Abstract
Objective
Experiments in animal models have shown a positive association between in utero exposure to pharmacologic sex hormones and offspring obesity. The developmental effects of such hormones on human obesity are unknown.
Methods
Using data from a large, prospective pregnancy cohort study (n=19,652), with linkage to a national prescription registry, we evaluated the association between use of hormonal contraceptives before and after conception (defined from dispensed prescription data and characterized by last date of use relative to conception, 12 – >4 months before (n=3,392), 4 – >1 months before (n=2,541), 1 – > 0 months before (n=2,997), and 0–12 weeks after (n=567)) in relation to offspring overweight or obesity at age 3 years.
Results
We observed a weak, inverse association between early pregnancy use of a combination oral contraceptive and offspring overweight or obesity at age 3 (adjusted OR: 0.75, 95% CI: 0.53, 1.08) and a positive, but imprecise, association with use of a progestin-only oral contraceptive in early pregnancy (adjusted OR: 1.26, 95% CI: 0.79, 2.02). In general, no association was observed between use of a hormonal contraceptive before conception and offspring overweight or obesity. A sensitivity analysis comparing combination oral contraceptive users in early pregnancy to other unplanned pregnancies without hormonal contraceptive use further strengthened the inverse association (adjusted OR: 0.70, 95% CI: 0.48, 1.02). Other sensitivity analyses were conducted to evaluate the robustness of the associations observed given varying assumptions.
Conclusion
Pharmacologic sex hormones in early pregnancy may be inversely or positively associated with offspring overweight or obesity at age 3, depending on the specific formulation used. The present study provides support for the potential for environmental sources of hormonally active agents to exert developmental effects.
Keywords: Hormonal contraceptives, Estrogen-mimicking compounds, Pediatric overweight and obesity, Developmental origins of health and disease, The Norwegian mother and child (MoBa) cohort study
Introduction
Worldwide, the prevalence of childhood overweight and obesity increased from 4.2 percent in 1990 to 6.7 percent in 2010.1 Children who are overweight or obese are more likely to be overweight in adulthood and to suffer from obesity-related morbidity and mortality.2, 3 The obesity epidemic has been primarily attributed to changes in dietary and physical activity behaviors,4, 5 but exposure to estrogen-mimicking compounds during developmentally sensitive periods may contribute.6, 7
Estrogenic agents can affect adipogenesis in vitro. 17-β estradiol has resulted in increased preadipocyte proliferation, likely though up-regulation of PPAR-γ.8 Preadipocyte formation can occur as early as the blastocyst stage,9 although upregulation of mesenchymal stem cell recruitment to preadipocytes is highest in the second trimester of pregnancy.10
In a previous study of the association between oral contraceptive (OC) and diethylstilbestrol (DES) use in pregnancy and offspring obesity,11 the strongest magnitude of association for OCs was in months 1 and 2 and, for DES, in months 3–4. This study was executed at a time when the potency of OCs was considerably stronger (1959–1974).
Experiments in animal models have shown a positive association between in utero and neonatal exogenous estrogen exposure and metabolic disruption in the offspring, including offspring overweight or obesity.6, 7 However, in utero exposure to androgens has also been associated with offspring obesity.12–14 Hormonal contraceptives can be androgenic, depending on the progestin component included.15 The developmental effects of exogenous sex hormones on growth may be sex-dependent, with associations primarily in male offspring.16
The maternal metabolic milieu is also associated with offspring overweight or obesity.17, 18 Hormonal contraceptives have, for many women, unintended metabolic effects, including elevated levels of very low-density lipoprotein cholesterol and total triglycerides.15, 19–21 Hormonal contraceptives increase plasma insulin and cortisol,21 and induce a state of insulin resistance.15, 19, 20 Some of these metabolic changes, including increased total cholesterol22 and insulin resistance,23 are similar to those in women who are overweight or obese.. Whether these metabolic effects persist after cessation of use is unclear; however more androgenic formulations may subsequently increase risk of gestational diabetes.24
The half-lives of hormonal contraceptives are generally <24 hours;25 however in some instances drug components may be detectable for several months post cessation. For example, Medroxyprogesterone Acetate has been detectable in serum at 8 months post cessation of administration.15 Previous hormonal contraceptive use may have long-term effects on endogenous hormone levels, including altered hormone levels both during pregnancy26 and after menopause.27
Given the data suggesting that hormonal compounds cause changes in follicular,28, 29 embryonic, and fetal development,30, 31 that they may cause an obesity-like metabolic milieu,32 and that they may exert long-term effects on endogenous sex hormone levels,27, 33 additional studies of hormonal contraceptive exposure and offspring development in humans are needed.
Because use of pharmacologic sex hormones in early pregnancy is relatively uncommon, most cohort studies lack the power to evaluate the association. Hormonal contraceptive failure occurs in about 3% of users.34 With over 40,000 children followed to age 3, the Norwegian Mother and Child Cohort Study (MoBa)35 offers an unusual opportunity to assess the influence of in utero exposure to exogenous sex hormones, through hormonal contraceptive use in early pregnancy, on childhood overweight or obesity. In the present study, through linkage of MoBa data with the Norwegian Prescription Registry (NorPD), we evaluated the association between hormonal contraceptive use and offspring overweight or obesity at 36 months of age.
Methods
MoBa study participants were recruited in Norway from 1999 through 2008, as described in detail elsewhere.35 Women were identified for eligibility when scheduling the routine prenatal ultrasound offered free of charge to all pregnant women in Norway at 17–20 weeks of gestation. Women were mailed an invitation to participate before the scheduled ultrasound, with informed consent and enrollment taking place at the ultrasound examination. Approximately 42 percent of all pregnant women in Norway were invited to participate in the study. Of these, 39 percent consented to participate. At enrollment, participants were asked to complete a self-administered questionnaire to collect data on demographic characteristics, reproductive health history, disease and medication history, lifestyle factors, and socioeconomic status. Follow-up is conducted through self-administered questionnaires.
Prescription data from NorPD contains individual-level data on all medications prescribed and dispensed through pharmacies to non-institutionalized individuals in Norway. By Norwegian law, as of January 1, 2004, all pharmacies must provide electronic data for all prescriptions dispensed. Data quality measures are in place for assuring the NorPD is accurate and complete.36 A validation study of hormonal contraceptive use in the NorPD was conducted in adolescents and indicated a sensitivity of 99% and a specificity of 76% for the NorPD as compared to self-reported use.37 In adolescents, hormonal contraceptives may be provided at no cost to the individual, but in adults, hormonal contraceptives are not a reimbursable prescription. This may increase the likelihood that a dispensed prescription will be used by the individual.
There were 107,308 MoBa pregnancies registered in the Medical Birth Registry of Norway (MBRN). All MBRN data are collected on a standardized birth notification form completed by the midwife or physician attending the birth. For the present analysis, we included pregnancies resulting in a singleton live birth, with no record of death in the first year of life, and with no documentation, on either the MoBa 17-week questionnaire or the MBRN, of having received infertility treatment for the index pregnancy. We additionally excluded pregnancies to women with pre-pregnancy chronic hypertension (n=527). As the NorPD registry was not initiated until January 1, 2004, we further restricted our study population to pregnancies of women enrolled at least 12 months after the date on which the NorPD registry began collection of data (n=48,615). For the primary analyses, we also excluded pregnancies with missing covariate data (n=3,966), and for loss to follow-up at age 3 (n=24,997). The final study population included 19,652 pregnancies to 18,759 women (17,867 women with 1 offspring in the cohort, 1,782 with 2, and 1 with 3 offspring) (Figure 1). The University of North Carolina at Chapel Hill, the National Institute of Environmental Health Sciences Institutional Review Board, and the Norwegian Southeastern Regional Ethics Committee reviewed and approved this study.
Figure 1.
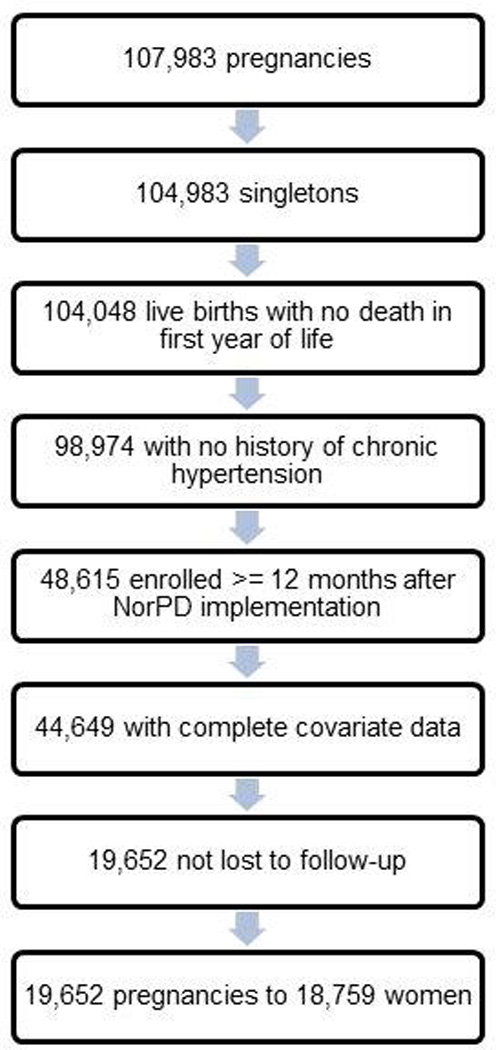
Study population selection for assessing association between hormonal contraceptives and offspring overweight or obesity in the Norwegian Mother Child Prospective Cohort Study (2004–2008)
Hormonal contraceptive use, before conception and in early pregnancy, was characterized according to the Anatomical Therapeutic Chemical (ATC) Classification System.38 We characterized exposure by type and route of administration (combination OC, progestin-only OC, vaginal ring, transdermal, injectable, implant, and hormonal-based intrauterine device) and by progestin formulation. All hormonal contraceptives with an estrogen component (combination OC, vaginal ring, and the transdermal contraceptive) contained ethinyl estradiol, but there were eight different progestin types used solely or in combination with ethinyl estradiol, including desogestrel, drospirenone, levonorgestrel, norelgestromin, norethisterone, lynestronol, medroxyprogesterone, and etonogestrel. Any exposures with fewer than 10 exposed cases we combined into a single “other” category (Supplementary Table S1).
Although our primary interest was to explore the association between exposure in early pregnancy and offspring overweight or obesity, we also characterized exposure into discrete windows of hormonal contraceptive exposure according to last date of use relative to conception, e.g. 12 – >4 months before, 4 – >1 months before, 1 – > 0 months before, and 0–12 weeks after. These periods of exposure were selected as they correspond to possibly distinct developmental periods of susceptibility, specifically the primordial follicular phase (FSH independent) (12 to 4 months before conception), the secondary to antral phase of follicular development (FSH dependent) (4 to 1 month before conception), the emergence of a dominant follicle and release of the oocyte (1 month before conception), and post conception, early pregnancy (weeks 0–12), when endogenous levels of estradiol and progesterone are still relatively low and exogenous sources of exposure may contribute a relatively higher dose (relative to endogenous levels).39
Date of conception was estimated by subtracting 17 days40 from the number of days of gestational length at birth (to account for the follicular phase prior to conception) and then subtracting this value from the date of birth. We used the last menstrual period (LMP)-based estimated gestational length unless the LMP-based gestational length was missing (5.1%) or ≥2 weeks from the ultrasound-based estimate of gestational length (5.2%), in which case we used the ultrasound based measure.41 We then constructed an exposure window for each hormonal contraceptive prescription filled using the date that the prescription was filled and the number of defined daily doses dispensed (day’s supply). Most OCs were dispensed in a 3 month supply (82%) or a 6 month supply (15%). For pregnancies with more than one type of hormonal contraceptive prescribed, we assigned exposure type according to the type of contraceptive used closest to the estimated date of conception. Because many women may choose to stop taking their hormonal contraceptive in order to achieve conception, we characterized women as exposed in early pregnancy only if they reported that the pregnancy was unplanned and had ≥ 1 day supply of hormonal contraceptive at or after the day of conception.
Offspring overweight or obesity was defined by first calculating the offspring body mass index (BMI) (kg/m2) at age 3 years from questionnaire-reported height and weight measures. In Norway, families are provided a health card to record information about their children. Mothers were asked to transcribe the health care data on height, weight, and date onto a MoBa questionnaire when the children were three years old. Offspring were characterized as overweight or obese using the age- and sex-specific cut points developed by the International Obesity Taskforce (IOTF) (17.89 kg/m2 for boys and 17.56 kg/m2 for girls).42 To evaluate the accuracy of the child height and weight data, we conducted a validation substudy. We assessed the correlation between BMI obtained from the questionnaire and BMI based on measures taken for the Bergen Growth Study.43 The correlation was examined among measures obtained within 90 days of one another.44
Covariate selection was informed through construction of a directed acyclic graph.45 The adjustment factors selected (and data source) were maternal age (MBRN) (14–19, 20–29, 30–39, 40–49), prepregnancy BMI (MoBa pregnancy questionnaire) (kg/m2)(<18.5, 18.5–24.9, ≥25.0), parity (MBRN) (0, 1, ≥2), smoking (composite from MoBa questionnaires and the MBRN) (none, quit during pregnancy, smoker), and education (MoBa pregnancy questionnaire) (>4 years of university or technical, 4 year university or technical degree, 3 years of college preparatory high school, 3 years of technical high school, 1–2 years of high school, <9 years of secondary school, other).
Primary analyses
Our primary analyses were concerned with assessing the association between early pregnancy hormonal contraceptive exposure, as compared to no use of a hormonal contraceptive in early pregnancy or the 12 months before pregnancy, and offspring overweight or obesity. We used generalized linear models with a logit link, and generalized estimation equations (GEE) with an independent correlation matrix46 to estimate robust standard errors and account for lack of independence between siblings. We used similar models to assess the association between hormonal contraceptive use prior to conception and offspring overweight or obesity. Finally, we evaluated the association between hormonal contraceptive use with BMI z-score, calculated from the World Health Organization growth standards for BMI.47
Subgroup analyses
In subgroup analyses, we explored the association between route of administration and type of progestin agent and offspring overweight or obesity. We also explored whether there was evidence of interaction between hormonal contraceptive use and offspring sex, maternal prepregnancy overweight/obese status (≥25.0 kg/m2; Yes/No), or maternal pre-pregnancy weight using interaction terms. A priori, we considered a p value <0.20 as evidence of potential interaction.48 Given evidence of possible interaction, we examined stratum-specific estimates. All analyses were conducted using SAS v9.3 (SAS Inc., Cary, North Carolina).
Sensitivity analyses
We conducted several sensitivity analyses to address the potential for residual confounding or confounding by indication (use of different comparator groups and additional adjustment factors), the potential for selection bias from loss to follow-up (use of both multiple imputation and inverse probability weighting), and the potential for exposure misclassification (consideration of self-reported use of hormonal contraceptives). We also assessed whether a log-binomial model, for estimating relative risks, generated estimates that were materially different from estimates obtained in the logit model estimating odds ratios. The methods for these analyses are described in Supplement I, with supporting details provided in Supplementary Tables S1–S3.
Results
In general, compared to all MoBa pregnancies with baseline data collected in pregnancy and at birth, the pregnancies included in the final study sample were to women who were older, less parous, more educated, and less likely to have smoked in pregnancy (Table 1). At 36 months of age, 2,653 (13.1%) children in the analysis met the IOTF definitions for overweight or obese. We identified 3,392 pregnancies exposed 12 – >4 months before, 2,541 4 – >1 months before, 2,997 1 – >0 month before, and 567 0 – 12 weeks after conception. For the validation substudy, 77 children had height and weight data at age 3 collected in both MoBa and the Bergen Growth study. These data were obtained no more than 90 days apart from the respective studies (mean difference: 32 days, std 33 days). The correlation of BMI between the two sources of data was high (Pearson r=0.86, 95% CI: 0.81, 0.90) and consistent with the expected correlation between BMI calculated from serial measures of height and weight in young children.44
Table 1.
Study and baseline population characteristics among women participating in the Norwegian Mother Child Prospective Cohort Study (2004–2008)
| Baseline population* | Study population | ||
|---|---|---|---|
| n=44,649 % |
n=19,652 % |
||
| Maternal age (years) | |||
| 14–19 | 0.9 | 0.4 | |
| 20–29 | 42.1 | 40.3 | |
| 30–39 | 54.9 | 57.1 | |
| 40–49 | 2.1 | 2.3 | |
| Maternal BMI (kg/m2) | |||
| <18.5 | 3.2 | 2.9 | |
| 18.5–24.9 | 66.1 | 67.2 | |
| 25.0–29.9 | 21.7 | 21.8 | |
| ≥30.0 | 9.0 | 8.1 | |
| Parity | |||
| 0 | 47.1 | 50.0 | |
| 1 | 35.5 | 34.3 | |
| 2 | 13.7 | 12.4 | |
| 3 | 2.8 | 2.4 | |
| 4 or more | 0.9 | 0.8 | |
| Maternal education | |||
| More than 4 years of university or technical | 27.2 | 29.9 | |
| 4 year university degree, regional technical | 40.8 | 43.9 | |
| 3 years high school, junior college | 13.5 | 12.0 | |
| Technical high school | 11.2 | 9.3 | |
| 1–2 years high school | 3.9 | 2.6 | |
| 9-year secondary | 2.2 | 1.1 | |
| Other | 1.4 | 1.4 | |
| Maternal smoking (at 17 weeks) | |||
| None | 79.3 | 82.8 | |
| Quit | 14.5 | 12.6 | |
| Daily | 1.4 | 1.2 | |
| Sometimes | 4.8 | 3.4 | |
Represents unique pregnancies with no use of IVF treatment, resulting in a singleton live birth and no death in the first year of life, with a date of birth ≥12 months after NorPD registry began (January 1, 2004)
Primary analyses
In early pregnancy, the combination OC was weakly, inversely associated with offspring overweight or obesity at age 3 (aOR: 0.75, 95% CI: 0.53, 1.08). The progestin-only OC was weakly, positively associated with overweight or obesity (aOR: 1.26, 95% CI: 0.79, 2.02) (Table 2). Use of a hormonal contraceptive before pregnancy was generally not associated with overweight or obesity, with the exception of use of a vaginal ring-type hormonal contraceptive, which was inversely associated, particularly for exposure estimated to have occurred 1 – >0 months before conception (aOR: 0.60, 95% CI: 0.35, 1.04) (Table 2). Data were too sparse to evaluate the association between early pregnancy use of the vaginal ring and subsequent offspring overweight or obesity. The direction of the estimates obtained when modeling BMI z-score as a continuous outcome were similar to those obtained when modeling BMI as a dichotomous outcome (data not shown).
Table 2.
Association between hormonal contraceptive use in early pregnancy* and offspring overweight or obese
| Exposure | Exposed (n) | Overweight or obese (n) |
Crude OR (95% CI) | Adjusted** OR (95% CI) |
|---|---|---|---|---|
| None† | 9,987 | 1,342 | referent | referent |
| Combination OC | 380 | 38 | 0.72 (0.51, 1.01) | 0.75 (0.53, 1.08) |
| Progestin only OC | 127 | 21 | 1.28 (0.80, 2.05) | 1.26 (0.79, 2.02) |
| Other‡ | 60 | 7 | 0.85 (0.39, 1.88) | 0.88 (0.40, 1.94) |
use within 12 weeks after conception as compared to no use of a hormonal contraceptive within the discrete categories of within 12, 4, and 1 month before conception and within 12 weeks after conception
adjusted for maternal age, maternal smoking at 17 weeks gestation, maternal pre-pregnancy BMI, and parity
no use of a hormonal contraceptive within the discrete categories of within12, 4, and 1 month before conception and within 12 weeks after conception
hormonal contraceptives with < 10 exposed cases were combined into an “other” category
Subgroup analyses
Among combination OC users, the association with overweight or obesity was similar across combination OCs with differing progestin components (Supplementary Tables S4–S7). In early pregnancy only, the desogestrel progestin-only OC was moderately associated with offspring overweight or obesity (aOR: 1.87, 95% CI: 1.06, 3.32) (Supplementary Table S4).
For early pregnancy use of a combination OC there was weak evidence of effect modification by offspring sex or maternal pre-pregnancy BMI. For exposure to the combination OC in early pregnancy, the observed association with overweight or obesity was present only in males (aOR: 0.56, 95% CI: 0.32, 0.97 in males vs aOR: 0.98, 95% CI: 0.63, 1.53 in females). The magnitude of association observed for use of the combination OC was also stronger in women characterized as normal BMI (BMI <25.0 kg/m2) (aOR: 0.64, 95% CI: 0.40, 1.02 in normal BMI women vs aOR: 0.95, 95% CI: 0.56, 1.62 in overweight or obese women), although confidence intervals of the strata overlapped considerably (Supplementary Table S8). There was no evidence of interaction between hormonal contraceptive use (of any type) and maternal pre-pregnancy weight (p for interaction term >0.20).
Sensitivity analyses
In early pregnancy, for the analyses evaluating the use of different comparator groups, the inverse association between the combination OC and offspring overweight or obesity was robust to restricting the comparator population to unplanned pregnancies (aOR: 0.70, 95% CI: 0.48, 1.02) (Table 3). This inverse relationship was also materially unchanged when comparing early pregnancy combination OC users to former users of the combination oral contraceptive and when comparing the combination OC users to the progestin-only OC users (aOR: 0.75, 95% CI: 0.52, 1.06) (Table 3). Similarly, the relationship between progestin use in early pregnancy and offspring overweight or obesity was robust to choice of comparator groups (Table 3).
Table 3.
Sensitivity analyses for early pregnancy exposure to hormonal contraceptives and offspring overweight or obesity
| n | OR (95% CI) | Adjusted** OR (95% CI) | ||
|---|---|---|---|---|
| Approach 1: Compared to former users Combination OC | ||||
| former* user of Combination OC | 6,146 | referent | referent | |
| *early pregnancy Combination OC user | 380 | 0.76 (0.54, 1.07) | 0.75 (0.52, 1.06) | |
| Progestin only OC | ||||
| former* user of Progestin OC | 1,962 | referent | referent | |
| *early pregnancy Progestin OC user | 127 | 1.20 (0.74, 1.94) | 1.24 (0.75, 2.04) | |
| Approach 2: Compared to unplanned pregnancies | ||||
| No hormonal contraception† | 2,264 | referent | referent | |
| Combination OC | 380 | 0.67 (0.46, 0.96) | 0.70 (0.48, 1.02) | |
| Progestin only OC | 127 | 1.19 (0.73, 1.93) | 1.22 (0.75, 1.98) | |
| Other‡ | 60 | 0.79 (0.36, 1.76) | 0.79 (0.35, 1.78) | |
| Approach 3: Compared to other oral hormonal contraceptive users | ||||
| Progestin only OC | 127 | referent | referent | |
| Combination OC | 380 | 0.46 (0.23, 0.91) | 0.56 (0.32, 1.00) | |
former use defined as within 12 months but not within 4 months of conception and early defined as within 12 weeks of conception
adjusted for maternal age, maternal smoking at 17 weeks gestation, maternal pre-pregnancy BMI, education, and parity
no use of a hormonal contraceptive within 12 months before conception and 12 weeks after conception
hormonal contraceptives with < 10 exposed cases were combined into an “other” category
The estimate comparing vaginal ring use to the combination OC (within 1 month prior to conception) was aOR 0.59 (95% CI: 0.33, 1.03) and consistent with estimates obtained when comparing vaginal ring users to non-users of a hormonal contraceptive (aOR: 0.60, 95% CI: 0.35, 1.04).
The magnitude of the estimates from models employing multiple imputation were somewhat attenuated (aOR: 0.85, 95% CI: 0.66, 1.11 for the combination OC) compared to those obtained in the primary analyses (aOR: 0.75, 95% CI: 0.53, 1.06) while estimates obtained using inverse probability weighting were somewhat strengthened (aOR: 0.68, 95% CI: 0.49, 0.99 for the combination OC) (Supplementary Tables S9–S10). Characterizing exposure by self-reported use in early pregnancy, as reported on the questionnaire administered during pregnancy, also attenuated the estimates observed and introduced additional imprecision (aOR: 0.88, 95% CI: 0.57, 1.35 for use of the combination OC and aOR 1.15, 95% CI 0.58, 2.28 for use of the progestin-only OC) (data not shown). Adjustment for diabetes type I or II, age in 5-year increments, income, and maternal weight did not substantively change the estimates (data not shown). Estimates obtained using a log-binomial model were also not materially different (Combination OC RR: 0.78 (95% CI: 0.57, 1.82) and Progestin-only OC RR: 1.23 (95% CI: 0.83, 1.82) for early pregnancy use).
Discussion
In our primary analysis of the association between early pregnancy hormonal contraceptive use and offspring overweight or obesity, we found that use of a combination OC was weakly, inversely associated with offspring overweight or obesity at age 3. Use of the progestin-only OC in early pregnancy was weakly, positively associated with offspring overweight or obesity. A moderate positive association for early pregnancy progestin-only OC use was observed for desogestrel. With the exception of an inverse association for the vaginal ring, there was no association with use of a hormonal contraceptive before pregnancy and offspring overweight or obesity. The absence of an association with use before pregnancy suggests that any developmental effect may be result of a direct effect on the embryo and fetus, as opposed to changes to follicular or oocyte development. The relevance of timing of use was further supported by an evaluation of self-reported duration which was unrelated to offspring overweight or obesity (data not shown).
All of the associations were qualitatively unchanged with selection of different comparator groups, suggesting that the characteristics of women using the contraceptive were not contributing to the estimates observed. The sensitivity analyses indicated that the results could have been affected somewhat by out-selection bias, but the direction of the bias was unclear. The sensitivity analyses also showed results were attenuated when based on self-reported hormonal contraceptive use. Self-reported data, however, lacked the detail of the NorPD data, and progestin specific associations could not be ascertained.
In experimental animal models, in utero and neonatal exposure to estrogenic agents (DES and 17β-estradiol) results in an initial period of depressed growth, followed by increasing adiposity at follow-up.6,16 Although the timing of exposure for these animal studies is somewhat different (from conception through birth for pregnancy and in early neonatal life), the weak, inverse association with the combination pill in the present study may be congruent with observations of an initial period of depressed growth in these experimental studies. The evidence for depressed growth from hormonal contraceptives in human studies has been mixed, with some studies indicating no association with birthweight or low-birth-weight and others indicating a weak, positive association with low-birth-weight. The number of exposed pregnancies in these studies has been small and unable to differentiate between contraceptive formulations.49–52 Additional follow-up of the MoBa cohort will allow investigation of associations at later ages to determine whether the growth pattern exhibited in animal models is relevant in humans.
We observed differences in association depending on the type of contraceptive and progestin used. The agents in different hormonal contraceptives vary with respect to their biding affinities for androgen, progestogen, and estrogen receptors. Some exert androgenic properties, others anti androgenic properties.15 To our knowledge, studies of offspring adiposity or growth following exposure to progestogenic compounds during early fetal development have not been conducted in animals or humans. However, endogenous serum progesterone levels in pregnancy have been positively associated with offspring birthweight53, 54 and birthweight has been associated with offspring weight at follow-up.55 Fetal exposure to androgenic agents has resulted in metabolic abnormalities, including a polycystic ovarian syndrome-like phenotype in animal models.12 Unopposed by ethinyl estradiol, desogestrel has high progestational and moderate androgenic activity relative to other progestin types, but when desogestrel is present in combination with ethinyl estradiol, the progestational and androgenic activities are substantively reduced.15 Norethisterone is only weakly androgenic. This property may explain the difference in association observed between desogestrel and norethisterone progestin-only contraceptives in this study.
The inverse association between use of the vaginal ring and offspring overweight or obesity may be attributable to the pharmacokinetic properties of the vaginal ring. The vaginal ring contains etonogestrel and ethinyl estradiol. Hormonal constituents of the vaginal ring are absorbed through the vaginal epithelium and provide steady release of etonogestrel and ethinyl estradiol for the three week period after the ring is inserted.15 Pharmacokinetic studies comparing the vaginal ring to the combination OC indicate that the dose of ethinyl estradiol, as measured in blood serum and represented by the area under the curve, is lower than that of doses experienced in combination OC users.56 The agents in the vaginal ring do not experience first pass metabolism. The ring provides a consistent release of hormones unaffected by dietary or gastrointestinal factors and is less subject to fluctuation in delivered dose when compared to oral or transdermal-administered forms of contraception.57
Sparse data limited the ability to assess variation in effects by different progestin types in early pregnancy. We found possible evidence of effect modification by offspring sex and maternal prepregnancy BMI for the combination OC; however, sample size limitations may have also precluded the ability to detect effect modification for the progestin-only formulation. The possible effect modification observed for early pregnancy is consistent with animal data suggesting that developmental effects of estrogenic compounds may be stronger among male offspring.16 The associations observed could be attributable to residual confounding or confounding by indication. Women may be prescribed different contraceptive formulations based on factors for which we cannot control in our data. Notwithstanding, estimates obtained from sensitivity analyses, conducted to assess the potential for confounding by indication, were robust to choice of comparator group. Use of methods to explore the potential for bias from loss to follow-up is effective only insofar as we have correctly assumed that we were able to successfully impute missing values from the covariates in our imputation models (multiple imputation approach) or correctly predict the probability of staying in the study from the covariates in our predicted probability models for generating weights (inverse probability weighting approach). There was also a potential for misclassification of the timing of exposure.
The overall proportion of overweight or obese children at age 3 in this study (13.1%) is relatively consistent with national prevalence estimates for overweight or obesity at age 3 that were obtained from height and weight data collected by research staff (11.3%).58 Nonetheless, BMI is less specific for identifying clinically relevant adiposity in children when compared to other measures of assessing childhood adiposity.59
Overweight and obesity may be influenced by developmental exposure to exogenous sex hormones. The present data suggest that pharmacologic sex hormone agents may be associated with offspring overweight or obesity at age 3. The direction of the relationships appears contingent upon hormone formulation. Little is known about long-term, formulation-specific effects on offspring weight status.
Data from experiments on animals suggest that early life exposure to hormonally-active agents may affect offspring growth. Given the evidence that early life anthropometric indicators are associated with adult adiposity,2, 3 the investigation of determinants of early life anthropometrics is warranted. The results presented provide support for the assertion that in utero exposure to hormonally active agents, during developmentally sensitive periods, may contribute to alterations in offspring growth.
Supplementary Material
Acknowledgements
We are grateful to all the participating families in Norway who take part in this ongoing cohort study.
This work was supported in part by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Environmental Health Sciences (NIEHS) (ES102985 to CJW), the University of North Carolina institutional training grant award for reproductive, perinatal, and pediatric epidemiology (grant T32HD052468 to ETJ), the National Cancer Institute (1K01CA172717-01 to WRR), and the Carolina Population Center (R24 HD050924 to WRR). The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health, contract N01-ES-75558 with the NIH/NIEHS, NIH/National Institute of Neurological Disorders and Stroke (grant 1 UO1 NS 047537-01), and the Norwegian Research Council/FUGE (grant 151918/S10).
TS receives investigator-initiated research funding and support as Principal Investigator (R01 AG023178) and Co-Investigator (R01 AG042845) from the National Institute on Aging (NIA), and as Co-Investigator (R01 CA174453) from the National Cancer Institute (NCI) at the National Institutes of Health (NIH), and as Principal Investigator of a Pilot Project from the Patient Centered Outcomes Research Institute (PCORI). He also received research funding as Principal Investigator of the UNC-DEcIDE center from the Agency for Healthcare Research and Quality. Dr. Stürmer does not accept personal compensation of any kind from any pharmaceutical company, though he receives salary support from the Center for Pharmacoepidemiology (current members: GlaxoSmithKline, UCB BioSciences, Merck) and research support from pharmaceutical companies (Amgen, Genentech, Merck, Sanofi) to the Department of Epidemiology, University of North Carolina at Chapel Hill.
Footnotes
Disclosures: None of the other authors have any disclosures.
Author contributions (all authors approved the final draft):
Jensen: Project conception, design, analyses, interpretation, manuscript draft
Daniels: Project conception, design, interpretation, critical manuscript review
Stürmer: Project design, interpretation, manuscript review
Robinson: Project design, interpretation, manuscript review
Williams: Project design, interpretation, manuscript review
Moster: Project design and manuscript review
Juliusson: Project design and manuscript review
Vejrup: Project design and manuscript review
Magnus: Project design and manuscript review
Longnecker: Project conception, design, interpretation, critical manuscript review
Supplementary information is available at the journal's website.
References
- 1.de Onis M, Blossner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr. 2010;92(5):1257–1264. doi: 10.3945/ajcn.2010.29786. [DOI] [PubMed] [Google Scholar]
- 2.Nader PR, O'Brien M, Houts R, Bradley R, Belsky J, Crosnoe R, et al. Identifying risk for obesity in early childhood. Pediatrics. 2006;118(3):e594–e601. doi: 10.1542/peds.2005-2801. [DOI] [PubMed] [Google Scholar]
- 3.Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes (Lond) 2011;35(7):891–898. doi: 10.1038/ijo.2010.222. [DOI] [PubMed] [Google Scholar]
- 4.Osei-Assibey G, Dick S, Macdiarmid J, Semple S, Reilly JJ, Ellaway A, et al. The influence of the food environment on overweight and obesity in young children: a systematic review. BMJ Open. 2012;2(6) doi: 10.1136/bmjopen-2012-001538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pearce J, Langley-Evans SC. The types of food introduced during complementary feeding and risk of childhood obesity: a systematic review. Int J Obes. 2013;37(4):477–485. doi: 10.1038/ijo.2013.8. [DOI] [PubMed] [Google Scholar]
- 6.Newbold RR, Padilla-Banks E, Snyder RJ, Jefferson WN. Perinatal exposure to environmental estrogens and the development of obesity. Molecular Nutrition & Food Research. 2007;51(7):912–917. doi: 10.1002/mnfr.200600259. [DOI] [PubMed] [Google Scholar]
- 7.Takai Y, Tsutsumi O, Ikezuki Y, Kamei Y, Osuga Y, Yano T, et al. Preimplantation exposure to bisphenol A advances postnatal development. Reproductive toxicology (Elmsford, N.Y) 2001;15(1):71–74. doi: 10.1016/s0890-6238(00)00119-2. [DOI] [PubMed] [Google Scholar]
- 8.Dieudonne MN, Pecquery R, Leneveu MC, Giudicelli Y. Opposite effects of androgens and estrogens on adipogenesis in rat preadipocytes: evidence for sex and site-related specificities and possible involvement of insulin-like growth factor 1 receptor and peroxisome proliferator-activated receptor gamma2. Endocrinology. 2000;141(2):649–656. doi: 10.1210/endo.141.2.7293. [DOI] [PubMed] [Google Scholar]
- 9.Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78(3):783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- 10.Poissonnet CM, Burdi AR, Garn SM. The chronology of adipose tissue appearance and distribution in the human fetus. Early Hum Dev. 1984;10(1–2):1–11. doi: 10.1016/0378-3782(84)90106-3. [DOI] [PubMed] [Google Scholar]
- 11.Jensen ETLM. Pharmacologic use of sex hormones in early pregnancy in relation to offspring obesity. Obesity (Silver Spring) doi: 10.1002/oby.20778. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbott DH, Tarantal AF, Dumesic DA. Fetal, infant, adolescent and adult phenotypes of polycystic ovary syndrome in prenatally androgenized female rhesus monkeys. Am J Primatol. 2009;71(9):776–784. doi: 10.1002/ajp.20679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Padmanabhan V, Veiga-Lopez A, Abbott DH, Recabarren SE, Herkimer C. Developmental programming: impact of prenatal testosterone excess and postnatal weight gain on insulin sensitivity index and transfer of traits to offspring of overweight females. Endocrinology. 2010;151(2):595–605. doi: 10.1210/en.2009-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veiga-Lopez A, Steckler TL, Abbott DH, Welch KB, MohanKumar PS, Phillips DJ, et al. Developmental programming: impact of excess prenatal testosterone on intrauterine fetal endocrine milieu and growth in sheep. Biology of reproduction. 2011;84(1):87–96. doi: 10.1095/biolreprod.110.086686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dickey RP. Managing contraceptive pill patients/drug patients. 14th edn. EMIS, Inc.; 2010. [Google Scholar]
- 16.Werner Fürst R, Pistek VL, Kliem H, Skurk T, Hauner H, Meyer HHD, et al. Maternal low-dose estradiol-17β exposure during pregnancy impairs postnatal progeny weight development and body composition. Toxicology and Applied Pharmacology. 2012;263(3):338–344. doi: 10.1016/j.taap.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115(3):e290–e296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 18.Fleming TP, Lucas ES, Watkins AJ, Eckert JJ. Adaptive responses of the embryo to maternal diet and consequences for post-implantation development. Reproduction, Fertility and Development. 2011;24(1):35–44. doi: 10.1071/RD11905. [DOI] [PubMed] [Google Scholar]
- 19.Frempong BA, Ricks M, Sen S, Sumner AE. Effect of low-dose oral contraceptives on metabolic risk factors in African-American women. J Clin Endocrinol Metab. 2008;93(6):2097–2103. doi: 10.1210/jc.2007-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen KR. Pharmacodynamic effects of oral contraceptive steroids on biochemical markers for arterial thrombosis. Studies in non-diabetic women and in women with insulin-dependent diabetes mellitus. Dan Med Bull. 2002;49(1):43–60. [PubMed] [Google Scholar]
- 21.Winkler UH, Sudik R. The effects of two monophasic oral contraceptives containing 30 mcg of ethinyl estradiol and either 2 mg of chlormadinone acetate or 0.15 mg of desogestrel on lipid, hormone and metabolic parameters. Contraception. 2009;79(1):15–23. doi: 10.1016/j.contraception.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Kannel WB, Wilson PW, Nam BH, D'Agostino RB. Risk stratification of obesity as a coronary risk factor. Am J Cardiol. 2002;90(7):697–701. doi: 10.1016/s0002-9149(02)02592-4. [DOI] [PubMed] [Google Scholar]
- 23.Pietilainen KH, Sysi-Aho M, Rissanen A, Seppanen-Laakso T, Yki-Jarvinen H, Kaprio J, et al. Acquired obesity is associated with changes in the serum lipidomic profile independent of genetic effects--a monozygotic twin study. PLoS One. 2007;2(2):e218. doi: 10.1371/journal.pone.0000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hedderson MM, Ferrara A, Williams MA, Holt VL, Weiss NS. Androgenicity of progestins in hormonal contraceptives and the risk of gestational diabetes mellitus. Diabetes Care. 2007;30(5):1062–1068. doi: 10.2337/dc06-2227. [DOI] [PubMed] [Google Scholar]
- 25.Orme ML, Back DJ, Ball S. Interindividual variation in the metabolism of ethynylestradiol. Pharmacol Ther. 1989;43(2):251–260. doi: 10.1016/0163-7258(89)90121-6. [DOI] [PubMed] [Google Scholar]
- 26.Mucci LA, Lagiou P, Hsieh CC, Tamimi R, Hellerstein S, Vatten L, et al. A prospective study of pregravid oral contraceptive use in relation to fetal growth. Bjog. 2004;111(9):989–995. doi: 10.1111/j.1471-0528.2004.00232.x. [DOI] [PubMed] [Google Scholar]
- 27.Chan M-F, Dowsett M, Folkerd E, Wareham N, Luben R, Welch A, et al. Past oral contraceptive and hormone therapy use and endogenous hormone concentrations in postmenopausal women. Menopause. 2008;15(2):332–339. doi: 10.1097/gme.0b013e31806458d9. [DOI] [PubMed] [Google Scholar]
- 28.Armenti AE, Zama AM, Passantino L, Uzumcu M. Developmental methoxychlor exposure affects multiple reproductive parameters and ovarian folliculogenesis and gene expression in adult rats. Toxicology and Applied Pharmacology. 2008;233(2):286–296. doi: 10.1016/j.taap.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gandolfi F, Pocar P, Brevini TAL, Fischer B. Impact of endocrine disrupters on ovarian function and embryonic development. Domestic Animal Endocrinology. 2002;23(1–2):189–201. doi: 10.1016/s0739-7240(02)00156-x. [DOI] [PubMed] [Google Scholar]
- 30.Leese HJ, Baumann CG, Brison DR, McEvoy TG, Sturmey RG. Metabolism of the viable mammalian embryo: quietness revisited. Mol Hum Reprod. 2008;14(12):667–672. doi: 10.1093/molehr/gan065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leese HJ, Sturmey RG, Baumann CG, McEvoy TG. Embryo viability and metabolism: obeying the quiet rules. Hum Reprod. 2007;22(12):3047–3050. doi: 10.1093/humrep/dem253. [DOI] [PubMed] [Google Scholar]
- 32.Jungheim ES, Schoeller EL, Marquard KL, Louden ED, Schaffer JE, Moley KH. Diet-induced obesity model: abnormal oocytes and persistent growth abnormalities in the offspring. Endocrinology. 2010;151(8):4039–4046. doi: 10.1210/en.2010-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott L, Xu X, Veenstra T, Tooze J, Wood C, Register T, et al. Past oral contraceptive use and current dietary soy isoflavones influence estrogen metabolism in postmenopausal monkeys (Macaca fascicularis) Cancer epidemiology, biomarkers & prevention. 2008;17(10):2594–2602. doi: 10.1158/1055-9965.EPI-08-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trussell J. Contraceptive failure in the United States. Contraception. 2011;83(5):397–404. doi: 10.1016/j.contraception.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa) International journal of epidemiology. 2006;35(5):1146–1150. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 36.Furu K, Wettermark Br, Andersen M, Martikainen J, Almarsdottir A, SÃfrensen H. The Nordic countries as a cohort for pharmacoepidemiological research. Basic Clin Pharmacol Toxicol. 2010;106(2):86–94. doi: 10.1111/j.1742-7843.2009.00494.x. [DOI] [PubMed] [Google Scholar]
- 37.Skurtveit S, Selmer R, Tverdal A, Furu K. The validity of self-reported prescription medication use among adolescents varied by therapeutic class. J Clin Epidemiol. 2008;61(7):714–717. doi: 10.1016/j.jclinepi.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 38.Methodology WCCfDS. Guidelines for ATC classification and DDD assignment. Norwegian Institute of Public Health; 2012. [Google Scholar]
- 39.Yen SSC, Jaffe RB. Reproductive endocrinology : physiology, pathophysiology, and clinical management. 3rd edn. Philadelphia: Saunders; 1991. [Google Scholar]
- 40.Jukic AM, Weinberg CR, Baird DD, Wilcox AJ. Lifestyle and reproductive factors associated with follicular phase length. J Womens Health (Larchmt) 2007;16(9):1340–1347. doi: 10.1089/jwh.2007.0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dietz PM, England LJ, Callaghan WM, Pearl M, Wier ML, Kharrazi M. A comparison of LMP-based and ultrasound-based estimates of gestational age using linked California livebirth and prenatal screening records. Paediatric and perinatal epidemiology. 2007;21(Suppl 2):62–71. doi: 10.1111/j.1365-3016.2007.00862.x. (Journal Article) [DOI] [PubMed] [Google Scholar]
- 42.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. Bmj. 2000;320(7244):1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Júlíusson PB, Roelants M, Hoppenbrouwers K, Hauspie R, Bjerknes R. Growth of Belgian and Norwegian children compared to the WHO growth standards: prevalence below −2 and >2 SD and the effect of breastfeeding. Archives of Disease in Childhood. 2009 doi: 10.1136/adc.2009.166157. (Journal Article). [DOI] [PubMed] [Google Scholar]
- 44.Cole TJ, Faith MS, Pietrobelli A, Heo M. What is the best measure of adiposity change in growing children: BMI, BMI %, BMI z-score or BMI centile? Eur J Clin Nutr. 2005;59(3):419–425. doi: 10.1038/sj.ejcn.1602090. [DOI] [PubMed] [Google Scholar]
- 45.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd edn. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 46.Sullivan Pepe M, Anderson GL. A cautionary note on inference for marginal regression models with longitudinal data and general correlated response data. Communications in Statistics - Simulation and Computation. 1994;23(4):939–951. [Google Scholar]
- 47.World Health Organization UNCsF. WHO child growth standards. 2009
- 48.Greenland S. Tests for interaction in epidemiologic studies: a review and a study of power. Statistics in medicine. 1983;2(2):243–251. doi: 10.1002/sim.4780020219. [DOI] [PubMed] [Google Scholar]
- 49.Ahn HK, Choi JS, Han JY, Kim MH, Chung JH, Ryu HM, et al. Pregnancy outcome after exposure to oral contraceptives during the periconceptional period. Hum Exp Toxicol. 2008;27(4):307–313. doi: 10.1177/0960327108092290. [DOI] [PubMed] [Google Scholar]
- 50.Pardthaisong T, Gray RH. In utero exposure to steroid contraceptives and outcome of pregnancy. American Journal of Epidemiology. 1991;134(8):795–803. doi: 10.1093/oxfordjournals.aje.a116152. [DOI] [PubMed] [Google Scholar]
- 51.Polednak AP, Janerich DT, Glebatis DM. Maternal exposure to exogenous sex hormones in relation to birth weight of offspring. Teratology. 1983;27(2):223–229. doi: 10.1002/tera.1420270210. [DOI] [PubMed] [Google Scholar]
- 52.Vessey M, Meisler L, Flavel R, Yeates D. Outcome of pregnancy in women using different methods of contraception. Br J Obstet Gynaecol. 1979;86(7):548–556. doi: 10.1111/j.1471-0528.1979.tb10808.x. [DOI] [PubMed] [Google Scholar]
- 53.Mucci L, Lagiou P, Tamimi R, Hsieh C-C, Adami H-O, Trichopoulos D. Pregnancy estriol, estradiol, progesterone and prolactin in relation to birth weight and other birth size variables (United States) Cancer Causes & Control. 2003;14(4):311–318. doi: 10.1023/a:1023966813330. [DOI] [PubMed] [Google Scholar]
- 54.Hartwig IRV, Pincus MK, Diemert A, Hecher K, Arck PC. Sex-specific effect of first-trimester maternal progesterone on birthweight. Hum Reprod. 2013;28(1):77–86. doi: 10.1093/humrep/des367. [DOI] [PubMed] [Google Scholar]
- 55.Weng SF, Redsell SA, Swift JA, Yang M, Glazebrook CP. Systematic review and meta-analyses of risk factors for childhood overweight identifiable during infancy. Archives of Disease in Childhood. 2012;97(12):1019–1026. doi: 10.1136/archdischild-2012-302263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van den Heuvel MW, van Bragt AJ, Alnabawy AK, Kaptein MC. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception. 2005;72(3):168–174. doi: 10.1016/j.contraception.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 57.Alexander NJ, Baker E, Kaptein M, Karck U, Miller L, Zampaglione E. Why consider vaginal drug administration? Fertil Steril. 2004;82(1):1–12. doi: 10.1016/j.fertnstert.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 58.Júlíusson PB, Eide GE, Roelants M, Waaler PE, Hauspie R, Bjerknes R. Overweight and obesity in Norwegian children: prevalence and socio-demographic risk factors. Acta Pædiatrica. 2010;99(6):900–905. doi: 10.1111/j.1651-2227.2010.01730.x. [DOI] [PubMed] [Google Scholar]
- 59.Freedman DS, Sherry B. The validity of BMI as an indicator of body fatness and risk among children. Pediatrics. 2009;124(Suppl 1):S23–S34. doi: 10.1542/peds.2008-3586E. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


