishment of epithelial polarity, and the role that membrane transport and polarity complexes play in this process. An overview of the formation of the apical junctional complexes is given in relation to the generation of distinct membrane domains characterized by the asymmetric distribution of phosphoinositides and proteins. The mechanisms and machinery utilized by the trafficking pathways involved in the generation and maintenance of this apical-basolateral polarization are expounded, highlighting processes of apical-directed transport. Furthermore, the current proposed mechanisms for the organization of entire networks of cells into a structured, polarized three-dimensional structure are described, with an emphasis on the proposed mechanisms for the formation and expansion of the apical lumen.
Keywords: apical protein, endocytic transport, epithelial cell, lumen, plasma membrane, polarization
INTRODUCTION
Epithelium is a tissue composed of polarized cells, which line the body’s organs and perform specialized functions, such as absorption, secretion and transcellular transport. Since epithelial cells often act as barriers, the significance of the polarization, or asymmetry, of this cell type is clear. Failure of epithelial cells to appropriately polarize leads to a variety of diseases, including but not limited to polycystic kidney disease, cystic fibrosis and certain metastatic cancers [1,2]. As a result, the polarization of epithelial cells is a highly regulated event that is conserved across various organisms and is the topic of this review, with particular focus on apical protein targeting and transport.
The PM (plasma membrane) of epithelial cells is divided into the apical and basolateral domains, which are distinct in both lipid and protein composition [3]. The apical domain faces the lumen, the basal domain faces the basement membrane or ECM (extra-cellular matrix), and the lateral domains of these cells interact with neighbouring cells (Figure 1). Specialized apical junctional complexes, the tight junction (zonula occludens) and the adherens junction (zonula adherens), maintain the integrity of these two discrete apical and basolateral domains. Additionally, a distinct ‘membrane domain’, which has only recently been characterized, is the primary cilium, which extends from the apical domain of most epithelial cells (Figure 1). This structure has recently been identified as a ‘Mecca’ of signalling regulation and has been comprehensively reviewed elsewhere [4]. Thus the ciliary targeting and transport are outside the scope of this review.
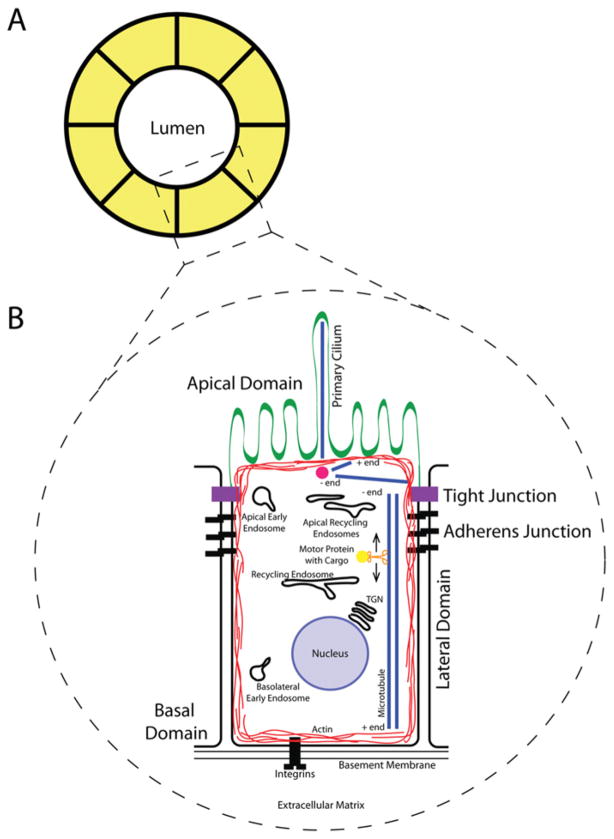
Figure 1. Structure of the mammalian epithelia.
(A) Cross-section of a polarized cyst or tubule. The apical domain of the PM faces the hollow lumen, and the basolateral domain faces the ECM. (B) Schematic representation of a single polarized epithelial cell. The apical domain faces the lumen and contains the specialized subdomain, the primary cilium. The tight junction separates the apical and basolateral domains and is composed primarily of occludins and claudins. Cadherins and nectins make up the adherens junction, which lies directly basal to the tight junction, and functions as a link to the actin cytoskeleton, which forms a cortex around the cell’s periphery. The lateral domain of the cell faces neighbouring cells in the monolayer, while the basal domain faces the basement membrane and interacts with the ECM via integrins. Microtubules are oriented with their plus end facing the apical domain and their minus end facing the basal domain. Motor proteins transport cargo via endocytic carriers along these microtubules.
The tight junction, via its adhesion proteins, occludins and claudins, acts as a barrier to paracellular transport [5], in addition to functioning as a ‘fence’ for the diffusion of lipid and protein components between membrane domains [6]. Tight junction formation is crucial for initiating the polarity programme of the cell and is regulated by the cell’s polarity complexes, as it marks the separation of the apical and lateral domains [7,8]. In mammalian cells, the adherens junction lies basally to the tight junction on the lateral domain of the epithelial cell and exists as an ‘adhesive belt’. This adhesive belt enfolds the cell and functions as the cell’s primary source of mechanical stability and linkage between neighbouring cells. These junctions are rich in calcium-dependent cadherin [9], nectin and nectin-like molecules [10]. Moreover, adherens junctions provide the membrane with a link to the actin cytoskeleton (Figure 1). ECM receptors, such as integrins, lie on the basal side of the cell and are capable of interacting with the basement membrane. One of the most interesting current areas of study within the field of epithelial cell biology is centred on the elucidation of the mechanisms regulating the formation of these polarized epithelial cells, and the cues that initiate this process of polarization.
POLARITY COMPLEXES
The classical model of epithelial polarization suggests that polarity initiating and driving cues come from the interaction of an epithelial cell with neighbouring cells [11]. These cues initiate the calcium-dependent trafficking of E-cadherin molecules to sites of cell–cell adhesion [12,13], thus spatially orienting apical–basolateral polarity. As adhesion molecules accumulate at these spot-like points of cell–cell adhesion, polarity complexes are recruited and initiate the formation of the adherens and tight junctions. Following this, lipids and protein are trafficked to the lateral membrane basal to the adherens junction, inducing the vertical growth of these cells, which results in a mature, polarized epithelium.
There exist three polarity complexes whose functions and regulations are dynamically intertwined with the creation of the epithelial junctional complexes and thus the establishment of apical–basolateral polarity in epithelial cells. These are the CRB (Crumbs) complex, the PAR (Partitioning-defective) complex and the SCRIB (Scribble) complex (Figure 2A). These polarity complexes were originally discovered in Caenorhabditis elegans and Drosophila melanogaster, but later were shown to be highly conserved in mammalian epithelia. All three complexes are well established as key regulators of the formation of the tight junction and the segregation of the apical and basolateral PM.
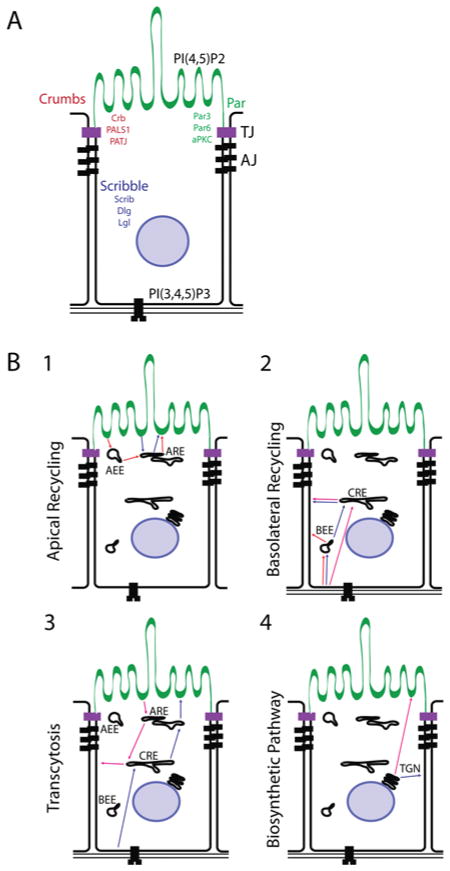
Figure 2. Polarity complexes and routes of polarized transport in epithelial cells.
(A) PIP2 is enriched on the apical PM domain, whereas PIP3 is found pre-dominantly on the basolateral domain. The Par3 protein, part of the apical PAR polarity complex, is localized to the tight junction. Upon the activation of the preformed Par6–aPKC complex by Cdc42, Par6–aPKC is recruited to Par3, where it forms the Par3–Par6–aPKC (PAR) polarity complex and marks the separation of the apical domain from the basolateral. Par6 recruits the CRB complex, which acts as a regulator of the formation and maintenance of the tight junction. The SCRIB complex is recruited by cadherin signalling at sites of cell–cell contacts, and mediates the formation of the basolateral domain. (B) (1) In the apical recycling pathway, cargo is endocytosed from the apical PM domain, and recycled back to the apical domain. (1) Cargo can be transported directly to the ARE, and then be returned directly from the ARE back to the apical PM domain. (2) Cargo can be transported first to the AEE, where it is passed along to the ARE, and then returned to the apical PM. (2) Basolateral recycling occurs when cargo is recycled from and back to the basolateral PM domain. (1) Proteins can be endocytosed to the BEEs and returned directly back to the basolateral PM. (2) Alternatively, cargo can be endocytosed to the BEE, after which it is transported to the CRE, which returns it to the basolateral PM. (3) Finally, cargo can be transported directly to the CRE, and returned to the apical PM. (3) Transcytosis is the transport of cargo from one PM domain to the other. The best-studied pathway of transcytosis is basolateral to apical trans-cytosis. (1) Cargo is internalized from the basolateral PM to the CRE (sometimes via the BEE), which sorts it to the ARE, and releases the cargo on the apical PM. (2) Apical to basolateral transcytosis occurs via the internalization of apical cargo to the ARE (sometimes via the AEE), which sorts the cargo to the CRE. The CRE then directs the cargo to the basolateral PM. (4) Newly synthesized proteins are transported via the biosynthetic pathway from the TGN to either the apical or basolateral domains. These pathways sometimes use endosomal sorting intermediaries; however, direct transport from the TGN to the PM also occurs.
While the CRB and PAR complexes have been well characterized in mammalian epithelia, the function of the SCRIB complex in vertebrate cells is much less understood. The SCRIB complex is composed of the scribble (scrib), discs large (dlg), and lethal giant larvae (lgl) proteins, all of which have been identified as tumour-suppressor genes regulating the establishment of apical-basolateral polarity [14]. This complex is thought to be recruited to sites of cell–cell adhesion in response to cadherin signalling [15,16], which will be discussed in the next section of this review. The SCRIB complex is required for the establishment of the basolateral domain, with recent evidence indicating that it regulates endocytic vesicle targeting to the basolateral domain via association with the tethering protein syntaxin 4 [17,18]. The PAR complex is composed PAR3, PAR6 and aPKC (atypical protein kinase C). The current model of the PAR-complex-dependent initiation of epithelial cell polarity is that PAR3 is localized to sites of contact between neighbouring cells before polarization, and that the binding of active Cdc42 (cell division control protein 42 homologue) to the pre-formed PAR6–aPKC complex results in the activation of the PAR3–PAR6–aPKC complex [19,20] at the site of the forming apical PM domain. At this site, the PAR complex marks the apical domain of the cell and results in the formation of the tight junctions and the separation of apical- and basolateral domain-initiating factors. Furthermore, PAR6 recruits the CRB complex, which is largely specific to epithelial cells [21,22]. The CRB complex consists of the CRB protein, PALS1 (protein associated with Lin Seven 1) and PATJ (PALS1-associated tight junction protein). The CRB complex functions as a unit of regulation for the formation of the tight junction [23–25] by concentrating at the site of the tight junction and demarking the point of separation between the apical and lateral domains. Furthermore, the size and maintenance of the established apical PM domain are regulated by the CRB complex [26].
One of the more elusive concepts in this model of the establishment of polarity is the identification of the signalling event that results in the recruitment and activation of these polarity complexes. Preliminary studies in mammalian cells imply that the asymmetric distribution of PIs (phosphoinositides) may recruit the PAR complex and initiate the polarization process [27–29]. However, this is an area of controversy, as the reverse seems to be true in Drosophila [30]. Additionally, the activation of aPKC by Cdc42 has been suggested to be a downstream result of the PI3K (phosphatidylinositide 3-kinase) signalling pathway [31], highlighting the complications arising from the interplay between the polarity complexes and PIs.
ROLE OF LIPIDS IN POLARIZED EPITHELIAL TRANSPORT
In addition to differential protein distribution, epithelial cells also display the polarization of various lipids. The composition of the inner leaflet of the PM is distinct between the apical and basolateral domains of the epithelial cell. While PIP2 (phosphatidylinositol 4,5-bisphosphate) localizes primarily to the apical domain, PIP3 (phosphatidylinositol 3,4,5-triphosphate) is concentrated on the basolateral domain of mammalian epithelial cells [32–34]. The segregation of these PIs into discrete membrane domains is necessary for the generation of apical–basolateral polarity. In part, this is due to the function of the PIs as apical and basolateral determinants that recruit specific proteins necessary for epithelial morphogenesis. The use of recombination techniques to mislocalize PIs to the opposite membrane domain results in the mistargeting of apical and basolateral proteins. Additionally, depletion of either factor from its appropriate domain is shown to inhibit the ability of these cells to undergo lumen morphogenesis [33].
One of the first theories of apical targeting, the ‘lipid raft hypothesis’ [35], centres on the self-aggregation of various lipids into distinct subdomains, which retain characteristics discrete from those of the basolateral membrane [36]. These lipid rafts were proposed to be detergent-resistant membrane domains, which are enriched in PIP2, cholesterol and glycosphingolipids. Lipid rafts also were suggested to be sorting sites for apical cargo exit at the level of the TGN (trans-Golgi network) [36]. While a mechanistic understanding of the role of lipid rafts in apical sorting has not yet been resolved and remains controversial, recent research indicates that lipid rafts might function as sites for the clustering and oligomerization of at least some apical proteins [37,38]. However, recent studies have demonstrated the existence of a ‘raft-independent’ TGN exit pathway. Indeed, many proteins, such as endolyn, are transported to the apical PM in a manner that does not require lipid rafts [39]. Thus current studies in this area are aimed towards determining the role of other potential clustering/sorting regulators, which differentially sort cargo to lipid raft-dependent and -independent transport pathways. A variety of candidate proteins have already been identified, including clathrin adaptor proteins [40,41] and carbohydrate-interacting proteins [42,43].
The association of the apical junctional complexes with the actin cytoskeleton is an important link, which is integral for the polarization of epithelial cells. Annexin2 is a PIP2-binding scaffolding protein that activates the PAR6–aPKC complex and initiates apical lumen morphogenesis, as well as the formation of the apical junctional complexes [34]. During the establishment of these cell–cell adhesions, PIP2 regulates epithelial differentiation through its ability to associate with numerous actin-binding proteins at the apical domain [44,45]. Another pathway of actin cytoskeletal regulation by PIP2 is via PIP2’s binding to ezrin through ezrin’s FERM (4.1/ezrin/radixin/moesin) binding domain [46], which allows PIP2 to regulate ezrin’s activity [47]. Through this interaction, PIP2 directly links actin filaments to the PM, and allows for the regulation of the orientation and shape of the apical domain during the maturation of the epithelial lumen.
While the mechanisms regulating the generation of the PIP2/PIP3 lipid asymmetry remain to be fully elucidated, phosphatases and kinases are known to be the tools through which this asymmetry is created. A series of phosphorylation events regulate the concentration and dynamics of these PIs within their respective membrane domains. One enzyme of interest is PTEN (phosphatase and tensin homologue deleted on chromosome 10), which generates PIP2 from PIP3. PTEN is shown to bind and be activated by PIP2 at the apical PM [48,49], where it is likely recruited by binding to PAR3. On depletion of PTEN in MDCK (Madin–Darby canine kidney) cells, which leads to a decrease in PIP2 levels, generation of the apical lumen is inhibited [33].
Another protein that is important for the generation of the polarized distribution of PIs in epithelial cells is PI3K, a key kinase that phosphorylates PIP2, thus increasing PIP3 levels in the basolateral PM. E-cadherin accumulation at the sites of cell–cell adhesion results in the recruitment of the human homologue of Disc-large protein [15], which in turn recruits and activates PI3K to stabilize the adherens junctions via linkages to the actin cytoskeleton [50–53]. After being recruited to cell–cell adhesion sites, PI3K not only acts as a stabilizing factor for the adherens junctions, but additionally functions as a predominant contributor to the supply of PIP3 for the lateral domain. The inhibition of PI3K results in a significant decrease in the height of epithelial cells, presumably by inhibiting growth of the lateral PM [54]. Interestingly, the fate of the apical and basolateral domains are intertwined, as apical PIP2 also has a role in regulating the endocytic routes of transport taken by the basolateral cadherins [55,56]. This allows for the disintegration and reorganization of cell–cell contacts to keep up with the dynamic needs of epithelial cells [57,58].
While lipid asymmetry and PI-dependent signalling events are critical for the establishment of distinct apical and basolateral domains, a comprehensive understanding of these PIs is not sufficient to explain the complexities of polarized transport. In addition to these membrane-initiated sorting and transport events, proper biosynthetic and endocytic transport relies on defined targeting signals embedded in the cargo itself as well as specific transport machinery that processes cargo through a series of sub-cellular sorting intermediaries, called endosomes, which are distinct for sorting to the apical and basolateral membrane domains.
ENDOCYTIC SORTING AND TRANSPORT IN EPITHELIAL CELLS
Similar to the PM, subcellular compartments within the epithelia are spatially and functionally distinct. A series of domain-specific endosomes act as intermediaries for endocytic transport, in which proteins are sorted and targeted to their appropriate PM domain [59] (Figure 2B). Endosomes are not only crucial sites for endocytic transport, but additionally play a role in the biosynthetic transport pathway, as many proteins are transported from the TGN to endosomes before reaching their final destination at the cell surface [60]. Cargo, delivered to endosomes for sorting, follow one of three possible pathways: (1) they are returned to the same domain from which they were endocytosed; (2) they are transported to the opposite PM domain (a process referred to as transcytosis); or (3) they become the constituents of a degradative pathway [61] (Figure 2B).
There are unique early endosomes for the apical and basolateral domains, known as the AEEs (apical early endosomes) and the BEEs (basolateral early endosomes), which are located adjacent to their respective PM domains (Figure 2B). The CREs (common recycling endosomes) are the centrally localized sites of polarized protein sorting, which receive cargo from, and are capable of transport to, both membrane domains. CREs are often characterized by the presence of both apical and basolateral markers. AREs (apical recycling endosomes) are spatially and functionally separate apical domain-specific organelles from the AEEs, and are marked by the presence of the small monomeric GTPase Rab11a/b, and mediate protein transport to the apical PM domain [62–65]. It remains to be determined whether an ARE is a subdomain of the CRE or a functionally distinct compartment [66].
Protein sorting and transport within and between endosomes is a complex process that is regulated by several families of proteins, which regulate endocytic carrier formation, transport, tethering and fusion with either the apical or the basolateral membrane. Additionally, the transport of cargo from endosomes to the correct PM domain of the cell at the appropriate time during epithelial morphogenesis requires the timed spatial delivery of specific cargo via a series of specialized motor proteins.
Kinesin-2 has emerged as a molecular motor required for polarized protein transport in epithelial cells. The KIF (kinesin superfamily of molecular motor proteins) consists of predominantly plus-end-directed microtubule motors, which co-ordinate the intracellular transport of a variety of proteins [67]. The Kinesin-2 subfamily consists of the KIF3A/B and KIF17 molecular motors [68,69]. KIF3A and KIF3B exist as heterodimers, often bound to an adaptor protein, such as KIF-associated protein 3, which associates the kinesin with the cargo to be transported. Alternatively, KIF17 exists as a homodimer and appears to bind cargo directly via its Kif17 tail domain. These Kinesin-2 motor proteins have been shown to regulate the formation and stability of cell–cell adhesions, and thus the polarity programme of these cells [70]. Furthermore, KIF17 motors were shown to mediate protein transport to the apical PM [70a], whereas KIF3A/B motors are known to function as protein transporters within the primary cilia [71,72].
In addition to transport, the correct sorting of cargo into various endocytic carriers also plays an important role in the establishment and maintenance of apical polarity. Several basolateral adaptor proteins have been identified and have been shown to associate with the clathrin-dependent endocytic transport pathway [73,74]. One of the proteins currently known to be involved in basolateral transport is AP1 (adaptor protein 1; specifically the AP1B variant which is expressed in many epithelial cells) [75–77]. It has been shown that the μ1b subunit of AP1B is responsible for directing the polarity of the tyrosine-based basolateral sorting signals of the cargo [76,77], which are thought to cluster into areas of clathrin-coated pit formation [78]. The sorting machinery dedicated for protein transport to the apical PM is much less understood, and does not appear to depend on specific adaptor proteins. Furthermore, it has been suggested that in some cases, apical transport may be a default pathway and may not require specialized sorting signals. This is consistent with the observation that mutation of basolateral sorting signals usually sends cargo to the apical PM. Apical PM proteins may rely more heavily on lipid-dependent sorting, directional transport and tethering to maintain their apical localization. Alternatively, they may simply depend on their retention signals, such as the PDZ domain, to keep them anchored to the actin cytoskeleton associated with the apical PM domain.
In addition to adaptor proteins and molecular motors, members of the Rab family of small monomeric GTPases have emerged as a group of key regulators of polarized transport in epithelial cells [79]. There are over 50 Rab GTPases identified in mammalian cells which are all thought to regulate distinct membrane transport pathways [80]. The Rab11 subfamily of GTPases is recognized as a key regulator of polarized endocytic sorting and transport in epithelial cells [81]. Rab11 has been implicated in the regulation of many transport steps, including the apical recycling pathway [82,83], basolateral-to-apical transcytosis [62], as well as in the delivery of biosynthetic proteins from the TGN to the apical and basolateral PM domains [59, 84–86]. In addition to Rab11, Rab8, and Rab10 also affect basolateral transport from the CRE, and potentially play a role in transcytosis in MDCK cells [87,88]. The functional role of Rab8 remains to be elucidated, as there are conflicting reports of an apical localization of Rab8 [89]. Finally, Rabs 4, 5 and 7 have been characterized in non-polarized cells [90] and are thought to mediate similar endocytic processes in polarized cells; however, these GTPases remain the focus of future inquiries.
One of the most pressing questions in the field is concerning how the specificity of each Rab is imparted and regulated. All small GTPases bind downstream effectors while in their active GTP-bound conformation. There is some evidence indicating that GTP-bound Rabs might recruit specific motor proteins. For example, Rab11a binds the actin molecular motor, myosin-Vb, to transport cargo to the apical domain [91], in addition to mediating the recruitment of Kinesin-2 to endocytic membranes [92]. While it is possible that this regulation of myosin and kinesin activity by Rabs may have a role in the spatial distribution of distinct organelle subpopulations within polarized cells, it is also thought that there are other downstream effector molecules that oversee Rab function and specificity. Rab11 FIPs (family interacting proteins) were identified as a family of proteins that bind specifically to Rab11 GTPases [81,93] and act as scaffolds for the recruitment of various factors involved in the regulation of endocytic transport. Because each FIP forms a mutually exclusive complex with Rab11 [94], it is possible that each individual Rab11–FIP complex is formed to specifically regulate individual pathways of endocytic transport. Consistent with this specificity-imparting and potential scaffolding function of FIPs, several FIP family members have been shown to interact with other regulators of membrane trafficking [95]. For example, FIP5/Rip11, a Rab11-binding protein involved in apical-directed transport, has been immunoprecipitated with KIF3A/B [92], while FIP2 binds to myosin-Vb and FIP1/RCP (Rab coupling protein) interacts with Golgin-97, [96].
In addition to the specificity imparted by Rabs, FIPs, and molecular motors, another set of proteins that make up an important part of the endocytic machinery are tethering and fusion proteins. The key example of this, being conserved from yeast to mammalian cells, is the Exocyst complex. The Exocyst complex is required for the polarization of epithelial cells [97], as it is integral for the transport of cargo from the Golgi or the endosomes to the PM [98]. This complex is composed of eight subunits, and is required for the establishment of epithelial polarization [99]. The assembly of this complex aids in driving the fusion of vesicular carriers with their target PM domain. The tethering function of the Exocyst complex delivers and drives the fusion of vesicles at sites of polarized development [97,100]. The localization and function of the Exocyst complex is regulated by a series of binding proteins, including Rabs and their binding proteins, which are thought to drive the assembly of the Exocyst complex (reviewed in [101]).
The fusion of transport vesicles with their appropriate target membrane is mediated by a group of tethering proteins known as SNAREs (soluble N-ethylmaleimide-sensitive fusion protein-attachment protein receptors), which are part of the exocyst machinery. There are two subclasses of SNAREs, v-SNAREs (vesicle-SNAREs) that are localized to the membrane of the endocytic carrier and t-SNAREs (target-SNAREs) that are located in the acceptor membrane. It is well established that distinct sets of SNAREs are present on the apical and the basolateral domains of the polarized cell. In MDCK cells, syntaxin 3 is a marker of the apical domain, whereas syntaxin 4 is a marker of the basolateral domain [102]. It has also been reported that different pathways of endocytosis function with the aid of unique v-SNAREs [103–105]. The binding of complementary v- and t-SNAREs and the resulting tethering and fusion of the membrane, is thought to impart a mechanism for the specificity of cargo transport [106].
These mechanisms of apical and basolateral targeting are regulated by the structure and sequence of the proteins themselves, as well as by the overarching polarity programme of the cell. This establishment of apical–basolateral polarity, which utilizes the described routes of endocytic transport via a variety of regulators, must be coupled by each individual cell to the polarity programme of the forming tissue for the successful generation of a polarized structure. In epithelial tissues, the resulting structure created by a network of polarized cells is a cylindrical tube made up of a single layer of epithelial cells connected on their lateral faces, which are oriented such that their apical domain lies facing the lumen, and the basolateral domain faces the ECM of this monolayer.
THE ROLES OF POLARITY COMPLEXES AND ENDOCYTIC TRANSPORT DURING EPITHELIAL LUMEN MORPHOGENESIS
Tissue morphogenesis requires the co-ordination of, and therefore communication between, entire groups of epithelial cells within three-dimensional space. The final result of these processes is the formation of epithelial tubes or end buds, in which all epithelial cells are properly oriented such that their apical domain faces the central lumen of the three-dimensional structure [107]. Since much of the work on epithelial polarity has been conducted using two-dimensional epithelial cell models, the process of epithelial polarization during the formation of three-dimensional structures remains poorly understood. The mechanisms regulating epithelial tube formation and branching involve very complex processes and have been extensively reviewed elsewhere [108,109]. Here, we will focus only on the mechanisms of apical lumen formation, since several recent reports have demonstrated the co-ordinated involvement of polarity complexes and polarized endocytic transport in the establishment and formation of the apical lumen.
The two prevailing models of lumen formation during the tubulogenesis of non-polarized precursor cells are the hollowing model and the cavitation model (Figure 3). In the hollowing model, it was proposed that specialized organelles called VACs (vacuolar apical compartments) are formed and exocytosed to the site of the forming lumen [110]. The fusion of these VACs with each other and with the PM is believed to initiate the formation of the apical central lumen [111]. VACs appear to be specialized endocytic carriers that are formed by the internalization of apically targeted proteins, lumen formation factors as well as some extracellular fluid [110]. These vacuoles are transported through the cell and are exocytosed at the meeting point of the dividing cells, and fuse to generate the initial apical luminal space, which is enlarged as the cyst grows (Figure 3). In MDCK cells, VACs are known to secrete both glycoproteins (such as gp135/podocalyxin) and polysaccharides into the lumen, thereby leveraging electrostatic repulsion and steric hindrance as driving forces for maintaining the luminal space and preventing self-association of the apical membrane, which would act to close the lumen [112–114].
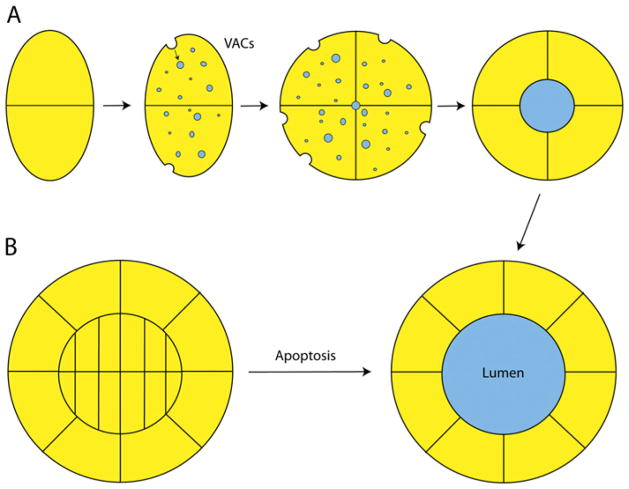
Figure 3. Models of epithelial lumen morphogenesis.
(A) In the hollowing model of lumen morphogenesis, newly polarizing cells divide, and, at the two-cell stage, the basolateral domain is the site of cell–cell contact, while the apical domain faces the ECM. Endocytosis of apical proteins and ECM fluids occurs through the use of specialized organelles called VACs, which are targeted to the meeting point of the dividing cells. These VACs accumulate and form the apical lumen at the centre of the forming cyst. Glycoproteins and polysaccharides are transported in these VACs and aid in the self-repulsion of the apical PM, allowing the lumen to remain open. (B) In the cavitation model, cells proliferate to form a solid cyst or tube. The outer cells of this structure, which are in contact with the ECM, then polarize. The cells internal to this monolayer then undergo apoptosis, resulting in the clearing of the apical lumen.
Rab8 and Rab11 have been found to regulate the formation of the lumen in vivo [89,115], and evidence has come into light that argues that Rab11 is capable of mediating the endocytosis of the polarity protein, Crumbs3a, and thereby demarks the site of the apical lumen membrane [116]. Results from that paper indicate that, in MDCK cells, the lumen is initiated during the first cell division of the forming cyst, via the trafficking of Crumbs3a in Rab11-positive VACs to the site of cytokinesis. The involvement of a CRB polarity complex protein, which is thought to recruit aPKC to the site of the forming lumen and thereby enable the polarity programming of the forming cyst, highlights the complexity of epithelial morphogenesis in the context of an entire tissue. While epithelial morphogenesis and the polarity programming of a single cell are still not completely understood, even less is known about the mechanisms used during organogenesis, and this is an area of great interest and debate within the field of epithelial cell biology.
The alternative model, the cavitation model of tube morphogenesis, arises from original studies conducted in the early mouse embryo [117], which propose that an aggregation of non-polarized cells is a precursor to the polarized epithelial tube. The cells on the outer periphery of this agglomeration, namely the cells that are in contact with the ECM, polarize as a cylindrical monolayer and separate from the cells in the centre of the forming tubule. The cells located in the middle of the forming tube then undergo caspase-dependent programmed cell death, called apoptosis, to evacuate and maintain the central luminal space (Figure 3). It is thought that the lack of contact between centrally located cells with the basement membrane acts as a large contributor to the susceptibility of these cells to apoptotic death. Evidence supporting the cavitation model of epithelial morphogenesis in mammalian cells has come from human mammary cells [118] and MDCK cells grown in a collagen matrix [119]. Moreover, the most striking proof for the cavitation model comes from in vivo studies of the mammary and salivary glands showing large numbers of apoptotic cells in the centre of the forming tubules within these glands [120,121].
While cavitation has been observed in epithelial morphogenesis as a mechanism that enables the clearing of the forming apical lumen, and thus the establishment of a differentiated epithelial tubule, apoptosis is not absolutely required for the creation of these tubules. Research conducted in MDCK cells reveals that, on overexpression of the apoptotic inhibitor, Bcl-2, lumen formation is delayed, but not altogether blocked [122]. Additionally, in vivo studies conducted in the mouse mammary gland reveal that the inhibition of apoptosis delays, but does not completely abrogate, lumen clearing [123]. One alternative to caspase-dependent cell death is anoikis, another form of cell death, which is also thought to be triggered by the loss of cell contact with the ECM [124–126]. Furthermore, initial evidence for lumen clearing via autophagy has been established [118,123]. These results highlight the importance of redundant mechanisms that act to ensure the formation of the apical lumen.
While these two models of apical lumen formation are classically distinct, it is likely that they are functionally intertwined, with a mixture of both hollowing and cavitation occurring during the morphogenesis of most three-dimensional epithelial structures. It is likely that the predominance of one mechanism in particular tissues is dependent on a series of regulatory factors. An example of the regulation of the balance between hollowing and cavitation during tubulogenesis lies in studies conducted in MDCK cells, where it has been shown that the chosen mechanism of lumen formation and clearing depends on the ability of the cells to quickly polarize [119,127]. When plated in an ECM that provides the appropriate factors for epithelial polarization, such as Matrigel, polarized cysts rapidly form without the use of apoptosis as a lumen-clearing mechanism. In this case, the secretion of luminal proteins, such as gp135, induces the separation of the apical membrane via the hollowing mechanism. However, when MDCK cells are plated in a stiffer, less-differentiated matrix such as collagen, there is a delay in polarization, which is likely affected by the necessity of the cells to now form their own ECM factors to allow polarization. This delay results in the accumulation of cells in the apical lumen upon polarization of the cyst, which leads to apoptosis of these excess cells, and thus cavitation [119]. Similarly, the preferential use of hollowing or cavitation has been shown to be involved in the morphogenesis of different tissues in vivo.
The evidence for the hollowing model of lumen formation in vivo in epithelial cells is largely derived from studies conducted under non-physiological conditions, such as calcium depletion [110] or the delay of cell polarization [119]. Only recently has evidence for the formation of VACs in epithelial cells in vivo come into light, through studies of lumen formation in the zebra-fish gut epithelium [128]. Moreover, further support for the hollowing model comes from the visualization of VACs in the development of endothelial blood vessels in zebrafish embryos [129]. Despite the visualization of VACs in vivo, there is a debate over the validity of the hollowing model due to the potential for the visualization of VACs or VAC-like structures as a result of alternative programmes of morphogenesis [130]. The primary example supporting the cavitation model of lumen clearing in vivo is in the mouse mammary gland, in which apoptotic clearing of luminal cells in the mammary duct has been visualized [120]. These results are supported by three-dimensional cell culture studies of mammary cells, which mimic these results [131].
All the levels of regulation imparted by the polarity complexes, the generation of the distinct membrane domains, and the regulated sorting and transport of endocytic vesicles discussed earlier in this review, play a large part in the generation of the epithelial lumen. A deeper understanding of the mechanism(s) used to create and maintain the apical luminal space during epithelial morphogenesis is highly important. The clinical impacts of this information would be far-reaching, as cancer and inflammation are associated with the filling or improper clearing of the apical luminal space.
CONCLUSIONS AND FUTURE OBJECTIVES
The development and maintenance of epithelial cell polarity is critical for the functioning of many of the body’s tissues and organs. While the co-ordination between individual epithelial cell polarization and tissue morphogenesis is a complex process involving many levels of regulation, much progress has been made in the field within the last decade. The advent of three-dimensional cyst-formation assays in vitro, along with organotypic and in vivo studies, have led to great advances in our understanding of epithelial morphogenesis, as well as lumen formation and maintenance. Nevertheless, there remain many gaps in our knowledge of epithelial polarization, especially in terms of integrating the roles of the cytoskeleton and polarity complexes with endocytic transport. Furthermore, the signalling machinery that initiates and maintains polarity during the formation of three-dimensional structures remains to be fully understood. However, the model systems that are currently being established will afford us the tools necessary to deepen our understanding of these processes, and fill the gaps in our understanding of epithelial morphogenesis.
Acknowledgments
We apologize to our colleagues for not being able to cite all work related to epithelial polarity, due to the focused nature of this review and space constraints.
FUNDING
Work in our laboratory was supported by the National Institutes of Health [grant number DK064380 (to R.P.)] and the Susan G. Komen Breast Cancer Research Foundation (to R.P.).
Abbreviations
- AEE
apical early endosome
- AP1
adaptor protein 1
- aPKC
atypical protein kinase C
- ARE
apical recycling endosome
- BEE
basolateral early endosome
- CRB
Crumbs
- CRE
common recycling endosome
- ECM
extracellular matrix
- FIP
family interacting protein
- KIF
kinesin superfamily of molecular motor proteins
- MDCK
Madin–Darby canine kidney
- PALS1
protein associated with Lin Seven 1
- PAR
Partioning-defective
- PI
phosphoinositide
- PI3K
phosphatidylinositide 3-kinase
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PIP3
phosphatidylinositol 3,4,5-triphosphate
- PM
plasma membrane
- PTEN
phosphatase and tensin homologue deleted on chromosome 10
- SCRB
Scribble
- SNARE
soluble N-ethylmaleimide-sensitive fusion protein-attachment protein receptor
- TGN
trans-Golgi network
- t-SNARE
target SNARE
- VAC
vacuolar apical compartment
- v-SNARE
vehicle SNARE
References
- 1.McConkey DJ, Choi W, Marquis L, Martin F, Williams MB, Shah J, Svatek R, Das A, Adam L, Kamat A, et al. Role of epithelial-to-mesenchymal transition (EMT) in drug sensitivity and metastasis in bladder cancer. Cancer Metastasis Rev. 2009;28:335–344. doi: 10.1007/s10555-009-9194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson PD. Epithelial cell polarity and disease. Am J Physiol. 1997;272:F434–F442. doi: 10.1152/ajprenal.1997.272.4.F434. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Boulan E, Kreitzer G, Musch A. Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol. 2005;6:233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- 4.Satir P, Pedersen LB, Christensen ST. The primary cilium at a glance. J Cell Sci. 2010;123:499–503. doi: 10.1242/jcs.050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diamond JM. Twenty-first Bowditch lecture. The epithelial junction: bridge, gate, and fence. Physiologist. 1977;20:10–18. [PubMed] [Google Scholar]
- 6.Dragsten PR, Blumenthal R, Handler JS. Membrane asymmetry in epithelia: is the tight junction a barrier to diffusion in the plasma membrane? Nature. 1981;294:718–722. doi: 10.1038/294718a0. [DOI] [PubMed] [Google Scholar]
- 7.Lin D, Edwards AS, Fawcett JP, Mbamalu G, Scott JD, Pawson T. A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat Cell Biol. 2000;2:540–547. doi: 10.1038/35019582. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki A, Yamanaka T, Hirose T, Manabe N, Mizuno K, Shimizu M, Akimoto K, Izumi Y, Ohnishi T, Ohno S. Atypical protein kinase C is involved in the evolutionarily conserved par protein complex and plays a critical role in establishing epithelia-specific junctional structures. J Cell Biol. 2001;152:1183–1196. doi: 10.1083/jcb.152.6.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyaguchi K. Ultrastructure of the zonula adherens revealed by rapid-freeze deep-etching. J Struct Biol. 2000;132:169–178. doi: 10.1006/jsbi.2000.4244. [DOI] [PubMed] [Google Scholar]
- 10.Nakanishi H, Takai Y. Roles of nectins in cell adhesion, migration and polarization. Biol Chem. 2004;385:885–892. doi: 10.1515/BC.2004.116. [DOI] [PubMed] [Google Scholar]
- 11.Ebnet K, Suzuki A, Ohno S, Vestweber D. Junctional adhesion molecules (JAMs): more molecules with dual functions? J Cell Sci. 2004;117:19–29. doi: 10.1242/jcs.00930. [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa M, Fukata M, Yamaga M, Itoh N, Kaibuchi K. Recruitment and activation of Rac1 by the formation of E-cadherin-mediated cell–cell adhesion sites. J Cell Sci. 2001;114:1829–1838. doi: 10.1242/jcs.114.10.1829. [DOI] [PubMed] [Google Scholar]
- 13.Wang Q, Chen XW, Margolis B. PALS1 regulates E-cadherin trafficking in mammalian epithelial cells. Mol Biol Cell. 2007;18:874–885. doi: 10.1091/mbc.E06-07-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wodarz A. Tumor suppressors: linking cell polarity and growth control. Curr Biol. 2000;10:R624–R626. doi: 10.1016/s0960-9822(00)00658-8. [DOI] [PubMed] [Google Scholar]
- 15.Reuver SM, Garner CC. E-cadherin mediated cell adhesion recruits SAP97 into the cortical cytoskeleton. J Cell Sci. 1998;111:1071–1080. doi: 10.1242/jcs.111.8.1071. [DOI] [PubMed] [Google Scholar]
- 16.Ide N, Hata Y, Nishioka H, Hirao K, Yao I, Deguchi M, Mizoguchi A, Nishimori H, Tokino T, Nakamura Y, et al. Localization of membrane-associated guanylate kinase (MAGI)-1/BAI-associated protein (BAP) 1 at tight junctions of epithelial cells. Oncogene. 1999;18:7810–7815. doi: 10.1038/sj.onc.1203153. [DOI] [PubMed] [Google Scholar]
- 17.Ludford-Menting MJ, Thomas SJ, Crimeen B, Harris LJ, Loveland BE, Bills M, Ellis S, Russell SM. A functional interaction between CD46 and DLG4: a role for DLG4 in epithelial polarization. J Biol Chem. 2002;277:4477–4484. doi: 10.1074/jbc.M108479200. [DOI] [PubMed] [Google Scholar]
- 18.Musch A, Cohen D, Yeaman C, Nelson WJ, Rodriguez-Boulan E, Brennwald PJ. Mammalian homolog of Drosophila tumor suppressor lethal (2) giant larvae interacts with basolateral exocytic machinery in Madin-Darby canine kidney cells. Mol Biol Cell. 2002;13:158–168. doi: 10.1091/mbc.01-10-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tabuse Y, Izumi Y, Piano F, Kemphues KJ, Miwa J, Ohno S. Atypical protein kinase C cooperates with PAR-3 to establish embryonic polarity in Caenorhabditis elegans. Development. 1998;125:3607–3614. doi: 10.1242/dev.125.18.3607. [DOI] [PubMed] [Google Scholar]
- 20.Qiu RG, Abo A, Steven Martin G. A human homolog of the C. elegans polarity determinant Par-6 links Rac and Cdc42 to PKCzeta signaling and cell transformation. Curr Biol. 2000;10:697–707. doi: 10.1016/s0960-9822(00)00535-2. [DOI] [PubMed] [Google Scholar]
- 21.Hurd TW, Fan S, Liu CJ, Kweon HK, Hakansson K, Margolis B. Phosphorylation-dependent binding of 14–3-3 to the polarity protein Par3 regulates cell polarity in mammalian epithelia. Curr Biol. 2003;13:2082–2090. doi: 10.1016/j.cub.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 22.Wang Q, Hurd TW, Margolis B. Tight junction protein Par6 interacts with an evolutionarily conserved region in the amino terminus of PALS1/stardust. J Biol Chem. 2004;279:30715–30721. doi: 10.1074/jbc.M401930200. [DOI] [PubMed] [Google Scholar]
- 23.Lemmers C, Michel D, Lane-Guermonprez L, Delgrossi MH, Medina E, Arsanto JP, Le Bivic A. CRB3 binds directly to Par6 and regulates the morphogenesis of the tight junctions in mammalian epithelial cells. Mol Biol Cell. 2004;15:1324–1333. doi: 10.1091/mbc.E03-04-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roh MH, Fan S, Liu CJ, Margolis B. The Crumbs3–Pals1 complex participates in the establishment of polarity in mammalian epithelial cells. J Cell Sci. 2003;116:2895–2906. doi: 10.1242/jcs.00500. [DOI] [PubMed] [Google Scholar]
- 25.Wang Q, Margolis B. Apical junctional complexes and cell polarity. Kidney Int. 2007;72:1448–1458. doi: 10.1038/sj.ki.5002579. [DOI] [PubMed] [Google Scholar]
- 26.Lu H, Bilder D. Endocytic control of epithelial polarity and proliferation in Drosophila. Nat Cell Biol. 2005;7:1232–1239. doi: 10.1038/ncb1324. [DOI] [PubMed] [Google Scholar]
- 27.Feng W, Wu H, Chan LN, Zhang M. Par-3-mediated junctional localization of the lipid phosphatase PTEN is required for cell polarity establishment. J Biol Chem. 2008;283:23440–23449. doi: 10.1074/jbc.M802482200. [DOI] [PubMed] [Google Scholar]
- 28.Takahama S, Hirose T, Ohno S. aPKC restricts the basolateral determinant PtdIns(3,4,5)P3 to the basal region. Biochem Biophys Res Commun. 2008;368:249–255. doi: 10.1016/j.bbrc.2008.01.083. [DOI] [PubMed] [Google Scholar]
- 29.von Stein W, Ramrath A, Grimm A, Muller-Borg M, Wodarz A. Direct association of Bazooka/PAR-3 with the lipid phosphatase PTEN reveals a link between the PAR/aPKC complex and phosphoinositide signaling. Development. 2005;132:1675–1686. doi: 10.1242/dev.01720. [DOI] [PubMed] [Google Scholar]
- 30.Pinal N, Goberdhan DC, Collinson L, Fujita Y, Cox IM, Wilson C, Pichaud F. Regulated and polarized PtdIns(3,4,5)P3 accumulation is essential for apical membrane morphogenesis in photoreceptor epithelial cells. Curr Biol. 2006;16:140–149. doi: 10.1016/j.cub.2005.11.068. [DOI] [PubMed] [Google Scholar]
- 31.Nakagawa H, Mutoh T, Kumano T, Kuriyama M. Tyrosine phosphorylation of the catalytic subunit p110 of phosphatidylinositol-3 kinase induced by HMG-CoA reductase inhibitor inhibits its kinase activity in L6 myoblasts. FEBS Lett. 2001;508:53–56. doi: 10.1016/s0014-5793(01)03021-6. [DOI] [PubMed] [Google Scholar]
- 32.Gassama-Diagne A, Yu W, ter Beest M, Martin-Belmonte F, Kierbel A, Engel J, Mostov K. Phosphatidylinositol-3,4,5-trisphosphate regulates the formation of the basolateral plasma membrane in epithelial cells. Nat Cell Biol. 2006;8:963–970. doi: 10.1038/ncb1461. [DOI] [PubMed] [Google Scholar]
- 33.Martin-Belmonte F, Gassama A, Datta A, Yu W, Rescher U, Gerke V, Mostov K. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell. 2007;128:383–397. doi: 10.1016/j.cell.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin-Belmonte F, Mostov K. Phosphoinositides control epithelial development. Cell Cycle. 2007;6:1957–1961. doi: 10.4161/cc.6.16.4583. [DOI] [PubMed] [Google Scholar]
- 35.Weisz OA, Rodriguez-Boulan E. Apical trafficking in epithelial cells: signals, clusters and motors. J Cell Sci. 2009;122:4253–4266. doi: 10.1242/jcs.032615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simons K, van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988;27:6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- 37.Paladino S, Sarnataro D, Pillich R, Tivodar S, Nitsch L, Zurzolo C. Protein oligomerization modulates raft partitioning and apical sorting of GPI-anchored proteins. J Cell Biol. 2004;167:699–709. doi: 10.1083/jcb.200407094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paladino S, Lebreton S, Tivodar S, Campana V, Tempre R, Zurzolo C. Different GPI-attachment signals affect the oligomerisation of GPI-anchored proteins and their apical sorting. J Cell Sci. 2008;121:4001–4007. doi: 10.1242/jcs.036038. [DOI] [PubMed] [Google Scholar]
- 39.Ihrke G, Bruns JR, Luzio JP, Weisz OA. Competing sorting signals guide endolyn along a novel route to lysosomes in MDCK cells. EMBO J. 2001;20:6256–6264. doi: 10.1093/emboj/20.22.6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D’Angelo R, Aresta S, Blangy A, Del Maestro L, Louvard D, Arpin M. Interaction of ezrin with the novel guanine nucleotide exchange factor PLEKHG6 promotes RhoG-dependent apical cytoskeleton rearrangements in epithelial cells. Mol Biol Cell. 2007;18:4780–4793. doi: 10.1091/mbc.E06-12-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vieira OV, Verkade P, Manninen A, Simons K. FAPP2 is involved in the transport of apical cargo in polarized MDCK cells. J Cell Biol. 2005;170:521–526. doi: 10.1083/jcb.200503078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delacour D, Gouyer V, Zanetta JP, Drobecq H, Leteurtre E, Grard G, Moreau-Hannedouche O, Maes E, Pons A, Andre S, et al. Galectin-4 and sulfatides in apical membrane trafficking in enterocyte-like cells. J Cell Biol. 2005;169:491–501. doi: 10.1083/jcb.200407073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morelle W, Stechly L, Andre S, Van Seuningen I, Porchet N, Gabius HJ, Michalski JC, Huet G. Glycosylation pattern of brush border-associated glycoproteins in enterocyte-like cells: involvement of complex-type N-glycans in apical trafficking. Biol Chem. 2009;390:529–544. doi: 10.1515/BC.2009.075. [DOI] [PubMed] [Google Scholar]
- 44.Hilpela P, Vartiainen MK, Lappalainen P. Regulation of the actin cytoskeleton by PI(4,5)P2 and PI(3,4,5)P3. Curr Top Microbiol Immunol. 2004;282:117–163. doi: 10.1007/978-3-642-18805-3_5. [DOI] [PubMed] [Google Scholar]
- 45.Yin HL, Janmey PA. Phosphoinositide regulation of the actin cytoskeleton. Annu Rev Physiol. 2003;65:761–789. doi: 10.1146/annurev.physiol.65.092101.142517. [DOI] [PubMed] [Google Scholar]
- 46.Pearson MA, Reczek D, Bretscher A, Karplus PA. Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell. 2000;101:259–270. doi: 10.1016/s0092-8674(00)80836-3. [DOI] [PubMed] [Google Scholar]
- 47.Kovacs EM, Goodwin M, Ali RG, Paterson AD, Yap AS. Cadherin-directed actin assembly: E-cadherin physically associates with the Arp2/3 complex to direct actin assembly in nascent adhesive contacts. Curr Biol. 2002;12:379–382. doi: 10.1016/s0960-9822(02)00661-9. [DOI] [PubMed] [Google Scholar]
- 48.Campbell RB, Liu F, Ross AH. Allosteric activation of PTEN phosphatase by phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2003;278:33617–33620. doi: 10.1074/jbc.C300296200. [DOI] [PubMed] [Google Scholar]
- 49.McConnachie G, Pass I, Walker SM, Downes CP. Interfacial kinetic analysis of the tumour suppressor phosphatase, PTEN: evidence for activation by anionic phospholipids. Biochem J. 2003;371:947–955. doi: 10.1042/BJ20021848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pece S, Chiariello M, Murga C, Gutkind JS. Activation of the protein kinase Akt/PKB by the formation of E-cadherin-mediated cell–cell junctions. Evidence for the association of phosphatidylinositol 3-kinase with the E-cadherin adhesion complex. J Biol Chem. 1999;274:19347–19351. doi: 10.1074/jbc.274.27.19347. [DOI] [PubMed] [Google Scholar]
- 51.Laprise P, Chailler P, Houde M, Beaulieu JF, Boucher MJ, Rivard N. Phosphatidylinositol 3-kinase controls human intestinal epithelial cell differentiation by promoting adherens junction assembly and p38 MAPK activation. J Biol Chem. 2002;277:8226–8234. doi: 10.1074/jbc.M110235200. [DOI] [PubMed] [Google Scholar]
- 52.Laprise P, Langlois MJ, Boucher MJ, Jobin C, Rivard N. Down-regulation of MEK/ERK signaling by E-cadherin-dependent PI3K/Akt pathway in differentiating intestinal epithelial cells. J Cell Physiol. 2004;199:32–39. doi: 10.1002/jcp.10432. [DOI] [PubMed] [Google Scholar]
- 53.Somasiri A, Wu C, Ellchuk T, Turley S, Roskelley CD. Phosphatidylinositol 3-kinase is required for adherens junction-dependent mammary epithelial cell spheroid formation. Differentiation. 2000;66:116–125. doi: 10.1046/j.1432-0436.2000.660206.x. [DOI] [PubMed] [Google Scholar]
- 54.Jeanes A, Smutny M, Leerberg JM, Yap AS. Phosphatidylinositol 3′-kinase signalling supports cell height in established epithelial monolayers. J Mol Histol. 2009;40:395–405. doi: 10.1007/s10735-010-9253-y. [DOI] [PubMed] [Google Scholar]
- 55.Roth MG. Phosphoinositides in constitutive membrane traffic. Physiol Rev. 2004;84:699–730. doi: 10.1152/physrev.00033.2003. [DOI] [PubMed] [Google Scholar]
- 56.Collins BM, McCoy AJ, Kent HM, Evans PR, Owen DJ. Molecular architecture and functional model of the endocytic AP2 complex. Cell. 2002;109:523–535. doi: 10.1016/s0092-8674(02)00735-3. [DOI] [PubMed] [Google Scholar]
- 57.Maddugoda MP, Crampton MS, Shewan AM, Yap AS. Myosin VI and vinculin cooperate during the morphogenesis of cadherin cell cell contacts in mammalian epithelial cells. J Cell Biol. 2007;178:529–540. doi: 10.1083/jcb.200612042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bryant DM, Stow JL. The ins and outs of E-cadherin trafficking. Trends Cell Biol. 2004;14:427–434. doi: 10.1016/j.tcb.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 59.Cresawn KO, Potter BA, Oztan A, Guerriero CJ, Ihrke G, Goldenring JR, Apodaca G, Weisz OA. Differential involvement of endocytic compartments in the biosynthetic traffic of apical proteins. EMBO J. 2007;26:3737–3748. doi: 10.1038/sj.emboj.7601813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Orzech E, Cohen S, Weiss A, Aroeti B. Interactions between the exocytic and endocytic pathways in polarized Madin-Darby canine kidney cells. J Biol Chem. 2000;275:15207–15219. doi: 10.1074/jbc.275.20.15207. [DOI] [PubMed] [Google Scholar]
- 61.Mostov KE, Verges M, Altschuler Y. Membrane traffic in polarized epithelial cells. Curr Opin Cell Biol. 2000;12:483–490. doi: 10.1016/s0955-0674(00)00120-4. [DOI] [PubMed] [Google Scholar]
- 62.Apodaca G, Katz LA, Mostov KE. Receptor-mediated transcytosis of IgA in MDCK cells is via apical recycling endosomes. J Cell Biol. 1994;125:67–86. doi: 10.1083/jcb.125.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barroso M, Sztul ES. Basolateral to apical transcytosis in polarized cells is indirect and involves BFA and trimeric G protein sensitive passage through the apical endosome. J Cell Biol. 1994;124:83–100. doi: 10.1083/jcb.124.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gibson A, Futter CE, Maxwell S, Allchin EH, Shipman M, Kraehenbuhl JP, Domingo D, Odorizzi G, Trowbridge IS, Hopkins CR. Sorting mechanisms regulating membrane protein traffic in the apical transcytotic pathway of polarized MDCK cells. J Cell Biol. 1998;143:81–94. doi: 10.1083/jcb.143.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown PS, Wang E, Aroeti B, Chapin SJ, Mostov KE, Dunn KW. Definition of distinct compartments in polarized Madin-Darby canine kidney (MDCK) cells for membrane-volume sorting, polarized sorting and apical recycling. Traffic. 2000;1:124–140. doi: 10.1034/j.1600-0854.2000.010205.x. [DOI] [PubMed] [Google Scholar]
- 66.van ISC, Hoekstra D. The subapical compartment: a novel sorting centre? Trends Cell Biol. 1999;9:144–149. doi: 10.1016/s0962-8924(99)01512-3. [DOI] [PubMed] [Google Scholar]
- 67.Hirokawa N, Noda Y. Intracellular transport and kinesin superfamily proteins, KIFs: structure, function, and dynamics. Physiol Rev. 2008;88:1089–1118. doi: 10.1152/physrev.00023.2007. [DOI] [PubMed] [Google Scholar]
- 68.Hirokawa N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science. 1998;279:519–526. doi: 10.1126/science.279.5350.519. [DOI] [PubMed] [Google Scholar]
- 69.Dagenbach EM, Endow SA. A new kinesin tree. J Cell Sci. 2004;117:3–7. doi: 10.1242/jcs.00875. [DOI] [PubMed] [Google Scholar]
- 70.Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, Igarashi P. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci USA. 2003;100:5286–5291. doi: 10.1073/pnas.0836980100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70a.Jaulin F, Kreitzer G. KIF17 stabilizes microtubules and contributes to epithelial morphogenesis by acting at MT plus ends with EB1 and APC. J Cell Biol. 2010;190:443–460. doi: 10.1083/jcb.201006044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fan S, Hurd TW, Liu CJ, Straight SW, Weimbs T, Hurd EA, Domino SE, Margolis B. Polarity proteins control ciliogenesis via kinesin motor interactions. Curr Biol. 2004;14:1451–1461. doi: 10.1016/j.cub.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 72.Hirokawa N, Tanaka Y, Okada Y. Left-right determination: involvement of molecular motor KIF3, cilia, and nodal flow. Cold Spring Harbor Perspect Biol. 2009;1:a000802. doi: 10.1101/cshperspect.a000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bonifacino JS. The GGA proteins: adaptors on the move. Nat Rev Mol Cell Biol. 2004;5:23–32. doi: 10.1038/nrm1279. [DOI] [PubMed] [Google Scholar]
- 74.Owen DJ, Collins BM, Evans PR. Adaptors for clathrin coats: structure and function. Annu Rev Cell Dev Biol. 2004;20:153–191. doi: 10.1146/annurev.cellbio.20.010403.104543. [DOI] [PubMed] [Google Scholar]
- 75.Ohno H, Tomemori T, Nakatsu F, Okazaki Y, Aguilar RC, Foelsch H, Mellman I, Saito T, Shirasawa T, Bonifacino JS. Mu1B, a novel adaptor medium chain expressed in polarized epithelial cells. FEBS Lett. 1999;449:215–220. doi: 10.1016/s0014-5793(99)00432-9. [DOI] [PubMed] [Google Scholar]
- 76.Gan Y, McGraw TE, Rodriguez-Boulan E. The epithelial-specific adaptor AP1B mediates post-endocytic recycling to the basolateral membrane. Nat Cell Biol. 2002;4:605–609. doi: 10.1038/ncb827. [DOI] [PubMed] [Google Scholar]
- 77.Folsch H, Ohno H, Bonifacino JS, Mellman I. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell. 1999;99:189–198. doi: 10.1016/s0092-8674(00)81650-5. [DOI] [PubMed] [Google Scholar]
- 78.Deborde S, Perret E, Gravotta D, Deora A, Salvarezza S, Schreiner R, Rodriguez-Boulan E. Clathrin is a key regulator of basolateral polarity. Nature. 2008;452:719–723. doi: 10.1038/nature06828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jordens I, Marsman M, Kuijl C, Neefjes J. Rab proteins, connecting transport and vesicle fusion. Traffic. 2005;6:1070–1077. doi: 10.1111/j.1600-0854.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- 80.Novick P, Zerial M. The diversity of Rab proteins in vesicle transport. Curr Opin Cell Biol. 1997;9:496–504. doi: 10.1016/s0955-0674(97)80025-7. [DOI] [PubMed] [Google Scholar]
- 81.Prekeris R. Rabs, Rips, FIPs, and endocytic membrane traffic. Scientific World J. 2003;3:870–880. doi: 10.1100/tsw.2003.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Casanova JE, Wang X, Kumar R, Bhartur SG, Navarre J, Woodrum JE, Altschuler Y, Ray GS, Goldenring JR. Association of Rab25 and Rab11a with the apical recycling system of polarized Madin-Darby canine kidney cells. Mol Biol Cell. 1999;10:47–61. doi: 10.1091/mbc.10.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang E, Brown PS, Aroeti B, Chapin SJ, Mostov KE, Dunn KW. Apical and basolateral endocytic pathways of MDCK cells meet in acidic common endosomes distinct from a nearly-neutral apical recycling endosome. Traffic. 2000;1:480–493. doi: 10.1034/j.1600-0854.2000.010606.x. [DOI] [PubMed] [Google Scholar]
- 84.Lock JG, Hammond LA, Houghton F, Gleeson PA, Stow JL. E-cadherin transport from the trans-Golgi network in tubulovesicular carriers is selectively regulated by golgin-97. Traffic. 2005;6:1142–1156. doi: 10.1111/j.1600-0854.2005.00349.x. [DOI] [PubMed] [Google Scholar]
- 85.Lock JG, Stow JL. Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Mol Biol Cell. 2005;16:1744–1755. doi: 10.1091/mbc.E04-10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Potter BA, Hughey RP, Weisz OA. Role of N- and O-glycans in polarized biosynthetic sorting. Am J Physiol. 2006;290:C1–C10. doi: 10.1152/ajpcell.00333.2005. [DOI] [PubMed] [Google Scholar]
- 87.Babbey CM, Ahktar N, Wang E, Chen CC, Grant BD, Dunn KW. Rab10 regulates membrane transport through early endosomes of polarized Madin-Darby canine kidney cells. Mol Biol Cell. 2006;17:3156–3175. doi: 10.1091/mbc.E05-08-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schuck S, Gerl MJ, Ang A, Manninen A, Keller P, Mellman I, Simons K. Rab10 is involved in basolateral transport in polarized Madin-Darby canine kidney cells. Traffic. 2007;8:47–60. doi: 10.1111/j.1600-0854.2006.00506.x. [DOI] [PubMed] [Google Scholar]
- 89.Sato T, Mushiake S, Kato Y, Sato K, Sato M, Takeda N, Ozono K, Miki K, Kubo Y, Tsuji A, et al. The Rab8 GTPase regulates apical protein localization in intestinal cells. Nature. 2007;448:366–369. doi: 10.1038/nature05929. [DOI] [PubMed] [Google Scholar]
- 90.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 91.Lapierre LA, Kumar R, Hales CM, Navarre J, Bhartur SG, Burnette JO, Provance DW, Jr, Mercer JA, Bahler M, Goldenring JR. Myosin vb is associated with plasma membrane recycling systems. Mol Biol Cell. 2001;12:1843–1857. doi: 10.1091/mbc.12.6.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schonteich E, Wilson GM, Burden J, Hopkins CR, Anderson K, Goldenring JR, Prekeris R. The Rip11/Rab11-FIP5 and kinesin II complex regulates endocytic protein recycling. J Cell Sci. 2008;121:3824–3833. doi: 10.1242/jcs.032441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tarbutton E, Peden AA, Junutula JR, Prekeris R. Class I FIPs, Rab11-binding proteins that regulate endocytic sorting and recycling. Methods Enzymol. 2005;403:512–525. doi: 10.1016/S0076-6879(05)03045-4. [DOI] [PubMed] [Google Scholar]
- 94.Meyers JM, Prekeris R. Formation of mutually exclusive Rab11 complexes with members of the family of Rab11-interacting proteins regulates Rab11 endocytic targeting and function. J Biol Chem. 2002;277:49003–49010. doi: 10.1074/jbc.M205728200. [DOI] [PubMed] [Google Scholar]
- 95.Hales CM, Vaerman JP, Goldenring JR. Rab11 family interacting protein 2 associates with myosin Vb and regulates plasma membrane recycling. J Biol Chem. 2002;277:50415–50421. doi: 10.1074/jbc.M209270200. [DOI] [PubMed] [Google Scholar]
- 96.Jing J, Junutula JR, Wu C, Burden J, Matern H, Peden AA, Prekeris R. FIP1/RCP binding to Golgin-97 regulates retrograde transport from recycling endosomes to the trans-Golgi network. Mol Biol Cell. 2010;21:3041–3053. doi: 10.1091/mbc.E10-04-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Munson M, Novick P. The exocyst defrocked, a framework of rods revealed. Nat Struct Mol Biol. 2006;13:577–581. doi: 10.1038/nsmb1097. [DOI] [PubMed] [Google Scholar]
- 98.Whyte JR, Munro S. The Sec34/35 Golgi transport complex is related to the exocyst, defining a family of complexes involved in multiple steps of membrane traffic. Dev Cell. 2001;1:527–537. doi: 10.1016/s1534-5807(01)00063-6. [DOI] [PubMed] [Google Scholar]
- 99.Wu H, Rossi G, Brennwald P. The ghost in the machine: small GTPases as spatial regulators of exocytosis. Trends Cell Biol. 2008;18:397–404. doi: 10.1016/j.tcb.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cai H, Reinisch K, Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell. 2007;12:671–682. doi: 10.1016/j.devcel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 101.Jahn R, Scheller RH. SNAREs – engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 102.Low SH, Chapin SJ, Weimbs T, Komuves LG, Bennett MK, Mostov KE. Differential localization of syntaxin isoforms in polarized Madin-Darby canine kidney cells. Mol Biol Cell. 1996;7:2007–2018. doi: 10.1091/mbc.7.12.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pocard T, Le Bivic A, Galli T, Zurzolo C. Distinct v-SNAREs regulate direct and indirect apical delivery in polarized epithelial cells. J Cell Sci. 2007;120:3309–3320. doi: 10.1242/jcs.007948. [DOI] [PubMed] [Google Scholar]
- 104.Steegmaier M, Lee KC, Prekeris R, Scheller RH. SNARE protein trafficking in polarized MDCK cells. Traffic. 2000;1:553–560. doi: 10.1034/j.1600-0854.2000.010705.x. [DOI] [PubMed] [Google Scholar]
- 105.Lafont F, Verkade P, Galli T, Wimmer C, Louvard D, Simons K. Raft association of SNAP receptors acting in apical trafficking in Madin-Darby canine kidney cells. Proc Natl Acad Sci USA. 1999;96:3734–3738. doi: 10.1073/pnas.96.7.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rothman JE, Warren G. Implications of the SNARE hypothesis for intracellular membrane topology and dynamics. Curr Biol. 1994;4:220–233. doi: 10.1016/s0960-9822(00)00051-8. [DOI] [PubMed] [Google Scholar]
- 107.O’Brien LE, Zegers MM, Mostov KE. Opinion: building epithelial architecture: insights from three-dimensional culture models. Nat Rev Mol Cell Biol. 2002;3:531–537. doi: 10.1038/nrm859. [DOI] [PubMed] [Google Scholar]
- 108.Andrew DJ, Ewald AJ. Morphogenesis of epithelial tubes: insights into tube formation, elongation, and elaboration. Dev Biol. 2010;341:34–55. doi: 10.1016/j.ydbio.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Michos O. Kidney development: from ureteric bud formation to branching morphogenesis. Curr Opin Genet Dev. 2009;19:484–490. doi: 10.1016/j.gde.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vega-Salas DE, Salas PJ, Rodriguez-Boulan E. Modulation of the expression of an apical plasma membrane protein of Madin-Darby canine kidney epithelial cells: cell–cell interactions control the appearance of a novel intracellular storage compartment. J Cell Biol. 1987;104:1249–1259. doi: 10.1083/jcb.104.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Davis GE, Bayless KJ. An integrin and Rho GTPase-dependent pinocytic vacuole mechanism controls capillary lumen formation in collagen and fibrin matrices. Microcirculation. 2003;10:27–44. doi: 10.1038/sj.mn.7800175. [DOI] [PubMed] [Google Scholar]
- 112.Orlando RA, Takeda T, Zak B, Schmieder S, Benoit VM, McQuistan T, Furthmayr H, Farquhar MG. The glomerular epithelial cell anti-adhesin podocalyxin associates with the actin cytoskeleton through interactions with ezrin. J Am Soc Nephrol. 2001;12:1589–1598. doi: 10.1681/ASN.V1281589. [DOI] [PubMed] [Google Scholar]
- 113.Takeda T, Go WY, Orlando RA, Farquhar MG. Expression of podocalyxin inhibits cell–cell adhesion and modifies junctional properties in Madin-Darby canine kidney cells. Mol Biol Cell. 2000;11:3219–3232. doi: 10.1091/mbc.11.9.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Meder D, Shevchenko A, Simons K, Fullekrug J. Gp135/podocalyxin and NHERF-2 participate in the formation of a preapical domain during polarization of MDCK cells. J Cell Biol. 2005;168:303–313. doi: 10.1083/jcb.200407072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li BX, Satoh AK, Ready DF. Myosin V, Rab11, and dRip11 direct apical secretion and cellular morphogenesis in developing Drosophila photoreceptors. J Cell Biol. 2007;177:659–669. doi: 10.1083/jcb.200610157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schluter MA, Pfarr CS, Pieczynski J, Whiteman EL, Hurd TW, Fan S, Liu CJ, Margolis B. Trafficking of Crumbs3 during cytokinesis is crucial for lumen formation. Mol Biol Cell. 2009;20:4652–4663. doi: 10.1091/mbc.E09-02-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Coucouvanis E, Martin GR. Signals for death and survival: a two-step mechanism for cavitation in the vertebrate embryo. Cell. 1995;83:279–287. doi: 10.1016/0092-8674(95)90169-8. [DOI] [PubMed] [Google Scholar]
- 118.Debnath J, Mills KR, Collins NL, Reginato MJ, Muthuswamy SK, Brugge JS. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell. 2002;111:29–40. doi: 10.1016/s0092-8674(02)01001-2. [DOI] [PubMed] [Google Scholar]
- 119.Martin-Belmonte F, Yu W, Rodriguez-Fraticelli AE, Ewald AJ, Werb Z, Alonso MA, Mostov K. Cell-polarity dynamics controls the mechanism of lumen formation in epithelial morphogenesis. Curr Biol. 2008;18:507–513. doi: 10.1016/j.cub.2008.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Humphreys RC, Krajewska M, Krnacik S, Jaeger R, Weiher H, Krajewski S, Reed JC, Rosen JM. Apoptosis in the terminal endbud of the murine mammary gland: a mechanism of ductal morphogenesis. Development. 1996;122:4013–4022. doi: 10.1242/dev.122.12.4013. [DOI] [PubMed] [Google Scholar]
- 121.Jaskoll T, Melnick M. Submandibular gland morphogenesis: stage-specific expression of TGF-α/EGF, IGF, TGF-β, TNF, and IL-6 signal transduction in normal embryonic mice and the phenotypic effects of TGF-β2, TGF-β3, and EGF-r null mutations. Anat Rec. 1999;256:252–268. doi: 10.1002/(SICI)1097-0185(19991101)256:3<252::AID-AR5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 122.Lin HH, Yang TP, Jiang ST, Yang HY, Tang MJ. Bcl-2 overexpression prevents apoptosis-induced Madin-Darby canine kidney simple epithelial cyst formation. Kidney Int. 1999;55:168–178. doi: 10.1046/j.1523-1755.1999.00249.x. [DOI] [PubMed] [Google Scholar]
- 123.Mailleux AA, Overholtzer M, Schmelzle T, Bouillet P, Strasser A, Brugge JS. BIM regulates apoptosis during mammary ductal morphogenesis, and its absence reveals alternative cell death mechanisms. Dev Cell. 2007;12:221–234. doi: 10.1016/j.devcel.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Meredith JE, Jr, Fazeli B, Schwartz MA. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993;4:953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Frisch SM, Francis H. Disruption of epithelial cell–matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ruoslahti E, Reed JC. Anchorage dependence, integrins, and apoptosis. Cell. 1994;77:477–478. doi: 10.1016/0092-8674(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 127.Martin-Belmonte F, Rodriguez-Fraticelli AE. Acquisition of membrane polarity in epithelial tube formation patterns, signaling pathways, molecular mechanisms, and disease. Int Rev Cell Mol Biol. 2009;274:129–182. doi: 10.1016/S1937-6448(08)02003-0. [DOI] [PubMed] [Google Scholar]
- 128.Bagnat M, Cheung ID, Mostov KE, Stainier DY. Genetic control of single lumen formation in the zebrafish gut. Nat Cell Biol. 2007;9:954–960. doi: 10.1038/ncb1621. [DOI] [PubMed] [Google Scholar]
- 129.Kamei M, Saunders WB, Bayless KJ, Dye L, Davis GE, Weinstein BM. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature. 2006;442:453–456. doi: 10.1038/nature04923. [DOI] [PubMed] [Google Scholar]
- 130.Blum Y, Belting HG, Ellertsdottir E, Herwig L, Luders F, Affolter M. Complex cell rearrangements during intersegmental vessel sprouting and vessel fusion in the zebrafish embryo. Dev Biol. 2008;316:312–322. doi: 10.1016/j.ydbio.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 131.Blatchford DR, Quarrie LH, Tonner E, McCarthy C, Flint DJ, Wilde CJ. Influence of microenvironment on mammary epithelial cell survival in primary culture. J Cell Physiol. 1999;181:304–311. doi: 10.1002/(SICI)1097-4652(199911)181:2<304::AID-JCP12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]