Unstructured Summary
We present a case of an adult patient with new-onset severe, idiopathic, protein-wasting enteropathy, in whom an extensive immunological workup was performed. We found a lack of dendritic cell (DC) subsets in the blood and bowel, as well as elevated circulating TGF-beta levels and decreased numbers of circulating FOXP3+ regulatory T cells with diminished CTLA4 expression. She failed to respond to glucocorticoids and infliximab, and instead developed a constellation of opportunistic infections, including CMV ileitis, Mucormycosis, and Pneumocystis carinii pneumonia, and ultimately passed away. While the cause of her lack of DC’s is unknown, this data suggests a key role for these cells in both regulating mucosal immunity and promoting effective cell-mediated immunity against pathogens in humans.
Keywords: “protein-losing enteropathy”, “plasmacytoid dendritic cell”, “myeloid dendritic cell”, FOXP3, “regulatory T cell”
Case Report
A previously healthy 58 year old female presented with 5 months of diarrhea and weight loss. Fecal studies were lactoferrin positive, but repeatedly negative for fat, C. difficile, giardia antigen, ova and parasites, or other enteric pathogens. Blood HIV ELISA, CMV PCR, and tuberculosis Quantiferon tests were all negative. Small bowel biopsies revealed mild villous blunting and intraepithelial lymphocytes, resembling celiac sprue, but sprue serologies were negative, and she failed to respond to a gluten-free diet. Loperamide, cholestyramine, mesalamine, budesonide, and prednisone were likewise ineffective. She started TPN for bowel rest and 5 mg/kg infliximab was administered. Diarrhea worsened within a week, accompanied by hypotension, dyspnea from pleural effusions, and anasarca with an albumin level of 1.2. She was admitted to the hospital and started on hydrocortisone, 100 mg IV TID, plus oral budesonide. Echocardiogram showed new apical ballooning with a 25–30% ejection fraction (previously 53%), so a second dose of 5 mg/kg infliximab was delayed until this spontaneously resolved, 3 weeks after the first dose. Severe diarrhea persisted, and 8–12 days after each infusion, her infliximab level was at or below the lower limit of detection (1.4 mcg/ml), with no detectable antibodies to infliximab. Therefore, a third dose of infliximab was administered at 12 mg/kg, two weeks after the second dose.
One week after the second dose, repeat EGD was grossly normal, with duodenal biopsies showing resolution of inflammation and regenerating villi, but severe diarrhea persisted. Capsule endoscopy showed diffuse villous blunting and severe ulceration of the ileum, so colonoscopy was repeated just before the third infliximab dose, revealing completely normal colon, but a severely ulcerated and inflamed ileum. Ileal biopsies showed normal T and B cell subsets on flow cytometry, to exclude enteropathy associated T cell lymphoma (EATL), but contained CMV inclusions (confirmed on IHC), so budesonide was discontinued, gancyclovir was started, and hydrocortisone taper was commenced. Repeat serum PCR showed 56,000 copies of CMV, which gradually decreased to a low of 41 copies over the following 3 weeks, without improvement in diarrhea.
The patient also developed a skin infection in her left ring finger shortly before her third infliximab dose, which grew fusarium, so voriconazole was started. Then, two weeks after her third infliximab dose, the patient developed a new cough. BAL revealed mucor, extended-spectrum beta lactamase-positive (ESBL+) Klebsiella pneumoniae, and Pneumocystis carinii pneumonia (PCP). IV bactrim, vancomycin, imipenem and amphotericin B were started, and both TPN and central venous access were discontinued. Attempted nasogastric feeding resulted in massive diarrhea, which markedly diminished when feeding was held. The patient was transiently intubated for respiratory distress, but pulmonary function gradually improved on the above regimen, and the patient expressed a desire for no reintubation. Nine weeks after her first infliximab, she had a sudden episode of acute hypoxia, possibly mucous plugging, which was reversed, but left her comatose with unequal pupils and a new bradycardia. The family decided to initiate comfort care, and the patient expired that evening. An autopsy was declined. A month later, anti-enterocyte antibody results came back negative.
Immunological Evaluation
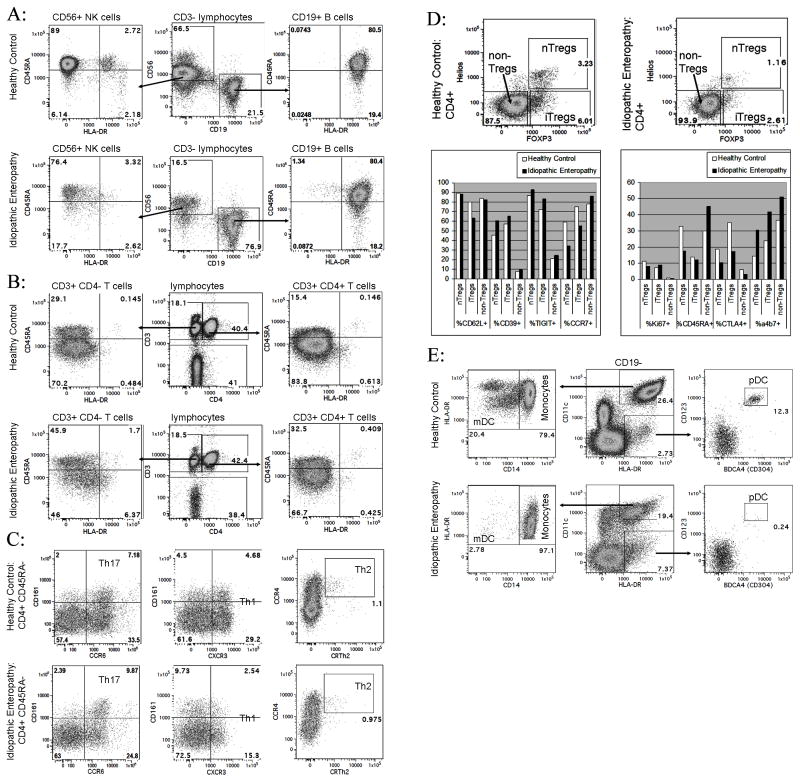
The patient and controls consented to participate in an Immune Mediated Disease registry and biorepository program, under the approval of the Institutional Review Board at Virginia Mason Medical Center. Blood drawn 1 week after the second infliximab dose revealed normal B cells, but diminished NK cells (figure 1a). Normal fractions of naïve and experienced T cells were seen (figure 1b), including presumed Th1 (CXCR3+), Th2 (CCR4+, CRTh2+), and Th17 cells (CCR6+, CD161+) (figure 1c). Both natural (Helios+ “nTregs”) and induced (Helios- “iTregs”) FOXP3+ regulatory T cells were decreased in frequency (figure 1d), but they (and FOXP3- T cells) had normal expression of the differentiation and proliferation markers (figure 1d). Expression of the inhibitory receptor CTLA4 was mildly diminished while the gut-homing integrin α4β7 was increased (figure 1d). Furthermore, copious CD4+ and FOXP3+ T cells were seen in intestinal biopsies, suggesting that a paucity of circulating Tregs could have been due to their mucosal sequestration.
Figure 1.
Blood leukocytes were collected from the case subject (“idiopathic enteropathy”) 3 weeks into her hospitalization, and from a healthy control. Cells were stained with panels of antibodies for the indicated markers (BD Biosciences, Beckman-Coulter, BioLegend, and eBioscience), and then analyzed on an LSR-II flow cytometer (BD Biosciences). A: Live CD3− lymphocytes gated to quantify CD56+ NK cells and CD19+ B cells. B: Live lymphocytes gated to quantify CD4+ and CD4− CD3+ T cells. C: CD45RA− CD4+ CD3+ T cells gated to estimate the frequency of Th1, Th2, and Th17 cells. D: Expression of FOXP3 and Helios by CD4+ CD3+ T cells. Expression of markers by cells within each of the indicated gates is graphed. E: Live CD19− cells gated to quantify monocytes, mDC, and pDC as shown. Similar results were also obtained with whole blood on the first day of hospital admission (data not shown).
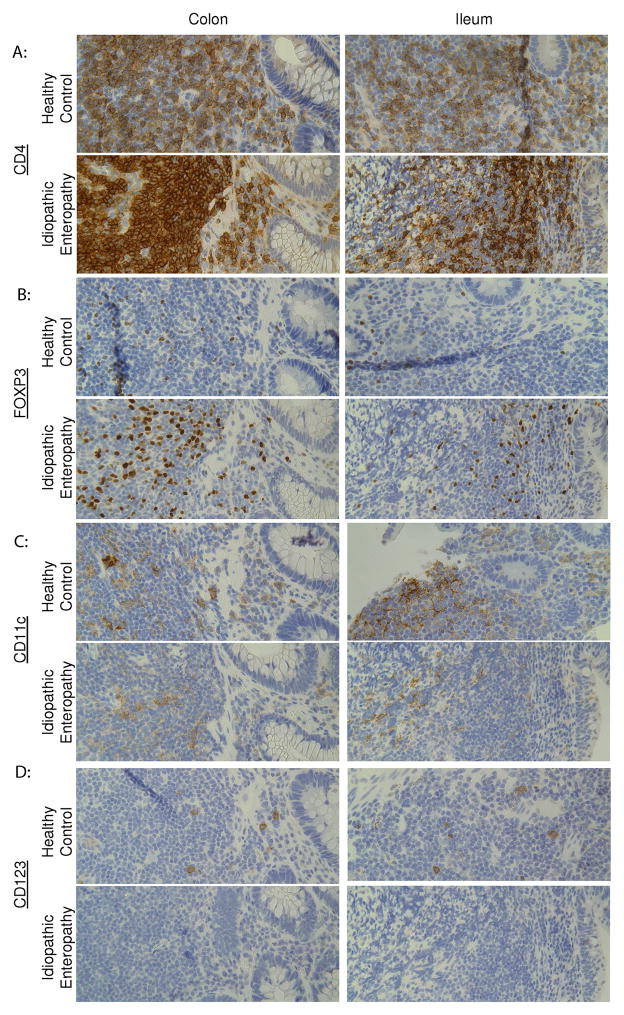
Blood showed relatively numbers of monocytes, but with diminished HLA-DR expression (figure 1e). However, almost no circulating myeloid (mDC) or plasmacytoid dendritic cells (pDC) were seen (figure 1e). IHC of biopsies showed weakly CD11c+ cells in the colon and small bowel mucosa (figure 2c), but no CD123+ cells (figure 2d), indicating that the absence of dendritic cells in the blood was not due to their sequestration at the site of inflammation.
Figure 2.
Serial sections of formalin-fixed, paraffin-embedded biopsies of the case subject’s colon and ileum from one month prior to hospital admission, as well as normal colon surgically resected from a patient with colon cancer and normal ileum surgically resected from a patient with FAP, were stained by immunohistochemistry for (A) CD4 (clone 4B12, Vector), (B) FOXP3 (clone 236A/E7, eBioscience), (C) CD11c (clone M1/70.15, Thermo Scientific), or (D) CD123 (clone 6H6, eBioscience). Similar results were seen with biopsies obtained 1 month after hospitalization (data not shown).
Luminex analyses (BioRad, Millipore) of serum drawn on the day of admission revealed levels of most cytokines (including TNF-α) below average relative to samples archived from healthy controls and IBD patients in the biorepository at the Benaroya Research Institute (table 1). Thus, rapid clearance of and nonresponse to infliximab was not simply due to extremely high circulating TNF-α levels saturating the drug. In contrast, levels of TGF-β1 and β2 were higher than any control cohorts (table 1). As TGF-β is associated with inhibition of the immune system, this may have contributed to her susceptibility to opportunistic infections. Serum also had decreased immunoglobin concentrations in all subsets (IgA 49, IgG 243, IgM 18, IgE 111), which paralleled hypoalbuminemia, and thus was presumed to be a nonspecific result of enteric protein loss, and not an intrinsic immunodeficiency.
Table 1.
| Conc (pg/ml): | Mean (+/− SD) | |||
|---|---|---|---|---|
| Normal (n=102) | Crohn’s (n=89) | UC (n=16) | Enteropathy Case | |
| IL-1β | 4.35 (+/− 8.05) | 4.19 (+/− 8.7) | 8.02 (+/− 24.55) | 0.52 |
| IL-2 | 12.58 (+/− 24.45) | 8.26 (+/− 16.41) | 8.25 (+/− 18.86) | 0.00 |
| IL-4 | 5.74 (+/− 8.49) | 6.58 (+/− 11.05) | 10.23 (+/− 22.07) | 2.33 |
| IL-5 | 5.98 (+/− 14.93) | 5.66 (+/− 10.49) | 8.79 (+/− 17.93) | 3.24 |
| IL-6 | 85.09 (+/− 112.88) | 58.25 (+/− 73.61) | 61.1 (+/− 73.33) | 5.67 |
| IL-7 | 87.76 (+/− 117.66) | 54.74 (+/− 71.21) | 65.71 (+/− 80.39) | 70.50 |
| IL-8 | 128.55 (+/− 557.9) | 38.98 (+/− 103.1) | 33.39 (+/− 50.49) | 24.07 |
| IL-10 | 11.4 (+/− 36.49) | 10.27 (+/− 33.79) | 4.53 (+/− 5.94) | 6.19 |
| IL-12(p70) | 18.53 (+/− 39.66) | 15.84 (+/− 29.1) | 19.99 (+/− 44.09) | 4.95 |
| IL-13 | 25.84 (+/− 42.19) | 21.86 (+/− 25.1) | 20.9 (+/− 19.8) | 30.69 |
| IL-17A | 2.89 (+/− 21.61) | 0.83 (+/− 2.61) | 0.36 (+/− 1.38) | 0.00 |
| G-CSF | 58.41 (+/− 81.28) | 46.41 (+/− 65.3) | 82.44 (+/− 151.53) | 8.06 |
| GM-CSF | 5.14 (+/− 10.57) | 4.61 (+/− 10.66) | 4.01 (+/− 11.36) | 0.00 |
| IFN-γ | 147.08 (+/− 217.29) | 124.71 (+/− 306.71) | 406.11 (+/− 736.56) | 91.09 |
| MCP-1(MCAF) | 69.15 (+/− 72.69) | 57.13 (+/− 45.35) | 50.92 (+/− 50.92) | 46.29 |
| MIP-1β | 11 (+/− 11.47) | 9.72 (+/− 10.7) | 10.3 (+/− 10.14) | 1.54 |
| TNF-α | 133.74 (+/− 218.63) | 92.48 (+/− 148.96) | 41.76 (+/− 43.37) | 5.68 |
| IL-9 | 3.41 (+/− 13.74) | 6.55 (+/− 22.82) | 8.22 (+/− 19.59) | 0.00 |
| IL-15 | 25.42 (+/− 39.66) | 15.12 (+/− 18.41) | 13.55 (+/− 20.13) | 0.00 |
| IFN-α2 | 81.01 (+/− 60.45) | 78.92 (+/− 59.41) | 99.64 (+/− 65.1) | 60.98 |
| TGF-β1 | 10,596.97 (+/− 8,115.59) | 11,675.1 (+/− 8,782.04) | 5,997.8 (+/− 2,951.58) | 25,809.31 |
| TGF-β2 | 97.94 (+/− 36.57) | 87.82 (+/− 34.11) | 88.43 (+/− 31.73) | 153.40 |
| TGF-β3 | 8.36 (+/− 6.48) | 6.99 (+/− 7.51) | 15.17 (+/− 2.16) | 0.00 |
| TSLP | 2.03 (+/− 6.49) | 184.23 (+/− 1044.26) | 0.38 (+/− 0.82) | 0.00 |
| IL-21 | 0.11 (+/− 0.51) | 0.55 (+/− 2.63) | 0.17 (+/− 0.68) | 0.00 |
| IL-22 | 3.05 (+/− 13.66) | 5.89 (+/− 29.62) | 0 (+/− 0) | 0.00 |
| IL-23 | 0.08 (+/− 0.36) | 1.21 (+/− 2.6) | 0.77 (+/− 1.92) | 0.00 |
Discussion
The presented case represents severe, refractory, acquired enteritis, with no features of Crohn’s disease, autoimmune enteropathy, celiac sprue, or other food intolerance. Although defects in regulatory T cells (Tregs) can lead to severe enteritis [1], and this case showed diminished circulating Tregs, their phenotype was fairly normal, and they were enriched in the inflamed mucosa.
In contrast, pDC were absent in both her blood and bowel. pDC promote antiviral immunity, through potent type-I interferon secretion [2], but may also have immunoregulatory roles [3]. mDC were also absent in her blood, but CD11c+ cells, which are normally predominantly mDC, were evident in her bowel. These cells could also be the CD14+ cells that were still present in her blood, as a CD14+ monocytic population has been described in the mucosa of Crohn’s patients and suggested to contribute to their inflammatory enteropathy[4,5].
Although mucor, Klebsiella, and fusarium are seen in neutropenic individuals, this case actually demonstrated a granulocytosis. Likewise, CMV, PCP are seen in the setting of T cell immunodeficiency, which was not evident in her blood or biopsies. We therefore hypothesize that her adaptive immunity to opportunistic pathogens may have been compromised by a lack of effective antigen-presenting cells (APC). As DC are potent and dynamic APC for T cells, their absence would limit T cell activation. Furthermore, decreased levels of the antigen-presenting molecule HLA-DR on peripheral CD14+ cells would likewise compromise their ability to stimulate immunity.
The opportunistic infections of this case may also have resulted from pharmacologic immunosuppression, particularly via glucocorticoids. An escalated (12 mg/kg), accelerated (week 5) third dose of infliximab could have also increased risk for opportunistic infection. However, infliximab levels fell to or below the limits of detection rapidly after the first two infusions, so any persisting immunodeficiency would presumably result from a durable effect of transient drug on the immune system, rather than ongoing blockade of TNF-α. It is possible that her rapid infliximab clearance was due to an ATI not detected through conventional testing, as the patient also developed HIT antibodies to heparin, perhaps representing a proclivity to mount humoral immunity against pharmaceuticals. This could in turn be a manifestation of whatever hyperimmune disorder caused her enteritis.
Death is an unusual direct consequence of chronic enteritis, but this case demonstrates how it can be the inevitable conclusion of severe, refractory disease, particularly when treatment options are limited by opportunistic complications.
Acknowledgments
Support: This work was funded through a grant from the NIDDK/NIH (5 K08 DK081659), the NIH/NIAID (5 U19 AI050864), and internal resources at the Benaroya Research Institute.
Funding: We wish to thank Melissa Peda for patient consent and phlebotomy, Mary Beauchamps for assistance with histology preparation, and K. Aru Arumuganathan for assistance with flow cytometry.
Footnotes
Notifications of Conflicts of Interest and Ethical Adherence: None of the authors report any conflicts of interest relevant to the submitted manuscript. All research was performed on consenting subjects in accordance with the Declaration of Helsinki, and was approved by an Institutional Review Board.
Contributor Information
Janice Chen, Translational Research Program, Benaroya Research Institute, Seattle, WA, U.S.A.
Richard A. Kozarek, Gastroenterology Division and Digestive Disease Institute, Virginia Mason Medical Center, Seattle, WA, U.S.A.
References
- 1.Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutation of FOXP3. Nature Genetics. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 2.Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V. Plasmacytoid dendritic cells: recent progress and open questions. Annu Rev Immunol. 2011 Apr 23;29:163–183. doi: 10.1146/annurev-immunol-031210-101345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goubier A, Dubois B, Gheit H, et al. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008 Sep 19;29:464–475. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamada N, Hisamatsu T, Okamoto S, et al. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J Clin Invest. 2008;118:2269–2280. doi: 10.1172/JCI34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamada N, Hisamatsu T, Honda H, et al. Human CD14+ macrophages in intestinal lamina propria exhibit potent antigen-presenting ability. J Immunol. 2009 Aug 1;183:1724–1731. doi: 10.4049/jimmunol.0804369. [DOI] [PubMed] [Google Scholar]