In 2011 Hazen and colleagues made the seminal discovery that three metabolites of dietary phosphatidylcholine (choline, trimethylamine N-oxide (TMAO) and betaine) predicted risk for cardiovascular disease in an independent large clinical cohort1. They also demonstrated that supplementing the diet with choline or TMAO promoted atherosclerosis in a mouse model1. The gut flora was shown to be required for the production of TMAO, and when the gut flora was suppressed, dietary-choline-enhanced atherosclerosis was inhibited1.
The robustness of TMAO derived from choline in dietary phosphatidylcholine as a predictor of cardiovascular risk was subsequently confirmed in a larger cohort2,3. It was also demonstrated that L-carnitine, a constituent of red meat, was another excellent substrate for gut flora to produce TMA, which was then converted to TMAO4. More recently, it was shown that following L-carnitine ingestion γ-butyrobetaine is produced as an intermediary metabolite by gut bacteria at a rate approximately 1,000-fold higher than the formation of TMA, and in mice is converted into TMA and TMAO and accelerates atherosclerosis5.
Other studies demonstrated that TMAO predicted risk in patients with heart failure6. Heart failure patients with high TMAO levels had increased long-term mortality independent of traditional risk factors and independent of cardiorenal indexes6. In these studies6 it was noted that there was an inverse correlation between TMAO levels and the estimated glomerular filtration rate (eGFR) (r = −0.55; p < 0.001). An accompanying editorial7 stated that “…the strong correlation between TMAO concentration and kidney function raises the following question: given the importance of the kidney in eliminating TMAO, is higher TMAO level just a marker of renal impairment (7)?” The reference8 cited in the editorial7 reported that TMA (which is produced in the intestine by gut bacteria and transported to the liver where it is acted upon by flavin monooxygenase family members9 to form TMAO), as well as TMAO itself were both elevated in the plasma from ten patients with end stage renal disease (ESRD) undergoing hemodialysis compared to ten healthy adults8. Moreover, the authors observed that the elevated levels in the ESRD patients were efficiently reduced during a single hemodialysis treatment8.
It has been recognized that chronic kidney disease (CKD) alters the intestinal microbial flora10–12. In a recent publication from the Framingham Heart Study liquid chromatography/mass spectrometry-based metabolite profiling on plasma from 1434 participants demonstrated that nine metabolites predicted the development of chronic kidney disease13. Interestingly, choline was one of three markers that remained significant after adjustment for eGFR, age, sex, diabetes, hypertension, and proteinuria at baseline13.
In this issue of Circulation Research, Tang et al.14 provide evidence that the TMAO pathway is not only a biomarker for renal disease, but likely contributes to the progression of renal disease and contributes to the risk of mortality in CKD.
The authors utilized samples collected from adults who underwent elective diagnostic coronary angiography for cardiac evaluation from 2001 – 2007 at the Cleveland Clinic2. They defined CKD as eGFR <60 mL/min/1.73m2, and they ascertained all-cause mortality at 5-years by telephone contact and chart review plus interrogation of the Social Security Death Index to the year 2011. Of the 3,687 subjects considered, 521 met the criteria for CKD leaving 3,166 subjects classified as not having CKD. The median TMAO level in the CKD group was 7.9 µM and the median value in the non-CKD group was 3.4 µM (p<0.001). Comparing those CKD subjects with TMAO levels in the highest quartile to those subjects with TMAO levels in the lowest quartile revealed a 2.8-fold increase in all-cause mortality at 5 years (p<0.001). After adjusting for traditional cardiovascular risk factors, those CKD subjects in the highest quartile still had a 1.9-fold poorer 5-year survival (p<0.05). Stratifying the subjects according to median TMAO levels (7.9 µM) showed that higher TMAO levels conferred a 1.7-fold increase in risk for all-cause mortality (p<0.001), and remained significant even after adjusting for high sensitivity C-reactive protein and cystatin C levels. Elevated TMAO levels were associated with a higher 5-year mortality risk among subjects with either normal or elevated cystatin C levels.
In mouse studies, six weeks after adding either 1.0% choline or 0.12% TMAO to a chemically defined diet comparable to normal mouse chow (which contains 0.08 gm% choline) there was a significant increase in TMAO to levels seen in the human CKD subjects. The elevated TMAO levels were associated with significant increases in tubulointerstitial fibrosis and collagen deposition. The phosphorylation of Smad3 which regulates the pro-fibrotic TGF-β/Smad3 signaling pathway was also significantly increased. Moreover, the mice fed diets to raise TMAO levels showed significant increases in kidney injury marker-1 levels. TMAO levels were significantly correlated with collagen in the kidney, the ratio of phosphorylated Smad3 to total Smad3, and the expression of kidney injury marker-1 suggesting causality. Extending the feeding of choline or TMAO to 16 weeks resulted in a significant increase in serum cystatin C levels.
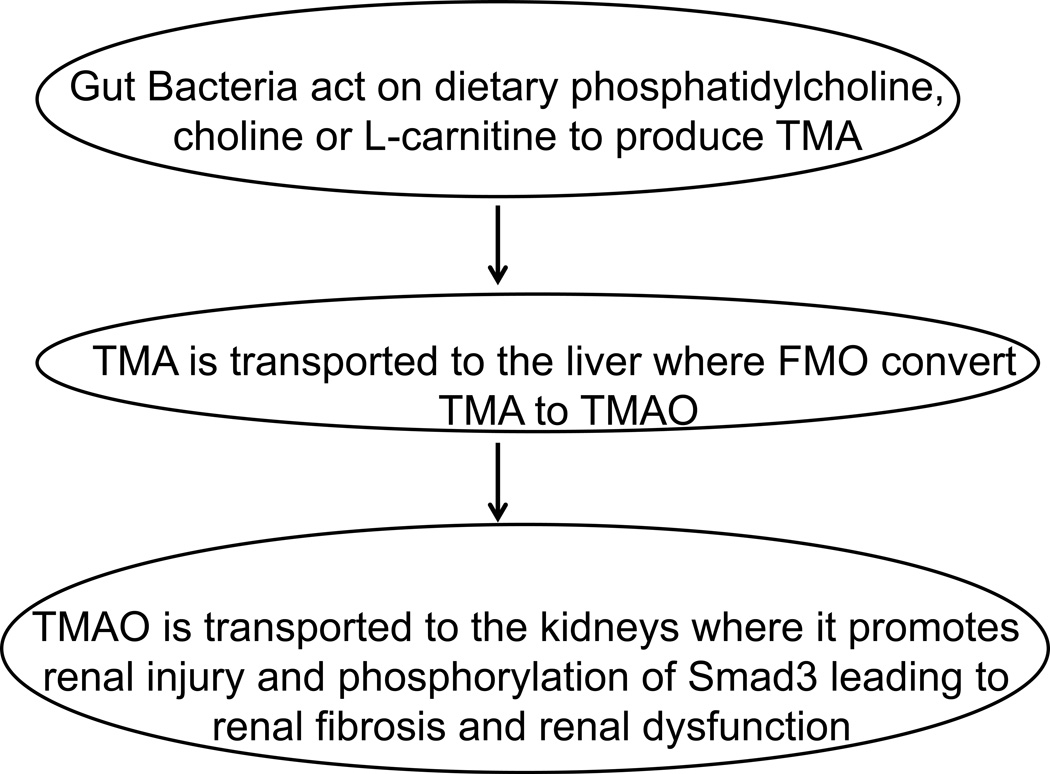
The sequence of events leading from the gut to the kidney is depicted schematically in the Figure. The discovery of this novel connection between the gut, the liver and the kidneys opens the possibility for new therapeutic approaches at each level. The diet may be targeted, or the gut flora may be manipulated. The metabolism of TMA in the liver may be altered, or the action of TMAO on the kidneys may be ameliorated. These discoveries by Hazen and colleagues present exciting new research opportunities for slowing or reversing the growing CKD epidemic.
Figure.
A schematic diagram of the formation of trimethylamine (TMA) in the gut, its conversion in the liver to trimethylamine N-oxide (TMAO) by members of the flavin monooxygenase (FMO) family, which results in TMAO-mediated renal injury, fibrosis and dysfunction.
Acknowledgments
Funding sources: USPHS grant HL-30568 and a Network Grant from the Leducq Foundation.
Footnotes
Disclosures: AMF is a principal and an officer in Bruin Pharma
REFERENCES
- 1.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, DuGar B, Feldstein AE, Britt EB, Fu X, Chung Y-M, Wu Y, Schauer P, Smith JD, Allayee H, Tang WHW, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–65. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang WHW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Z, Tang WHW, Buffa JA, Fu X, Britt EB, Koeth RA, Levison BS, Fan Y, Wu Y, Hazen SL. Prognostic value of choline and betaine depends on intestinal microbota-generated metabolite trimethlamine-N-oxide. Eur Heart J. 2014;35:904–910. doi: 10.1093/eurheartj/ehu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WHW, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–587. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koeth RA, Levison BS, Culley MK, Buffa JA, Wang Z, Gregory JC, Org E, Wu Y, Li L, Smith JD, Tang WHW, DiDonato JA, Lusis AJ, Hazen SL. γ-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab. 2014;20:799–812. doi: 10.1016/j.cmet.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang WHW, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM, Wu Y, Hazen SL. Prognositic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure. Refining the gut hypothesis. J Am Coll Cardiol. 2014;64:1908–1914. doi: 10.1016/j.jacc.2014.02.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cannon JA, McMurray JV. Gut feelings about heart failure. J Am Coll Cardiol. 2014;64:1915–1916. doi: 10.1016/j.jacc.2014.04.088. [DOI] [PubMed] [Google Scholar]
- 8.Bain MA, Faull R, Fornasini G, Milne RW, Evans AM. Accumulation of trimethylamine and trimethylamine-N-oxide in end-stage renal disease patients undergoing haemodialysis. Nephrol Dial Transplant. 2006;21:1300–1304. doi: 10.1093/ndt/gfk056. [DOI] [PubMed] [Google Scholar]
- 9.Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, Allayee H, Lee R, Graham M, Crooke R, Edwards PA, Hazen SL, Lusis AJ. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17:49–60. doi: 10.1016/j.cmet.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, Ni Z, Nguyen T-H, Andersen GL. Chronic kidney disease alters intestinal microbial flora. Kidney International. 2013;83:308–315. doi: 10.1038/ki.2012.345. [DOI] [PubMed] [Google Scholar]
- 11.Anders H-J, Andersen K, Stecher B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney International. 2013;83:1010–1016. doi: 10.1038/ki.2012.440. [DOI] [PubMed] [Google Scholar]
- 12.Ramezani A, Raj DS. The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol. 2014;25:657–670. doi: 10.1681/ASN.2013080905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhee EP, Clish CB, Ghorbani A, Larson MG, Elmariah S, McCabe E, Yang Q, Cheng S, Pierce K, Deik A, Souza AL, Farrell L, Domos C, Yeh RW, Palacios I, Rosenfield K, Vasan RS, Florez JC, Wang TJ, Fox CS, Gerszten RE. A combined epidemiologic and metabolomics approach improves CKD prediction. J Am Soc Nephrol. 2013;24:1330–1338. doi: 10.1681/ASN.2012101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang WHW, Wang Z, Kennedy DJ, Wu Y, Buffa J, Agatisa-Boyle B, Li XS, Levison BS, Hazen SL. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2014 doi: 10.1161/CIRCRESAHA.116.305360. XX; XXX-XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]