Abstract
Purpose.
We investigated the progressive degeneration of retinal and superior collicular functions in a mouse model of sustained ocular hypertension.
Methods.
Focal laser illumination and injection of polystyrene microbeads were used to induce chronic ocular hypertension. Retinal ganglion cell (RGC) loss was characterized by in vivo optical coherence tomography (OCT) and immunohistochemistry. Retinal dysfunction was also monitored by the full-field ERG. Retinal ganglion cell light responses were recorded using a 256-channel multielectrode array (MEA), and RGC subtypes were characterized by noncentered spike-triggered covariance (STC-NC) analysis. Single-unit extracellular recordings from superficial layers of the superior colliculus (SC) were performed to examine the receptive field (RF) properties of SC neurons.
Results.
The elevation of intraocular pressure (IOP) lasted 4 months in mice treated with a combination of laser photocoagulation and microbead injection. Progressive RGC loss and functional degeneration were confirmed in ocular hypertensive (OHT) mice. These mice had fewer visually responsive RGCs than controls. Using the STC-NC analysis, we classified RGCs into ON, OFF, and ON-OFF functional subtypes. We showed that ON and OFF RGCs were more susceptible to the IOP elevation than ON-OFF RGCs. Furthermore, SC neurons of OHT mice had weakened responses to visual stimulation and exhibited mismatched ON and OFF subfields and irregular RF structure.
Conclusions.
We demonstrated that the functional degeneration of RGCs is subtype-dependent and that the ON and OFF pathways from the retina to the SC were disrupted. Our study provides a foundation to investigate the mechanisms underlying the progressive vision loss in experimental glaucoma.
Keywords: ocular hypertension, retinal ganglion cells, superior colliculus, neural degeneration, multielectrode array
Sustained ocular hypertension induced progressive degeneration of retinal and superior collicular function in mice.
Introduction
Glaucoma is characterized by progressive degeneration of RGCs and vision loss.1–5 Because elevated IOP is the most important risk factor for the development of glaucoma, different animal models with acute or chronic ocular hypertension have been established to investigate the characteristics of RGC loss.6,7 Among the models are episcleral venous occlusion, which induces IOP elevation for several days8,9; injection of polystyrene beads, which leads to IOP elevation for 2 to 4 weeks10–14; and laser photocoagulation of the outflow of aqueous humor,15,16 which—in our hands—results in IOP elevation for 2 months in mice.17,18
Progressive RGC loss has been demonstrated in these different models of experimental glaucoma, and many studies suggest that different types of RGCs may respond to the glaucomatous insult differently.12,14,18–20 In mice, there are more than 20 types of RGCs, each with a unique dendritic morphology and function,21,22 making it challenging to profile how each RGC type degenerates and dies in experimental glaucoma. Based on their responses to light onset and offset, RGCs can be classified into three functional subtypes: ON, OFF, and ON-OFF.23–25 Spike-triggered average (STA) analysis has been used to classify RGCs into ON or OFF subtypes,26 and Della Santina and colleagues14 used this technique to show that OFF-transient RGCs degenerate more rapidly than other types in glaucomatous mice. However, STA analysis struggles to identify and characterize the RF properties of ON-OFF RGCs.24 To address this deficiency, we developed noncentered spike-triggered covariance (STC-NC) analysis, which maintains the simplicity of STA but is capable of characterizing RGCs of the ON-OFF center variety.24
In glaucoma, RGC degeneration and death are followed by morphologic and functional changes in the higher visual centers.27,28 Functional magnetic resonance imaging (fMRI) studies indicate that glaucoma patients suffer a significant loss of visual function in the primary visual cortex.27 In monkeys with ocular hypertension, neurons in the lateral geniculate nucleus (LGN) exhibit decreased soma and dendritic field sizes.28–31 In rodents, the SC is the most prominent target of RGC axons, and each superficial SC neuron receives converging inputs from balanced ON- and OFF-centered RGCs with overlapping RFs, an arrangement believed to be important for detecting object salience.32–35 While it has been reported that the RFs of SC neurons were larger in a rat model of experimental glaucoma,36 there has been little study of SC neuronal responses in glaucomatous mice.
In this study, we combined laser illumination with injection of polystyrene microbeads to achieve sustained ocular hypertension for several months. We confirmed the RGC loss by OCT imaging and immunohistochemistry. We recorded the light responses of different subtype RGCs with a 256-channel MEA system and characterized their RF properties using STA and STC-NC analysis, permitting inclusion of ON-OFF RGCs. We also investigated the physiological consequences of retinal degeneration on the RF properties of SC neurons.
Materials and Methods
All animal procedures were approved by the Institutional Animal Care and Use Committee at Northwestern University and conformed to the guidelines on the Use of Animals from the NIH and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Induction of IOP Elevation
Intraocular pressure (IOP) elevation was induced by laser photocoagulation and injection of microbeads into the eyes of wild-type (WT) and Thy1-YFP transgenic mice with the C57BL/6 background (Line H; Jackson Laboratory, Bar Harbor, ME, USA). We induced ocular hypertension in right eyes and used the left eyes of the same animals as controls. The procedure of laser illumination has been described previously.17,18 In brief, 2- to 3-month-old mice were anesthetized by an intraperitoneal injection of ketamine (100 mg/kg; Butler Schein Animal Health, Dublin, OH, USA) and xylazine (10 mg/kg; Lloyd, Inc., Iowa, Shenandoah, IA, USA). Approximately 80 to 100 laser spots (514-nm wavelength and 200-μm diameter, Ultima 2000 SE; Coherent, Inc., Santa Clara, CA, USA) were applied to the corneal limbus of the right eye.17,18 To achieve the long-term ocular hypertension (>10 weeks), we combined laser photocoagulation with microbead injection. One microliter of polystyrene microbeads (10-μm diameter, 14.4 × 106 beads/mL in PBS; Life Technologies, Eugene, OR, USA) was immediately injected into the anterior chamber of the same eye by a Hamilton syringe right after laser illumination.11,12 The left eyes of the same animals were untreated or injected with saline (1 μL; Molecular Toxicology, Inc., Boone, NC, USA) to serve as controls.
The intraocular pressure of the right eyes was measured (TonoLab; Colonial Medical Supply, Franconia, NH, USA) every 2 weeks for the first month and then every 2 to 4 weeks until the mice were killed for experiments.17,18,37 The left eyes of the same animals were measured at the same time as baseline controls. Mice with IOP of the right eyes constantly more than 50% above baseline (i.e., left eyes) were considered as OHT mice. Mice with a transient IOP elevation (i.e., IOP elevated for the first 1 or 2 weeks but which then dropped back to the baseline) were excluded from this study. For electroretinogram measurements, mice were repeatedly tested following IOP elevation. Some of these mice were used for the in vivo electrophysiological recording experiments described below.
Optical Coherence Tomography (OCT)
The optical coherence tomography system was custom built by the Zhang lab at Northwestern University.38 The laser beam (SuperK versa, ~500–620 nm; NKT Photonics, Birkerød, Denmark) was divided into a sample arm and a reference arm by a cube beam splitter ([BS] CM1-BS013; Thorlabs, Inc., Newton, NJ, USA) and then delivered onto the eye.38 The light reflected from the reference and sample arms was recombined at the BS and delivered to a linear CCD camera (sp2k; Basler AG, Ahrensburg, Schleswig-Holstein, Germany) after passing through an optical grating for spectral acquisition at an A-line rate of 70 kHz. The axial (z-axis) and lateral (x- and y-axis) resolutions of the OCT system were 0.8 and 15 μm in the retina, respectively.38 After the three-dimensional (3D) image of the mouse retina was taken, the fundus image was obtained through mean intensity projection of the z-stacked image.38,39 Five to ten cross-section images were then obtained from each retina. The thickness of nerve fiber layer (NFL) and ganglion cell layer (GCL, labeled as NFL+GCL) and the inner plexiform layer (IPL) and inner nuclear layer (INL, labeled as IPL+INL) and the total thickness (which included retina, pigment epithelium, and choroid) were measured manually from the cross-section images in ImageJ (http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA).
Immunohistochemistry
Whole-mount retinas were prepared for immunostaining as described previously.18,40,41 The primary antibodies used in this study included mouse anti-Brn3a (MAB1585, 1:400; Millipore Corp., Billerica, MA, USA); goat anti-Brn3b (sc-6026, 1:1000; Santa Cruz Biotechnology, Dallas, TX, USA); and AlexaFluor-conjugated secondary antibodies were used (1:1000; Invitrogen, Carlsbad, CA, USA). After immunostaining, images were captured with a confocal microscope (Zeiss Pascal; Zeiss, Thornwood, NY, USA). For cell counting, 16 to 20 micrographs per retina were used to quantify the cell density (Imaris; Bitplane AG, Zürich, Switzerland).18
Full-Field ERG Recordings
Electroretinogram recordings were performed using a commercial platform (Micron III; Phoenix Research Laboratories, Pleasanton, CA, USA). Mice were dark adapted overnight prior to recording, and all procedures were performed under dim red light. The pupils of anesthetized mice were dilated by 1% tropicamide (Akorn, Lake Forest, IL, USA) and 2.5% phenylephrine (Bausch & Lomb, Tampa, FL, USA). A gold-tipped corneal electrode was placed in contact with the cornea with ophthalmic solution (Goniovisc 2.5%; HUB Pharmaceuticals, Rancho Cucamonga, CA, USA). The reference electrode was placed subcutaneously between the ears and the ground electrode inserted into the base of the tail. The mice were placed on a heated platform (Phoenix Research Laboratories, Pleasanton, CA, USA) during recording and recovery.
Full-field ERGs were obtained for flashing white light of a range of intensities (−2.0, −0.97, 0.51, 2.13, and 3.53 log cd × s/m2). The light pulse was 10 ms in duration, and the stimulus repeated five times. The electrical signals from the retinas were bandpass filtered between 0.5 Hz and 2 kHz over a 300-ms period and analyzed using customized programs (MATLAB; MathWorks, Natick, MA, USA). Electroretinogram waveforms were averaged from five trials at each intensity level. The amplitude of the a-wave was measured from the baseline to the maximum negative-valued peak (a-wave peak), and the amplitude of the b-wave was from the a-wave peak to the maximum positive-valued peak (b-wave peak). The waveform between the a- and b-wave peaks was then bandpass filtered between 50 and 300 Hz to reveal the oscillatory potentials (OPs16,42). The amplitude of OP was measured as the difference between the maximum positive- and negative-valued peaks from the filtered waveform.
MEA Recordings
Multielectrode array (MEA) recordings were performed as described previously.18,24 In brief, ocular hypertension was induced in mice and, after 5 to 7 weeks, the eyes were removed and retinas dissected.18 Retinal ganglion cells from the OHT eyes and age-matched control eyes were recorded using a 256-channel MEA (MEA-200/30iR-ITO; Multi Channel Systems MCS GmbH, Reutlingen, Germany). Voltage signals from the MEA were sampled at 25 kHz, and spike trains detected using a 5.5-SD voltage threshold in MC_Rack (Multi Channel Systems MCS GmbH, Reutlingen, Germany), sorted into units (Offline Sorter; Plexon, Dallas, TX, USA), and exported to computing software (MATLAB; MathWorks) for further analysis.18,24
We used a spatiotemporal Gaussian white-noise stimulus, which appeared as a flickering grayscale checkerboard with random spatial and temporal structure (100 × 100-μm square checker).24 The firing rate of the RGCs in response to the stimulus was defined as the ratio of the total number of spikes to the total stimulation time period (60 minutes). To characterize the RF properties of RGCs, we performed the computational analysis described next.18,24
Step 1: STA.
The spike-triggered average (STA) provides an estimate of the linear spatiotemporal RF properties of RGCs. It was calculated by averaging all stimulus frames in a 1-second window preceding each spike, generating a sequence of the mean effective stimulus (30 frames) for triggering a spike. The spike-triggered average was then normalized by the mean and SD of the STA elements (normalized STA = [STA − mean]/SD). Spike-triggered average contrast was defined as the maximum absolute value of the normalized STA elements.18
Step 2: STA Thresholding.
We defined visual responsiveness using the STA contrast. Cells with STA contrast greater than five were classified as visually responsive cells. A retinal ganglion cell was deemed unresponsive if no element of its STA exceeded 5 SD.18,24
Step 3: STC-NC.
We further performed STC-NC analysis in order to identify three functional subtypes (ON, OFF, and ON-OFF).24 Traditionally, principal component analysis (PCA) was performed by the Eigen decomposition of the mean-centered covariance matrix. Instead, we used Eigen decomposition on a noncentered second moment matrix, which simplifies the computational analysis but is capable of characterizing different RGC subtypes.24
Step 4: Threshold STC-NC.
The noncentered spike-triggered covariance contrast measures how strong the RF center signal was compared with the surrounding noise. It was calculated as the maximum magnitude of the STC-NC vector divided by the SD of the outermost ring of pixels in the STC-NC. We set 5 SD as the threshold of STC-NC contrast for further analysis.
Step 5: Static Nonlinearity.
For retinal ganglion cells with STC-NC contrast larger than 5 SD, the corresponding static nonlinearities were recovered by using the projection of the spike-triggered ensemble (STE) onto the STC-NC. STC-NC bias of the cell was then determined as the scalar value:
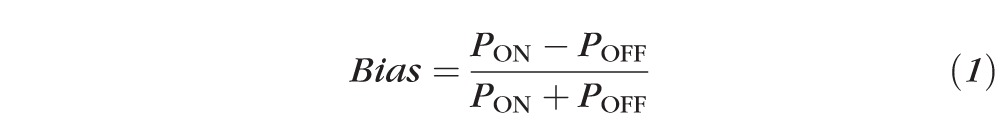 |
where PON and POFF represent integrals of the static nonlinearity over the positive or negative ranges of contrast, respectively.
Step 6: Cell-Type Classification.
We classified RGCs into ON, OFF, and ON-OFF subtypes based on the value of bias from step 5. For cells with STC-NC contrast larger than 5 SD, we classified a cell as ON with STC-NC bias ≥ 0.6, OFF with bias ≤ −0.6, and ON-OFF with −0.6 < bias < 0.6. Cells with STC-NC contrast less than 5 SD but STA above 5 SD were also included in our sample as they were light-responsive cells; they were separated into ON or OFF cells based on the polarity of the maximum value of the normalized STA elements.24 The spatial extent of the RF center was computed from the STA. The peak frame of the STA containing the maximal deviation from the mean was determined, and a bivariate Gaussian distribution was fit to this frame to delineate the RF center. The receptive field center size was calculated as the size of the ellipse defined by the contour of the Gaussian at 1 SD.
In Vivo Single-Unit Electrophysiology
The receptive field structures of superficial SC neurons were measured following our published protocol.33,43 Urethane (1.2–1.3 g/kg in 10% saline solution, intraperitoneal injection) supplemented with chlorprothixene (10 mg/kg in 4-mg/mL water solution, intramuscular injection) was used to anesthetize the animals because of the long-lasting effect of urethane for stable recordings of neural activity.44 Atropine (0.3 mg/kg) and dexamethasone (2.0 mg/kg) were injected subcutaneously to minimize mucus secretion and edema, respectively. A craniotomy (4–8 mm2) was performed on the left hemisphere to expose the brain for recording with 5 to 10-MΩ tungsten microelectrodes (FHC, Inc., Bowdoinham, ME, USA). The electrode was inserted vertically through the cortex into the SC. Neurons within 300 μm below the SC surface were included in our analysis, corresponding to the superficial retinal recipient layers of the SC.33,43 Amplified voltage signals, filtered between 0.5 and 7 kHz and sampled at 25 kHz by a commercial workstation (System 3; Tucker Davis Technologies, Alachua, FL, USA) were recorded, and the spikes sorted into single units by commercial software (OpenSorter; Tucker Davis Technologies).
Visual stimuli were generated using the extensions (MathWorks) as described previously.45–47 In brief, the stimuli were displayed on a flat panel cathode ray tube monitor (G225f, 60 Hz refresh rate, ~35 cd/m2 mean luminance; ViewSonic, Walnut, CA, USA) placed 25 cm from the animal, and delivered to the right eye while the left eye was occluded.33,43,47 To determine RF structures of SC neurons, 5 × 5° white squares were flashed at different locations on 13 × 13 or 11 × 11 grids. The flashed spot stayed on for 0.5 seconds on a gray background (ON) followed by the background luminance for 0.5 seconds (OFF). The spontaneous firing rate was calculated when no flash was displayed.33,43,48
A cell was considered light responsive at a given grid location when there were more spikes than the 2 SD above the mean spontaneous rate in more than 40% of the total trials.43,48 After subtracting the spontaneous rate, the cell's firing rate at each responsive grid location was calculated by averaging spikes within a 200-ms time window (starting from 50 ms after flash onset or offset) from all trials. The peak firing rate of a cell was defined as the maximal value from all grid locations, and mean firing rate as the mean value from all grid locations.
Peak and mean firing rates for ON and OFF responses were measured, and the following response ratio calculated:
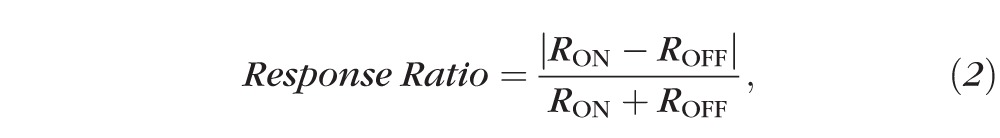 |
where RON and ROFF represent the peak or mean firing rate of ON and OFF responses. The response ratio ranges from zero to one, with a value of zero indicating an identical firing rate for ON and OFF responses and one indicating the cell has a pure ON or pure OFF response.
The receptive field was mapped based on the firing rates from all grid locations where visual responses were evoked by the flashing spot. We first fitted the RFs to a two-dimensional (2D) Gaussian function to model its structure. In order to get more accurate fits, the RFs were processed by linear interpolation from 11 × 11 (or 13 × 13) to 55 × 55 (or 65 × 65) grids to generate more data points and then fitted with the 2D Gaussian in the coordinate system defined by the axes of the response field:
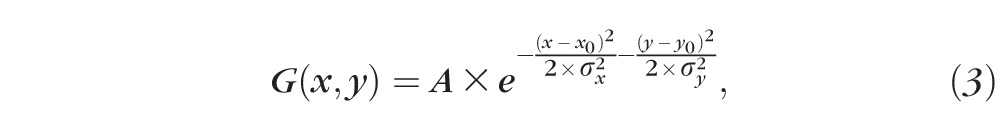 |
where A is the amplitude of the Gaussian, x0 and y0 refer to the center location where the maximum response was observed, and σx and σy are the SDs. The value of R2 was used to quantify the goodness of fit, with R2 ≥ 0.8 representing a normal RF structure. When the value of R2 was less than 0.8, the RF could not be fitted by a 2D Gaussian and exhibited an irregular structure with multiple peaks. The ON and OFF subfields were mapped similarly based on grid locations with either ON or OFF responses.
Because some RF structures could not be fitted by 2D Gaussians, we next measured and compared the RF sizes by counting responsive squares regardless of ON or OFF polarity. Similarly, the sizes of the ON and OFF subfields were calculated by counting squares with either ON or OFF responses. The overlap between ON and OFF subfields was characterized by the overlap ratio, the number of grids having both ON and OFF responses divided by the RF size. The overlap ratio ranges from 0 to 1, with a value of 1 indicating complete ON-OFF overlap and 0 no overlap.
Statistics
The Student's t-test and Kolmogorov–Smirnov (K-S) test were used to examine the difference between paired samples and the shapes of distributions of continuous-valued variables, respectively. A two-sample χ2 test was used to compare distributions of categorical variables (such as the distributions of RF size). A one-way ANOVA test was applied to compare the IOP values among control and experimental groups at a particular time point and a two-way ANOVA test to compare the ERG amplitudes recorded at all light intensities among different groups.
Results
Sustained Ocular Hypertension Is Enhanced by Combining Photocoagulation With Microbead Injection
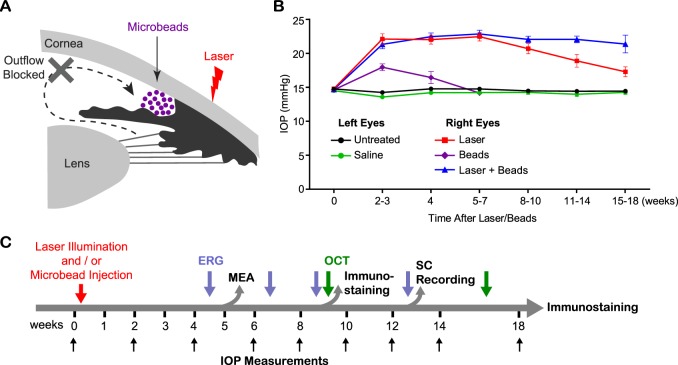
We compared the effects on IOP elevation by laser photocoagulation, microbead injection, and the combination of the two methods (Fig. 1). Elevation of IOP was induced in the right eyes (experimental group) and compared with the left eyes (control group) of the same animals. Laser illumination–induced IOP elevation lasts for approximately 2 months but decreases from then on (Fig. 1B), as shown previously.18 Microbead injections achieved a milder IOP elevation, which also lasted a shorter time period (Fig. 1B). At 2 to 3 weeks after the induction of IOP elevation, the mean IOP of right eyes treated with laser illumination was 22.2 ± 0.8 mm Hg (n = 57), significantly higher than untreated eyes (14.3 ± 0.1 mm Hg, n = 127; P < 0.001, one-way ANOVA, Tukey's posttest; Fig. 1B). By contrast, the IOP elevation induced by microbead injection (18.0 ± 0.5 mm Hg, n = 12) was lower than the laser-treated eyes (P < 0.01), though significantly higher than saline-injected eyes (13.7 ± 0.2 mm Hg, n = 19; P < 0.05, one-way ANOVA, Tukey's posttest). Furthermore, the IOP elevation induced by microbead injection fell back to baseline by the end of 7 weeks (14.2 ± 0.3 mm Hg; P = 0.97, one-way ANOVA, Tukey's posttest). Note that the saline-injected eyes exhibited normal IOP (14.2 ± 0.1 mm Hg), similar to the untreated control eyes (14.6 ± 0.1 mm Hg; P = 0.50, two-way ANOVA, Tukey's posttest; Fig. 1B).
Figure 1.
Sustained ocular hypertension was induced by laser photocoagulation and injection of microbeads. (A) Schematic diagram of the laser illumination and microbead injection to induce ocular hypertension. (B) Intraocular pressure measurement of right eyes treated with laser illumination (red), microbead injection (purple), or combined laser illumination with microbead injection (blue). Left eyes of the same animals were used as controls either untreated (black) or injected with saline (green). (C) Schematic diagram of the experimental design.
In order to boost long-term IOP elevation (>10 weeks), we injected microbeads immediately after laser illumination. The elevation of IOP induced by laser illumination itself or in combination with microbead injection showed no difference for the first 10 weeks (P = 0.99, two-way ANOVA, Tukey's posttest; Fig. 1B). Therefore, we employed laser illumination only for experimental groups with IOP elevation for shorter than 10 weeks. At 11 to 14 weeks post laser treatment, the mean IOP of the laser-treated eyes started to decrease (19.0 ± 0.9 mm Hg, n = 24), and dropped further at 15 to 18 weeks (17.4 ± 0.7 mm Hg, n = 24; Fig. 1B). By contrast, the IOP of the eyes treated with the laser and microbead combination remained above 20 mm Hg (11–14 weeks: 22.1 ± 0.5 mm Hg, n = 57; 15–18 weeks: 21.5 ± 1.3 mm Hg, n = 12), which was significantly higher than those of laser-treated eyes (P < 0.001, one-way ANOVA, Tukey's posttest; Fig. 1B). Thus, by combining laser photocoagulation with microbead injections, IOP elevation was maintained for a longer period, making this the preferred technique for studies of the long-term effect of IOP elevation on functional changes of the retina and higher visual centers. We used the laser and microbead combination to induce the chronic ocular hypertension longer than 10 weeks.
OCT Imaging and Immunohistochemistry Confirmed RGC Loss in Mice With Sustained IOP Elevation
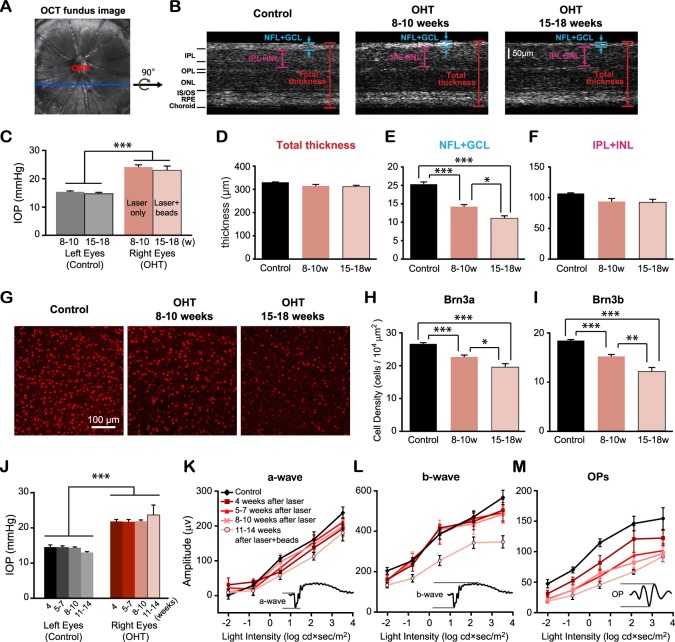
We had previously shown that chronic ocular hypertension induces progressive RGC loss in mice with laser-induced ocular hypertension.18 Here, we confirmed the degeneration of the RGCs following chronic IOP elevation by two methods: in vivo OCT imaging and immunohistochemistry (Figs. 2A–I). Intraocular pressure was measured right before experiments (Fig. 2C), and OCT images were taken to compare the retinal thickness in the OHT (right) and control eyes (left) of the same animals (Figs. 2A–F). First, we measured the total thickness from OHT eyes and controls and found that they were comparable (P > 0.05, Student's t-test; Fig. 2D). Next, we quantified the changes of the NFL+GCL (Fig. 2E). At 8 to 10 weeks post laser illumination, the thickness of NFL+GCL in OHT eyes (n = 4 retinas) was reduced 29.9% compared with control eyes (n = 5 retinas, P < 0.001, Student's t-test; Fig. 2E). At 15 to 18 weeks post laser and microbeads, the thickness of NFL+GCL decreased further to be just 54.4% of controls (n = 3, P < 0.001 in Student's t-test; Fig. 2E). We also measured the thickness of the IPL and INL and found the trend of decrease following chronic IOP elevation, though the change was not significant (P = 0.07 at 8–10 weeks and P = 0.10 at 15–18 weeks, Student's t-test; Fig. 2F).
Figure 2.
Retinal ganglion cell loss following chronic IOP elevation. (A) The fundus image obtained by the OCT imaging. ONH, optic nerve head. (B) The cross-sections of retinas from a control eye (left) and OHT eyes at 8 to 10 weeks (center) and 15 to 18 weeks (right). OPL, outer plexiform layer; ONL, outer nuclear layer; IS, inner segment; OS, outer segment. (C) The mean IOP from OHT eyes measured right before OCT was significantly higher than that of control eyes. (D–F) The thickness of the NFL and GCL decreased significantly post IOP elevation (E), but not the total thickness (D) or the thickness of the IPL and INL (F). (G) Flat-mounted retinas immunostained by Brn3b antibody from one control and two OHT eyes. (H, I) The density of RGCs labeled by Brn3a (H) and Brn3b (I) decreased following chronic ocular hypertension. (J) The mean IOP measured right before ERG recordings. (K–M) The mean amplitudes of OPs of laser-treated eyes continued to decrease with time (P < 0.001, two-way ANOVA, Tukey's posttest), but not a- and b-wave for the first 10 weeks (P > 0.7). The mean amplitudes of a- and b-wave were significantly reduced at 11 to 14 weeks following chronic IOP elevation (P < 0.05 for a-wave, and P < 0.001 for b-wave). Insets: Representative ERG waveforms of the a-wave, b-wave, and OPs. For (C–F) and (H–J): *P < 0.05. **P < 0.01. ***P < 0.001 in Student's t-test.
We next confirmed the progressive loss of RGCs in OHT eyes with immunohistochemistry. Retinal ganglion cells were immunolabeled with two markers, Brn3a and Brn3b (Figs. 2G–I). At 8 to 10 weeks after the induction of IOP elevation, Brn3a densities of OHT eyes decreased 14.9% compared with the left control eyes (n = 10, P < 0.001 in Student's t-test; Fig. 2H). At 15 to 18 weeks, we observed a 26.7% reduction in Brn3a cell density (n = 5, P < 0.001 in Student's t-test; Fig. 2H). Similar reductions were also found in the Brn3b density (reduced by 17.5% and 34.2% at 8–10 and 15–18 weeks post IOP elevation, respectively; P < 0.001 in Student's t-test; Fig. 2I).
We also probed the functional changes of the retinal neurons by ERG recordings. The amplitudes of the a-wave, b-wave, and OPs were compared between OHT and control eyes after the induction of IOP elevation (Figs. 2J–M). At 4 weeks post laser illumination, the mean amplitude of OPs was significantly smaller in OHT eyes (n = 5 for OHT and n = 8 for control; P < 0.01, two-way ANOVA, Tukey's posttest; Fig. 2M). The mean amplitudes of OPs continued to decrease at 5 to 7 (n = 6), 8 to 10 (n = 5), and 11 to 14 (n = 5) weeks post IOP elevation (P < 0.001, two-way ANOVA, Tukey's posttest; Fig. 2M). By contrast, the amplitudes of the a- and b-wave were unchanged for the first 10 weeks with ocular hypertension (P > 0.7, two-way ANOVA, Tukey's posttest; Figs. 2K, 2L). At 11 to 14 weeks post laser illumination and microbead injection, the mean amplitudes of the a- and b-wave exhibited significant reductions (P < 0.05 for a-wave, P < 0.001 for b-wave; Figs. 2K, 2L). We confirmed that saline injection had no effect on the amplitudes of the a-wave, b-wave, and OPs at 11 to 14 weeks post ocular hypertension (n = 9; P = 0.93 for a-wave, P = 0.41 for b-wave, and P = 0.65 for OPs, two-way ANOVA, Tukey's posttest; Supplementary Figs. S1A–D). Taken together, our results confirmed that sustained ocular hypertension induced progressive RGC loss and retinal functional loss.
Functional Degeneration of RGCs Is Subtype-Dependent
We next characterized the visual response properties of individual RGCs using MEA recordings. We examined the RGC response properties at the time based on our ERG measurements, when the inner but not the outer retinal neurons showed significantly reduced responses (e.g., when IOP had been elevated for 5–7 weeks). In total, we recorded 1377 cells in OHT eyes (n = 10 retinas, labeled as OHT) and 2673 cells in age-matched controls (n = 18 retinas, labeled as C in Figs. 3–8). A 2D Gaussian white-noise stimulus of appropriate checker size was used to evoke RGC light responses, and their RF properties were determined through STA and STC-NC analysis (Fig. 3A; also see Materials and Methods).18,24 A cell was considered visually responsive if its STA contrast exceeded 5 SD (Fig. 3B). The spike-triggered average analysis indicates that fewer RGCs were visually responsive in OHT eyes (67%) than in the controls (76%; P < 0.001 in χ2 test; Fig. 3B), consistent with our previous finding.18 In addition, the average firing rate of RGCs that were responsive to the white-noise stimulation was lower in OHT eyes than in controls. This was true whether we considered RGCs with STA contrast greater than 5 SD (OHT eyes: 3.57 ± 0.16 Hz, n = 926; controls: 4.11 ± 0.15 Hz, n = 2025; P < 0.05; Figs. 3B, 3C) or RGCs with STC-NC contrast greater than 5 SD (OHT eyes: 4.19 ± 0.20 Hz, n = 696; controls: 4.93 ± 0.18 Hz, n = 1472; P < 0.05, Student's t-test; Figs. 3D, 3E). The analysis of STC-NC is significantly more computationally intensive than STA analysis, typically requiring more action potentials to obtain a reliable result. Consequently, this analysis has an inherent bias toward cells with higher firing rates. In spite of this bias, the average firing rate of RGCs from OHT eyes was still significantly below that of cells from control eyes (Fig. 3E).
Figure 3.
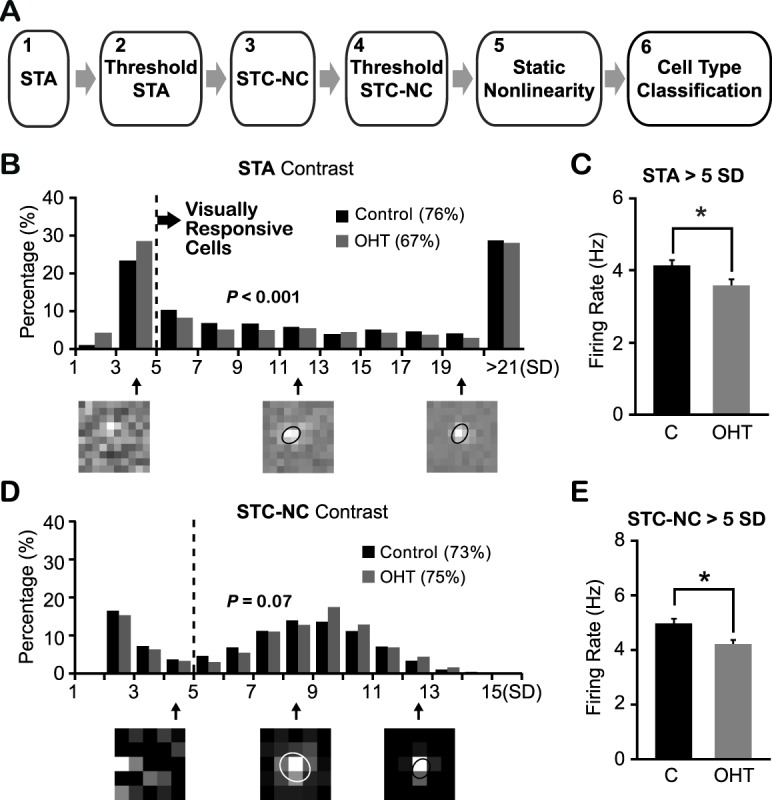
Ocular hypertensive eyes had fewer visually responsive RGCs than controls. (A) Schematic diagram of the characterization of different functional subtypes of RGCs. (B) Distributions of STA contrast of RGCs from OHT eyes and age-matched controls. Dashed line indicates the cutoff value (5 SD) to define visual responsiveness. Three examples of STA peak frame shown underneath and the cell with STA contrast below 5 SD lacked a defined RF structure. The ellipses illustrate the RF center locations and sizes (with 1-SD Gaussian contour). P < 0.001 in χ2 test. (C) Mean firing rate of visually responsive RGCs was smaller in OHT eyes than controls. *P < 0.05 in Student's t-test. (D) Distributions of STC-NC contrast of visually responsive RGCs from hypertensive and control eyes. Dashed line: The cutoff value (5 SD) for static nonlinearity analysis (step 5) and cell-type classification (step 6). P = 0.07 in χ2 test. Bottom: Examples of STC-NC peak frame. (E) The mean firing rate of RGCs with STC-NC contrast larger than 5 SD from OHT eyes was significantly smaller than that of controls. *P < 0.05 in Student's t-test.
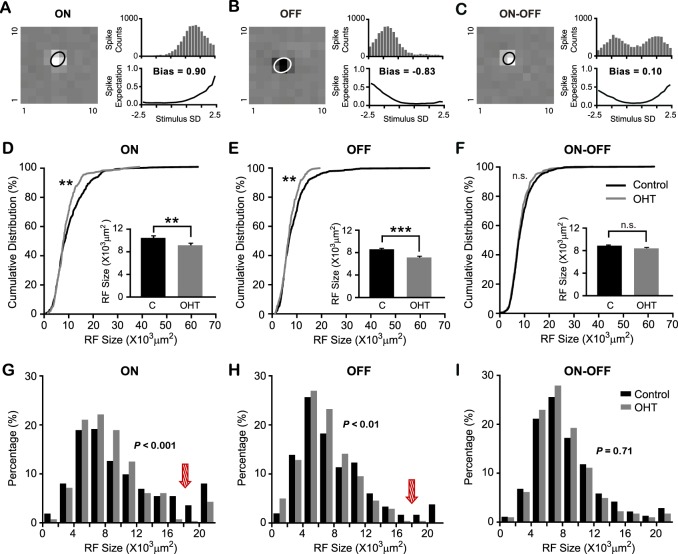
Figure 4.
The functional degeneration of RGCs is subtype dependent in OHT mice. (A–C) Noncentered spike-triggered covariance analysis was used to classify RGC into three functional subtypes: ON (A), OFF (B), and ON-OFF (C). Left: STA peak frame. Right: 1D STE projection onto the STC-NC directions (top) and resulting static nonlinearity (bottom). Cells with an STC-NC bias > 0.6 were defined as ON cells (A), cells with an STC-NC bias < −0.6 as OFF cells (B), and those with intermediate values as ON-OFF cells ([C]; see Materials and Methods). (D–F) Receptive field center sizes of ON (D) and OFF (E) RGCs from OHT eyes were smaller than controls, but not ON-OFF RGCs (F). **P < 0.01. ***P < 0.001. n.s., not significant (P > 0.05) in K-S test and Student's t-test. (G–I) Distributions of RF center sizes of ON (G), OFF (H), and ON-OFF (I) RGCs from OHT eyes and controls. The arrows point to the decrease of percentages in ON and OFF RGCs with large RF sizes in OHT mice. Values of P were calculated in the χ2 test.
Figure 5.
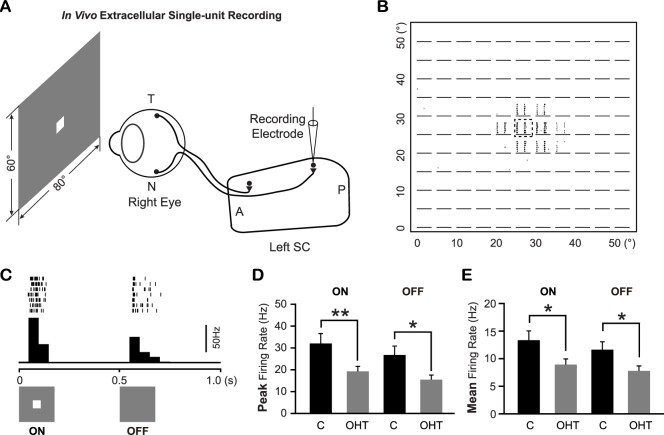
Superior colliculus neurons in OHT mice exhibited lower responses to spot flashed on their RFs than controls. (A) Schematic diagram of the in vivo extracellular single-unit recording of SC neurons in mice. T, temporal; N, nasal; A, anterior; P, posterior. (B, C) The representative raster plots of the spikes recorded from an SC neuron in response to a 5° spot flashed at different locations (11 × 11 grids). The details of the raster plot marked by a dashed square were shown in (C) with the peristimulus time histogram shown below. (D, E) The peak firing rate (D) and mean firing rate (E) of ON and OFF responses of SC neurons in OHT mice and age-matched controls. *P < 0.05. **P < 0.01 in Student's t-test.
Figure 6.
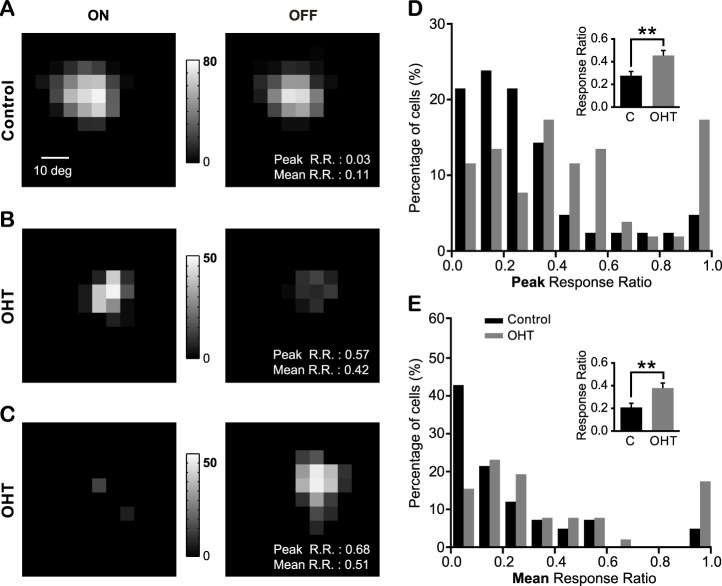
The balance of the ON and OFF responses of individual SC neurons was disrupted in OHT mice. (A–C) Representative maps of responses to spot flashed on the RF of one control SC cell (A) and two cells from OHT mice (B, C). The gray scales illustrate the neurons' firing rates (spikes/s). (D, E) The distribution of the peak response ratio (D) and mean response ratio (E) from OHT and control mice. Zero indicates identical firing rates to both ON and OFF stimulation, and one indicates a preferred response to either ON or OFF. Inserts, mean of the peak and mean response ratio. **P < 0.01 in Student's t-test.
Figure 7.
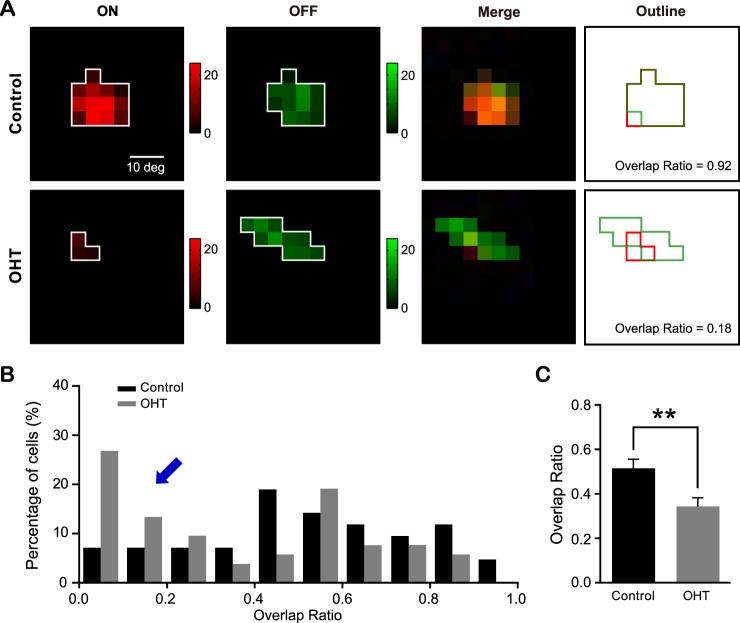
The ON and OFF subfields of individual SC neurons were mismatched in OHT mice. (A) ON (red) and OFF (green) subfields of SC neurons from a control (top) and an OHT (bottom) mouse. (B) The distributions of overlap ratio from OHT mice and age-matched controls. The overlap ratio ranges from 0 to 1, with a value of 1 indicating complete ON-OFF overlap and 0 no overlap. The blue arrow points to the increased number of SC neurons with low or no overlap of ON and OFF subfields in OHT mice. (C) Mean overlap ratio from OHT mice was smaller than age-matched controls. **P < 0.01 in Student's t-test.
Figure 8.
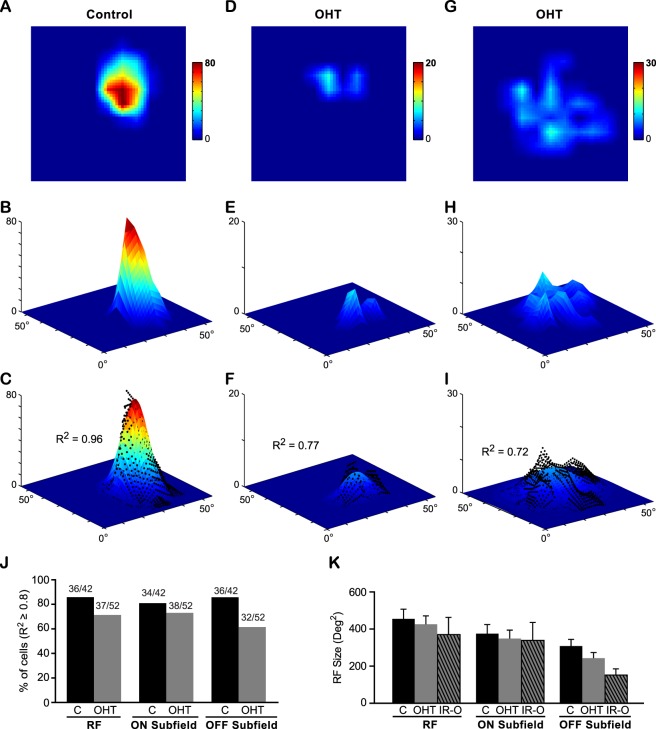
More SC neurons in OHT mice exhibited a patchy RF structure compared with the age-matched controls. (A–I) The RF structure of SC neurons from a control mouse (A–C) and two OHT mice (D–I). (A, D, G) The RFs were smoothed by linear interpolation from 11 × 11 grids to 55 × 55 grids. The color scales represent the neurons' firing rates (spikes/s). Note the firing rates were much lower in OHT mice (D, G). (B, E, H) 3D plots of the RF (with the same color scales shown above, and z-axis also represents the firing rates). (C, F, I) The RF was fitted by a 2D Gaussian function. The black dots represent the original RF map from (B, E, H). The value of R2 was used to quantify the goodness of fit, with R2 ≥ 0.8 corresponding to a Gaussian-shaped RF structure and R2 < 0.8 to a patchy RF structure. (J) The percentages of cells with R2 ≥ 0.8. More cells in the OHT mice showed poor 2D Gaussian function fits. (K) The receptive field size of SC neurons in OHT mice and age-matched controls were not significantly changed. IR-O, cells with irregular RF structure (R2 < 0.8) from OHT mice. P > 0.05 in one-way ANOVA test.
We next used STC-NC analysis to classify RGCs into ON, OFF, and ON-OFF subtypes,24 permitting us to investigate how these subtypes might be affected differentially by hypertension. Cells with the STC-NC bias larger than 0.6 were identified as ON RGCs (Fig. 4A), cells with STC-NC bias smaller than −0.6 were identified as OFF RGCs (Fig. 4B), and those with the bias between −0.6 and 0.6 as ON-OFF RGCs (Fig. 4C; also see details in Materials and Methods). The receptive field center sizes from each RGC subtype were measured and compared between OHT and control eyes. Our analysis revealed that ON RGCs in OHT eyes had smaller RFs (9.0 ± 0.3 × 103 μm2, n = 280) than controls (10.3 ± 0.3 × 103 μm2, n = 475, P < 0.01 in Student's t-test and K-S test; Fig. 4D), as did OFF RGCs (OHT eyes: 6.9 ± 0.2 × 103 μm2, n = 241; controls: 8.3 ± 0.2 × 103 μm2, n = 713, P < 0.001 in Student's t-test and P < 0.01 K-S test; Fig. 4E). By contrast, ON-OFF RGCs had similar RF sizes in OHT eyes (8.2 ± 0.2 × 103 μm2, n = 405) and controls (8.7 ± 0.2 × 103 μm2, n = 837; P = 0.08 in Student's t-test and P = 0.16 in K-S test; Fig. 4F).
From the cumulative distributions of RF size for ON and OFF RGCs, it seems that cells with large RF centers are proportionately fewer in OHT eyes. We further plotted their histograms and confirmed that the numbers of large RGCs of these subtypes were reduced in OHT eyes (Figs. 4G, 4H). For ON and OFF RGCs, the proportion of cells with large RFs (e.g., >12 × 103 μm2) was smaller, and the proportion of cells with small RFs was larger (e.g., <12 × 103 μm2) in OHT eyes than in controls (ON: P < 0.001 in χ2 test; OFF: P < 0.01 in χ2 test; Figs. 4G, 4H). In other words, fewer large ON and OFF RGCs survived following the ocular hypertensive insult. By contrast, the change of RF size distribution for ON-OFF RGCs was not significant (P = 0.71 in χ2 test; Fig. 4I). Together, our data show that OHT mice had fewer visually responsive RGCs; ON and OFF RGCs were more susceptible to IOP elevation than ON-OFF cells; and fewer ON and OFF RGCs had large RF sizes, suggesting that large RGCs of these subtypes may be more susceptible to the hypertensive insult.
ON and OFF Balance of Individual SC Neurons Was Disrupted in Ocular Hypertensive Mice
We next consider the physiological consequences of OHT-induced retinal degeneration on the SC, where the vast majority of RGC axons terminate in mice. We performed in vivo extracellular single-unit recording of superficial SC neurons (Fig. 5A). Ocular hypertension was induced by laser illumination and microbead injection, IOP was measured every 2 weeks, and, at 11 to 14 weeks post laser and microbeads, mice with sustained IOP elevation were used for SC recordings. Age-matched WT mice were used as controls. We confirmed that the overall morphology of the superficial SC was largely normal in OHT mice (Supplementary Fig. S2). A white square (5 × 5°), which flashed at different locations (on an 11 × 11 or 13 × 13 grid; Figs. 5A, 5B), was used as the visual stimulus. For all visually responsive SC neurons, OHT mice had a much lower firing rate compared with controls (Figs. 5D, 5E). The peak firing rate of the ON response from OHT mice was 19.1 ± 2.3 Hz (n = 52 from 11 mice), significantly lower than controls (32.0 ± 4.5 Hz, n = 42 from eight mice; P < 0.01 in Student's t-test; Fig. 5D). Similarly, OFF responses also exhibited a lower peak firing rate in OHT mice (15.4 ± 2.2 Hz, n = 52) compared with controls (26.8 ± 4.1 Hz, n = 42; P < 0.05 in Student's t-test; Fig. 5D). The reduction in response magnitude in OHT mice was also observed when mean firing rates were analyzed for both ON and OFF responses (P < 0.05 in Student's t-test; Fig. 5E).
We measured the relative strength of ON and OFF responses of individual neurons by computing their response ratios. The range of response ratios is from 0 to 1, with 0 indicating balanced ON and OFF responses and 1 indicating a preferred response to either ON or OFF (Figs. 6A–C; also see Materials and Methods). In control mice, the ON-OFF response ratios, whether by analyzing the peak response (0.28 ± 0.04; Fig. 6D) or the mean response (0.21 ± 0.04; Fig. 6E), were closer to 0 than to 1—that is, most neurons exhibited balanced responses to both ON and OFF. By contrast, OHT mice had a higher ON-OFF response ratio (peak: 0.45 ± 0.04; mean: 0.38 ± 0.04, n = 52; Figs. 6D, 6E). In addition, our previous studies showed that nearly all SC neurons responded to both ON and OFF in WT mice.33 As expected, we found that control mice had just one cell responding to ON and another cell to OFF out of a total of 42 cells. By contrast, more cells exhibited pure ON or pure OFF responses in OHT mice (ON: five out of 52 cells; OFF: four out of 52 cells). These results showed that SC neurons in OHT mice had less balanced ON and OFF responses.
We further examined ON and OFF subfield properties of individual SC neurons. We calculated the ON-OFF overlap ratio as the ratio of the number of grid locations that showed both ON and OFF responses over the total number of responsive locations regardless of ON or OFF polarity (see Materials and Methods). The overlap ratio ranges from 0 to 1, with a value of 1 indicating complete ON-OFF overlap and 0 no overlap (Fig. 7A). In OHT mice, more cells exhibited smaller overlap ratio (Fig. 7B), and the mean ON-OFF overlap ratio (0.34 ± 0.04, n = 52) was also reduced compared with controls (0.52 ± 0.04, n = 42; P < 0.01 in Student's t-test; Fig. 7C), demonstrating that the ON subfield was less well matched to the OFF subfield in OHT mice.
In addition, we noticed that many SC neurons exhibited irregular RFs in OHT mice. In age-matched control mice, 86% of neurons had elliptical RFs with a single peak (Figs. 8A–C) that could be fitted well by a 2D Gaussian function (36 out of 42 cells, R2 ≥ 0.8; Fig. 8J). By contrast, only 71% of cells (37 out of 52 cells) recorded from OHT mice could be fitted by a 2D Gaussian function (Figs. 8D–J). In other words, more cells from OHT mice showed irregular/abnormal RF structures with multiple peaks (R2 < 0.8). Next, we separated the ON and OFF subfields and analyzed their structure. For ON subfields, 27% of cells exhibited abnormal structure (14 out of 52 cells, R2 < 0.8) compared with 19% in age-matched controls (8 out of 42 cells; Fig. 8J). For OFF subfields, 38% of cells (20 out of 52 cells) had abnormal structure in OHT mice, significantly higher than controls (14%, six out of 42 cells; Fig. 8J). Our data showed that more SC neurons had abnormal ON or OFF subfields in OHT mice.
We compared the RF sizes of SC neurons in OHT mice and age-matched controls. Although the visual responses were weak in OHT mice, the RF sizes of all neurons showed no significant difference between controls (mean: 456 ± 52 degree2) and OHT mice (mean: 425 ± 45 degree2; P = 0.90, one-way ANOVA, Tukey's posttest; Fig. 8K). We also measured neurons with irregular RF structure in OHT mice separately (labeled as “IR-O” in Fig. 8K) and found no change in their RF sizes (373 ± 91 degree2; P = 0.65, one-way ANOVA, Tukey's posttest). We next examined the ON and OFF subfields separately and found no significant change among the three groups (P = 0.90 for ON and P = 0.08 for OFF, one-way ANOVA, Tukey's posttest; Fig. 8K).
Taken together, our data showed that SC neurons in OHT mice were less responsive to the visual stimulation than controls, that the balance of ON and OFF responses of individual neurons was disrupted, and that more cells from OHT mice exhibited irregular RF structure with mismatched ON and OFF subfields.
Discussion
Correlation of Functional and Morphological Degeneration of RGCs
Progressive RGC loss has been reported in different rodent models of glaucoma.9,12,14,18,49 However, the RGC loss induced by ocular hypertension varied substantially among different studies. In the microbead-induced ocular hypertensive mouse model, a mild reduction of 11% was reported for retrogradely labeled RGCs at 3 months after microbead injection.49 But another study showed 43% loss of Brn3a-labeled RGCs at just 1 month after injection of microbeads.14 In DBA mice, axon density was found reduced by 13% in 13-month-old mice compared with 3-month-old mice, whereas the retrogradely labeled RGC density decreased by 75%.50 Here, we found that Brn3a and Brn3b density decreased ~16% at 2 months and ~30% at 4 months following chronic ocular hypertension (Figs. 2H, 2I). In addition, we tracked the morphologic changes of the retinas with in vivo OCT imaging and found that the thickness of the NFL and GCL in OHT eyes was reduced ~30% and ~45% at 2- and 4-month post ocular hypertension, respectively (Fig. 2E). Different labeling methods and various IOP levels among different studies might contribute to the differences observed in RGC loss.9,12,18,51 Clearly, there is a need for better standardization in the quantification of the extent of RGC injury and loss.
Degeneration of retinal function measured by ERG recordings has been reported (e.g., in mice and rat).16,49,52–54 Analysis of the ERG components (e.g., amplitudes of a-wave, b-wave, OPs) is an effective strategy for monitoring retinal function in vivo. We noticed decreases in the a- and b-wave at 11 to 14 weeks after the induction of IOP elevation, similar to those previously reported in mouse models of glaucoma,16,55 but not typical of primate models and human glaucoma.56 The reductions of the a- and b-wave could be due to retinal ischemia induced by laser or ocular hypertension, which would affect all layers of the retina.57,58 We also observed decreased amplitudes of OPs with time post ocular hypertension, consistent with previous studies in mouse and rat models of experimental glaucoma.16,53,54 Because OPs reflect the extracellular electrical currents generated by negative feedback pathways among bipolar cells, amacrine cells, and RGCs,42 it does not specifically identify the functional changes of RGCs. To probe this, we examined RGC function at the single-cell level using MEA recordings.
Some recent studies have suggested that RGC degeneration is subtype dependent in mouse models of experimental glaucoma.14,18,20 Retinal ganglion cells can be classified into more than 20 subtypes based on a combination of many parameters such as soma size, dendritic field size, and branching pattern and their stratification in the IPL.21,22,40 Yet only a limited number of RGC types have been examined due to the technical challenges of classifying individual RGC types in mice. A recent study found that the spontaneous activity of ON- and OFF-sustained RGCs decreased after optic nerve crush.59 Another study has shown that the OFF-transient RGCs exhibited a more rapid decline in RF size and dendritic field size than OFF-sustained cells in OHT mice.14 Taking advantage of the STC-NC analysis we developed,24 we have demonstrated here that the RF size of ON and OFF RGCs decreases following chronic IOP elevation, but not the RF size of ON-OFF RGC, a result consistent with our previous finding that the degeneration of RGC dendritic structure is also subtype dependent.18 We further showed that OHT mice had fewer ON and OFF RGCs with large RFs, again consistent with our earlier results for dendritic tree size.18 This result also lends support to the notion that larger RGCs are selectively sensitive to the IOP elevation at the early stage of glaucoma.1,20,60
The morphologic changes of RGCs may not always correlate with functional degeneration. For example, ON-sustained and OFF-sustained RGCs showed reduced light responses in glaucoma but had dendritic field sizes like controls.14 These differences may be clinically important. Retinal ganglion cell death in glaucomatous eyes is preceded by morphologic alterations to dendrites and changes in RF properties,14,18,60,61 so characterizing the early signs of RGC damage before death may provide new diagnostics that could guide treatment earlier. The transgenic tools available to label specific RGC types coupled with improved in vivo physiological recording techniques make the study of subtype-dependent functional and morphologic degeneration of RGCs in glaucomatous mice an attractive experimental objective.
RGC Axonal Transport Deficits and Visual Field Defects at Higher Visual Centers
Early signs of RGC damage have been detected at the axon terminals, including metabolic stress, disruption of axonal transport, and downregulation of specific genes.10,50,62,63 Deficits in retrograde axonal transport were observed at an early stage of glaucoma in DBA/2 mice,50 and it seems to follow a retinotopic pattern that resembles vision loss in glaucoma.10 One of the critical questions is whether the deficits in axonal transport precede the dendritic and functional degeneration of the RGCs. A study of the DBA/2 mouse model showed that RGCs with dramatic dendritic shrinkage were not retrogradely labeled from the SC, suggesting that the dendritic changes happen either concurrently with or following a failure of retrograde transport.51
In addition to the degeneration observed in RGCs and their axonal terminals, deficits of neurons in higher visual centers have also been reported in glaucoma.27,28,64,65 In glaucoma patients, a significant loss of visual function in the primary visual cortex was detected by fMRI and correlated with visual field loss.27 In monkey models of experimental glaucoma, a number of studies revealed significant degenerative changes in LGN, which included shrinkage and loss of neurons as well as loss of dendrites, suggesting that deficits of pre-synaptic neurons in the retina led to degeneration of postsynaptic neurons in higher visual centers.29–31,64 Oxidative injury and glutamate excitotoxicity were implicated in this process.2,5,66 However, a study in the DBA mouse showed that the volume of the superficial SC remained unchanged with aging, suggesting little or no loss of SC neurons.10 Although we also observed a largely normal morphology of the superficial SC in OHT mice (Supplementary Fig. S2), SC neurons exhibited multiple functional deficits (Figs. 5–8). For example, SC neurons had reduced light responses (Fig. 5), which could be directly induced by the reduced light responses of RGCs (Fig. 3). These results suggest that the deficits of SC neurons were likely due to the fewer or weaker inputs from RGCs in our mouse model of ocular hypertension.
In mice, it is estimated that at least 70% of mouse RGCs project to the superficial SC.32 Each SC neuron receives convergent input from approximately six RGCs.67 Converging onto SC neurons is balanced synaptic drive from ON- and OFF-centered RGCs with overlapping RFs, which is believed to be important for detecting object salience, irrespective of contrast.33,34 Here, we found mismatched ON and OFF subfields of SC neurons in OHT mice (Fig. 7), though they had comparable RF sizes to those of controls (Fig. 8K). Some SC neurons showed significantly smaller ON or OFF subfields (Figs. 6B, 6C, 8K). At the same time, there were examples where RF sizes might have increased (one example in Fig. 8G). One possibility is that the dendritic arbors of the SC neurons may form random contacts with nearby surviving RGCs searching for substitutes for lost inputs.36 This may partly account for the patchy RF structure observed in OHT mice. Recent studies have just begun to reveal the structure and function of the SC during normal development.33,35,43,68 More work is needed to better characterize when and how retinal degeneration leads to the functional defects in higher visual centers.5,28,65,69
In summary, here we report that the functional degeneration of RGCs is subtype dependent and that the balance of ON and OFF of SC neurons is disrupted. Our findings provide a foundation to examine the early visual impairments induced by the neuronal degeneration not only in the retina but also in the higher visual centers in experimental glaucoma.
Acknowledgments
We thank Genn Suyeoka for mouse husbandry.
Supported by the Dr. Douglas H. Johnson Award for Glaucoma Research from BrightFocus Foundation (XL); the Illinois Society for the Prevention of Blindness (HC); William & Mary Greve Special Scholar Award from the Research to Prevent Blindness (XL), an unrestricted RPB grant to the Department of Ophthalmology, Northwestern University; Northwestern Memorial Foundation/Brinson Foundation (XL); Midwest Eye-banks Research Grants (XL); Juvenile Diabetes Research Foundation Postdoctoral Fellowship (JY); National Natural Science Foundation of China (PL, NSFC No. 31471054); the Program of Introducing Talents of Discipline to Universities (PL, No. B08020); and NIH Grants R21EB004200 (JBT), R01EY01995 (HFZ), EY020950 (JC), and R01EY019034 (XL).
Disclosure: H. Chen, None; Y. Zhao, None; M. Liu, None; L. Feng, None; Z. Puyang, None; J. Yi, None; P. Liang, None; H.F. Zhang, None; J. Cang, None; J.B. Troy, None; X. Liu, None
References
- 1. Quigley HA. Neuronal death in glaucoma. Prog Retin Eye Res. 1999; 18: 39–57. [DOI] [PubMed] [Google Scholar]
- 2. Gupta N, Yucel YH. Glaucoma as a neurodegenerative disease. Curr Opin Ophthalmol. 2007; 18: 110–114. [DOI] [PubMed] [Google Scholar]
- 3. Nickells RW. From ocular hypertension to ganglion cell death: a theoretical sequence of events leading to glaucoma. Can J Ophthalmol. 2007; 42: 278–287. [PubMed] [Google Scholar]
- 4. Wax MB, Tezel G. Immunoregulation of retinal ganglion cell fate in glaucoma. Exp Eye Res. 2009; 88: 825–830. [DOI] [PubMed] [Google Scholar]
- 5. Calkins DJ. Critical pathogenic events underlying progression of neurodegeneration in glaucoma. Prog Retin Eye Res. 2012; 31: 702–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johnson TV, Tomarev SI. Rodent models of glaucoma. Brain Res Bull. 2010; 81: 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McKinnon SJ, Schlamp CL, Nickells RW. Mouse models of retinal ganglion cell death and glaucoma. Exp Eye Res. 2009; 88: 816–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shareef SR, Garcia-Valenzuela E, Salierno A, Walsh J, Sharma SC. Chronic ocular hypertension following episcleral venous occlusion in rats. Exp Eye Res. 1995; 61: 379–382. [DOI] [PubMed] [Google Scholar]
- 9. Fu CT, Sretavan D. Laser-induced ocular hypertension in albino CD-1 mice. Invest Ophthalmol Vis Sci. 2010; 51: 980–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crish SD, Sappington RM, Inman DM, Horner PJ, Calkins DJ. Distal axonopathy with structural persistence in glaucomatous neurodegeneration. Proc Natl Acad Sci U S A. 2010; 107: 5196–5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sappington RM, Carlson BJ, Crish SD, Calkins DJ. The microbead occlusion model: a paradigm for induced ocular hypertension in rats and mice. Invest Ophthalmol Vis Sci. 2010; 51: 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen H, Wei X, Cho KS, et al. Optic neuropathy due to microbead-induced elevated intraocular pressure in the mouse. Invest Ophthalmol Vis Sci. 2011; 52: 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kalesnykas G, Oglesby EN, Zack DJ, et al. Retinal ganglion cell morphology after optic nerve crush and experimental glaucoma. Invest Ophthalmol Vis Sci. 2012; 53: 3847–3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Della Santina L, Inman DM, Lupien CB, Horner PJ, Wong RO. Differential progression of structural and functional alterations in distinct retinal ganglion cell types in a mouse model of glaucoma. J Neurosci. 2013; 33: 17444–17457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aihara M, Lindsey JD, Weinreb RN. Experimental mouse ocular hypertension: establishment of the model. Invest Ophthalmol Vis Sci. 2003; 44: 4314–4320. [DOI] [PubMed] [Google Scholar]
- 16. Grozdanic SD, Betts DM, Sakaguchi DS, Allbaugh RA, Kwon YH, Kardon RH. Laser-induced mouse model of chronic ocular hypertension. Invest Ophthalmol Vis Sci. 2003; 44: 4337–4346. [DOI] [PubMed] [Google Scholar]
- 17. Feng L, Chen H, Suyeoka G, Liu XA. Laser-induced mouse model of chronic ocular hypertension to characterize visual defects. J Vis Exp. 2013; 78 doi:10.3791/50440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feng L, Zhao Y, Yoshida M, et al. Sustained ocular hypertension induces dendritic degeneration of mouse retinal ganglion cells that depends on cell type and location. Invest Ophthalmol Vis Sci. 2013; 54: 1106–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weber AJ, Harman CD. Structure-function relations of parasol cells in the normal and glaucomatous primate retina. Invest Ophthalmol Vis Sci. 2005; 46: 3197–3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Filippopoulos T, Danias J, Chen B, Podos SM, Mittag TW. Topographic and morphologic analyses of retinal ganglion cell loss in old DBA/2NNia mice. Invest Ophthalmol Vis Sci. 2006; 47: 1968–1974. [DOI] [PubMed] [Google Scholar]
- 21. Sun W, Li N, He S. Large-scale morphological survey of mouse retinal ganglion cells. J Comp Neurol. 2002; 451: 115–126. [DOI] [PubMed] [Google Scholar]
- 22. Volgyi B, Chheda S, Bloomfield SA. Tracer coupling patterns of the ganglion cell subtypes in the mouse retina. J Comp Neurol. 2009; 512: 664–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tian N, Copenhagen DR. Visual stimulation is required for refinement of ON and OFF pathways in postnatal retina. Neuron. 2003; 39: 85–96. [DOI] [PubMed] [Google Scholar]
- 24. Cantrell DR, Cang J, Troy JB, Liu X. Non-centered spike-triggered covariance analysis reveals neurotrophin-3 as a developmental regulator of receptive field properties of ON-OFF retinal ganglion cells. PLoS Comput Biol. 2010; 6: e1000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen H, Liu X, Tian N. Subtype-dependent postnatal development of direction- and orientation-selective retinal ganglion cells in mice. J Neurophysiol. 2014; 112: 2092–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chichilnisky EJ. A simple white noise analysis of neuronal light responses. Network. 2001; 12: 199–213. [PubMed] [Google Scholar]
- 27. Duncan RO, Sample PA, Weinreb RN, Bowd C, Zangwill LM. Retinotopic organization of primary visual cortex in glaucoma: Comparing fMRI measurements of cortical function with visual field loss. Prog Retin Eye Res. 2007; 26: 38–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Imamura K. Central Changes in Glaucoma: Neuroscientific Study Using Animal Models. Rijeka, Croatia: InTech; 2011: 307–330. [Google Scholar]
- 29. Weber AJ, Chen H, Hubbard WC, Kaufman PL. Experimental glaucoma and cell size, density, and number in the primate lateral geniculate nucleus. Invest Ophthalmol Vis Sci. 2000; 41: 1370–1379. [PubMed] [Google Scholar]
- 30. Gupta N, Ly T, Zhang Q, Kaufman PL, Weinreb RN, Yucel YH. Chronic ocular hypertension induces dendrite pathology in the lateral geniculate nucleus of the brain. Exp Eye Res. 2007; 84: 176–184. [DOI] [PubMed] [Google Scholar]
- 31. Sasaoka M, Nakamura K, Shimazawa M, Ito Y, Araie M, Hara H. Changes in visual fields and lateral geniculate nucleus in monkey laser-induced high intraocular pressure model. Exp Eye Res. 2008; 86: 770–782. [DOI] [PubMed] [Google Scholar]
- 32. Hofbauer A, Drager UC. Depth segregation of retinal ganglion cells projecting to mouse superior colliculus. J Comp Neurol. 1985; 234: 465–474. [DOI] [PubMed] [Google Scholar]
- 33. Wang L, Sarnaik R, Rangarajan K, Liu X, Cang J. Visual receptive field properties of neurons in the superficial superior colliculus of the mouse. J Neurosci. 2010; 30: 16573–16584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Knudsen EI. Control from below: the role of a midbrain network in spatial attention. Eur J Neurosci. 2011; 33: 1961–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cang J, Feldheim DA. Developmental mechanisms of topographic map formation and alignment. Annu Rev Neurosci. 2013; 36: 51–77. [DOI] [PubMed] [Google Scholar]
- 36. King WM, Sarup V, Sauve Y, Moreland CM, Carpenter DO, Sharma SC. Expansion of visual receptive fields in experimental glaucoma. Vis Neurosci. 2006; 23: 137–142. [DOI] [PubMed] [Google Scholar]
- 37. Rangarajan KV, Lawhn-Heath C, Feng L, Kim TS, Cang J, Liu X. Detection of visual deficits in aging DBA/2J mice by two behavioral assays. Curr Eye Res. 2011; 36: 481–491. [DOI] [PubMed] [Google Scholar]
- 38. Yi J, Wei Q, Liu W, Backman V, Zhang HF. Visible-light optical coherence tomography for retinal oximetry. Opt Lett. 2013; 38: 1796–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang Q, Cho KS, Chen H, et al. Microbead-induced ocular hypertensive mouse model for screening and testing of aqueous production suppressants for glaucoma. Invest Ophthalmol Vis Sci. 2012; 53: 3733–3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu X, Grishanin RN, Tolwani RJ, et al. Brain-derived neurotrophic factor and TrkB modulate visual experience-dependent refinement of neuronal pathways in retina. J Neurosci. 2007; 27: 7256–7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yoshida M, Feng L, Grimbert F, et al. Overexpression of neurotrophin-3 stimulates a second wave of dopaminergic amacrine cell genesis after birth in the mouse retina. J Neurosci. 2011; 31: 12663–12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wachtmeister L. Oscillatory potentials in the retina: what do they reveal. Prog Retin Eye Res. 1998; 17: 485–521. [DOI] [PubMed] [Google Scholar]
- 43. Liu M, Wang L, Cang J. Different roles of axon guidance cues and patterned spontaneous activity in establishing receptive fields in the mouse superior colliculus. Front Neural Circuits. 2014; 8: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cang J, Kalatsky VA, Lowel S, Stryker MP. Optical imaging of the intrinsic signal as a measure of cortical plasticity in the mouse. Vis Neurosci. 2005; 22: 685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997; 10: 433–436. [PubMed] [Google Scholar]
- 46. Pelli DG. The Video Toolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997; 10: 437–442. [PubMed] [Google Scholar]
- 47. Niell CM, Stryker MP. Highly selective receptive fields in mouse visual cortex. J Neurosci. 2008; 28: 7520–7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sarnaik R, Wang BS, Cang J. Experience-dependent and independent binocular correspondence of receptive field subregions in mouse visual cortex. Cereb Cortex. 2013; 24: 1658–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Frankfort BJ, Khan AK, Tse DY, et al. Elevated intraocular pressure causes inner retinal dysfunction before cell loss in a mouse model of experimental glaucoma. Invest Ophthalmol Vis Sci. 2013; 54: 762–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Buckingham BP, Inman DM, Lambert W, et al. Progressive ganglion cell degeneration precedes neuronal loss in a mouse model of glaucoma. J Neurosci. 2008; 28: 2735–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jakobs TC, Libby RT, Ben Y, John SW, Masland RH. Retinal ganglion cell degeneration is topological but not cell type specific in DBA/2J mice. J Cell Biol. 2005; 171: 313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kong YX, Crowston JG, Vingrys AJ, Trounce IA, Bui VB. Functional changes in the retina during and after acute intraocular pressure elevation in mice. Invest Ophthalmol Vis Sci. 2009; 50: 5732–5740. [DOI] [PubMed] [Google Scholar]
- 53. Bayer AU, Danias J, Brodie S, et al. Electroretinographic abnormalities in a rat glaucoma model with chronic elevated intraocular pressure. Exp Eye Res. 2001; 72: 667–677. [DOI] [PubMed] [Google Scholar]
- 54. Fortune B, Bui BV, Morrison JC, et al. Selective ganglion cell functional loss in rats with experimental glaucoma. Invest Ophthalmol Vis Sci. 2004; 45: 1854–1862. [DOI] [PubMed] [Google Scholar]
- 55. Bayer AU, Neuhardt T, May AC, et al. Retinal morphology and ERG response in the DBA/2NNia mouse model of angle-closure glaucoma. Invest Ophthalmol Vis Sci. 2001; 42: 1258–1265. [PubMed] [Google Scholar]
- 56. Colotto A, Falsini B, Salgarello T, Iarossi G, Galan ME, Scullica L. Photopic negative response of the human ERG: losses associated with glaucomatous damage. Invest Ophthalmol Vis Sci. 2000; 41: 2205–2211. [PubMed] [Google Scholar]
- 57. Block F, Schwarz M. The b-wave of the electroretinogram as an index of retinal ischemia. Gen Pharmacol. 1998; 30: 281–287. [DOI] [PubMed] [Google Scholar]
- 58. Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res. 2004; 23: 91–147. [DOI] [PubMed] [Google Scholar]
- 59. Stutzki H, Leibig C, Andreadaki A, Fischer D, Zeck G. Inflammatory stimulation preserves physiological properties of retinal ganglion cells after optic nerve injury. Front Cell Neurosci. 2014; 8: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Leung CK, Weinreb RN, Li ZW, et al. Long-term in vivo imaging and measurement of dendritic shrinkage of retinal ganglion cells. Invest Ophthalmol Vis Sci. 2011; 52: 1539–1547. [DOI] [PubMed] [Google Scholar]
- 61. Morgan JE, Datta AV, Erichsen JT, Albon J, Boulton ME. Retinal ganglion cell remodelling in experimental glaucoma. Adv Exp Med Biol. 2006; 572: 397–402. [DOI] [PubMed] [Google Scholar]
- 62. Soto I, Oglesby E, Buckingham BP, et al. Retinal ganglion cells downregulate gene expression and lose their axons within the optic nerve head in a mouse glaucoma model. J Neurosci. 2008; 28: 548–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Baltan S, Inman DM, Danilov CA, Morrison RS, Calkins DJ, Horner PJ. Metabolic vulnerability disposes retinal ganglion cell axons to dysfunction in a model of glaucomatous degeneration. J Neurosci. 2010; 30: 5644–5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yucel YH, Zhang Q, Weinreb RN, Kaufman PL, Gupta N. Effects of retinal ganglion cell loss on magno-, parvo-, koniocellular pathways in the lateral geniculate nucleus and visual cortex in glaucoma. Prog Retin Eye Res. 2003; 22: 465–481. [DOI] [PubMed] [Google Scholar]
- 65. Calkins DJ, Horner PJ. The cell and molecular biology of glaucoma: axonopathy and the brain. Invest Ophthalmol Vis Sci. 2012; 53: 2482–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yucel Y, Gupta N. Glaucoma of the brain: a disease model for the study of transsynaptic neural degeneration. Prog Brain Res. 2008; 173: 465–478. [DOI] [PubMed] [Google Scholar]
- 67. Chandrasekaran AR, Shah RD, Crair MC. Developmental homeostasis of mouse retinocollicular synapses. J Neurosci. 2007; 27: 1746–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sarnaik R, Chen H, Liu X, Cang J. Genetic disruption of the On visual pathway affects cortical orientation selectivity and contrast sensitivity in mice. J Neurophysiol. 2014; 111: 2276–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Almasieh M, Wilson AM, Morquette B. Cueva Vargas JL, Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012; 31: 152–181. [DOI] [PubMed] [Google Scholar]