Abstract
Background
Perphenazine is a treatment option in postoperative nausea and vomiting (PONV) prophylaxis. Chronic administration and high dose are known to cause extrapyramidal system (EPS) dysfunction at a frequency of 8%, but the incidence of acute EPS after a single 4 or 8 mg dose is unknown.
Objective
A retrospective analysis of patient medication billing data and departmental quality records was performed (January 2001 – 10 July 2012) to identify patients who experienced EPS dysfunction after oral perphenazine.
Design
Retrospective analysis.
Setting
Surgical outpatients presenting to any one of ten hospitals in the area of Pittsburgh, PA, USA.
Patients, other participants
Overall, 45,766 patients received 4 or 8 mg of perphenazine before same-day surgery.
Main Outcome Measures
EPS dysfunction was defined as acute dystonia, akathisia, or pseudoparkinsonism. Records were reviewed to determine the (i) likely number of reactions to perphenazine, (ii) nature of these reactions, and (iii) impact on patient care.
Results
There were four “likely” cases of EPS dysfunction, and two “possible” cases. Five reported events were consistent with akathisia, with the sixth being a dystonic reaction. All six patients had resolution of symptoms, with five receiving intravenous diphenhydramine for treatment. The incidence of EPS dysfunction was 1.3 events per 10,000 patients (95% CI: 0.4, 3.0, based on 6 events). All patients who experienced reactions preoperatively were able to proceed to surgery without complications or delay. One patient required unplanned admission and 3-hour observation due to sedation from diphenhydramine. The incidence of EPS dysfunction after oral perphenazine is low. Reactions that did occur were mild and easily treated.
Conclusions
Given the infrequent side-effects, this single, low-dose of perphenazine should be encouraged as a low-risk adjunct to any multimodal PONV prophylaxis regimen, based on the selection criteria described.
Introduction
Perphenazine, an antipsychotic phenothiazine, possesses strong anti-emetic properties because of its dopaminergic receptor antagonism. It is a treatment option in postoperative nausea and vomiting (PONV) prophylaxis,1,2 and its lack of sedation makes it suitable for ambulatory surgery where a single-dose is favoured.3 A rare adverse reaction of low-dose oral perphenazine is extrapyramidal system (EPS) dysfunction, which is perhaps surprising since the EPS depends upon dopamine as a neurotransmitter. When EPS dysfunction does occur, it is thought to be associated with significant patient discomfort.4 Despite its clinical popularity and apparently low side-effect profile, a large scale audit of EPS dysfunction following a single-dose of perphenazine for PONV prophylaxis, to our knowledge, has not yet been performed.
With chronic use of doses ranging from 8 to 32 mg daily (average 20.8 mg daily) the incidence of EPS dysfunction is significant, with one study revealing that 8% of patients discontinue perphenazine due to motor side effects.5 However, the incidence of EPS dysfunction secondary to a single oral dose of perphenazine 4 or 8 mg is unknown. As the use of low-dose prophylactic oral perphenazine in all patients at risk for PONV is based on the assumption that serious adverse events are rare, the incidence of such events must be quantified to permit an analysis of risk versus benefit. We therefore performed a retrospective analysis of an 11-year period (January 2001 – July 10, 2012) at our university health system’s acute care hospitals and surgical centres, with the specific aim of identifying the incidence and outcome of, and risk factors for EPS dysfunction among patients who received perphenazine for PONV prophylaxis. This study period extends from the first use of perphenazine at a single hospital in the system, to a more widespread administration to our ambulatory and orthopaedic patients at our larger centre and other affiliated institutions within our multi-hospital network.
Methods
Ethical approval for this study (IRB #PRO12050188) was considered by the University of Pittsburgh Institutional Review Board, University of Pittsburgh, Pittsburgh, Pennsylvania, USA on 24 July 2012, who confirmed that the project met all criteria for exemption under section 45 CFR 46.101(b)(4), and was designated as “exempt”. A search was made of our pharmacy PharmNet data system patient billing records to determine the number of outpatient and ambulatory/same-day-surgery patients presenting to any of ten hospitals in the general metropolitan area of Pittsburgh, Pennsylvania, USA, who received PONV prophylaxis with single-dose oral perphenazine 4 or 8 mg. This was given to each patient on arrival at the Same Day Surgery unit, typically within two hours of surgery. In our institution, perphenazine 8 mg is not recommended for PONV prophylaxis for patients (i) older than 70 years, (ii) younger than 12 years, (iii) weighing less than 45 kg, (iv) with a history of cerebral palsy or Parkinson’s disease, (v) with any history of extrapyramidal reactions to phenothiazines, and/or (vi) on any Class III antidysrhythmic medication. The concomitant use of metoclopramide is actively discouraged.
Patient records indicating PONV prophylaxis with single-dose oral perphenazine were then examined for possible adverse reactions to perphenazine 4 or 8 mg, using the following identifiers: (i) administration of diphenhydramine or benztropine (for treatment of EPS dysfunction) within 24 h of perphenazine administration; (ii) a “stop medication” order written for perphenazine; (iii) an allergy to perphenazine noted in the medical record; or (iv) a complication secondary to perphenazine. The cases identified as having one of the above markers of adverse drug reaction formed the study cohort.
The medical records from each case identified were thoroughly reviewed to (i) verify that perphenazine was given, (ii) determine the indication for diphenhydramine or benztropine administration, and (iii) locate entries relating to movement disorders, restlessness, agitation, or altered mental status. Examples of sources for such information included anaesthesia records, preoperative, intraoperative, and postoperative reports. The clinical notes and data from any documented adverse event were compared to the diagnostic criteria suggestive of EPS symptoms that were developed in collaboration with a staff neurologist (Department of Neurology Comprehensive Movement Disorder Clinic, University of Pittsburgh Medical Center [UPMC]). These criteria included acute dystonia, akathisia, and parkinsonism.4 The definition of each subtype was standardised. Acute dystonia was defined as a sustained posture produced by continuous muscular contraction. Akathisia was defined as a subjective feeling of internal motor restlessness. Parkinsonism was defined as a clinical triad of symptoms, rigidity, bradykinesia, and resting tremor.
Each case identified during our review was classified as acute dystonia, akathisia, or parkinsonism and categorised as “likely” or “possible” according to how closely the described physical findings met our definitions. In addition, personal details were extracted to identify any possible risk factors, including age, sex, medical history, and concurrent medications at the time of perphenazine administration.
Given the expected rarity of EPS dysfunction following administration of a single dose of oral perphenazine, the Department of Anesthesiology’s Quality Improvement (QI) data from each of our affiliated hospitals included in the primary pharmacy query were reviewed to improve sensitivity. For all sites which use perphenazine, QI reports of pre-operative, intra-operative, and post-operative events are generated after each anaesthetic either in writing, or by electronic forms. Although reporting of all adverse events is required, reporting relies on voluntary disclosure by anaesthesiologists, hands-on providers in the operating room, and nurses. If details within the QI report indicated a possible case of EPS dysfunction, the corresponding patient’s chart was thoroughly reviewed (i) to confirm that the patient had received perphenazine, and (ii) to elucidate further details regarding the possible reaction. As with the cases identified by the review of pharmacy records, cases identified by review of QI data were categorised as dystonia, akathisia, or parkinsonism, and determined to be either “likely” or “possible”.
The available documents for each case identified by the pharmacy record and QI data reviews were evaluated by multiple authors who were in agreement regarding the categorisation of “likely” and “possible” cases. The exact binomial confidence interval was then calculated for both the “likely” cases and the combined total “likely” and “possible” cases. There were no a priori statistical determinations of sample size based on this retrospective review.
Results
During the 11-year study period (January 2001 – July 10, 2012), 45,766 patients received either 4 or 8 mg of oral perphenazine as PONV prophylaxis. Of the these, 96% received the 8mg oral perphenazine dose, with the remaining 4% receiving the 4mg dose. None of the patients that encountered the described symptoms were co-administered metoclopramide. In total, 4 likely cases and 2 possible cases of EPS dysfunction were identified (Table 1). All six of these patients received 8 mg perphenazine. Five demonstrated akathisia, one did not. The sixth patient (likely) demonstrated dystonia localised to the upper back and upper extremities. No reactions consistent with parkinsonism were found. Three patients (two likely and one possible) had onset of symptoms before surgery, but surgery was neither delayed nor otherwise affected. Two patients (one likely and one possible) experienced symptoms in the Post-Anaesthesia Care Unit (PACU) following surgery, while one patient (likely) did not have symptoms until several hours after discharge from the PACU. All four likely reactions and one of the two possible reactions were treated with diphenhydramine; only one of these diphenhydramine-treated patients received a separate dose of benztropine. The diphenhydramine-benztropine-treated patient showed complete resolution of symptoms with no recurrence. The one possible patient who did not receive treatment showed spontaneous resolution of symptoms by the end of surgery. There were no unplanned admissions; one patient’s discharge was delayed for a 3 h observation period due to sedation from diphenhydramine.
Table 1. Summary of Possible/Likely Reactions to oral Perphenazine 8 mg.
| Case | Age | Sex | BMI | Type of reaction |
Treatment | Past Medical History |
Home Medications |
Likelihoo d of Reaction |
Method of Detection |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 19 | M | 25.4 | Akathisia | Diphenhydramine 25 mg i.v., Benztropine 4 mg i.v. |
asthma, allergies | Albuterol Cetirizine |
Likely | PharmNet |
| 2 | 54 | M | 37.6 | Akathisia | Diphenhydramine 25 mg i.v. × 3 |
Several beers/day, hypertension, 60 pack-year tobacco history, current smoker |
Lisinopril, Furosemide, Potassium Chloride, Hydrocodone , Lorazepam, Albuterol |
Likely | QI/ PharmNet |
| 3 | 40 | M | 29.8 | Akathisia | Diphenhydramine 25 mg i.v. |
Thalassemia | None | Likely | QI/ PharmNet |
| 4 | 61 | M | 31.1 | Dystonia | Diphenhydramine 25 mg i.v. |
Hypertension, hypothyroidism |
Levothyroxine, Hydrochloro- thiazide |
Likely | QI |
| 5 | 30 | M | 25.9 | Akathisia | Diphenhydramine 25 mg i.v. |
1/2 pack-per-day smoker |
None | Possible | PharmNet |
| 6 | 18 | M | 25.5 | Akathisia | None | Parotid mass | None | Possible | QI |
There were no reported or discernible reactions to oral perphenazine 4 mg, based on the described review of records.
None of these patients were given metoclopramide.
BMI = Body mass index (kg m−2), M = male
PharmNet = our institution's electronic pharmacy record system,
QI = Department of Anesthesiology Quality Improvement records
Based on these findings, the incidence of extrapyramidal side effects following a single dose of oral perphenazine 8 mg or 4 mg is either 0.008% (95% CI: 0.002%, 0.02%), or 0.013% (95% CI: 0.004%, 0.03%), depending on whether 4 or 6 events out of 45,766 patients are counted. If these confidence intervals are applied to our sample of 45,766, the number of events is 4 (95% CI: 1.1, 10.2), or 6 (95% CI: 2.2, 13.0) events. Expressed in terms of events per 10,000 patients, this is 0.8 (95% CI: 0.2, 2.0), or 1.3 (95% CI: 0.4, 3.0) events per 10,000.
One likely case and one possible case of EPS dysfunction were identified using our primary strategy of a pharmacy query followed by detailed chart review (Table 1, Cases 1 and 5). Two additional likely cases were flagged in an initial pharmacy query, but there was insufficient evidence in the medical records to assess the likelihood of extrapyramidal reactions until information from the associated QI reports became available (Table 1, Cases 2 and 3). Using our secondary strategy of reviewing QI reports followed by detailed chart review, one likely case and one possible case of EPS dysfunction were identified (Table 1, Cases 4 and 6). Of these two cases who were not identified as EPS dysfunction during the PharmNet query, but who were later identified from the QI data, Case 4 was not detected during the pharmacy review due to incorrect charting and charging of diphenhydramine. Case 6 would not have been expected to be identified through the pharmacy query since the patient never received treatment nor had any allergy reported.
Discussion
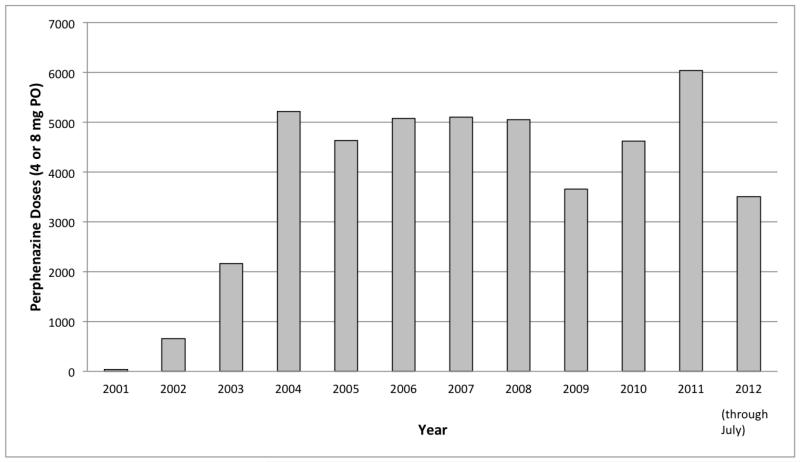
Single-dose oral perphenazine has become accepted at our institution as a non-sedating, inexpensive, and effective PONV prophylactic. Multiple studies support its effectiveness. Schnabel et al. systematically reviewed four randomised controlled trials of prophylactic perphenazine 5 mg (oral, intramuscular or i.v.) in adults, published between 1965 and 1999.6 Perphenazine was shown to be effective for early PONV in all four studies, reducing risk of PONV to close to 30%, which is similar to that of dexamethasone 4 mg, ondansetron 4 mg, or droperidol 1.25 mg.7 Despite our understanding of the effectiveness of perphenazine, limited information exists on the side effects of low dose, short-term use or single-dose use. Year on year, perphenazine administration at our institution has increased significantly (Figure 1). Despite its increasing use, EPS-related side effects have been extremely infrequent, at about 1 in 10,000 patients.
Figure 1. Doses of perphenazine (oral 4-8 mg single doses) for individual patients as postanaesthesia nausea and vomiting (PONV) prophylaxis per year in the authors’ hospital system.
Given how few patients were identified with likely EPS dysfunction, it is difficult to make conclusions regarding risk factors. Our policy is to screen for extremes of age (<14, >70), disorders such as cerebral palsy and Parkinson’s disease, and history of extrapyramidal reactions to phenothiazines, prior to administering perphenazine for PONV prophylaxis, so patients expected to be high risk for reactions do not receive the drug. Case 2 did have a history of (i) akathisia secondary to prochlorperazine several years earlier, (ii) daily alcohol use of “several beers per day”, and (iii) hydrocodone use. All of these factors probably increased risk for this individual. He unfortunately did not share his history with any member of the health care team until he began experiencing akathisia after perphenazine.
With respect to this study’s limitations, there may have been more cases of extrapyramidal dysfunction than we have identified. QI reports are voluntary so there may be patients that experienced events that did not have QI reports filed (such as the two identified via the pharmacy review). However, it is reasonable to believe that if a patient had a very significant reaction that (i) a report would have been made or (ii) the patient would have had treatment and/or an allergy recorded that would have been detected by our pharmacy record review. We acknowledge that there may have been a limited number of patients detected by pharmacy review that could have had reactions, but with insufficient details in the medical record to determine that an event occurred. There were two patients that appeared in an initial pharmacy review of cases that warranted further investigation that were only included as likely and possible reactions, once information from their associated QI reports became available. There may have been other similar cases for which we were simply unable to locate confirmation of a reaction. Again, if significant reactions did indeed occur, it would be reasonable to expect that more information about that reaction would be present in either the patient’s medical record or the QI system, so it is unlikely that many patients can be categorised as such. Another potential way for cases of EPS dysfunction to be missed is through improper and inaccurate charting and billing of perphenazine, diphenhydramine, and benztropine. We know this occurred in at least one instance for a patient identified in the QI review that we would have expected to find in the PharmNet query as well. Estimating the frequency at which this type of error might have occurred over the 11-year study period would be a labour intensive undertaking not warranting the required resources to do so. Our upper boundary of the 95% confidence interval for likely or possible events was 3 in 10,000. With five of the six cases being easily treated (and the other case not requiring treatment), it is reasonable to consider this 8 mg oral dose for PONV prophylaxis as essentially (but not absolutely) risk-free. This statement is subject to the dose being given before surgery, the recognition of side effects before same-day discharge home, and resolution through simple remedies. Since this study is a retrospective review, we fully acknowledge that there may be minor reactions to perphenazine that we did not detect. A prospective study would better enable us to identify these potential mild cases and evaluate further for risk factors. However, based on our analysis we are confident that it is unlikely the incidence of clinically significant events is greater than 3 in 10,000 patients.
It should be recognised that the incidence of extrapyramidal symptoms with other low-dose anti-dopaminergic agents may also be low. Two identical randomised clinical trials were conducted in which patients received droperidol 0.625 mg, droperidol 1.25 mg, ondansetron 4 mg, or placebo. In these trials, over 1,000 patients received droperidol for prevention of PONV, and there was a similar frequency of adverse events (beyond EPS reactions) reported in the droperidol groups compared to the ondansetron or placebo group. In both clinical trials, the most common adverse event reported was headache, with the incidence of headache reported following droperidol being much less than that of ondansetron.8 Another low dose anti-dopaminergic agent used for the prevention of PONV is haloperidol. In a review of 12 clinical trials, two placebo-controlled clinical trials presented data on EPS symptoms following administration of 0.25 to 4 mg of haloperidol i.v. The clinical trials surveyed 1,842 surgical patients; in these trials 806 patients received haloperidol. Less than 1% reported extrapyramidal symptoms, with the only case described as “a puckering of the lips”, which was interpreted as an adverse event by the study team.9 Neither of these reports (involving droperidol or haloperidol) could match our study for the number of patients.
As a single-dose component of multimodal antiemetic prophylaxis therapy, we believe that single dose perphenazine administration is justified in adults who meet our criteria. Avoiding co-administration of metoclopramide, we believe, is critically important for preventing a dopaminergic-related drug interaction. As rare as the side effects of perphenazine are, they appear to be easily treated, and can generally be detected before an ambulatory surgery patient would be considered for discharge home the same day.
Acknowledgments
Assistance with the study: none.
Financial support and sponsorship: Assistance with IRB submission and statistical analysis for this project was supported by the National Institutes of Health through Grant Numbers UL1 RR024153 and UL1TR000005.
Footnotes
Contribution: John Henao helped design the study and prepare the manuscript. He helped conduct the study and collect and analyze data obtained from pharmacy records.
Attestation: John Henao approved this final manuscript. John Henao reviewed the original study data and data analysis and attests to the integrity of the original data and the analysis reported in this manuscript. John Henao is the archival author.
Contribution: Katherin Peperzak helped prepare the manuscript. She helped collect and analyze data obtained from quality improvement data
Attestation: Katherin Peperzak approved this final manuscript. Katherin Peperzak reviewed the original study data and data analysis and attests to the integrity of the original data and the analysis reported in this manuscript.
Contribution: Alicia Lichvar helped collect and analyze data obtained from pharmacy records
Attestation: Alicia Lichvar approved this final manuscript.
Contribution: Steven Orebaugh helped design and conduct the study.
Attestation: Steven Orebaugh approved this final manuscript.
Contribution: Susan Skledar served as a mentor to Alicia Lichvar and helped collect and analyze data obtained from pharmacy records
Attestation: Susan Skledar approved this final manuscript.
Contribution: Michael Pippi assisted in preparation of the manuscript, including literature review
Attestation: Michael Pippi approved this final manuscript.
Contribution: Brian Williams assisted in preparation of the manuscript. He helped design and conduct the study as well as analyze all data
Attestation: Brian Williams approved this final manuscript.
Name of Department(s) and Institution(s): Department of Anesthesiology, University of Pittsburgh School of Medicine, A-1305 Scaife Hall, 200 Lothrop Street, Pittsburgh, Pennsylvania, 15213, USA
IRB: IRB #PRO12050188 (exempt protocol). Contact Information: Christopher Ryan, 3500 Fifth Avenue, Pittsburgh, PA, 15213, (412) 383-1480
Conflict of interest: none
Presentation: Data were presented as a poster at the Annual Meeting of the American Society of Anesthesiologists, October 2013, San Francisco, CA, United States.
Contributor Information
John P. Henao, Department of Anesthesiology, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, United States, johnpaulhenao@gmail.com.
Katherin A. Peperzak, Department of Anesthesiology, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, United States, kpeperza@wpahs.org.
Alicia B. Lichvar, School of Pharmacy, University of Pittsburgh, Pittsburgh, Pennsylvania, United States, lichvarab@upmc.edu.
Steven L. Orebaugh, Department of Anesthesiology, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, United States, OrebaughSL@anes.upmc.edu.
Susan J. Skledar, School of Pharmacy, University of Pittsburgh, Pittsburgh, Pennsylvania, United States, skledarsj@upmc.edu.
Michael A. Pippi, Department of Anesthesiology, University of Pittsburgh, Pittsburgh, Pennsylvania, USA, pippima@upmc.edu.
Brian A. Williams, Department of Anesthesiology, Veterans Affairs Pittsburgh Healthcare System, Pittsburgh, PA, United States.
References
- 1.Gan TJ, Meyer TA, Apfel CC, Chung F, Davis PJ, Habib AS, Hooper VD, Kovac AL, Kranke P, Myles P, Philip BK, Samsa G, Sessler DI, Temo J, Tramer MR, Kolk CV, Watcha M. Society for Ambulatory Anesthesia guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2007;105(6):1615–1628. doi: 10.1213/01.ane.0000295230.55439.f4. [DOI] [PubMed] [Google Scholar]
- 2.Williams BA, Kentor ML, Skledar SJ, Orebaugh SL, Vallejo MC. Routine multimodal antiemesis including low-dose perphenazine in an ambulatory surgery unit of a university hospital: A 10-year history. TheScientificWorldJOURNAL. 2007;7:978–986. doi: 10.1100/tsw.2007.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desilva P, Darvish AH, McDonald SM, Cronin MK, Clark K. The efficacy of prophylactic ondansetron, droperidol, perphenazine, and metoclopramide in the prevention of nausea and vomiting after major gynecologic surgery. Anesth Analg. 1995;81:139–143. doi: 10.1097/00000539-199507000-00028. [DOI] [PubMed] [Google Scholar]
- 4.Pierre JM. Extrapyramidal symptoms with atypical antipsychotics: incidence, prevention and management. Drug Safety. 2005;28(3):191–208. doi: 10.2165/00002018-200528030-00002. [DOI] [PubMed] [Google Scholar]
- 5.Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins CO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–23. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 6.Schnabel A, Eberhart LH, Muellenbach R, Morin AM, Roewer N, Kranke P. Efficacy of perphenazine to prevent postoperative nausea and vomiting: a quantitative systematic review. Eur J Anaesthesiol. 2010;27:1044–51. doi: 10.1097/EJA.0b013e32833b7969. [DOI] [PubMed] [Google Scholar]
- 7.Apfel CC, Korttila K, Abdalla M, Kerger H, Turan A, Vedder I, Zernak C, Danner K, Jokela R, Pocock SJ, Trenkler S, Kredel M, Biedler A, Sessler DI, Roewer N, IMPACT Investigators A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med. 2004;350:2441–2451. doi: 10.1056/NEJMoa032196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fortney JT, Gan TJ, Graczyk S, Wetchler B, Melson T, Khalil S, et al. S3A-409 and S3A-410 Study Groups A comparison of the efficacy, safety, and patient satisfaction of ondansetron versus droperidol as antiemetics for elective outpatient surgical procedures. Anesth Analg. 1998;86(4):731–8. doi: 10.1097/00000539-199804000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Buttner M, Walder B, von Elm E, Tramer MR. Is low-dose haloperidol a useful antiemetic?: A meta-analysis of published and unpublished randomized trials. Anesthesiology. 2004;101(6):1454–63. doi: 10.1097/00000542-200412000-00028. [DOI] [PubMed] [Google Scholar]