Abstract
Purpose.
To investigate the expression of platelet factor-4 variant (PF-4var/CXCL4L1) in epiretinal membranes from patients with proliferative diabetic retinopathy (PDR) and the role of PF-4var/CXCL4L1 in the regulation of blood–retinal barrier (BRB) breakdown in diabetic rat retinas and human retinal microvascular endothelial cells (HRMEC).
Methods.
Rats were treated intravitreally with PF-4var/CXCL4L1 or the anti-vascular endothelial growth factor (VEGF) agent bevacizumab on the first day after diabetes induction. Blood–retinal barrier breakdown was assessed in vivo with fluorescein isothiocyanate (FITC)-conjugated dextran and in vitro in HRMEC by transendothelial electrical resistance and FITC-conjugated dextran cell permeability assay. Occludin, vascular endothelial (VE)-cadherin, hypoxia-inducible factor (HIF)-1α, VEGF, tumor necrosis factor (TNF)-α, receptor for advanced glycation end products (RAGE), caspase-3 levels, and generation of reactive oxygen species (ROS) were assessed by Western blot, enzyme-linked immunosorbent assays, or spectrophotometry.
Results.
In epiretinal membranes, vascular endothelial cells and stromal cells expressed PF-4var/CXCL4L1. In vitro, HRMEC produced PF-4var/CXCL4L1 after stimulation with a combination of interleukin (IL)-1β and TNF-α, and PF-4var/CXCL4L1 inhibited VEGF-mediated hyperpermeability in HRMEC. In rats, PF-4var/CXCL4L1 was as potent as bevacizumab in attenuating diabetes-induced BRB breakdown. This effect was associated with upregulation of occludin and VE-cadherin and downregulation of HIF-1α, VEGF, TNF-α, RAGE, and caspase-3, whereas ROS generation was not altered.
Conclusions.
Our findings suggest that increasing the intraocular PF-4var/CXCL4L1 levels early after the onset of diabetes protects against diabetes-induced BRB breakdown.
Keywords: diabetic retinopathy, blood-retinal barrier, platelet factor-4 variant (PF-4var CXCL4L1)
Increasing the intraocular PF-4var/CXCL4L1 levels early after the onset of diabetes protects against diabetes-induced blood–retinal barrier breakdown.
Introduction
Diabetic retinopathy (DR), a common and serious complication of diabetes, is one of the leading causes of blindness. Increased vascular permeability caused by the breakdown of the blood–retinal barrier (BRB), a characteristic sign of early DR, results in diabetic macular edema, which is a major cause of visual impairment in diabetic patients.1 However, current treatments to prevent or decrease diabetic macular edema have only limited efficacy.
Streptozotocin (STZ) destroys pancreatic island β cells and is used to induce experimental diabetes in rodents. Adult rats treated with a single dose of STZ demonstrating hyperglycemia within 48 hours are widely used as a model of insulin-dependent diabetes mellitus. Streptozotocin-induced diabetic rats demonstrate characteristics of the nonproliferative DR that occurs in humans, such as increased vascular permeability resulting from breakdown of the BRB.2–8 Breakdown of the BRB was observed in this animal model as early as 2 weeks following diabetes induction.3,7–9
Several cellular mechanisms and molecular pathways activated by hyperglycemia and/or hypoxia have been demonstrated to be involved in promoting BRB breakdown in diabetes. First, chronic, low-grade subclinical inflammation is responsible for many of the vascular lesions of DR. Increased retinal leukostasis is observed within few days of developing STZ-induced diabetes and correlates with the increased expression of retinal intercellular adhesion molecule-1 (ICAM-1) and the leukocyte integrin CD18.10–12 Expression of the proinflammatory cytokine tumor necrosis factor (TNF)-α is increased in the diabetic retina as early as 1 week after induction of diabetes and is involved in diabetes-induced retinal endothelial cell apoptosis and vascular leakage.13,14 Endothelial apoptosis leads to BRB breakdown and formation of acellular capillaries.13,14 Second, upregulation of vascular endothelial growth factor (VEGF), a potent angiogenic and vascular permeability factor, is a major contributor to BRB breakdown in diabetes.2–4 Third, downregulation of the tight junction and the adherence junction proteins in retinal endothelial cells is a major mechanism in BRB breakdown in STZ-induced diabetes.5–8,15,16 Fourth, oxidative stress plays an important role in retinal vascular endothelial dysfunction in diabetes.17 Finally, the inhibitors of receptor for advanced glycation end products (RAGE) ameliorate BRB breakdown, leukostasis, expression of ICAM-1, and capillary degeneration in the retina of diabetic animals,11,12 revealing the roles of activation of RAGE in promoting BRB breakdown.
Platelets play a key role in hemostasis, and accumulating evidence has highlighted a central role for platelets in inflammatory responses. Platelets, by virtue of their large numbers and their ability to rapidly release a broad spectrum of immunomodulatory cytokines, chemokines, and other mediators, participate in assisting and modulating inflammatory reactions and immune responses.18 A previous report demonstrated the accumulation of platelet-containing microthrombi in the retinal vasculature of the rat within 2 weeks of experimental diabetes that increased with duration of diabetes and that suppressed BRB breakdown.19 Among the platelet-derived products are various chemokines that are secreted upon platelet activation.18 The chemokine family can be subdivided into CXC and CC chemokine ligands (CXCL, CCL).20,21 The CXC chemokine platelet factor-4 (PF-4/CXCL4) is a major constituent of platelet α-granules and is released in high amounts upon platelet activation. PF-4/CXCL4 was the first chemokine described to inhibit neovascularization.22,23 In a previous report, we demonstrated that PF-4/CXCL4 is significantly upregulated in the vitreous fluid from patients with proliferative diabetic retinopathy (PDR) and that diabetes induced increased PF-4/CXCL4 expression in rat retinas. Furthermore, PF-4/CXCL4 inhibited VEGF-induced signal transduction in human retinal microvascular endothelial cells (HRMEC) and inhibited their migration.24 These findings suggest that the levels of the angiostatic chemokine PF-4/CXCL4 are not always high enough to counteract the neovascular outgrowth and progression of PDR. Recently, Struyf et al.25 isolated and identified the platelet factor-4 variant (PF-4var/CXCL4L1) from thrombin-stimulated platelets, differing from authentic PF-4/CXCL4 in three carboxy-terminally located amino acids. Human PF-4var/CXCL4L1 is a nonallelic gene variant from human PF-4/ CXCL4. The gene arose rather late in evolution, probably through gene duplication. Therefore the PF-4var/CXCL4L1 gene exists only in primates, not in rodents. Surprisingly, PF-4var/CXCL4L1 is a more potent inhibitor of endothelial cell chemotaxis in vitro and tumor angiogenesis and growth than PF-4/CXCL4.25–28
Although the role of the potent angiostatic chemokine PF-4var/CXCL4L1 has been investigated as a candidate anticancer drug,26,27 its role as regulator of the BRB in diabetes has not been investigated previously. The aim of this study was, therefore, to examine the expression of PF-4var/CXCL4L1 in epiretinal membranes from patients with PDR and to evaluate the effects of intravitreal PF-4var/CXCL4L1 therapy on the BRB.
Materials and Methods
Epiretinal Membrane Specimens
Epiretinal fibrovascular membranes were obtained from 14 patients with PDR during pars plana vitrectomy for the repair of tractional retinal detachment. Membranes were fixed in 10% formalin solution and embedded in paraffin. The study was conducted according to the tenets of the Declaration of Helsinki. All the patients were candidates for vitrectomy as a surgical procedure. All patients signed a preoperative informed written consent and approved the use of the excised epiretinal membranes for further analysis and clinical research. The study design and the protocol were approved by the Research Centre and Institutional Review Board of the College of Medicine, King Saud University.
Immunohistochemical Staining
Antigen retrieval was performed by boiling the sections (20 minutes) in citrate buffer, pH 5.9 to 6.1 (BOND Epitope Retrieval Solution 1; Leica, Buffalo Grove, IL, USA), or Tris/EDTA buffer, pH 9 (BOND Epitope Retrieval Solution 2), for CD34 and PF-4var/CXCL4L1 detection, respectively. Next, sections were incubated (60 minutes) with mouse monoclonal anti-CD34 (1:50; BD Biosciences, San Jose, CA, USA) or rabbit polyclonal anti-PF-4var/CXCL4L128 antibodies, respectively. Immune complexes were visualized by the Leica BOND Polymer Refine Red Detection Kit, resulting in bright-red immunoreactive sites. The slides were faintly counterstained with Mayer's hematoxylin. Omission or substitution of the primary antibody with an irrelevant antibody from the same species and staining with chromogen alone were used as negative controls.
Cell Culture and Induction Experiments
Human retinal microvascular endothelial cells (Cell Systems, Kirkland, WA, USA) were cultured in endothelial basal medium-2 (EBM-2) enriched with endothelial growth medium-2 MV Bulletkit (Lonza, Verviers, Belgium). Three days after seeding, cell growth medium was replaced by EBM-2 containing 3% fetal calf serum and phorbol myristate acetate (PMA; Sigma-Aldrich Corp., St. Louis, MO, USA), and human recombinant VEGF (R&D Systems, Minneapolis, MN, USA), interleukin (IL)-1β (Peprotech, Rocky Hill, NY, USA), TNF-α (Peprotech), transforming growth factor (TGF)-β1 (Peprotech), or combinations thereof were added. After 4 days of stimulation, cell supernatants were harvested for analysis of PF-4var/CXCL4L1 content by enzyme-linked immunosorbent assay (ELISA).28
Animals
Diabetes was induced in rats (male Sprague-Dawley, 200–220 g; Experimental Animal Care Center, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia) with STZ (55 mg/kg body weight). Rats were considered diabetic if blood glucose levels exceeded 250 mg/dL. Age-matched normal rats served as control. After 2 weeks the animals were euthanized by pentobarbital overdose, and the retinas were removed and snap frozen in liquid nitrogen and stored at −80°C. All experiments were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the King Saud University's Animal Care and Use Committee Guidelines.
Intravitreal Injection of PF-4var/CXCL4L1
Immediately after determining blood glucose levels, animals were kept under deep anesthesia, and 5 μL (10 ng/μL) sterilized solution of recombinant human PF-4var/CXCL4L1 (produced as described previously26) or 5 μL (3.75 μg/μL) bevacizumab (Avastin; Genentech, Inc., South San Francisco, CA, USA) was injected into the vitreous of the right eye; the left eye received an equal volume of sterile phosphate-buffered saline (PBS) as a control.The animals were euthanized 2 weeks after diabetes induction and the retinas were carefully dissected, snap frozen in liquid nitrogen, and stored at −80°C to be analyzed by Western blot or ELISA.
Measurement of Blood–Retinal Barrier Breakdown
Retinas were analyzed for BRB breakdown 2 weeks after diabetes induction using fluorescein isothiocyanate (FITC)-conjugated dextran as described previously.3,19 Briefly, rats were deeply anesthetized, and then FITC-conjugated dextran (3–5 kDa; Sigma-Aldrich Corp.) was injected intravenously (50 mg/kg body weight). After 30 minutes, a blood sample was collected, and each rat was then perfused with PBS. The retinas were carefully removed, weighed, and homogenized to extract the FITC-conjugated dextran. Fluorescence was measured using a spectraMax Gemini-XPS microplate reader (Molecular Devices, Sunnyvale, CA, USA) with excitation and emission wavelengths of 485 nm and 538 nm, respectively, with PBS as a blank. Corrections were made by subtracting the autofluorescence of retinal tissue from rats without FITC-conjugated dextran injection. The amount of FITC-conjugated dextran in each retina was calculated from a standard curve of FITC-conjugated dextran in water. For normalization, the retinal FITC-conjugated dextran amount was divided by the retinal weight and by the concentration of FITC-conjugated dextran in the plasma. Blood–retinal barrier breakdown was calculated using the following equation, with the results being expressed in μL/g/h.
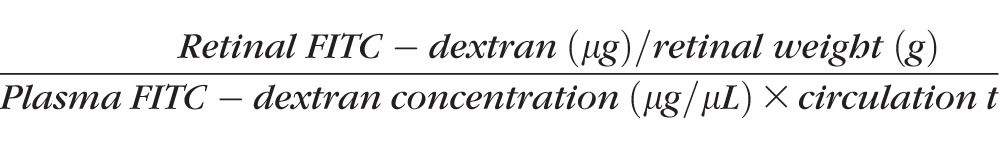 |
Western Blot Analysis
Retinas were homogenized in Western blot lysis buffer (30 mM Tris-HCl, pH 7.5, 5 mM EDTA, 1% Triton X-100, 250 mM sucrose, 1 mM sodium vanadate, and protease inhibitor cocktail); the lysate was centrifuged and supernatants were collected. Equal amounts of protein (30–50 μg) were separated on SDS-polyacrylamide gels and transferred to nitrocellulose membranes for Western blot analysis as described previously.29 Immunodetection was performed using the antibodies indicated in the Table. Membranes were stripped and reprobed with β-actin-specific antibody to evaluate equal loading.
Table. .
Antibodies Used for Western Blot Analysis
|
Antibody |
Dilution |
Source |
| Anti-RAGE | 1:400 | Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA |
| Anti-VE-cadherin | 1:2000 | Abcam, Cambridge, UK |
| Anti-occludin | 1:400 | Santa Cruz Biotechnology, Inc. |
| Anti-caspase-3 | 1:400 | Santa Cruz Biotechnology, Inc. |
| Anti-TNF-α | 1:400 | Santa Cruz Biotechnology, Inc. |
| Anti-HIF-1α | 1:2000 | R&D Systems, Minneapolis, MN, USA |
| Anti-β-actin | 1:2000 | Santa Cruz Biotechnology, Inc. |
Enzyme-Linked Immunosorbent Assay for VEGF and PF-4var/CXCL4L1
The concentration of PF-4var/CXCL4L1 in the HRMEC-conditioned media was determined by a specific ELISA as described elsewhere.28 Vascular endothelial growth factor was measured by the Quantikine VEGF kit (cat no. RRV00; R&D Systems) according to the manufacturer's instructions. Tissue homogenate (150 μg) in lysis buffer was analyzed in the VEGF ELISA. Each assay was performed in duplicate.
Measurement of Reactive Oxygen Species (ROS)
Reactive oxygen species generation was measured in retinal tissue homogenates using 2′-7′-dichlorofluorescein-diacetate (DCFH-DA).30 Retinas were homogenized in PBS in the presence of protease inhibitor with the use of a glass homogenizer. Samples containing 20 μg protein diluted in PBS were incubated (15 minutes) in 5 μM DCFH-DA in the dark. Fluorescence was measured every 15 minutes for 1 hour with excitation and emission wavelengths of 488 nm and 525 nm, respectively.
Measurement of Transendothelial Electrical Resistance (TER)
Transendothelial electrical resistance in cultured HRMEC was measured as described.31 Human retinal microvascular endothelial cells (5 × 104 cells/well) were seeded in plates coated with cystein, collagen, and fibronectin. The electric currents passing through fully confluent monolayers were measured by the Electrical Cell–Substrate Impedance Sensing equipment (ECIS from Applied Biophysic, Inc., Troy, NY, USA). Cells were starved for 24 hours and then treated with vehicle or VEGF with or without PF-4var/CXCL4L1. Transendothelial electrical resistance was recorded over the experimental time course (24 hours). Resistance values for each chamber were normalized at each time point as the ratio of measured resistance to baseline resistance and plotted as a function of time.
Cell Permeability Assay
The FITC-conjugated dextran cell permeability assay was performed as described previously.31 Human retinal microvascular endothelial cells were seeded on collagen/fibronectin-coated membranes with 0.4-μm pores (Transwell; Corning Life Sciences, Acton, MA, USA) in normal glucose medium. After becoming completely confluent, cultures were shifted to 1% serum media overnight, then were treated by vehicle or VEGF (50 ng/mL) in the presence or absence of PF-4var/CXCL4L1 (50 ng/mL) in the upper chambers for 4 hours. Fluorescein isothiocyanate-conjugated dextran (1 mg/mL) was then added to the upper compartments followed by obtaining aliquots from the lower and upper compartments at different time points. The amount of FITC-conjugated dextran was determined (excitation wavelength, 485 nm; emission wavelength, 530 nm) and corrected to the fluorescence reading of samples from the upper compartment. Permeability is given as the amount of FITC-conjugated dextran passing from the top to the bottom compartment.
Statistical Analysis
Data are presented as the mean ± standard deviation (SD) or standard error of mean (SEM). When comparing three or more independent groups, the Kruskal-Wallis test was performed. When the across-group difference was statistically significant, the Mann-Whitney test was used to compare means from two independent groups. A P value less than 0.05 indicated statistical significance. SPSS version 19.0 (IBM, Inc., Chicago, IL, USA) was used for the statistical analyses.
Results
Immunohistochemical Analysis of PDR Fibrovascular Epiretinal Membranes
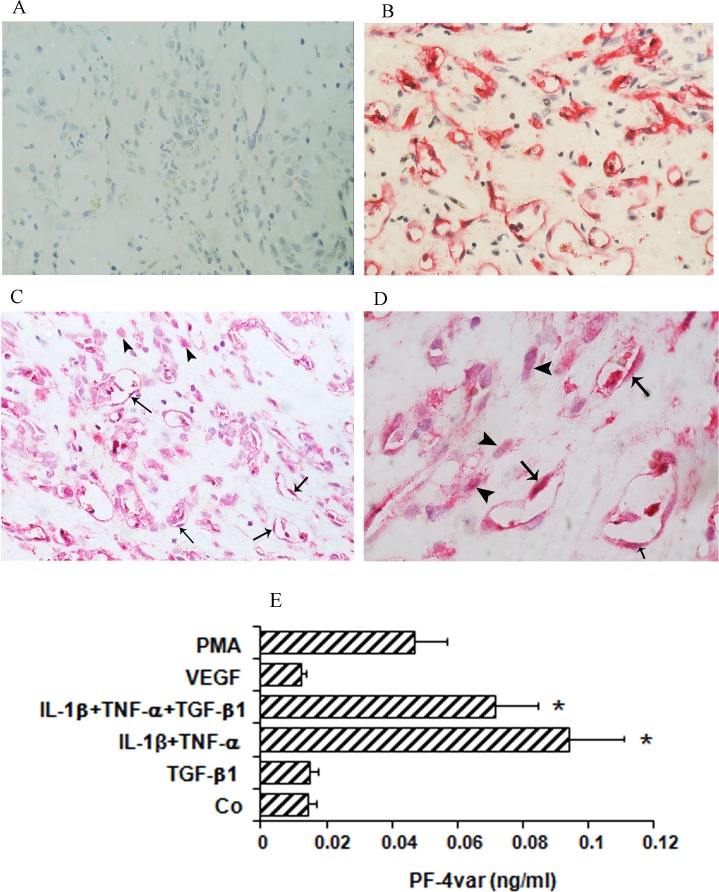
No staining was observed in the negative control slides. Figure 1A shows the isotype control staining for the monoclonal anti-CD34 antibody. Other controls (omission of the primary antibodies, staining with irrelevant antibodies, and staining with chromogen alone) were also performed and did not reveal any specific reactions (data not shown). All membranes showed blood vessels positive for the panendothelial cell marker CD34 (Fig. 1B). Immunoreactivity for PF-4var/CXCL4L1 was present in all membranes and was noted in the cytoplasm of vascular endothelial and stromal cells (Figs. 1C, 1D).
Figure 1.
Expression of PF-4var/CXCL4L1 in proliferative diabetic retinopathy (PDR) and stimulated human retinal microvascular endothelial cells (HRMEC). Epiretinal membranes from patients with PDR were subjected to immunohistochemistry using antibodies against CD34 and PF-4var/CXCL4L1. (A) Control slide that was treated with an irrelevant isotype control antibody showing no labeling. (B) Immunohistochemical staining for CD34 showing blood vessels positive for CD34. (C, D) Immunohistochemical staining for PF-4var/CXCL4L1 showing immunoreactivity in vascular endothelial cells (arrows) and stromal cells (arrowheads). Low power (C) (original magnification ×40) and high power (D) (original magnification ×100). To verify whether HRMEC can produce PF-4var/CXCL4L1 in vitro as well, HRMEC were incubated for 96 hours with 100 ng/mL phorbol myristate acetate (PMA), 10 ng/mL VEGF, 10 ng/mL TGF-β1, 10 ng/mL IL-1β plus 30 ng/mL TNF-α, or 10 ng/mL IL-1β plus 30 ng/mL TNF-α plus 10 ng/mL TGF-β1 or were left untreated (Co) as described in Materials and Methods (E). Results represent the mean (± SEM) PF-4var/CXCL4L1 concentration measured by ELISA (*P < 0.05) (n = 5).
Human Retinal Microvascular Endothelial Cells Produce PF-4var/CXCL4L1 In Vitro
To confirm observed production of PF-4var/CXCL4L1 by endothelial cells, we performed induction experiments on retinal endothelial cells with inducers relevant in the context of DR and combinations thereof. Human retinal microvascular endothelial cells were stimulated for 4 days with VEGF, TGF-β1, IL-1β plus TNF-α, or IL-1β plus TNF-α plus TGF-β1, and the resulting conditioned media were analyzed for the presence of PF-4var/CXCL4L1 (Fig. 1E). PF-4var/CXCL4L1 was not spontaneously produced, nor could VEGF or TGF-β1 trigger gene expression of PF-4var/CXCL4L1 in HRMEC. However, HRMEC produced low, but significant, levels of the angiostatic chemokine in response to the combined treatment of IL-1β plus TNF-α. Addition of TGF-β1 did not change the IL-1β/TNF-α-induced PF-4var/CXCL4L1 production levels.
Effect of PF-4var/CXCL4L1 and the Anti-VEGF Agent Bevacizumab on Blood–Retinal Barrier Breakdown in STZ-Induced Diabetic Rats
Two weeks after induction of diabetes with a single high dose of STZ, the body weights of the diabetic rats were lower and their blood glucose levels were more than 4-fold higher compared with age-matched normal control rats (174 ± 19 vs. 249 ± 27 g and 449 ± 29 vs. 115 ± 12 mg/dL, respectively).
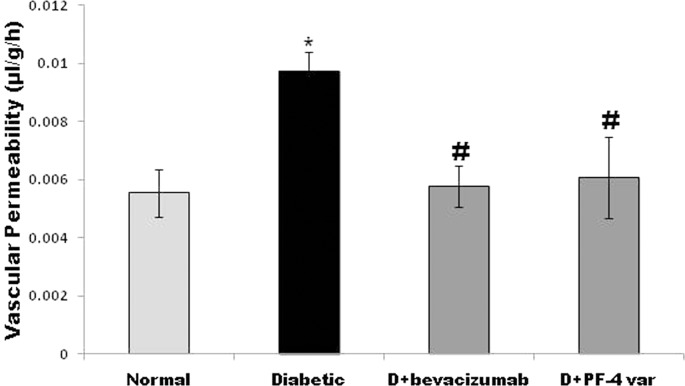
Fluorescein isothiocyanate-conjugated dextran was used to determine the extent of vascular permeability. In STZ-diabetic rats, retinal vascular permeability was significantly increased by 75% when compared with nondiabetic rats. Treatment with intravitreal PF-4var/CXCL4L1 (50 ng) or bevacizumab (18.75 μg) significantly attenuated the effect of STZ treatment on BRB breakdown and reduced vascular leakage by approximately 70% and 73%, respectively, when compared with PBS-treated diabetic eyes (Fig. 2).
Figure 2.
PF-4var/CXCL4L1 prevents diabetes-induced blood–retinal barrier (BRB) breakdown. Treatment with 50 ng intravitrial PF-4var/CXCL4L1 in one eye and 18.75 μg bevacizumab in the contralateral eye in diabetic rats. The BRB breakdown was quantified with the FITC-conjugated dextran technique. Bars represent the mean ±SD of six or seven rats in each group. Diabetic, PBS-injected diabetic eye; D+ bevacizumab, bevacizumab-injected diabetic eye; D+PF-4var/CXCL4L1, PF-4var/CXCL4L1-injected diabetic eye. Each retina was subsampled and analyzed at least three times. Results are expressed as mean ± SD of at least six rats in each group. *P < 0.05 compared to normal, #P < 0.05 compared to diabetes.
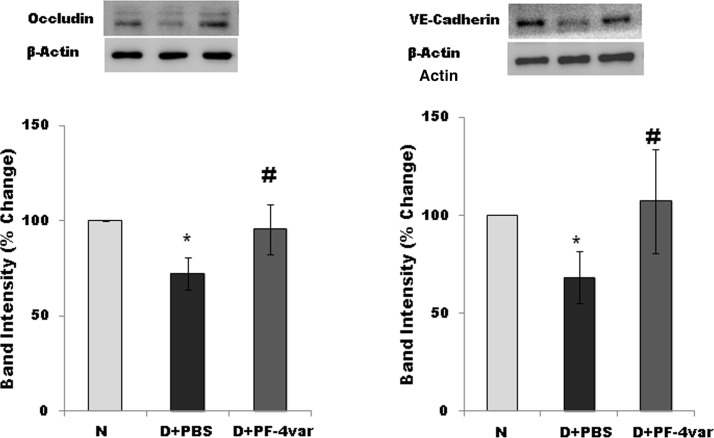
Effect of PF-4var/CXCL4L1 on the Expression of the Tight Junction Protein Occludin and the Adherens Junction Protein VE-Cadherin in Retina of STZ-Induced Diabetic Rats
Diabetes significantly reduced the retinal level of occludin and VE-cadherin (major components of the BRB) by 30% and 33%, respectively, when compared with the retina of nondiabetic rats (Fig. 3). Treatment with intravitreal PF-4var/CXCL4L1 significantly increased the level of retinal occludin and VE-cadherin in STZ-induced diabetic rats by 28% and 38%, respectively, when compared with the values obtained from the contralateral diabetic eye that received PBS alone. These results indicate that PF-4var/CXCL4L1 may exert its stabilizing effect on the BRB by upregulating the level of tight junction and adherens junction proteins.
Figure 3.
PF-4var/CXCL4L1 upregulates the level of retinal occludin and VE-cadherin in diabetic rat retinas. Western blot analysis of occludin and VE-cadherin level in normal and diabetic rat eyes that received intravitreal injection of PBS or PF-4var/CXCL4L1. Equal loading in each lane was confirmed via detection of β-actin. Quantitation of the signals was done by densitometric analysis and results were normalized to β-actin content, followed by normalization to values of normal eyes. Results (mean ± SD) for seven or eight rats in each group are shown. N, healthy eye; D+PBS, PBS-injected diabetic eye; D+PF-4var/CXCL4L1, PF-4var/CXCL4L1-injected diabetic eye. *P < 0.05 compared to normal, #P < 0.05 compared to diabetes.
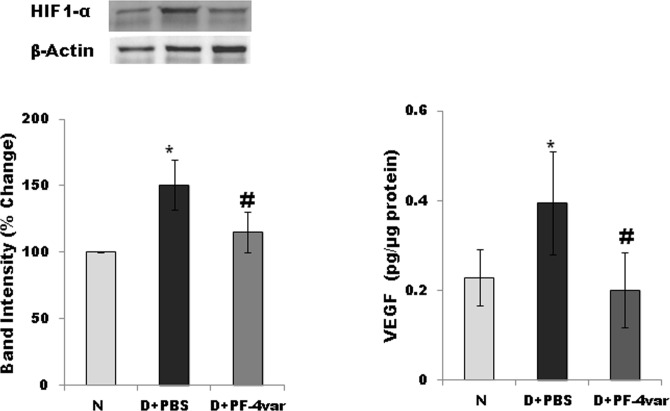
Effect of PF-4var/CXCL4L1 on the Expression of the Transcription Factor HIF-1α and Its Downstream Target VEGF in Retina of STZ-Induced Diabetic Rats
Retinal hypoxia-inducible factor (HIF)-1α protein expression was measured by Western blot analysis, whereas ELISA was used to measure retinal VEGF protein expression. Diabetes significantly increased the retinal expression of HIF-1α and VEGF proteins by 51% and 72%, respectively, when compared with the retinas of nondiabetic control rats at 2 weeks after the induction of diabetes. Treatment with intravitreal PF-4var/CXCL4L1 significantly reduced the expression of retinal HIF-1α and VEGF proteins in STZ-induced diabetic rats by 24% and 50%, respectively, when compared with the values obtained from the PBS-treated contralateral eye (Fig. 4).
Figure 4.
PF-4var/CXCL4L1 reduces the expression of HIF-1α and VEGF in diabetic rat retinas. The expression of HIF-1α was quantified by Western blot analysis and adjusted to the expression of β-actin in each sample, followed by normalization to values of normal eyes. Total VEGF levels were measured in rat retinal tissue homogenates by sandwich ELISA with 150 μg protein. Each sample was measured in duplicate, and eight or more rats were included in each group. N, normal; D+PBS, PBS-injected diabetic eye; D+PF-4var/CXCL4L1, PF-4var/CXCL4L1-injected diabetic eye. *P < 0.05 compared to normal, #P < 0.05 compared to diabetes.
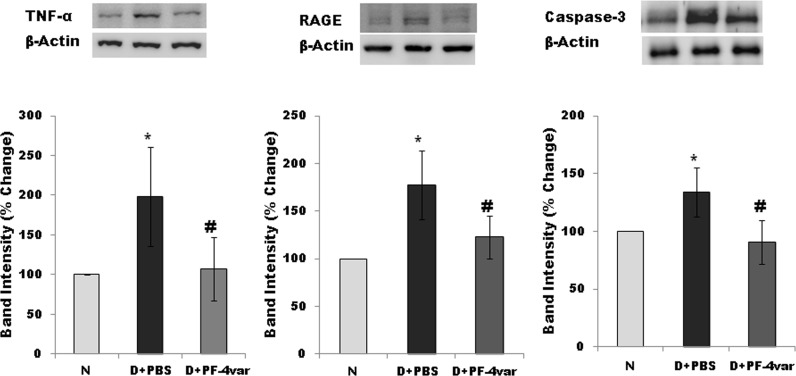
Effect of PF-4var/CXCL4L1 on the Expression of the Inflammatory Mediators TNF-α and RAGE and the Apoptosis Executer Enzyme Caspase-3 in Retina of STZ-Induced Diabetic Rats
Blood–retinal barrier breakdown, a characteristic of DR, is believed to depend on inflammation and apoptosis. To study if PF-4var/CXCL4L1 protected the BRB via regulation of retinal inflammation and apoptosis, we measured by Western blot analysis the expression of the proinflammatory factors TNF-α and RAGE and the apoptosis executer enzyme caspase-3. Our results showed that expression levels of TNF-α, RAGE, and caspase-3 were significantly increased by 95%, 70%, and 35%, respectively, in the retina of STZ-induced diabetic rats at 2 weeks after the induction of diabetes when compared with the retina of nondiabetic rats. Treatment with intravitreal PF-4var/CXCL4L1 effectively attenuated the expression of TNF-α, RAGE, and caspase-3 by 90%, 80%, and 37%, respectively, when compared with the values obtained from the contralateral PBS-treated diabetic eye (Fig. 5).
Figure 5.
PF-4var/CXCL4L1 attenuates the expression of TNF-α, RAGE, and caspase-3 in diabetic rat retinas. TNF-α, RAGE, and caspase-3 expression in rat retinas was studied by Western blot analysis. Relative expression levels to β-actin as housekeeping control were calculated, followed by normalization to values of normal eyes. Each group comprised seven or eight animals, and values represent mean ± SD. *P < 0.05 compared to normal, #P < 0.05 compared to diabetes.
Effect of PF-4var/CXCL4L1 on the Generation of Reactive Oxygen Species in Retina of STZ-Induced Diabetic Rats
Oxidative stress plays an important role in retinal vascular endothelial dysfunction in diabetes.17 To assess whether PF-4var/CXCL4L1 reduces diabetes-induced BRB breakdown through a reduction in ROS generation, we measured ROS retinal levels. Compared with the retina of nondiabetic animals, the retina of diabetic animals demonstrated a 73% increase in ROS generation. The ROS generation in diabetic animals treated with PF-4var/CXCL4L1 did not differ significantly from the levels in the untreated diabetic animals (Fig. 6).
Figure 6.
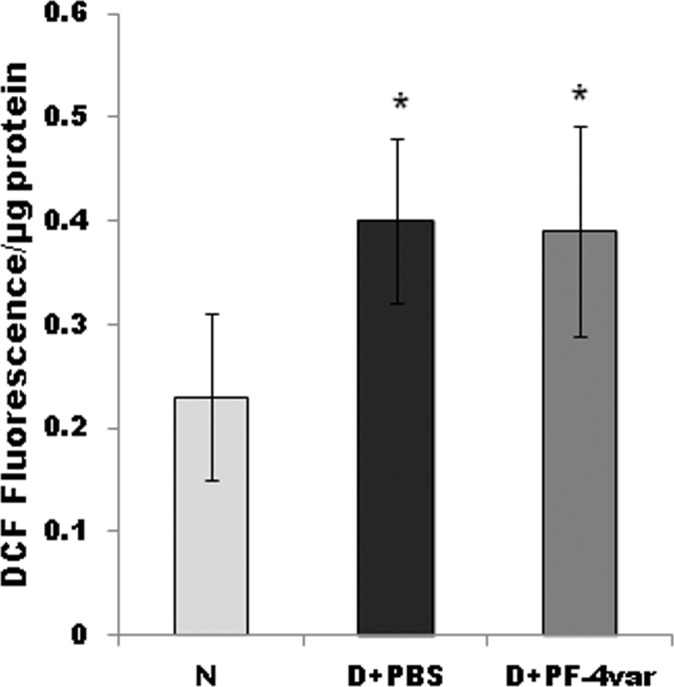
PF-4var/CXCL4L1 has no effect on the generation of reactive oxygen species in diabetic rat retinas. Freshly prepared rat retinal homogenates were incubated with 2′-7′-dichlorofluorescein-diacetate, and 2′-7′-dichlorofluorescein (DCF) fluorescence intensity was quantitated. N, normal; D+ PBS, PBS-injected diabetic eye; D+ PF-4var/CXCL4L1, PF-4var/CXCL4L1 injected diabetic eye. Each group comprised seven or eight animals, and values represent mean ± SD. *P < 0.05 compared to normal.
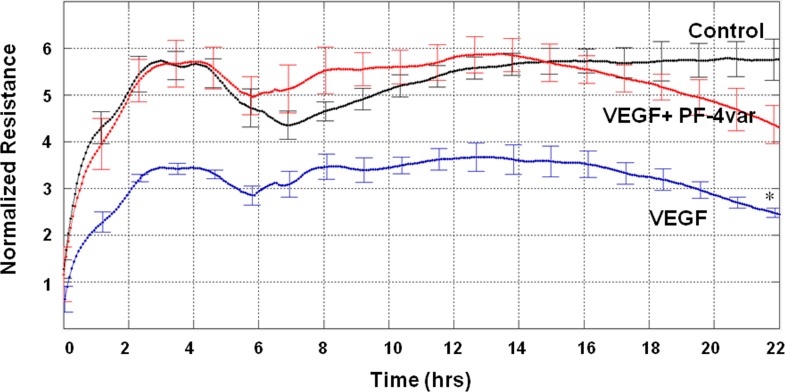
Effect of PF-4var/CXCL4L1 on VEGF-Induced Hyperpermeability in Human Retinal Microvascular Endothelial Cells
The antipermeable effect of PF-4var/CXCL4L1 on HRMEC was evaluated via testing TER. An increase in the endothelial permeability is accompanied by a reduction in TER. Treatment of HRMEC with VEGF (50 ng/mL) significantly reduced TER. Cotreatment of HRMEC with VEGF and PF-4var/CXCL4L1 (50 ng/mL) inhibited VEGF-induced hyperpermeability and reversed TER to control values (Fig. 7).
Figure 7.
PF-4var/CXCL4L1 promotes transcellular electrical resistance (TER) in human retinal microvascular endothelial cells (HRMEC). Monolayers of HRMEC were treated with 50 ng/mL VEGF or 50 ng/mL VEGF in combination with 50 ng/mL PF-4var/CXCL4L1, and changes in TER were monitored. Experiments were repeated two times in triplicate. *P < 0.05 versus control, n = 6.
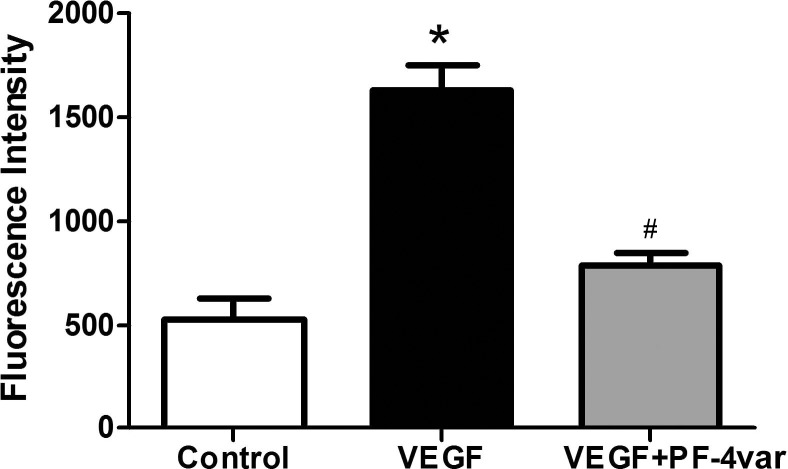
The FITC-conjugated dextran flux assay through an endothelial cell layer grown in transwells was used to examine the effects of PF-4var/CXCL4L1 on VEGF-induced permeability. Vascular endothelial growth factor significantly increased the leakage of FITC through the confluent monolayer of HRMEC after 30 minutes. However, concomitant treatment of HRMEC with PF-4var/CXCL4L1 and VEGF significantly reduced VEGF-induced HRMEC permeability (Fig. 8).
Figure 8.
PF-4var/CXCL4L1 neutralizes permeability induced by VEGF in human retinal microvascular endothelial cells. HRMEC were seeded in transwells until confluency and were starved overnight in 1% serum-containing media. Cells were treated with vehicle, VEGF (50 ng/mL), or VEGF (50 ng/mL) plus 50 ng/mL PF-4var/CXCL4L1. FITC-conjugated dextran (1 mg/mL) was then added, and aliquots were assessed for fluorescence intensity using a fluorescence plate reader. n = 4 to 6, *P < 0.05.
Discussion
Vascular endothelial growth factor is a hypoxia-induced angiogenic factor and is a potent vascular permeability factor that has emerged as a key mediator of BRB breakdown in the diabetic retina.3,4,32 The efficacy and safety of intravitreal anti-VEGF as therapy for diabetic macular edema have recently been proven by various clinical trials providing significantly positive visual and anatomical results.33 In the present study, we demonstrated that diabetes induced significant upregulation of retinal expression of VEGF and that treatment with intravitreal PF-4var/CXCL4L1 normalized retinal VEGF expression. In addition, PF-4var/CXCL4L1 was as potent as the anti-VEGF agent bevacizumab in attenuating the increase in retinal vascular leakage and BRB breakdown in STZ-induced diabetic rats. Moreover, PF-4var/CXCL4L1 blocked VEGF-induced increases in HRMEC permeability. PF-4var/CXCL4L1 is a recently described natural nonallelic gene variant of the angiostatic CXC chemokine PF-4/CXCL4.25–28 PF-4/CXCL4 is angiostatically more active than its homologue PF-4/CXCL4 in various test systems and inhibited tumor growth in animal models of several types of cancers more efficiently than PF-4/CXCL4.25–27 Other investigators have shown that authentic PF-4/CXCL4 inhibits both expression34 and the function of VEGF by disrupting the binding of VEGF to its receptor35 and disrupting the VEGF-induced intracellular signaling cascade.36
Several studies provided evidence that activation of the transcription factor HIF-1α, a known activator of the VEGF promoter,37,38 is associated with BRB breakdown and retinal inflammation induced by diabetes.39–40 In this study, we demonstrated that intravitreal PF-4var/CXCL4L1 normalized retinal HIF-1α expression. Our findings suggest that increasing intraocular PF-4var/CXCL4L1 levels early after the onset of diabetes would antagonize HIF-1α/VEGF signaling and inhibit BRB breakdown. Indeed, intravitreal injection of PF-4var/CXCL4L1 attenuated diabetes-induced downregulation of the tight junction protein occludin and the adherens junction protein VE-cadherin, known to be downregulated by VEGF16,41,42 and TNF-α,15 in the retinal endothelial cells. Therefore, PF-4var/CXCL4L1 therapy may be effective for diabetic macular edema. In addition, we demonstrated that diabetes induced significant upregulation of retinal RAGE expression, consistent with previous reports,43,44 and that treatment with intravitreal PF-4var/CXCL4L1 downregulated RAGE levels. Previous studies already demonstrated that inhibition of RAGE ameliorated BRB breakdown, leukostasis, and expression of ICAM-1 in the retina of diabetic animals.11,12
Angiogenesis, the growth of new vascular networks from preexisting ones, is regulated by a dynamic balance between angiogenic stimulators and inhibitors.45 Similarly, within the ocular microenvironment the balance between angiogenic and angiostatic factors also determines blood vessel formation, which is essential for progression of PDR.24 To our knowledge, this study demonstrates for the first time the in situ localization of the expression of the angiostatic chemokine PF-4var/CXCL4L1 in endothelial cells and stromal cells in epiretinal membranes from patients with PDR. We additionally showed that treatment of HRMEC with IL-1β plus TNF-α induced PF-4var/CXCL4L1 production. These results indicate that HRMEC are a cellular source of PF-4var/CXCL4L1 in PDR. These findings suggest that PF-4var/CXCL4L1 can contribute to the angiostatic counterbalance in PDR progression and that PF-4var/CXCL4L1 contributes to the spontaneous regression of new blood vessels in patients with end-stage PDR.
In conclusion, we demonstrated that intravitreal administration of the angiostatic chemokine PF-4var/CXCL4L1 significantly reversed increased retinal vascular permeability in 2-week STZ-diabetic rats. These data confirm the clinical potential of the platelet-derived PF-4var/CXCL4L1 for the treatment of diabetes-induced retinal vasculopathy.
Acknowledgments
The authors thank Noëmie Pörtner and Isabelle Ronsse for excellent technical assistance and Connie B. Unisa-Marfil for secretarial work.
Supported by Dr Nasser Al-Rashid Research Chair in Ophthalmology (AMAE-A), the Fund for Scientific Research of Flanders (FWO-Vlaanderen project G.0764.14 and G.0773.13), the Interuniversity Attraction Poles Programme initiated by the Belgian Science Policy Office (I.A.P. project P7/40), the Concerted Research Actions of the Regional Government of Flanders (GOA13/014) (SS, GO, JVD), and National Institutes of Health Grant (1R01EY023315-01) (MA-S).
Disclosure: A.M. Abu El-Asrar, None; G. Mohammad, None; M.I. Nawaz, None; M. Abdelsaid, None; M.M. Siddiquei, None; K. Alam, None; K. Van den Eynde, None; G. De Hertogh, None; G. Opdenakker, None; M. Al-Shabrawey, None; J. Van Damme, None; S. Struyf, None
References
- 1. Ferris FL III, Patz A. Macular edema. A complication of diabetic retinopathy. Surv Ophthalmol. 1984; 28 (suppl): 452–461. [DOI] [PubMed] [Google Scholar]
- 2. Kusari J, Zhou SA, Padillo E, Clarke KG, Gil DW. Inhibition of vitreoretinal VEGF elevation and blood-retinal barrier breakdown in streptozotocin-induced diabetic rats by brimonidine. Invest Ophthalmol Vis Sci. 2010; 51: 1044–1051. [DOI] [PubMed] [Google Scholar]
- 3. Ishida S, Usui T, Yamashiro K, et al. VEGF164 is proinflammatory in the diabetic retina. Invest Ophthalmol Vis Sci. 2003; 44: 2155–2162. [DOI] [PubMed] [Google Scholar]
- 4. Qaum T, Xu Q, Joussen AM, et al. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci. 2001; 42: 2408–2413. [PubMed] [Google Scholar]
- 5. Yu H, Chen L, Jiang J. Administration of pigment epithelium-derived factor delivered by adeno-associated virus inhibits blood-retinal barrier breakdown in diabetic rats. Mol Vis. 2010; 16: 2384–2394. [PMC free article] [PubMed] [Google Scholar]
- 6. Leal EC, Martins J, Voabil P, et al. Calcium dobesilate inhibits the alterations in tight junction proteins and leukocyte adhesion to retinal endothelial cells induced by diabetes. Diabetes. 2010; 59: 2637–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Navaratna D, Menicucci G, Maestas J, Srinivasan R, McGuire P, Das A. A peptide inhibitor of the urokinase/urokinase receptor system inhibits alteration of the blood-retinal barrier in diabetes. FASEB J. 2008; 22: 3310–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Navaratna D, McGuire PG, Menicucci G, Das A. Proteolytic degradation of VE-cadherin alters the blood-retinal barrier in diabetes. Diabetes. 2007; 56: 2380–2387. [DOI] [PubMed] [Google Scholar]
- 9. Poulaki V, Joussen AM, Mitsiades N, Mitsiades CS, Iliaki EF, Adamis AP. Insulin-like growth factor-1 plays a pathogenetic role in diabetic retinopathy. Am J Pathol. 2004; 165: 457–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Joussen AM, Poulaki V, Le ML, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004; 18: 1450–1452. [DOI] [PubMed] [Google Scholar]
- 11. Kaji Y, Usui T, Ishida S, et al. Inhibition of diabetic leukostasis and blood-retinal barrier breakdown with a soluble form of a receptor for advanced glycation end products. Invest Ophthalmol Vis Sci. 2007; 48: 858–865. [DOI] [PubMed] [Google Scholar]
- 12. Li G, Tang J, Du Y, Lee CA, Kern TS. Beneficial effects of a novel RAGE inhibitor on early diabetic retinopathy and tactile allodynia. Mol Vis. 2011; 17: 3156–3165. [PMC free article] [PubMed] [Google Scholar]
- 13. Joussen AM, Doehmen S, Le ML, et al. TNF-alpha mediated apoptosis plays an important role in the development of early diabetic retinopathy and long-term histopathological alterations. Mol Vis. 2009; 15: 1418–1428. [PMC free article] [PubMed] [Google Scholar]
- 14. Huang H, Gandhi JK, Zhong X, et al. TNFalpha is required for late BRB breakdown in diabetic retinopathy, and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. Invest Ophthalmol Vis Sci. 2011; 52: 1336–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li J, Wang JJ, Chen D, et al. Systemic administration of HMG-CoA reductase inhibitor protects the blood-retinal barrier and ameliorates retinal inflammation in type 2 diabetes. Exp Eye Res. 2009; 89: 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Antonetti DA, Barber AJ, Khin S, Lieth E, Tarbell JM, Gardner TW. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells. Penn State Retina Research Group. Diabetes. 1998; 47: 1953–1959. [DOI] [PubMed] [Google Scholar]
- 17. Frey T, Antonetti DA. Alterations to the blood-retinal barrier in diabetes: cytokines and reactive oxygen species. Antioxid Redox Signal. 2011; 15: 1271–1284. [DOI] [PubMed] [Google Scholar]
- 18. Von Hundelshausen P, Weber C. Platelets as immune cells: bridging inflammation and cardiovascular disease. Circ Res. 2007; 100: 27–40. [DOI] [PubMed] [Google Scholar]
- 19. Yamashiro K, Tsujikawa A, Ishida S, et al. Platelets accumulate in the diabetic retinal vasculature following endothelial death and suppress blood-retinal barrier breakdown. Am J Pathol. 2003; 163: 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Keeley EC, Mehrad B, Strieter RM. Chemokines as mediators of tumor angiogenesis and neovascularization. Exp Cell Res. 2011; 317: 685–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vandercappellen J, Van Damme J, Struyf S. The role of CXC chemokines and their receptors in cancer. Cancer Lett. 2008; 267: 226–244. [DOI] [PubMed] [Google Scholar]
- 22. Maione TE, Gray GS, Petro J, et al. Inhibition of angiogenesis by recombinant human platelet factor-4 and related peptides. Science. 1990; 247: 77–79. [DOI] [PubMed] [Google Scholar]
- 23. Vandercappellen J, Van Damme J, Struyf S. The role of the CXC chemokines platelet factor-4 (CXCL4/PF-4) and its variant (CXCL4L1/PF-4var) in inflammation, angiogenesis and cancer. Cytokine Growth Factor Rev. 2011; 22: 1–18. [DOI] [PubMed] [Google Scholar]
- 24. Nawaz MI, Van Raemdonck K, Mohammad G, et al. Autocrine CCL2, CXCL4, CXCL9 and CXCL10 signal in retinal endothelial cells and are enhanced in diabetic retinopathy. Exp Eye Res. 2013; 109: 67–76. [DOI] [PubMed] [Google Scholar]
- 25. Struyf S, Burdick MD, Proost P, Van Damme J, Strieter RM. Platelets release CXCL4L1, a nonallelic variant of the chemokine platelet factor-4/CXCL4 and potent inhibitor of angiogenesis. Circ Res. 2004; 95: 855–857. [DOI] [PubMed] [Google Scholar]
- 26. Struyf S, Burdick MD, Peeters E, et al. Platelet factor-4 variant chemokine CXCL4L1 inhibits melanoma and lung carcinoma growth and metastasis by preventing angiogenesis. Cancer Res. 2007; 67: 5940–5948. [DOI] [PubMed] [Google Scholar]
- 27. Struyf S, Salogni L, Burdick MD, et al. Angiostatic and chemotactic activities of the CXC chemokine CXCL4L1 (platelet factor-4 variant) are mediated by CXCR3. Blood. 2011; 117: 480–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vandercappellen J, Liekens S, Bronckaers A, et al. The COOH-terminal peptide of platelet factor-4 variant (CXCL4L1/PF-4var47-70) strongly inhibits angiogenesis and suppresses B16 melanoma growth in vivo. Mol Cancer Res. 2010; 8: 322–334. [DOI] [PubMed] [Google Scholar]
- 29. Mohammad G, Vandooren J, Siddiquei MM, Martens E, Abu El-Asrar AM, Opdenakker G. Functional links between gelatinase B/matrix metalloproteinase-9 and prominin-1/CD133 in diabetic retinal vasculopathy and neuropathy. Prog Retin Eye Res. 2014; 43: 76–91. [DOI] [PubMed] [Google Scholar]
- 30. Dasari B, Prasanthi JR, Marwarha G, Singh BB, Ghribi O. Cholesterol-enriched diet causes age-related macular degeneration-like pathology in rabbit retina. BMC Ophthalmol. 2011; 11: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Othman A, Ahmad S, Megyerdi S, et al. 12/15-Lipoxygenase-derived lipid metabolites induce retinal endothelial cell barrier dysfunction: contribution of NADPH oxidase. PLoS One. 2013; 8: e57254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Funatsu H, Yamashita H, Ikeda T, et al. Angiotensin II and vascular endothelial growth factor in the vitreous fluid of patients with diabetic macular edema and other retinal disorders. Am J Ophthalmol. 2002; 133: 537–543. [DOI] [PubMed] [Google Scholar]
- 33. Ho AC, Scott IU, Kim SJ, et al. Anti-vascular endothelial growth factor pharmacotherapy for diabetic macular edema: a report by the American Academy of Ophthalmology. Ophthalmology. 2012; 119: 2179–2188. [DOI] [PubMed] [Google Scholar]
- 34. Yang L, Du J, Hou J, Jiang H, Zou J. Platelet factor-4 and its p17-70 peptide inhibit myeloma proliferation and angiogenesis in vivo. BMC Cancer. 2011; 11: 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jouan V, Canron X, Alemany M, et al. Inhibition of in vitro angiogenesis by platelet factor-4-derived peptides and mechanism of action. Blood. 1999; 94: 984–993. [PubMed] [Google Scholar]
- 36. Sulpice E, Contreres JO, Lacour J, Bryckaert M, Tobelem G. Platelet factor 4 disrupts the intracellular signalling cascade induced by vascular endothelial growth factor by both KDR dependent and independent mechanisms. Eur J Biochem. 2004; 271: 3310–3318. [DOI] [PubMed] [Google Scholar]
- 37. Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 1998; 95: 7987–7992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yamakawa M, Liu LX, Date T, et al. Hypoxia-inducible factor-1 mediates activation of cultured vascular endothelial cells by inducing multiple angiogenic factors. Circ Res. 2003; 93: 664–673. [DOI] [PubMed] [Google Scholar]
- 39. Lin M, Chen Y, Jin J, et al. Ischaemia-induced retinal neovascularization and diabetic retinopathy in mice with conditional knockout of hypoxia-inducible factor-1 in retinal Müller cells. Diabetologia. 2011; 54: 1554–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Poulaki V, Qin W, Joussen AM, et al. Acute intensive insulin therapy exacerbates diabetic blood-retinal barrier breakdown via hypoxia-inducible factor-1alpha and VEGF. J Clin Invest. 2002; 109: 805–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim JH, Kim JH, Lee YM, Ahn EM, Kim KW, Yu YS. Decursin inhibits VEGF-mediated inner blood-retinal barrier breakdown by suppression of VEGFR-2 activation. J Cereb Blood Flow Metab. 2009; 29: 1559–1567. [DOI] [PubMed] [Google Scholar]
- 42. Wisniewska-Kruk J, Hoeben KA, Vogels IM, et al. A novel co-culture model of the blood-retinal barrier based on primary retinal endothelial cells, pericytes and astrocytes. Exp Eye Res. 2012; 96: 181–190. [DOI] [PubMed] [Google Scholar]
- 43. Mohammad G, Siddiquei MM, Othman A, Al-Shabrawey M. Abu El-Asrar AM. High-mobility group box-1 protein activates inflammatory signaling pathway components and disrupts retinal vascular-barrier in the diabetic retina. Exp Eye Res. 2013; 107: 101–109. [DOI] [PubMed] [Google Scholar]
- 44. Zong H, Ward M, Madden A, et al. Hyperglycaemia-induced pro-inflammatory responses by retinal Müller glia are regulated by the receptor for advanced glycation end-products (RAGE). Diabetologia. 2010; 53: 2656–2666. [DOI] [PubMed] [Google Scholar]
- 45. Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996; 86: 353–364. [DOI] [PubMed] [Google Scholar]