Abstract
We investigated the relationship between vascular disease and risk factors versus cognitive decline cross-sectionally and longitudinally in normal older control (NC), mild cognitive impairment (MCI), and mild Alzheimer’s disease (AD) dementia subjects. 812 participants (229 NC, 395 MCI, 188 AD) underwent cognitive testing, brain magnetic resonance imaging, and clinical evaluations at baseline and over a period of 3 years. General linear, longitudinal mixed effects, and Cox proportional hazards models were used. Greater homocysteine level and white matter hyperintensity (WMH) volume were associated with processing speed impairment (homocysteine: p=0.02; WMH: p<0.0001); greater vascular index score was associated with memory impairment (p=0.007); and greater number of apolipoprotein E ε4 (APOE4) alleles was associated with global cognitive impairment (p=0.007) at baseline. APOE4 was associated with greater rate of increase in global cognitive impairment (p=0.002) and processing speed impairment (p=0.001) over time, while higher total cholesterol was associated with greater rate of increase in global cognitive impairment (p=0.02) and memory impairment (p=0.06) over time. These results suggest a significant association of increased vascular disease and risk factors with cognitive impairment at baseline and over time in the AD spectrum in a sample that was selected to have low vascular burden at baseline.
Keywords: Alzheimer’s disease, processing speed, memory, mild cognitive impairment, vascular risk factors
INTRODUCTION
Vascular risk factors such as hyperlipidemia, diabetes, and smoking, and vascular disease, such as stroke, have been shown to alter the biologic processes associated with Alzheimer’s disease (AD)1. For example, elevated cholesterol intake increases amyloid-beta deposition in the brains of transgenic mice expressing human amyloid precursor protein2. Apolipoprotein E ε4 (APOE4) is a susceptibility gene for AD that has been associated with increased deposition and decreased clearance of amyloid-beta and has been shown to predict progression from mild cognitive impairment (MCI) to AD dementia3-6. Additionally, APOE4 has been shown to be a risk factor for development of cerebrovascular disease though this association has not been demonstrated as consistently7. Vascular disease, specifically prior stroke, and vascular risk factors, primarily hypertension and diabetes, have been associated with both the occurrence and progression of AD dementia; however, the risk for the individual is modest when compared to the risk at the population level8-10. It has also been found that vascular disease and risk factors increase the risk of MCI and are associated with progression from MCI to AD dementia; this appears to be primarily driven by prior stroke, but some studies have also implicated hypertension, diabetes, and hyperlipidemia11-13. Thus, it is likely that vascular risk factors and vascular disease modify the risk of cognitive decline in the AD spectrum. However, it remains unclear whether vascular risk factors, such as hypertension and diabetes, contribute to cognitive impairment and disease progression in AD separately from cerebrovascular disease, which could directly lead to cognitive impairment regardless of the presence of AD.
Carmichael et al. looked at the relationship of white matter hyperintensities (WMH) to vascular risk factors and cognition with a 1-year follow-up period in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) study14. A more recent study by Lo et al. used ADNI data to primarily assess the relationship between cardiovascular risk scores, WMH, and AD biomarkers over 3 years15. That study also assessed the relationship of cardiovascular risk scores and WMH with global cognition and executive function within individual diagnostic groups, finding variable associations mostly with poorer executive function. Building on the results of these studies, we aimed to take advantage of the 3-year follow-up period like the Lo study to investigate the same as well as other vascular disease and risk factor elements (assessing both prior history of and currently present vascular elements), multiple important covariates (including medication use), and clinical outcomes not previously explored (including memory performance). Moreover, the earlier study14 had only a 1-year follow-up period, while our study had a 3-year follow-up period. Although the more recent study15 also had a 3-year follow-up period, it assessed effects within each diagnostic group in separate models, while our study looked at effects across diagnostic groups and within each group within the same model. The goal of the current study was to investigate the relationship between vascular disease and risk factors and cognitive decline cross-sectionally and longitudinally in normal older control (NC), amnestic MCI, and mild AD dementia subjects. The analyses performed here accounted for various factors, which have not always been controlled for in other studies. A comprehensive pool of vascular disease and risk factors consisting of multiple elements available in the ADNI database was assessed: 1) prior history of vascular disease and risk factors was summarized by a Vascular index score; 2) current physiologic measures included serum glucose, serum total cholesterol, plasma homocysteine level, systolic blood pressure, and body mass index (BMI); 3) genetic testing included APOE4 carrier status; and 4) magnetic resonance imaging (MRI) measures included WMH volume. Our outcome measures included tests of processing speed, memory, and global cognition, and disease progression. We hypothesized that vascular disease and risk factors at baseline would be associated with greater cognitive decline cross-sectionally and longitudinally across the AD spectrum. Many population-based studies of aging and dementia have assessed the relationship between vascular disease and risk factors and cognitive impairment and disease progression. The subjects in ADNI are different from those in population-based studies in that they are selected to have limited vascular burden at baseline and to include individuals at early stages of AD or at risk for AD similar to subjects entering early AD treatment trials. We therefore used the available ADNI variables to explore the relevance of multiple vascular risk factors and modest existing vascular disease to typical AD progression measured by cognition and disease stage. As such, our study could inform future clinical trials in early AD.
METHODS
Subjects
The data used in the preparation of this article were obtained from the ADNI database (www.loni.ucla.edu\ADNI) (see Supplemental Digital Content)16. 812 subjects (229 NC, 395 MCI, 188 AD dementia) participating in ADNI underwent cognitive testing, brain MRI, and clinical evaluations at baseline and up to 5 more times over a period of 3 years. Mean follow-up time was 2.3±0.9 years. At baseline subjects were ages 55-91 (inclusive), in good health, had a Modified Hachinski Ischemic Score17≤4, and a Geriatric Depression Scale (short form)18<6. Subjects did not have a cortical stroke, multiple lacunar strokes, or a lacunar stroke in a critical memory structure on screening MRI, other neurological conditions, or active psychiatric disorders. Subjects with non-AD dementia (including vascular dementia) at baseline were excluded.
Subjects were assigned to one of three diagnostic groups at baseline (NC, amnestic MCI, mild AD dementia) as previously described (see Supplemental Digital Content)16.
The local Institutional Review Boards (IRB) of each participating site approved this study. After all study procedures and risks were explained in detail, in accordance with local IRB guidelines, written informed consent was obtained from all subjects and study partners.
Clinical assessments
The Wechsler Adult Intelligence Scale-Revised Digit Symbol19 was used to assess processing speed, complex attention, and visual scanning (lower scores indicate greater impairment; possible range 0-110, in current analyses 0-87); the Rey Auditory Verbal Learning Test (RAVLT)20 Total Learning score (i.e., words recalled over 5 learning trials) was used to assess episodic memory performance (lower scores indicate greater memory impairment; range 0-75); the Total Learning score reflects memory encoding which relates to aspects of executive function and is more likely to be associated with vascular disease and risk factors than memory storage; the Alzheimer Disease Assessment Scale Cognitive Subscale (ADAS-Cog) 13 item version21 was used to assess global cognition (higher scores indicate greater impairment; range 0-85); and the American National Adult Reading Test22 (AMNART) intelligence quotient (IQ), was used to provide an estimate of premorbid verbal intelligence and serve as a proxy of cognitive reserve.
A Vascular index score was created to be similar in concept to the Framingham Study Stroke Risk profile23 using the available information in the ADNI database. However, our intention was to create an index that specifically focuses on prior history of various vascular related conditions unlike the Framingham profile which also includes current physiologic measurements, which we assessed separately (see Supplemental Digital Content). In the Vascular index score one point was given for each of the following conditions if present at baseline or in the past: hypertension, hyperlipidemia, diabetes, myocardial infarction, atrial fibrillation, smoking, and stroke (range 0-7). Other vascular disease and risk factors used in the analyses included: systolic blood pressure, serum glucose and total cholesterol, BMI, APOE4 carrier status (non-carrier, heterozygous carrier, homozygous carrier), WMH volume, and plasma homocysteine level. Missing values data for key predictors and dependent variables are provided in Supplemental Digital Content.
WMH were detected on co-registered sets of T1, T2, and proton density MRI images using an automated validated method yielding volume measurements corrected for cranial size14. The WMH measurement method used in our study was consistent with the recently published recommended neuroimaging standards24. WMH volume was available for 632 of the 812 subjects at the time the ADNI database was queried. Of the remaining 180 subjects, 170 subjects had adequate MRI scans; however, WMH volume data was not available for those subjects. When subjects with WMH volume data were compared to subjects without that data across all relevant baseline demographics and clinical variables, those with WMH volume data performed significantly differently (better) than those without only on Digit Symbol (p=0.0009 after Sidak and False Discovery Rate corrections for multiple comparisons). The analyses outlined below were performed using the whole sample and repeated using the reduced sample with WMH volume data.
Duration of AD dementia symptoms (in years) was used as a covariate. It was available only for AD dementia subjects and was set as zero for NC and MCI subjects in order to be able to include them in the analyses. Use of aspirin, antihypertensive drugs, and lipid lowering drugs at baseline were each reported as dichotomous variables (present/absent) and were included as covariates.
Statistical Analyses
All analyses were performed using SAS Version 9.2. As part of initial descriptive univariate statistical analyses, associations between diagnostic groups versus demographics and baseline characteristics of subjects were assessed using analysis of variance with Bonferroni adjusted post hoc pairwise group mean comparison tests for continuous variables and the chi-square test for categorical variables, except for systolic blood pressure, serum glucose and total cholesterol which had positively skewed distributions and were analyzed using non-parametric tests (Kruskal-Wallis). All of these associations are illustrated in Table 1.
Table 1.
Baseline demographics and characteristics of subjects.
| Group | All subjects | NC | MCI | AD dementia |
|---|---|---|---|---|
| n | 812 | 229 | 395 | 188 |
| Age (years) | 75.3±6.9 | 76.0±5.0 | 74.8±7.5 | 75.3±7.5 |
| Sex (% male) | 57.9‡‡ | 52.0 | 64.3 | 51.6 |
| Education (years) | 15.5±3.1‡ | 16.0±2.9 | 15.7±3. 1 | 14.7±3.1 |
| AMNART IQ | 117.2±11.6 †† | 121.1±10. 6 | 116.6±11.5 | 114.0±11.7 |
|
AD dementia symptom
duration (years) |
3.5±2.5 | |||
| MMSE | 26.8±2.7* | 29.1±1.0 | 27.0±1.8 | 23.3±2.0 |
| CDR sum of boxes | 1.8±1.8* | 0.0±0.1 | 1.6±0.9 | 4.3±1.6 |
|
ADAS-Cog (13 item
version) |
18.4±9.3* | 9.50±4.2 | 18.6±6.3 | 29.0±7.6 |
| Digit Symbol | 36.9±13.4* | 45.8±10.2 | 36.8±11.3 | 26.5±13.2 |
| RAVLT Total Learning | 32.5±11.5* | 43.1±10.0 | 30.8±9.0 | 23.2±7.6 |
| Vascular index Score | 1.3±1.0 | 1.2±0.9 | 1.3±1.0 | 1.4±1.0 |
| Systolic Blood Pressure | 135.5±17.6 | 134.5±16.9 | 135.2±18.2 | 137.2±17.1 |
| Serum Glucose | 101.7±25.6 | 102.6±22.6 | 101.9±27.8 | 100.4±24.1 |
| Serum Total Cholesterol | 197.9±46.0 | 193.7±41.3 | 198.8±46.9 | 201.2±49.5 |
| Body Mass Index | 26.2±4.1** | 26.7±4.4 | 26.1±4.0 | 25.6±3.9 |
|
APOE4 (% non-
carrier/heterozygous carrier/homozygous carrier) |
51.2/38.2/10.6† | 73.2/24.6/2.2 | 46.7/41.9/11.4 | 34.0/46.8/19.2 |
| WMH volume | 3.0±4.3*** | 2.8±3.0 | 2.7±2.8 | 3.4±3.3 |
|
Homocysteine level
(mg/L) |
10.4±3.0‡‡‡ | 9.9±2.9 | 10.6±2.9 | 10.7±3.2 |
| Aspirin use (% present) | 47.9 | 52.4 | 47.3 | 43.6 |
|
Antihypertensive drug
use (% present) |
40.6 | 40.6 | 40.0 | 42.0 |
|
Lipid Lowering drug
use (% present) |
38.6 | 34.5 | 41.5 | 37.2 |
AD (Alzheimer’s disease), ADAS-Cog (Alzheimer Disease Assessment Scale Cognitive Subscale), AMNART IQ (American National Adult Reading Test intelligence quotient), APOE4 (Apolipoprotein E ε4), CDR (Clinical Dementia Rating), MCI (mild cognitive impairment), MMSE (Mini-Mental State Examination), NC (normal older control), RAVLT (Rey Auditory Verbal Learning Test), WMH (white matter hyperintensity). All values (except n, sex, APOE4, aspirin use, antihypertensive drug use, and lipid lowering drug use) represent mean ± standard deviation.
p<0.0001 for NC vs. MCI, NC vs. AD and MCI vs. AD.
p<0.01 for NC vs. MCI, NC vs. AD and MCI vs. AD.
p<0.05 for NC vs. MCI, NC vs. AD and MCI vs. AD.
p<0.001 for NC vs. AD and MCI vs. AD.
p<0.01 for NC vs. MCI and MCI vs. AD.
p<0.05 for NC vs. MCI and NC vs. AD.
p<0.05 for NC vs. AD.
p<0.05 for MCI vs. AD.
Cross-sectional analyses
A general linear model approach was employed with backward elimination of predictors (p<0.01 cut-off for all predictor terms, including interaction terms). Digit Symbol, RAVLT Total Learning, and ADAS-Cog scores were the dependent variables in 3 separate analyses. The predictors for these models were: Vascular disease and risk factors and the interaction of each of these variables with diagnosis, sex, diagnosis, the interaction of diagnosis and sex, age (linear/quadratic effects), duration of AD dementia symptoms, AMNART IQ, use of aspirin, antihypertensive drugs, and lipid lowering drugs. P values, partial unstandardized regression coefficient estimates (β) with confidence intervals (CI), estimates of percent variance in the dependent variable accounted for by the model as a whole (R2), and the portion of this variance uniquely accounted for by each predictor term individually (unbiased population estimate, adjusting for the other predictors, ω2) were reported.
The inclusion of the interaction of vascular disease and risk factors with diagnosis allowed us to test for any differential relation of any given vascular variable to a given cognitive measure across diagnostic groups (see Supplemental Digital Content).
Longitudinal analyses: Mixed Effects Models
Mixed random and fixed coefficient longitudinal regression models were employed. A backward elimination procedure (p=0.05 cut-off) was used on a large initial pool of fixed predictors and variances/covariances of random terms. The fixed predictors were vascular disease and risk factors and their interaction with time (in years), the covariates used in the cross-sectional analyses, and the baseline dependent variable and its interaction with time. The random predictors were correlated intercepts and linear slopes of time. Longitudinal Digit Symbol, RAVLT Total Learning, and ADAS-Cog scores were the dependent variables in 3 separate analyses. The squared correlations of predicted values from fixed and random predictor sets versus actual values were used to indicate the percent of variance of the dependent variable linearly accounted for by the predictors.
Longitudinal analyses: Cox Proportional Hazards Models
We employed two separate Cox proportional hazards models to assess time to change in diagnosis from a baseline diagnosis of MCI to an endpoint diagnosis of AD dementia and from NC to MCI (see Supplemental Digital Content). 13 subjects who progressed from a more impaired diagnosis to a less impaired diagnosis (MCI to NC) were excluded from the analyses. Subjects who remained stable at the specified baseline diagnosis were treated in the analyses as “censored” observations and the partial information they provided on time to change in diagnosis was used. Predictors were tested in a backward elimination algorithm (p<0.05 cut-off). Predictors included vascular disease and risk factors, age, sex, AMNART IQ, use of aspirin, antihypertensive drugs, and lipid lowering drugs. Hazard ratios (HR) were reported. The assumption of proportional hazards was tested, and if it appeared questionable, results were confirmed with nonparametric survival analyses.
RESULTS
Table 1 displays demographics and characteristics for all subjects, as well as for the three diagnostic groups (see Supplemental Digital Content).
Cross-sectional Analyses
Digit Symbol score
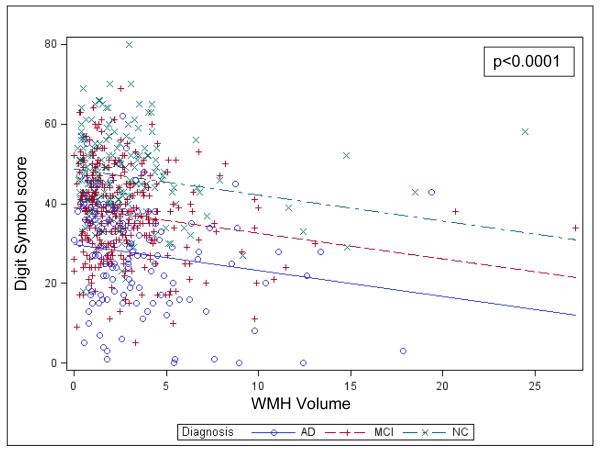
Greater homocysteine level was marginally associated with lower Digit Symbol score, representing greater processing speed impairment (β=−0.33, %Variance Total=0.4, p=0.02). In a second model including WMH volume, greater WMH volume was also associated with lower Digit Symbol score (β=−0.71, %Variance Total=2.4, p<0.0001), see Figure 1. Covariates that were significantly associated with Digit Symbol score were diagnostic group (NC subjects had greater Digit Symbol score than MCI and MCI greater than AD dementia) and AMNART IQ (greater AMNART IQ score was associated with greater Digit Symbol score) (R2=0.29, p<0.0001 for model), see Table e-1. The interaction of homocysteine or WMH volume with diagnostic group was not significant, suggesting that the relation of homocysteine and WMH volume to Digit Symbol score was not conditional on diagnostic group.
Figure 1.
Values predicted from general linear model of Digit Symbol score regressed on diagnostic group and WMH Volume. The lines indicate the predicted values for Digit Symbol and the symbols denote corresponding actual values (overlapping observations at the same coordinates are sometimes hidden). The final model included a number of additional partialed significant predictors, but to simplify the visual display, they were not included in the model producing the predicted values in the figures (including them had a negligible effect on the relations seen). AD (Alzheimer’s disease), MCI (mild cognitive impairment), NC (normal older control), WMH (white matter hyperintensity).
RAVLT Total Learning
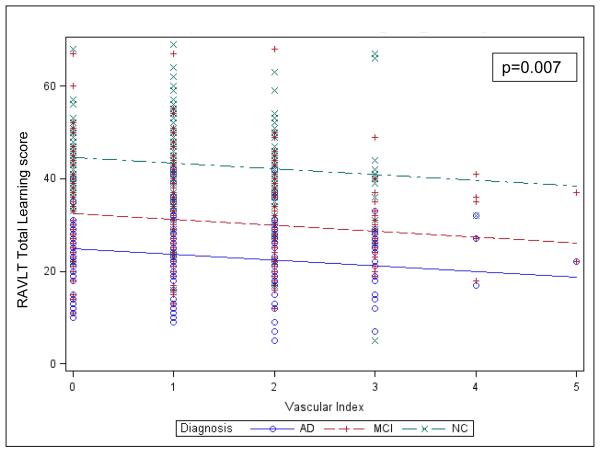
Greater Vascular index score was significantly associated with lower RAVLT Total Learning, representing greater memory impairment (β=−0.86, %Variance Total=0.4, p=0.007), see Figure 2. Covariates that were significantly associated with RAVLT Total Learning were diagnostic group (NC subjects had greater RAVLT Total Learning than MCI and MCI greater than AD dementia), sex (females higher), age (greater age was associated with lower RAVLT Total Learning), and AMNART IQ (greater AMNART IQ score was associated with greater RAVLT Total Learning) (R2=0.45, p<0.0001 for model), see Table e-2. The interaction of Vascular index score with diagnostic group was not significant.
Figure 2.
Values predicted from general linear model of RAVLT Total Learning regressed on diagnostic group and Vascular Index score. The lines indicate the predicted values for RAVLT Total Learning and the symbols denote corresponding actual values. AD (Alzheimer’s disease), MCI (mild cognitive impairment), NC (normal older control), RAVLT (Rey Auditory Verbal Learning Test).
ADAS-Cog score
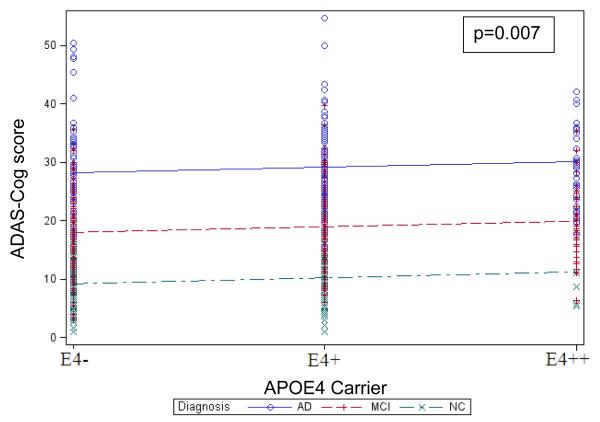
Greater number of APOE4 alleles was significantly associated with greater ADAS-Cog score, representing greater global cognitive impairment (β=0.89, %Variance Total=0.3, p<0.007), see Figure 3. Covariates that were significantly associated with ADAS-Cog score were diagnostic group (NC subjects had greater ADAS-Cog score than MCI and MCI greater than AD dementia), duration of AD dementia symptoms (greater duration was associated with greater ADAS-Cog score), and AMNART IQ (lower AMNART IQ score was associated with greater ADAS-Cog score) (R2=0.59, p<0.0001 for overall model), see Table e-3. The interaction of APOE4 with diagnostic group was not significant.
Figure 3.
Values predicted from general linear model of ADAS-Cog score, representing global cognitive impairment, regressed on diagnostic group and number of APOE4 alleles. The lines indicate the predicted values for ADAS-Cog and the symbols denote corresponding actual values. AD (Alzheimer’s disease), ADAS-Cog (Alzheimer Disease Assessment Scale Cognitive Subscale), APOE4 (Apolipoprotein E ε4), MCI (mild cognitive impairment), NC (normal older control).
Longitudinal Analyses: Mixed Effects Models
Digit Symbol score
Greater number of APOE4 alleles was significantly associated and greater WMH volume was marginally associated (at a trend-level) with a decrease in Digit Symbol score over time (β=−0.84, p=0.001 for interaction of APOE4 with time in the study; β=−0.10, p=0.06 for interaction of WMH with time). Additional significant fixed effect predictors of secondary interest were interaction of baseline diagnostic group with time (AD dementia subjects compared to MCI and MCI compared to NC were associated with a decrease in Digit Symbol score over time) and a linear effect of baseline Digit Symbol score (greater baseline Digit Symbol score was associated with higher Digit Symbol score over time) (R2=0.76, p<0.0001 for fixed effects model; R2=0.92 including random terms), see Table e-4.
RAVLT Total Learning
Higher serum total cholesterol was marginally associated (at a trend-level) with a decrease in RAVLT Total Learning over time (β=−0.006, p=0.06), see Figure e-1. Additional significant fixed effect predictors of secondary interest were APOE4 (greater number of APOE4 alleles was associated with a decrease in RAVLT Total Learning), baseline RAVLT Total Learning (greater baseline RAVLT Total Learning was associated an increase in RAVLT Total Learning), and the interaction of baseline diagnostic group with time (AD dementia subjects compared to MCI and MCI compared to NC were associated with a decrease in RAVLT Total Learning over time) (R2=0.69 p<0.0001 for fixed effects model; R2=0.90 including random terms), see Table e-5.
ADAS-Cog score
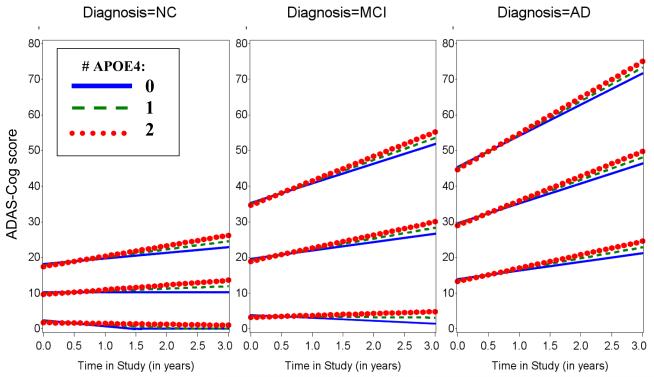
Greater number of APOE4 alleles and higher serum total cholesterol were significantly associated with greater rate of increase in ADAS-Cog score over time (β=0.65, p=0.002 for interaction of APOE4 with time; β=0.007, p=0.02 for interaction of total cholesterol with time), see Figure 4. Additional significant fixed effect predictors of secondary interest were interaction of baseline ADAS-Cog score with time (greater baseline ADAS-Cog score was associated with greater rate of increase in ADAS-Cog score over time), interaction of baseline diagnostic group with time (AD dementia subjects compared to MCI and MCI compared to NC were associated with greater rate of increase in ADAS-Cog score over time), and a linear effect of WMH volume (greater WMH volume was associated with an increase in ADAS-Cog score), serum glucose (lower glucose was associated with an increase in ADAS-Cog score), and age (greater age was associated with an increase in ADAS-Cog score) (R2=0.75, p<0.0001 for overall model fixed effects; R2=0.95 including random terms), see Table e-6.
Figure 4.
Predicted values from fixed effects of best fitting longitudinal model of ADAS-Cog score across time in the study by number of APOE4 alleles at selected baselines by diagnostic groups. WMH volume was set to equal its grand mean of 3 and sex was set to equal female. NC (Left), MCI (Middle), and AD dementia (Right). AD (Alzheimer’s disease), ADAS-Cog (Alzheimer Disease Assessment Scale Cognitive Subscale), APOE4 (Apolipoprotein E ε4), MCI (mild cognitive impairment), NC (normal older control).
Residuals for all models, cross-sectional and longitudinal, conformed reasonably to normality assumptions.
Longitudinal Analyses: Cox Proportional Hazards Models
One hundred and fifty six out of 365 (42.7%) subjects with a baseline diagnosis of MCI progressed to AD dementia over the three year follow-up period, while 11 out of 223 (4.9%) subjects progressed from NC to MCI.
MCI to AD dementia progression
Greater number of APOE4 alleles indicated significantly greater hazard to change from MCI to AD dementia (HR=1.60, p<0.0001, 95% CI for HR=1.29, 1.98). There were no other significant predictors (see Supplemental Digital Content).
NC to MCI progression
Greater homocysteine level, greater number of APOE4 alleles, and higher serum glucose indicated significantly greater hazard to change from NC to MCI (Homocysteine: HR=1.22, p=0.02, 95% CI for HR=1.01, 1.41; APOE4: HR=3.07, p=0.04, 95% CI for HR=1.02, 8.67; Glucose: HR= 1.03, p=0.01, 95% CI for HR= 1.00, 1.05). There were no other significant predictors.
DISCUSSION
In a cross-sectional and longitudinal analysis of 812 well-characterized subjects, we found a significant association between increased vascular disease and risk factors (including APOE4 carrier status, homocysteine level, WMH volume, serum total cholesterol and glucose, and Vascular index score) and cognitive impairment at baseline and over time in the AD spectrum after adjusting for diagnostic group, demographics, and medication use in a sample that was selected to have low vascular burden at baseline.
In the cross-sectional analyses, several types of vascular disease and risk factors were related to cognitive impairment. However, longitudinally, APOE4 carrier status appeared to be the main predictor of cognitive impairment and progression to a more impaired diagnostic group. APOE4 carriers are a particularly vulnerable group because of their high risk of development of AD dementia and the younger age at which AD manifests6. APOE4 has been shown to biologically relate to AD through its association with the metabolism of amyloid-beta, as well as being a risk factor for small vessel ischemic disease, microinfarcts, and cerebral amyloid angiopathy5-7,25. As such, APOE4 appears to contribute to cognitive decline and AD dementia through multiple mechanisms26. That said, we found an association between other aspects of vascular disease and risk factors and cognitive decline after adjusting for APOE4, especially cross-sectionally.
Our cross-sectional analyses demonstrated an association between greater WMH volume, greater homocysteine level, and slower processing speed; greater Vascular index score, representing a sum of 7 common vascular disease and risk factor elements obtained by history, and greater memory impairment; and APOE4 carrier status and greater global cognitive impairment. These results are in line with prior studies across the AD spectrum, including the two ADNI studies, demonstrating an association between WMH volume, poor executive function, and global cognitive impairment at baseline and over time14,15,27,28. Those studies did not show an association between a combination of cardiovascular risk factors (similar to the Vascular index score) and cognitive impairment at baseline or over time except for with poor executive function in MCI subjects only, but those studies did not look specifically at memory (separate from global cognition) as we did in the current study. The Rotterdam Scan Study, which is a population-based study, showed an association between WMH and poor memory performance, in addition to poor processing speed, executive function, and global cognition29. It is possible that we were unable to see an association between WMH, memory, and global cognition due to the ADNI sample having low vascular burden at baseline and being enriched for AD pathology. Another recent population-based study showed that regional (parietal) WMH was strongly associated with incidence of AD dementia30. A meta-analysis similarly indicated that greater baseline WMH were associated with increased risk of dementia; however, when looking at a sub-set of high risk population studies focused on MCI, there was no increased risk of dementia27. The latter is in line with our results and other recent analyses of the ADNI data, which did not show an association between greater WMH and disease progression15.
A recent longitudinal study with over 20,000 participants free of stroke and significant cognitive impairment at baseline showed that the Framingham Stroke Risk Profile (similar to the vascular index score we used), hypertension, and left ventricular hypertrophy predicted future cognitive decline using a screening cognitive test31. We had the advantage of more sensitive cognitive assessments and more carefully characterized subjects but had significantly fewer subjects. Elevated homocysteine level has been previously shown to be a risk factor for cerebrovascular disease, AD dementia, and cognitive impairment32-34. However, treatment with vitamin B12, B6, and folic acid, which successfully reduced homocysteine levels in subjects with already mild-moderate AD dementia, did not improve their global cognition35. In the current study, elevated homocysteine level was associated with slower baseline processing speed and risk of progression from NC to MCI.
We also found an association between higher baseline total cholesterol and greater memory and global cognitive impairment over time after adjusting for baseline use of lipid lowering drug use. These results are in agreement with recent studies demonstrating an association between hypercholesterolemia and worsening global cognition and disease progression in MCI and AD dementia13,36,37. Within our own set of analyses, we also showed an association between baseline memory performance and prior history of vascular disease and risk factors captured by the Vascular index score, which included a history of hyperlipidemia, suggesting a similar longitudinal association to the one found with the baseline measurement of serum total cholesterol and memory performance over time. Two potential explanations for the association between cholesterol, memory, and global cognition in the AD spectrum are the link between elevated cholesterol, specifically triglycerides, and microvascular disease in the brain37, and the link between elevated cholesterol, specifically low-density lipoprotein, and cortical amyloid deposition38.
The two vascular risk factors in our study that were not associated with any cognitive measures at baseline or over time were BMI and systolic blood pressure. This is consistent with studies showing a lack of association between increased late-life BMI and cognitive decline, in contrast to increased mid-life BMI being a significant risk factor for AD and vascular dementia39,40. Furthermore, increased late-life BMI has been associated with reduced risk of AD dementia41. In our study we did not find an association between systolic blood pressure and cognitive function or disease progression. However, a prior large population study of community-dwelling individuals did show an association between elevated baseline systolic blood pressure and incident cognitive impairment31,42. Similarly, a smaller study of individuals residing in assisted living facilities showed that a diagnosis of hypertension in those with a global CDR of 0.5 (similar to MCI) was associated with faster cognitive decline42.
Different associations between vascular disease and risk factors and cognitive measures were obtained. These varied with regard to the cognitive measure assessed and the type of analysis (cross-sectional general linear model, longitudinal mixed effects model, or longitudinal Cox proportional hazards model). This could be in part due to the sample available for each type of analysis. The cross-sectional analyses had the largest sample size and all 3 diagnostic groups were represented and their effect was examined closely by including interaction terms between vascular variables and diagnosis. In the longitudinal mixed effects analyses, those interactions were not included because interactions between vascular variables and time were more important. As such, the over-represented MCI group could have driven the results of the longitudinal mixed effects analyses, leading to similar results as those obtained from the Cox regression model assessing progression from MCI to AD dementia, where APOE4 carrier status showed a significant influence on progression. On the other hand, processing speed was associated with WMH volume both cross-sectionally and longitudinally.
There were a few limitations of our study. Subjects participating in ADNI were very carefully selected to fit into the diagnostic groups of normal cognition for age, amnestic MCI, and mild AD dementia. Subjects with significant cerebrovascular disease, psychiatric disorders, or major health issues were excluded. NC subjects had a higher than usual proportion of APOE4 carriers. Thus, this sample was not representative of the general population. Furthermore, since ADNI was not designed to investigate the relationship between vascular disease and risk factors and cognitive decline, the variables making up the Vascular index score were recorded as dichotomous values (present/absent) and severity, duration, and timing of vascular risk factors were not accounted for. Therefore, there may have been underreporting or non-specific reporting of vascular risk factors, which may have led to an underestimation of the effects of vascular risk factors on cognitive decline. The sample predominantly consisted of subjects with MCI, and therefore it is possible that this diagnostic group drove the results. However, the various models included interaction terms of vascular disease and risk factors with diagnostic group and those were not significant suggesting that diagnostic group did not modify the relationship between vascular disease and risk factors and cognitive impairment. Finally, due to our large sample, certain statistically significant results were not necessarily clinically relevant. Therefore, various designations of effect size were provided in order to better interpret the results.
In conclusion, these results suggest that there is a significant association between increased vascular disease and risk factors and cognitive impairment in the AD spectrum in the absence of significant baseline cerebrovascular disease. Future longitudinal observational biomarker studies, including the various extensions of ADNI, and clinical trials will help determine whether treatment of vascular disease and risk factors can prevent AD or slow down its progression. This will also help disentangle the vascular versus non-vascular effects of APOE4.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by R01 AG027435, K23 AG033634, K24 AG035007, Gilbert Foundation/AFAR New Investigator Awards in Alzheimer’s disease, Massachusetts Alzheimer’s Disease Research Center (P50 AG005134), Harvard Aging Brain Study (P01 AGO36694), and ADNI (NIH Grant U01 AG024904). We would also like to thank Dr. Deborah Blacker for her help in the initial interpretation of the results of the study (see Supplemental Digital Content). NL has no conflicts of interest to report. AV has two conflicts of interest to report, the following grants/funds: NIH/NIA2P50AG05134-26 and 1K23AG028726-01A2. JJL has no conflicts of interest to report. GAM has no conflicts of interest to report. DMR has no conflicts of interest to report. RAS has no conflicts of interest to report. KAJ has the following conflicts of interest to report: Massachusetts General Hospital, Brigham and Women’s Hospital, Harvard Medical School, NIA/NIH funds/grants, a consultant fro GEHC, Piramal, Avid/Lilly and Siemems.
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles. This research was also supported by NIH grants P30 AG010129 and K01 AG030514.
Abbreviations
- ADAS-Cog
Alzheimer Disease Assessment Scale Cognitive Subscale
- AD
Alzheimer’s disease
- ADNI
Alzheimer’s Disease Neuroimaging Initiative
- AMNART
American National Adult Reading Test
- APOE4
Apolipoprotein E ε4
- CDR
Clinical Dementia Rating
- CI
Confidence intervals
- HR
Hazard ratios
- IRB
Institutional Review Boards
- IQ
Intelligence quotient
- LM-IIa
Logical Memory IIa
- MRI
Magnetic resonance imaging
- BMI
Mass index
- MCI
Mild cognitive impairment
- MMSE
Mini-Mental State Examination
- NC
Normal older control
- β
Partial unstandardized regression coefficient estimates
- RAVLT
Rey Auditory Verbal Learning Test
- WMS-R
Wechsler Memory Scale-Revised
- WMH
White matter hyperintensity
REFERENCES
- 1.Hayden KM, Zandi PP, Lyketsos CG, et al. Vascular risk factors for incident Alzheimer disease and vascular dementia: the Cache County study. Alzheimer Dis Assoc Disord. 2006;20:93–100. doi: 10.1097/01.wad.0000213814.43047.86. [DOI] [PubMed] [Google Scholar]
- 2.Refolo LM, Malester B, LaFrancois J, et al. Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol Dis. 2000;7:321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- 3.Carter DB. The interaction of amyloid-beta with ApoE. Subcell Biochem. 2005;38:255–272. doi: 10.1007/0-387-23226-5_13. [DOI] [PubMed] [Google Scholar]
- 4.Petersen RC, Smith GE, Ivnik RJ, et al. Apolipoprotein E status as a predictor of the development of Alzheimer’s disease in memory-impaired individuals. JAMA. 1995;273:1274–1278. [PubMed] [Google Scholar]
- 5.Poirier J. Apolipoprotein E and Alzheimer’s disease. A role in amyloid catabolism. Ann N Y Acad Sci. 2000;924:81–90. doi: 10.1111/j.1749-6632.2000.tb05564.x. [DOI] [PubMed] [Google Scholar]
- 6.Saunders AM, Strittmatter WJ, Schmechel D, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 7.Yip AG, McKee AC, Green RC, et al. APOE, vascular pathology, and the AD brain. Neurology. 2005;65:259–265. doi: 10.1212/01.wnl.0000168863.49053.4d. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Zhang M, Xu ZQ, et al. Vascular risk aggravates the progression of Alzheimer’s disease in a Chinese cohort. J Alzheimers Dis. 2010;20:491–500. doi: 10.3233/JAD-2010-1383. [DOI] [PubMed] [Google Scholar]
- 9.Viswanathan A, Rocca WA, Tzourio C. Vascular risk factors and dementia: how to move forward? Neurology. 2009;72:368–374. doi: 10.1212/01.wnl.0000341271.90478.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de la Torre JC. Is Alzheimer’s disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol. 2004;3:184–190. doi: 10.1016/S1474-4422(04)00683-0. [DOI] [PubMed] [Google Scholar]
- 11.Di Carlo A, Lamassa M, Baldereschi M, et al. CIND and MCI in the Italian elderly: frequency, vascular risk factors, progression to dementia. Neurology. 2007;68:1909–1916. doi: 10.1212/01.wnl.0000263132.99055.0d. [DOI] [PubMed] [Google Scholar]
- 12.Solfrizzi V, Panza F, Colacicco AM, et al. Vascular risk factors, incidence of MCI, and rates of progression to dementia. Neurology. 2004;63:1882–1891. doi: 10.1212/01.wnl.0000144281.38555.e3. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Wang YJ, Zhang M, et al. Vascular risk factors promote conversion from mild cognitive impairment to Alzheimer disease. Neurology. 2011;76:1485–1491. doi: 10.1212/WNL.0b013e318217e7a4. [DOI] [PubMed] [Google Scholar]
- 14.Carmichael O, Schwarz C, Drucker D, et al. Longitudinal changes in white matter disease and cognition in the first year of the Alzheimer disease neuroimaging initiative. Arch Neurol. 2010;67:1370–1378. doi: 10.1001/archneurol.2010.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo RY, Jagust WJ. Vascular burden and Alzheimer disease pathologic progression. Neurology. 2012;79:1349–1355. doi: 10.1212/WNL.0b013e31826c1b9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiner MW, Veitch DP, Aisen PS, et al. The Alzheimer’s Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement. 2012;8:S1–68. doi: 10.1016/j.jalz.2011.09.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosen WG, Terry RD, Fuld PA, et al. Pathological verification of ischemic score in differentiation of dementias. Ann Neurol. 1980;7:486–488. doi: 10.1002/ana.410070516. [DOI] [PubMed] [Google Scholar]
- 18.Sheikh JIYJ. Clinical Gerontology: A Guide to Assessment and Intervention. The Haworth Press; New York: 1986. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version; pp. 165–173. [Google Scholar]
- 19.Wechsler D. Manual for the Wechsler Adult Intelligence Scale - Revised. Psychological Corporation; New York: 1981. [Google Scholar]
- 20.Rey A. L’examen clinique en psychologie. Presses Universitaires de France; Paris: 1964. [Google Scholar]
- 21.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 22.Nelson HE, O’Connell A. Dementia: the estimation of premorbid intelligence levels using the New Adult Reading Test. Cortex. 1978;14:234–244. doi: 10.1016/s0010-9452(78)80049-5. [DOI] [PubMed] [Google Scholar]
- 23.D’Agostino RB, Wolf PA, Belanger AJ, et al. Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study. Stroke. 1994;25:40–43. doi: 10.1161/01.str.25.1.40. [DOI] [PubMed] [Google Scholar]
- 24.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viswanathan A, Greenberg SM. Cerebral amyloid angiopathy in the elderly. Ann Neurol. 2011;70:871–880. doi: 10.1002/ana.22516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Packard CJ, Westendorp RG, Stott DJ, et al. Association between apolipoprotein E4 and cognitive decline in elderly adults. J Am Geriatr Soc. 2007;55:1777–1785. doi: 10.1111/j.1532-5415.2007.01415.x. [DOI] [PubMed] [Google Scholar]
- 27.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brickman AM, Honig LS, Scarmeas N, et al. Measuring cerebral atrophy and white matter hyperintensity burden to predict the rate of cognitive decline in Alzheimer disease. Arch Neurol. 2008;65:1202–1208. doi: 10.1001/archneur.65.9.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol. 2000;47:145–151. doi: 10.1002/1531-8249(200002)47:2<145::aid-ana3>3.3.co;2-g. [DOI] [PubMed] [Google Scholar]
- 30.Brickman AM, Provenzano FA, Muraskin J, et al. Regional white matter hyperintensity volume, not hippocampal atrophy, predicts incident Alzheimer disease in the community. Arch Neurol. 2012;69:1621–1627. doi: 10.1001/archneurol.2012.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Unverzagt FW, McClure LA, Wadley VG, et al. Vascular risk factors and cognitive impairment in a stroke-free cohort. Neurology. 2011;77:1729–1736. doi: 10.1212/WNL.0b013e318236ef23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lehmann M, Gottfries CG, Regland B. Identification of cognitive impairment in the elderly: homocysteine is an early marker. Dement Geriatr Cogn Disord. 1999;10:12–20. doi: 10.1159/000017092. [DOI] [PubMed] [Google Scholar]
- 33.McCaddon A, Hudson P, Davies G, et al. Homocysteine and cognitive decline in healthy elderly. Dement Geriatr Cogn Disord. 2001;12:309–313. doi: 10.1159/000051275. [DOI] [PubMed] [Google Scholar]
- 34.Morris MS. Homocysteine and Alzheimer’s disease. Lancet Neurol. 2003;2:425–428. doi: 10.1016/s1474-4422(03)00438-1. [DOI] [PubMed] [Google Scholar]
- 35.Aisen PS, Schneider LS, Sano M, et al. High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial. JAMA. 2008;300:1774–1783. doi: 10.1001/jama.300.15.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakurai H, Hanyu H, Sato T, et al. Vascular risk factors and progression in Alzheimer’s disease. Geriatr Gerontol Int. 2011;11:211–214. doi: 10.1111/j.1447-0594.2010.00669.x. [DOI] [PubMed] [Google Scholar]
- 37.Altman R, Rutledge JC. The vascular contribution to Alzheimer’s disease. Clin Sci (Lond) 2010;119:407–421. doi: 10.1042/CS20100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reed B, Villeneuve S, Mack W, et al. Associations Between Serum Cholesterol Levels and Cerebral Amyloidosis. JAMA Neurol. 2013 doi: 10.1001/jamaneurol.2013.5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Driscoll I, Espeland MA, Wassertheil-Smoller S, et al. Weight change and cognitive function: findings from the Women’s Health Initiative Study of Cognitive Aging. Obesity (Silver Spring) 2011;19:1595–1600. doi: 10.1038/oby.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whitmer RA, Gunderson EP, Quesenberry CP, Jr., et al. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res. 2007;4:103–109. doi: 10.2174/156720507780362047. [DOI] [PubMed] [Google Scholar]
- 41.Hughes TF, Borenstein AR, Schofield E, et al. Association between late-life body mass index and dementia: The Kame Project. Neurology. 2009;72:1741–1746. doi: 10.1212/WNL.0b013e3181a60a58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wysocki M, Luo X, Schmeidler J, et al. Hypertension is associated with cognitive decline in elderly people at high risk for dementia. Am J Geriatr Psychiatry. 2012;20:179–187. doi: 10.1097/JGP.0b013e31820ee833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.