Abstract
Human Papillomavirus (HPV) infection is commonly found in the genital tract of men and women with or without any clinical lesion. The association of HPV DNA with several different ano-genital cancers other than cervical has been reported for the vulva, vagina, anus and penis. HPV DNA has also been identified in head and neck cancers in the oral cavity, the oropharynx and the larynx in both sexes. In men, 80–85% of anal cancers and close to 50% of penile cancers are associated with HPV infection. In women, HPV DNA is prevalent in 36–40 % vulvar cancer cases and close to 90 % of vaginal cancers. There is limited data available on the natural history and HPV-related diseases in the genital tract in men, although studies are ongoing. Efficacy of HPV vaccines in the prevention of HPV infection and disease among men also remains unknown. Among HPV DNA positive ano-genital cancer cases, HPV-16 is the most frequently found followed distantly by HPV-18. In benign HPV-related diseases such as genital warts or recurrent respiratory papillomatosis HPV-6 and 11, the two most frequent non-oncogenic types, are the predominant types detected. Oncogenic types are rarely detected. In this article we summarize and review studies describing the natural history of HPV infections among men and its impact on HPV related disease in women. We summarize the evidence linking HPV in the epidemiology and etiology of cancers of the vulva, vagina, anus and oropharynx and present recent estimates of the burden of and HPV type distribution in genital warts and in cases of HPV infection of the airways.
Keywords: cancer, HPV, men, women, infection, genital warts, respiratory papillomatosis, anogenital cancer
1.0 INTRODUCTION
Among men and women, cancers of the ano-genital tract and their precursor lesions have been strongly linked to infection with sexually transmitted Human Papillomavirus (HPV) [1]. In men, HPV infection has been strongly associated with anal cancer and is associated with approximately 85% of the anal squamous cell cancers that occur annually worldwide. Likewise, approximately 50% of cancers of the penis, between 33 and 72% of oropharyngeal cancers, and 10% of cancers of the larynx may be attributed to HPV infection (Table 1) [Table 5: a1–a5]. Studies are underway to estimate the proportion of non-melanoma skin cancer also attributable to infections with the cutaneous branches of the HPV phylogeny.
Table 1.
Epidemiological traits of HPV and cancers other than cervical.
| Site and histological type |
Incidence (per 100.000) |
HPV DNA prevalence range (%) |
HPV-16 among HPV+ (%) |
|---|---|---|---|
| VULVA | 0.0 – 3.5 | ||
| VIN3 | 72 – 100 | 65 – 93 | |
| Vulva warty-basaloid | 75 – 100 | NA | |
| Vulva squamous | 2 – 23 | 71 | |
| VAGINA | 0.0 – 1.5 | ||
| VAIN | 82–100 | 67 – 75 | |
| Vagina squamous | 64 – 91 | 70 – 88 | |
| PENIS | 0.0 – 3.7 | ||
| PIN | 90 | 41 | |
| Penis warty-basaloid | 46– 100 | 60 – 88 | |
| Penis squamous verrucous | 31 – 35 | 60 – 75 | |
| ANUS | 0.1 – 2.8 males / 0.0 – 2.2 females | ||
| AIN | NA | NA | |
| Anus squamous | >80 | 76 | |
| OROPHARYNX & TONSILS | 0.3–21.5 males/0.0–2.8 females | 33 – 72 | 72 – 87 |
HPV+: subjects positive to HPV DNA.
Oropharynx incidence includes codes: C09 - Tonsil, C10 - other oropharynx, C12 and C13 – hypopharynx.
AIN: Anal intraepithelial neoplasia; PIN: Penile intraepithelial neoplasia; VAIN: Vaginal intraepithelial neoplasia; VIN: Vulvar intraepithelial neoplasia.
Sources of data: [Table 5: a1–a5].
Table 5.
Summary of epidemiological studies and reviews on HPV-related cancers other than cervical, in men and in benign conditions.
| Code | 1st author, Journal Year; Vol.: pages |
|---|---|
| References from Table 1: Epidemiological traits of HPV and cancers other than cervical | |
| a1 | IARC. Cancer Incidence in Five Continents, Vol. IX. IARC Scientific Publications No.160, Lyon, IARC. 2007 |
| a2 | IARC Monographs on the evaluation of carcinogenic risk to humans. Human Papillomavirus. Vol. 90, Lyon, IARC. 2007 |
| a3 | Lont AP et al, Int J Cancer. 2006;119(5):1078-81 |
| a4 | D’Souza G et al, N Engl J Med. 2007;356(19):1944-56 |
| a5 | Kreimer AR et al, Cancer Epidemiol Biomarkers Prev. 2005;14(2):467-75 |
| References from Table 2: Risk of vulvar and vaginal cancer associated with HPV-16,-18, -33 antibodies: case-control studies of type-specific HPV seropositivity and pre-cancer and cancer | |
| b1 | Carter JJ et al, Cancer Res. 2001;61(5):1934-40 |
| b2 | Madeleine MM et al, J Natl Cancer Inst. 1997;15;89(20):1516-23 |
| b3 | Hildesheim A et al, Obstet Gynecol. 1997;90(5):748-54 |
| b4 | Sun Y et al, Gynecol Oncol. 1996;63(2):200-3 |
| b5 | Daling JR et al, Gynecol Oncol. 2002;84(2):263-70 |
| b6 | Bjorge T et al, BMJ 1997;315:646–9 |
| References from Table 3: HPV DNA prevalence in ano-genital warts | |
| c1 | Grce M et al, Anticancer Res. 2000;20(3B):2097-102 |
| c2 | Skerlev M et al, Clin Dermatol. 2002;20(2):173-8 |
| c3 | Labropoulou V et al, J Med Virol. 1994;42(3):259-63 |
| c4 | Potocnik M et al, Acta Dermatovenerol Alp Panonica Adriat. 2007;16(3):91-6, 98 |
| c5 | Luk CH et al, Abstract PS8-01. 24th International Papillomavirus Conference, Beijing, China, 2007. Abstracts book |
| c6 | Greer CE et al, J Clin Microbiol. 1995;33(8):2058-63 |
| c7 | De Marco F et al, J Exp Clin Cancer Res. 2001;20(3):377-83 |
| c8 | Brown DR et al, J Clin Microbiol. 1999;37(10):3316-22 |
| C9 | Sanclemente G et al, J Eur Acad Dermatol Venereol. 2007;21(8):1054-60 |
| c10 | Cavazza Porro ME et al, Abstract PS12-04. 24th International Papillomavirus Conference, Beijing, China, November 2007. Abstracts book |
| References from Table 4. HPV DNA type distribution in subjects with recurrent respiratory papillomatosis | |
| d1 | Gomez MA 1995;553: 213–217 et al., 1995 |
| d2 | Levi JE et al, Am J Pathol. 1989;135(6):1179-84 |
| d3 | Draganov P et al, Int J Pediatr Otorhinolaryngol. 2006;70(3):469-73 |
| d4 | Fu M et al, Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2000;14(7):317-9 |
| d5 | Sun AK, Zhonghua Er Bi Yan Hou Ke Za Zhi. 1993;28(5):297-9, 315 |
| d6 | Lindeberg J et al, Clin Otolaryngol Allied Sci. 1990;15(4):367-71 |
| d7 | Dickens P et al, J Pathol. 1991;165(3):243-6 |
| d8 | Velyvyte D et al, Medicina (Kaunas). 2002;38(5):499–504 |
| d9 | Peñaloza-Plascencia M et al, Arch Otolaryngol Head Neck Surg. 2000;126(9):1119-23 |
| d10 | Obchinnikov IuM et al, Vestn Otorinolaringol. 2004;(3):29-33. |
| d11 | Padayachee A et al, Int J Pediatr Otorhinolaryngol. 1993;26(2):141-7. |
| d12 | Szeps M et al, J Gen Virol. 2005;86(Pt 6):1695-702. |
| d13 | Ushikai M et al, Jpn J Cancer Res. 1994;85(7):699–703. |
| d14 | Maloney EM et al, Arch Otolaryngol Head Neck Surg. 2006;132(7):711-5. |
| d15 | Rabah R et al, Pediatr Dev Pathol. 2001;4(1):68–72. |
| d16 | Pou AM et al, Ann Otol Rhinol Laryngol. 1995;104(10 Pt 1):758–62. |
| d17 | Bello de Afford M et al, RFM. 2001, vol.24, no.1, p.62–65 |
There is a growing body of literature focusing on HPV infection and risk of anal cancer among homosexual men. These studies have documented the rate of acquisition and clearance of anal HPV infections, and their association with squamous anal intraepithelial lesions (AIN). Anal cancer and cervical cancer share a common feature which is a strong association with HPV infection [2]. Collectively, several human immunodeficiency virus (HIV)/HPV cohort studies have shown that the histology of anal dysplasia is similar to cervical squamous intraepithelial lesions (SIL). In these studies, anal HPV infection has been shown to be associated with a history of receptive anal sex, as well as testing HIV-positive [3–6].
In recent years, there has been increased interest in understanding the burden of HPV infection among predominantly heterosexual men. Much of this interest has been focused on the role of men in the transmission of HPV to women and its contribution to the burden of cervical cancer [7–9]. As such, the focus of heterosexual male HPV infection is at the anatomic sites that would contribute to a transmission event, primarily the genital regions of the penile skin and scrotum. More recently there has been an interest in understanding male HPV infection as it relates to disease burden in men, such as cancers of the oral cavity and head and neck region (reviewed in [10]).
2.0 NATURAL HISTORY OF THE HPV INFECTIONS IN EXTERNAL MALE GENITALIA
Early studies of male HPV infection of the penis used visual inspection with aceto-whitening, with or without a magnifying lens, as a diagnostic marker for HPV infection. While HPV is significantly associated with penile aceto-white lesions (e.g., papular, spiked, and flat whitish or greyish epithelial lesions), many other benign genital conditions are also associated with these lesions, resulting in poor specificity of peniscopy for detection of HPV in male genitalia. To accurately assess HPV infection in men, exfoliated cytology or biopsy specimens and molecular techniques (e.g., polymerase chain reaction (PCR)) must be employed. Among studies that utilized molecular methods for HPV DNA detection, reported rates of HPV infection in men vary widely. This variation has been related to sampling of different anatomic sites, single versus multiple sites, use of different analytical methods, and the selection criteria of the populations studied. Few studies assessed the HPV prevalence using multiple specimens from different anatomic sites, now understood to be relevant in estimating HPV prevalence in males [11]. Poor cellularity and beta-globin negative inadequate samples for HPV detection has been identified as a limitation of studies in men.
2.1 Studies using HPV DNA detection and typing
The information to date regarding penile HPV infection is primarily derived from studies that examined husbands of female cervical cancer cases, cross-sectional studies of selected populations such as individuals with sexually transmitted infections (STI) and military recruits and small prospective studies.
HPV infection in the genital tract has been detected in up to 73% of healthy men. Among Finnish army recruits HPV infection was detected in 16.5% using PCR. Among young Mexican men where the coronal sulcus of the penis and the distal urethra were sampled, 42.7% were HPV-positive. Among STI clinic attendees, the prevalence of HPV has varied from 30.5% among Swedish men to 48% of men from Greenland and 49% of Danish men. Among 34 men whose partners were diagnosed with cervical dysplasia, HPV was detected in 64% of the ones whose partner had cervical intraepithelial neoplasia (CIN) grade 1, 16% of those whose partner had CIN2 and 50% among those whose partner had CIN3. Finally, in a British study of 43 couples, 69% of penile biopsy samples were HPV-positive (reviewed in [12]).
In more recent published reports from the United States of America (USA) where HPV DNA detection was systematically evaluated at several anatomic sites in the genital tract of men, 51.2% of individuals were positive for at least one known oncogenic or non-oncogenic HPV type and an additional 14.3% were positive for an unclassified HPV infection [13]. Among asymptomatic heterosexual men the penile shaft, the coronal sulcus/glans penis (including prepuce in uncircumcised men), and the scrotum are the sites that contribute to more than 95% of genital HPV infection detected [11]. Similar findings were reported by Weaver BA et al. in a study conducted among university male students [14]. Results from multi site sampling studies indicate that both the semen and the urethra samples contribute little to the estimate of HPV prevalence. As a result, current recommendations for estimating HPV prevalence among men include sampling the penile shaft, the glans, the coronal sulcus and the scrotum. In addition to these genital sites, collection of a specimen from the anal canal is also recommended in the context of the research projects [11]. Several different methods of male genital sampling have been utilized, two of which have been shown to result in reproducible and adequate samples for HPV DNA detection in men. The method of Weaver BA et al. includes abrading the genital skin prior to sampling with a Dacron swab and collection of the exfoliated cells in standard transport medium (STM). The method of Giuliano AR et al. utilizes direct sampling of the genital epithelium with a saline pre-wetted Dacron swab followed by collection in STM [11,14,15].
Few studies have determined the prevalence of specific types of HPV or the correlates of HPV infections in men [12,13]. Study results from an ongoing international prospective study of HPV infection in men found HPV DNA in 50.5% of them. The proportion of low-risk types was 20.7% and unclassified infections were found in an additional 14.7%. [16].
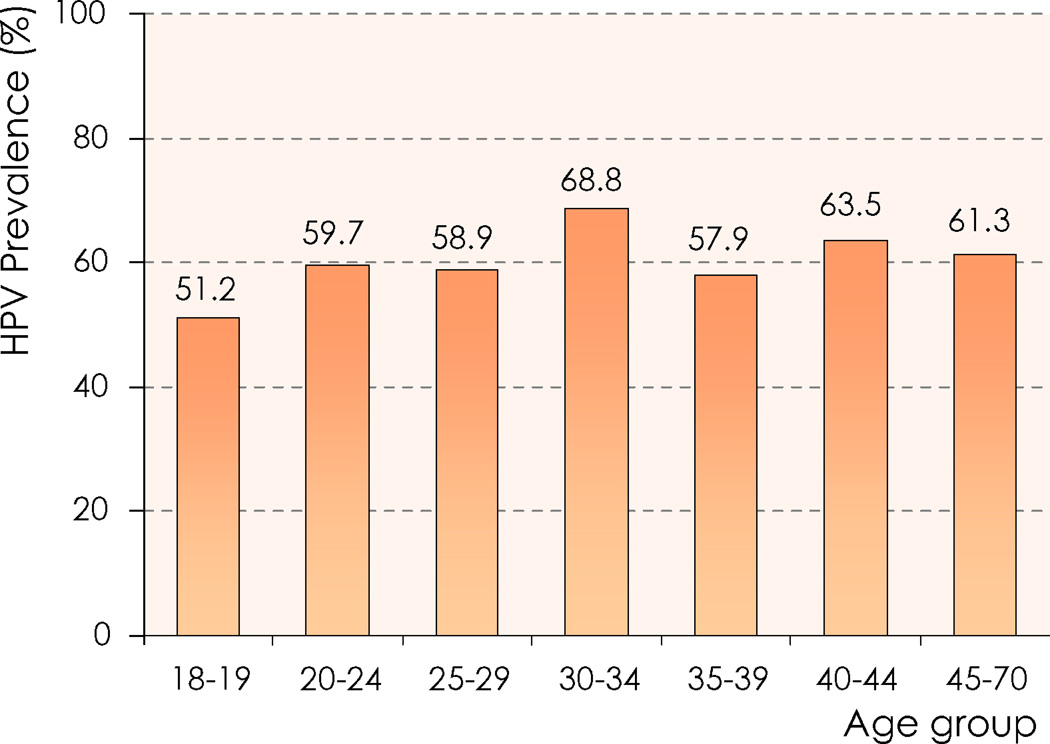
As correlates of HPV infections in men, a consistent positive association has been observed between measures of sexual history, including lifetime and recent number of sexual partners and sexual frequency and HPV detection. Circumcision has been consistently (five of seven studies) associated with reduced detection of HPV infection in men [8,12,17,18]. Less consistently (in two of six studies) condom use has been associated with reduced risk of HPV detection in men [12,13]. Most studies find no association between age and HPV detection in men (Figure 1)[16,19].
Figure 1. HPV prevalence (any type) by age group among men residing in Brazil, Mexico, and the USA and participating in the HIM Study.
HIM Study: Natural History of HPV Infection in Men. Source of data: Adapted from [19].
Like other STIs, HPV may be transmitted more readily from men to women than from women to men. In a small study comparing HPV status in the cervix and semen of heterosexual sex partners, Kyo S. et al. demonstrated that 75% of women whose male partners were HPV-positive had HPV DNA in their cervix, while only 39% of the men whose partners were HPV-positive carried HPV DNA in their semen [20]. Campion MJ et al. examined women whose sexual partners had penile condyloma, and found that 76% of them had genital HPV signs including 36% with abnormal cervical cytology and 27.7% with cervical HPV DNA detected by hybridization [21].
Small prospective studies have reported rates of HPV acquisition and clearance in sexually active men, however, these have not yielded precise estimates of HPV incidence, duration of infections and antibody response to HPV infections in men (reviewed in [12]). In a recently completed prospective study of heterosexual men ages 18–44 years, the probability of acquiring an HPV infection was 0.29 per year, an estimate similar to that reported for young males attending university [22] and females of a similar age range [23]. Unlike what has been observed among women, no clear age pattern is detected in rates of HPV acquisition in men [16].
HPV infections may be less likely to persist in men than in women. In a Dutch study, persistent infection (defined as detection of HPV DNA of a specific type at two consecutive visits over a one year period) was observed more frequently in women than in men (odds ratio (OR): 3.88). Twenty percent of HPV-positive women and 6% of men manifested persistent infections, while short-lasting infections with clearance of HPV were found in 49% of infected men and 31% of infected women [24]. Overall, shorter estimates of viral persistency have been observed for oncogenic infections in men, compared to women. Among men, the clearance rate of oncogenic and non-oncogenic infection appear similar with a global estimate of 75% of approximately HPV DNA clearance at one year [18].
2.2 Studies that used HPV antibody presence in men
Few studies have been conducted to understand the HPV antibody responses in men [25,26]. In a seroprevalence survey, antibodies to HPV type -16 virus-like particles was significantly lower in men than in women from the same geographic area [25]. In a cross-sectional study among bisexual and homosexual men, the presence of HPV-6 capsid antibodies correlated with the detection of anal HPV-6/11 DNA and anal warts. However, the detection of HPV-16 capsid antibodies did not correlate with the detection of HPV-16 DNA in tissue [27]. In another cross-sectional study conducted among high-risk men and women attending an STI clinic in New Orleans, men were less likely to be HPV type-6/11 and 16 antibody positive compared with women. Factors associated with presence of HPV antibodies were different for HPV-6/11 and HPV-16. In multivariate models, the factors significantly associated with HPV-16 antibody status in men were increasing age and a history of syphilis. Furthermore, ten or more sexual partners in the past 12 months, a history of genital warts and herpes simplex virus type 2 positivity (HSV-2) were significantly associated with HPV-6/11 antibody status.
There is a need for prospectively conducted studies among men to answer basic questions related to the incidence and persistence of HPV infection and the kinetics of the humoral response to genital HPV infection in men. These studies are currently underway internationally by several different research groups. It is unclear what the progression rate of genital HPV infection to disease is in men. Currently there are no clinically licensed tests for HPV detection among men and therefore there are no recommendations for HPV screening or for treatment of HPV infection among men.
3.0 THE IMPLICATIONS OF HPV IN OTHER CANCERS IN MEN AND WOMEN
3.1. Female genital cancers other than cervical
Screening efforts have traditionally focused attention to cervical pre-cancerous lesions. In spite of the complex logistics of repeated gynecological examinations in hundreds of thousands of women, the rates of vulvar and vaginal cancers have not benefited substantially by ancillary early diagnosis and treatment. These are rare cancers for which formal epidemiological studies are scarce. However, there is growing evidence that HPV is implicated in a variable fraction of them and HPV vaccine trials have shown that prevention of infection of HPV-16 and 18 results in an almost complete reduction of the precursor lesions, vulvar intraepithelial neoplasia (VIN) grade 2/3 and vaginal intraepithelial neoplasia (VAIN) grade 2/3 in a relatively young population (with a mean age at diagnosis of less than 55 years of age). The HPV vaccination clinical trials with the quadrivalent vaccine (Gardasil® Merck & Co., Whitehouse Station, NJ USA) have already shown an efficacy of 100% (95% confidence interval (CI): 72–100) against HPV-16 or HPV- 18 related-VIN2/3 or VAIN2/3 among the per-protocol susceptible population. No cases were observed among the 7,811 women of the vaccinated cohort versus 15 cases among the 7,785 unvaccinated cohort [28].
3.2 Vulvar cancer and preneoplastic lesions
3.2.1 Natural history and epidemiology of vulvar cancer and preneoplastic lesions
Vulvar cancer is uncommon and affects approximately 26,800 women worldwide, accounting for approximately 3% of all gynecologic cancers in 2002 [29]. Squamous cell carcinomas constitute the most common (90%) histologic type of primary vulvar cancer, followed by melanoma, Bartholin gland carcinoma, basal cell carcinoma, verrucous carcinoma, and Paget’s disease [30].
Approximately 60% of all cases of vulvar cancer worldwide occur in developed countries [29,30], signaling the limited impact of cervical cancer screening programs in the prevention of vulvar and vaginal cancers. Vulvar cancer is more common among older women and approximately 66% of patients are diagnosed at ages 70 and older. In the USA, higher incidence rates are observed among whites (2,3 per 100,000 women), intermediate rates among African-Americans and Hispanics (1.7–1,9 per 100,000 women), and lower rates among Asian/Pacific Islanders (0.7 per 100,000 women) [31].
VIN lesions and its classification remains controversial [32–35]. There are currently three grading systems for VIN: 1) the World Health Organization (WHO) three grade system of VIN1-3; 2) a Bethesda-like system of low- and high- grade vulvar intraepithelial lesions; and 3) the revised International Society for the Study of Vulvovaginal Disease (ISSVD) classification, which has no grading of VIN, introduced in 2004 [36,37]. By 1960, the body of clinical evidence had firmly established VIN3 as a precursor of invasive vulvar cancer. Studies of VIN3 patients identified: 1) a decreasing trend in the age at diagnosis of vulvar lesions; 2) recurrence rates strongly related to the presence of positive margins and treatment procedures; 3) low progression rates to invasive carcinoma (6%); 4) higher risk of progression among women (40 years and older); and 5) mean time to progression to invasive cancer of approximately four years (range: 1.1 – 7.3 years). However, coinciding with the increasing frequency of the condition from the 1970s, there has been a significant shift in opinion with respect to the neoplastic potential of the condition recognizing that the ’low progression rates’ do not reflect the real natural history of VIN but rather the outcome of treated patients included in these studies [30,32,33,38].
The ISSVD has recommended the elimination of the term VIN1 lesion as part of the spectrum of vulvar neoplasia and the use of the term VIN only for those lesions recognized as immediate precursors of invasive squamous cell carcinomas (VIN3 or in situ lesions). These recommendations are based on the lack of morphologic or biologic evidence to support the continuum of VIN1-3 lesions, the lack of reproducibility of the histological diagnosis of VIN1, and the lack of data supporting VIN1 as risk factors for invasive cell squamous carcinomas. Furthermore, the predominance of low-risk HPV types in VIN1 lesions supports the notion that these lesions are the (acute) result of a viral infection and not a genuine precancerous lesions [35].
Two recent case series study further document this issue based on the HPV profile of VIN lesions [34,39]. Srodon M. et al. observed a 67% prevalence of low-risk HPV types and 42% prevalence of high-risk types in VIN1 lesions, but only 6% of the HPV DNA positive specimens were so for HPV-16 [34]. By contrast, 100% of VIN3 lesions were high-risk HPV type positive and most of them (91%) were HPV-16 positive. These findings have prompted the suggestion of three different possible explanations: 1) VIN1 lesions might be distinct lesions without progressing capacity to VIN3; 2) only those VIN 1 lesions associated to HPV-16 might progress to VIN3; and 3) only a subset of VIN1 lesions might have a rapid progression to VIN3 lesions [34]. Skapa P. et al. observed that although VIN1 was a rare disorder in their series, it was associated with high-risk HPV infection [39].
The incidence of VIN has been reported to increase at a fast rate during the last decade, whereas the incidence of invasive vulvar cancer has increased at a lower rate or remained stable. In the USA the incidence of carcinoma in situ (VIN3) of the vulva increased by 411% between 1973 and 2000, whereas the incidence of invasive vulvar cancer increased by only 20% during this period [40]. This increasing trend has been observed in other developed countries including the United Kingdom (UK), Switzerland, Norway, New Zealand and Austria [40–43]. The increase has been reported primarily among women aged less than 50 years and it has been linked to a similar increase in the precursor VIN lesions in young women. In the UK, the proportion of cases of invasive vulvar cancer in women under 50 years has risen from 6% in 1975 to 11% in 2004 amounting to 0.4% of all cancers [43].
The overall five-year relative survival rate for vulvar cancer in the USA for the period 1988–2001 was estimated at 76.4% [44]. In the UK, the five-year survival rate for vulvar and vaginal cancers combined was 58% and it has increased over time [43].
In the early 1990’s, two different histological patterns of vulvar cancer with two distinct etiologic pathways were described: 1) the basaloid/warty, and 2) the keratinizing types [45]. Basaloid/warty lesions are more common in young women, are frequently found adjacent to VIN, are very often associated with HPV DNA detection (75 – 100%), and have a similar risk factor profile as cervical cancer [45,46]. Keratinizing vulvar carcinomas represent the majority of the vulvar lesions (60 + %). These lesions develop from non-HPV related chronic vulvar dermatoses especially lichen sclerosus and/or squamous hyperplasia, their immediate cancer precursor lesion is differentiated VIN, they occur more often in older women, and are rarely associated with HPV (2 – 23%) or to any of the other risk factors typical of cervical cancer [36,45–47].
Most epidemiologic studies of HPV and vulvar cancer and VIN have relied on serologic assays for a limited number of HPV types, mainly HPV-16. These studies have consistently shown that HPV antibody positive women are at higher risk of vulvar neoplasia compared with HPV antibody negative women, had a stronger association with vulvar cancer in situ (CIS) than with invasive vulvar carcinoma, and had a different association profile between HPV and basaloid/warty and keratinizing carcinomas (Table 2) [Table 5: b1–b6]. Results from studies in which HPV DNA testing has been conducted are consistent with the findings from seroprevalence studies. Higher HPV DNA prevalence has been observed in pre-invasive than invasive vulvar lesions and HPV-16 has been the most frequently type detected in these lesions [1,48,49]. In the case series included in the recently published International Agency for Research on Cancer (IARC) monograph (data published up to 2005), the HPV DNA positivity has ranged from 72 to 100% for VIN3 lesions and from 27.3 to 100% for vulvar cancer (3.9 – 6.3% in keratinizing vulvar cancers), HPV-16 was the most common type (65 – 93% in VIN and 71% for vulvar cancer) followed by HPV- 18. Other types when tested for were detected in a very small proportion of cases (Table 1) [1, Table 5: a1–a5]. Similarly, results from the meta-analysis by Nascimento C. et al. show a prevalence of HPV DNA of 76% for VIN lesions and 36% for vulvar cancer [48].
Table 2.
Risk of vulvar and vaginal cancer associated with HPV-16, 18, 33 antibodies: case-control studies of type-specific HPV seropositivity and pre-cancer and cancer.
| Site and lesion | Serology | Reference code (see Table 5) |
|||||
|---|---|---|---|---|---|---|---|
| HPV-16 | HPV-18 | HPV-33 | |||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| VULVA | |||||||
| Preneoplastic vulvar lesions | 4.5 | (3.1 – 6.4) | 3.0 | (0.9 – 10.2) | - | - | [Table 5: b1] |
| VIN3/Vulvar in situ | 3.6 | (2.6 – 4.8) | 0.9 | (0.6 – 1.5) | - | - | [Table 5: b2] |
| 13.4 | (3.9 – 46.5) | - | - | - | - | [Table 5: b3] | |
| VIN not other specified | 5.4 | (1.7 – 18.0) | - | - | - | - | [Table 5: b4] |
| Invasive vulvar cancer | 2.9 | (0.9 – 8.7) | - | - | - | - | [Table 5: b3] |
| 2.8 | (1.7 – 4.7) | 1.2 | (0.5 – 2.7) | - | - | [Table 5: b2] | |
| 3.7 | (1.5 – 9.1) | - | - | - | - | [Table 5: b1] | |
| Warty-basaloid | 3.8 | (0.8 – 18.9) | - | - | - | - | [Table 5: b3] |
| 4.5 | (1.2 – 16.0) | - | - | - | - | [Table 5: b4] | |
| Keratinising squamous | 1.6 | (0.4 – 7.4) | - | - | - | - | [Table 5: b3] |
| 1.3 | (0.3 – 4.9) | - | - | - | - | [Table 5: b4] | |
| Vulvar neoplasia (both pre and invasive) | 4.4 | (3.1 – 6.2) | 3.8 | (1.2 – 11.7) | - | - | [Table 5: b1] |
| 4.5 | (3.0 – 6.8) | - | - | - | - | [Table 5: b2] | |
| 5.3 | (2.5 – 11.1) | - | - | - | - | [Table 5: b3] | |
| VAGINA | |||||||
| Preneoplastic vaginal lesions | 13.1 | (5.6–30.4) | - | - | - | - | [Table 5: b1] |
| 4.2 | (2.8–6.4) | 2.1 | (1.3 – 3.2) | - | - | [Table 5: b5] | |
| Invasive vaginal cancer | 6.3 | (1.6–25.6) | - | - | - | - | [Table 5: b1] |
| 4.3 | (2.8–8.7) | 1.6 | (0.7 – 3.6) | - | - | [Table 5: b5] | |
| Vaginal neoplasia (both pre- and invasive) | 10.9 | (5.3–22.4) | - | - | - | - | [Table 5: b1] |
| 4.3 | (3.0–6.2) | 2 | (1.3 – 2.9) | - | - | [Table 5: b5] | |
| VULVA AND VAGINA | |||||||
| Preneoplastic vulvar and vaginal lesions | 7.8 | (1.4 – 80.0) | 12.0 | (1.2 – 590.0) | 4.5 | (0.5 – 54.0) | [Table 5: b6] |
| Invasive vulvar and vaginal cancer | 5.5 | (1.5 – 25.0) | 1.5 | (0.3 – 7.5) | 3.3 | (0.5 – 23.0) | [Table 5: b6] |
CI: Confidence interval; OR: Odds ratio; VIN: Vulvar intraepithelial neoplasia.
Sources of data: [Table 5: b1–b6].
3.2.2 Risk factors for preneoplastic lesions and vulvar cancer
Early epidemiologic studies conducted before the introduction of HPV testing technologies identified similar risk factors for vulvar cancer as for cervical cancer, including number of sexual partners, smoking, history of STI’s, history of abnormal cytological examinations or prior gynecologic cancer, oral contraception use, dietary factors, smoking and HSV-2 seropositivity (Tables 2) [Table 5: b1–b6]. After controlling for HPV status, women with three or more lifetime sexual partners had a 3.4-fold increased risk for vulvar carcinomas [50], women seropositive for HSV-2 had a 1.5 to 3.2-fold increased risk of vulvar carcinomas (both in situ and invasive carcinomas) [50;51], and current smokers had a 6.4-fold increased risk of CIS and a 3-fold increased risk for invasive carcinomas [51]. Among HPV-16 DNA positive women, current smoking was associated with a 7.1-fold increased risk of vulvar carcinoma. A possible interaction between HPV-16 seropositivity and smoking has been suggested [50,51]. Hildesheim A. et al. reported an 8.5-fold increased risk for vulvar cancer among women reporting to be current smokers and HPV-16 seropositive as compared to a 2.9-fold increase among non-smoker-HPV 16 seropositive women [50]; while Madeleine MM. et al. observed an 18.8-fold and a 3.4-fold increased risk, respectively [51].
3.3. Vaginal cancer
Cancer of the vagina is a rare condition. In 2002 approximately 13,200 women worldwide were diagnosed with vaginal cancer accounting for less than 2% of all gynecologic cancers [29]. Squamous cell carcinomas are the most common histological type (~90%), followed by clear cell adenocarcinomas and melanoma [30]. Similar to cervical cancer, the majority (68%) of vaginal cancer cases are reported in developing countries. Base d on data from Cancer Incidence in Five Continents, in 2002 only few regional registries reported incidence rates ≥ 1.0 per 100,000 women [52]. Incidence rates in the range of 0.4 to 0.7 per 100,000 women are reported from the USA, the UK and other European countries. In some settings, metastatic cervical cancer can be misclassified as primary cancer of the vagina.
An increasing trend in incidence of VAIN was reported during the last 25 years or more, while the incidence rate of invasive vaginal cancer remained stable [41] or it has decreased [53]. This neoplasm tends to be diagnosed in older women - median age at diagnosis 69 years - and it is very rare in women under the age 45 [31,41,54]. Rates of invasive vaginal cancer increase substantially after age 65 and increases steadily with age, whereas the incidence of CIS of the vagina reaches a peak between ages 55 and 70 [31].
The overall five-year relative survival rate for vaginal cancer in the USA for the period 1988–2001 was estimated at 49.4% for women aged 20 years and over [54]. In the UK, the five-year relative survival rate for vaginal and vulvar cancers combined was 58% and it has increased overtime [43].
It is currently accepted that vaginal and cervical cancers have similar risk factor profiles, including the central role of HPV infection [55,56]. However, this is based on limited epidemiologic evidence. Most of the current knowledge on the epidemiology of vaginal cancer comes from only two large population-based, case-control studies conducted in the USA [55,57]. In support of a similar etiology for vaginal and cervical cancer, these two tumors are frequently diagnosed simultaneously. Women with vaginal cancer are more likely to have a history of other ano-genital cancers, particularly cervical. HPV DNA and HPV antibodies are identified in up to 91% of invasive vaginal carcinomas and 82% of VAIN3 lesions, and HPV-16 - as in cervical cancer - is the most prevalent type [1,50,56,58]. In addition to HPV, other risk factors associated with cervical cancer have also been reported in association with vaginal cancer: 1) lower education; 2) income levels; 3) increased number of lifetime sexual partners (five or more); 4) early age first sexual intercourse (less than 17 years of age); 5) HSV-2 seropositivity; and 6) current smoking [50,56,59,60].
3.4 Anal cancer
Anal cancers are rare malignancies arising in the anal canal, largely in the transitional zone of the epithelium. Worldwide approximately 99,000 new cases of anal cancer were reported in 2002, 40% of cases in men and 60% in women. Despite the rarity of this malignancy, over the last five decades an increasing trend in incidence has been reported for both men and women. During the period 1973 to 2000, the incidence of anal cancer in the USA increased twice as fast among men (160%) than among women (78%) [61]. Incidence is particularly high amongst homosexual males and among HIV infected men and women [62].
Similar to cervical cancer, the majority of invasive anal carcinomas (65%) are squamous cell carcinomas (SCC) that develop from precancerous AIN lesions and most have also been linked to HPV infection. HPV has been detected in 80% or more of cancerous lesions and HPV-16 is the most common type detected (up to 87% of HPV-positive tumors) [1]. HPV-18 is the second most commonly detected HPV type and only found in approximately 9% of the cases; while other types are detected more rarely. HPV DNA is also detected in the majority of AIN lesions and the prevalence of HPV in these lesions increases with the severity of the lesion: 75% in AIN1, 86% in AIN2, and 94% in AIN3 lesions [63]. Results from studies using serologic measures for antibodies against HPV-16 and 18 have been consistent with the findings from DNA studies [1].
3.5 Penile cancer
Cancer of the penis accounts for less than 0.5% of cancers in men. In Western countries incident rates are generally less than one new case per 100,000 men, with greater incidence in countries from Latin America such as Brazil, Peru and Colombia where incidence rates range from1,5 to 3,7 per 100,000, followed by Uganda with 2,8 and 1,7 in specific regions of Thailand and India [64]. The geographical correlation between incidence of penile and cervical cancers and the concordance of these two cancers in married couples prompted the suggestion of a common etiology [65]. HPV DNA is detectable in about 40–50% of all penile cancers, and serological studies have confirmed the role of HPV-16 and 18 [1]. Likewise, HPV positivity is higher in penile intraepithelial neoplasias (PIN1/2/3) and in the basaloid histological type, ranging from 75 to 80% decreasing to a range between 30 to 60% in invasive SCC. Cancers of the penis are largely SCC. It has been demonstrated that some cases of penile SCC are HPV DNA negative. HPV DNA positivity strongly correlates histopathological with either basaloid or warty changes (47%) or purely basaloid changes (75%), and is only weakly associated (11%) with the usual type of keratinizing SCC. HPV-16 and, to a lesser extent, HPV-18 are the most frequent viral types associated [1].
3.6 Head and neck cancers
Head and neck cancer commonly refers to SCCs arising in the upper aerodigestive tract (oral cavity, oropharynx, hypopharynx, and larynx). In 2002, approximately 405,000 new cases of head and neck tumors and 211,000 deaths occurred worldwide. As a group, head and neck cancers represent the sixth most common cancer rubric and is particularly prevalent in South-central Asia [62]. Most head and neck cancers are associated with tobacco and alcohol exposures and occur in the sixth and seventh decade of life; however about 15–20% of cases do not report significant exposures to these risk factors [66]. A rising proportion of these cancers, particularly oropharyngeal cancers, are occurring in young adults and in people who have never smoked; while incidence rates of oral cavity, hypopharynx and larynx cancer are falling with the decline in cigarette smoking in the USA [67]. In the past decade, up to 60% of oropharyngeal cancers have been found to be associated with oncogenic HPV types, while other head and neck cancer sites have a much lower seroprevalence and/or HPV DNA prevalence (typically less than 20%). The latest systematic review of head and neck tumors reported an overall prevalence of HPV DNA of 25.9% (23.5% for oral cavity tumors, 24.0% for larynx tumors and 35.6% for oropharynx tumors) [10]. Similar results are reported in recent case-controls studies [68–70]: 1) Pintos J. et al. observed a 19% prevalence of HPV DNA in exfoliated cells of oral and oropharyngeal cancers [69]; 2) Soares RC. et al. reported a 24% prevalence of HPV DNA in paraffin-embedded oral cancer tissue [68]; and 3) D’Souza G. et al. observed a 37% prevalence of HPV DNA in exfoliated cells of oropharyngeal cancer patients [70]. Further, this study showed convincing evidence that oral cavity HPV DNA was related to sexual behavior, including the practice of oral sex.
Similar to all other HPV-related neoplasias, HPV-16 is the most common HPV type identified in head and neck lesions followed by HPV-18 [1,10,69]. Among HPV-positive head and neck tumors HPV-16 is reported in 90% of oropharyngeal cancers, 69% of laryngeal cancers and 68% of oral cavity cancers; whereas, HPV-18 is higher among oral cavity cancers (8%), followed by laryngeal cancers (4%) and oropharyngeal cancers (1%) [10]. Likewise, Pintos J. et al. found 93% of HPV-positive cases were positive for HPV-16 (13 of 14 cases), and D’Souza G. et al. found 86% of HPV-16 among HPV-positive cases [70]. An interesting finding from this study was the consistency of results using several biomarkers and specimens. HPV-16 prevalence was 72% in paraffin-embedded tumor tissue by in-situ hybridization and 64% seropositivity to HPV-16 E6 and E7 [70].
Although using a centralized protocol Herrero R. et al. did not find geographic differences in the prevalence of HPV in oral cavity and oropharyngeal cancers [71], a systematic review by Kreimer AR. et al. reported differences in the prevalence of HPV infection according to geographic region and tumor site [10]. These findings are supported by the IARC monograph on the evaluation of the carcinogenicity of HPV to humans [1]. In the IARC review, the average prevalence of HPV infection in oral cavity cancer was approximately 25% with HPV-16 being detected in about 70% of HPV-positive cases. The average prevalence of HPV among oropharyngeal cancers was higher than that observed in oral cavity cancers (35%), and HPV-16 was detected in approximately 80% of HPV-positive cases [1]. However, the data for larynx cancer was less consistent and a limited association between larynx cancer and HPV-16 and 18 was observed [1].
4.0 GENITAL WARTS IN MEN AND WOMEN
Genital HPV infection can cause a broad spectrum of lesions ranging from cancer to benign genital warts. Although having genital warts is not associated with mortality, the lesions are often associated with both clinical symptoms (burning, bleeding and pain) and psychosocial problems (embarrassment, anxiety and decreased self-esteem) [72]. More than 90% of genital warts are related to HPV types -6 and 11 (Table 3) [Table 5: c1–c10] and, although in general these HPV types give rise to benign changes, they can in rare cases be associated with malignant lesions such as the rare Buschke-Lowenstein tumors. In addition, in some studies [73] women with a history of genital warts have been shown to have an increased risk of CIN and cancer which is most likely explained by a higher risk of having other oncogenic HPV types present. This observation is supported by a recent finding that 20–50% of genital warts, in addition to HPV-6/11, also contained co-infection with oncogenic HPV types [74].
Table 3.
HPV DNA prevalence and type distribution in ano-genital warts.
| Country/ Region |
Study Population |
Age | Sex | No. of subjects |
HIV status |
HPV DNA detection |
HPV prevalence N (%) | Reference code (see Table 5) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any HPV |
Multiple HPV |
HPV- 6 |
HPV- 11 |
HPV- 6/11 |
HPV- 16 |
HPV- 18 |
HPV- 31 |
HPV -33 |
HPV- 35 |
HPV- 39 |
HPV- X |
Other HPV (N, %) |
||||||||
| Croatia | Cohort of 171 men with 175 samples tested:115 were CA, 17 CP and 36 HPV-associated lesions |
NA | Males | 175 | NA | PCR (primer not given) |
140 (80%) | 10 (6%) | 88 (50%) | 10 (6%) | 92 (54%) | 8 (5%) | 3 (2%) | - | 1 (0,6%) | - | - | 26 (15%) |
NA | [Table 5: c1] |
| Coatia (Zagreb) |
Specimens were collected from HPV-induced, clinically visible lesions (CA, CP, B-L tumour) through an STD clinic and medical school university |
2–68 | Males | 100 | NA | PCR TS primers 6/11, 16, 18, 31, 33 |
100 (100%) |
3 (3%) | NA | NA | 82 (82%) | 12 (12%) | 7 (7%) | 1 (1%) | 1 (1%) | - | - | - | NA | [Table 5: c2] |
| Greece | NA | NA | Females males |
26 | NA | SBH/ PCR (primer not given) |
26 (100%) | 4 (21%) | 19 (74%) | 4 (5%) | 19 (74%) | - | - | 1(4%) | - | 1(4%) | 1(4%) | '- | - | [Table 5: c3] |
| Slovenia | Patients included in study had at least 5 exophytic genital warts on glans of the penis, coronal sulcus or foreskin |
17–36 | Males | 55 | All HIV- negative |
PCR MY09-11, CPI/CPII |
55 (100%) | 12 (22%) | 43 (78%) | 10 (18%) | 53 (96%) | 2 (4%) | 0 | 1 (2%) | 0 | 0 | 0 | 12 (22%) |
HPV-44 (1, 2%) | [Table 5: c4] |
| HPV-53 (2, 4%) | ||||||||||||||||||||
| HPV-55 (1, 2%) | ||||||||||||||||||||
| HPV-62 (2, 4%) | ||||||||||||||||||||
| HPV-66 (2, 4%) | ||||||||||||||||||||
| HPV-73 (4, %) | ||||||||||||||||||||
| HPV-84 (2, 4%) | ||||||||||||||||||||
| HPV-91 (1, 2%) | ||||||||||||||||||||
| Hong Kong | 140 patients attending a public STD clinic for the management of genital warts. 100 HPV typed |
NA | Males | 100 | NA | PCR (primer not given) |
98 (98%) | NA | 54 (54%) | 38 (38%) | - | - | - | - | - | - | - | - | Non HPV-6/11 (13, 13%) |
[Table 5: c5] |
| USA (New Mexico and California) |
Patients attending a private dermatology clinic and a cohort of New Mexico |
NA | Female males |
39 | NA | PCR MY09/11 |
37 (100%) | 8 (22%) | 35 (94%) | 3 (8%) | >35 (100%) |
- | - | - | - | - | - | 9 (24%) | HPV-54 (3; 8%) | [Table 5: c6] |
| HPV-58 (3, 8%) | ||||||||||||||||||||
| Presence of immunosupresion in any subject | ||||||||||||||||||||
| Italy | Longitudinal study of patients with ano-genital condylomata |
40 | 1 HIV- positive |
PCRMY09 /11, TS- primers |
40 (100%) | NA | 31 (78%) | 7 (18%) | 38 (95%) | 0 | 0 | 0 | 0 | 0 | 0 | 2 (5%) | HPV-61 (1, 3%) in HIV+ man |
[Table 5: c7] | ||
| USA | Patients evaluated in an STD clinic, hospital-based gynecology outpatient clinic, a hospital-based surgical outpatient clinic or in a transplant unit |
14–62 | Females males |
65 | 41 HIV- negative |
PCR MY09/11 |
41 (100%) | 28 (68%) | 37 (90%) | 13 (42%) | 41 (100%) | 10 (24%) | 6 (15%) | 5 (12%) | 0 | 0 | 1 (2%) |
- | NA | [Table 5: c8] |
| 17–41 | 16 HIV- positive and 8 TRP |
24 (100%) | 24 (100%) | 7 (29%) | 17 (71%) | 22 (92%) | 10 (42%) | 2 (8%) | 5 (21%) | 0 | 0 | 2 (8%) |
- | HPV-42 (4, 17%) | ||||||
| HPV-45 (5, 21%) | ||||||||||||||||||||
| HPV-53 (6; 25%) | ||||||||||||||||||||
| HPV-54 (3, 13%) | ||||||||||||||||||||
| HPV-55 (7, 29%) | ||||||||||||||||||||
| HPV-56 (3,13%) | ||||||||||||||||||||
| HPV-59 (6, 25%) | ||||||||||||||||||||
| HPV-83 (5, 21%) | ||||||||||||||||||||
| Colombia (Medellín) |
Cohort study of 37 men with ano-genital warts attending a dermatology clinic- 84% were receiving HAART |
>18 | Males | 37 men; 43 samples |
37 HIV- positive |
PCR MY09/11 |
43 (100%) | 12 (28%) | 16 (37%) | 16 (37%) | 32 (74%) | 1 (2%) | 2 (4%) | 3 (7%) | 0 | 1 (2%) | 0 | - | HPV-56 (1,2%) | [Table 5: c9] |
| HPV-62 (1, 2%) | ||||||||||||||||||||
| HPV-68 (1, 2%) | ||||||||||||||||||||
| HPV-69 (1,2%) | ||||||||||||||||||||
| HPV-72 (1, 2%) | ||||||||||||||||||||
| HPV-81 (1,2%) | ||||||||||||||||||||
| HPV-86 (1,2%) | ||||||||||||||||||||
| Venezuela | Patients with genital warts in the genital area. Of the 90 biopsies 72 (80%) were histological CA and 18 other pathologies |
NA | NA | 72 | 1 HIV- positive |
PCR (primer not given) |
72 (100%) | 3 (4%) | 42 (58%) | 29 (40%) | - | 1 (1%) | - | - | - | - | - | 4 (6%) | NA | [Table 5: c10] |
Any HPV infection: all those typed.
CA: Condyloma acuminatum; CP: Condyloma plana; GW: Genital warts, HAART: Highly active antiretroviral therapy; HIV: Human immunodeficiency virus; LB: Löwen Bürkenstein; NA: Not applicable; PCR: Polymerase chain reaction; SBH: Southern blot hybridisation; STD: Sexually transmitted disease; TRP: Patients with transplants.
Sources of data: [Table 5: c1–c10].
Genital warts represent a significant public health problem. Population-based data from the UK among women 16–44 years [75] and from Australia among women 16–59 years [76] have shown a prevalence of genital warts of about 4%. The National Health and Nutrition Examination Survey (1999–2004) carried out in the USA in almost 8,500 sexually active men and women aged 18 to 59 years reported an overall 5.6% having ever been diagnosed with genital warts. A higher percentage was reported among women (7.2%; 95% CI: 6.2% – 8.4%) than in men (4%; 95% CI: 3.2% – 5.0%) with a peak of diagnosis among 25- to 34-year old women (10.4%) and 35- to 44-year old men (6.0%) [77]. A recent study including some 70,000 women from four Nordic countries (18–45 years) found a prevalence of genital warts of nearly 11% [78]. Furthermore, 48 out of 2,279 women in the placebo cohort of the HPV vaccines clinical trials presented with condylomata during follow-up, representing an incidence rate of 0.9 per 100 person-years at risk [79].
Several earlier studies have suggested that the occurrence of genital warts has been increasing over time [80]. In line with this, more recently published data from the USA on visits for genital warts to private physicians (first or repeated visits) showed a 4-fold increase in number of cases from 1966 to 2004 [81]. The total number of cases has shown a more than 10-fold increase since 1971 [82]. In addition, an increase in the estimated age-specific incidence of genital warts among younger birth cohorts was observed in the Nordic study [78]. Some of the increase in genital warts over time may be explained by an increased awareness, but changes in the sexual behavior at the population level has been suggested as a more important explanation [74,78].
More than 90% of genital warts contain HPV DNA types -6 and 11 among immunocompetent individuals (Table 3). HPV-6 is the most frequently observed infection (55 – 90% of cases) followed by HPV-11 (5 – 42% of cases). Among immunosuppressed individuals, the prevalence of HPV-11 is higher (similar to that of HPV-6), more lesions contain infection with multiple HPV types, the detection of oncogenic types of HPV is higher, and the diagnosis of genital warts without the presence of HPV-6 or 11 detected is increased.
Genital warts represent not only a problem for the individual but also imply a significant healthcare costs for society. A study from the USA estimated an annual cost of US$140 million for treatment of genital warts in private health plans [83] and in another American study the direct costs of genital warts was estimated as of US$200 million [84]. In the UK, a study had estimated a healthcare cost of around £10 million for the management of new cases of genital warts during one year [85]. Finally, using the estimated costs for successful treatment of one episode of genital warts and the genital warts prevalence figures from STI clinics in the UK, Lacey CJN et al. calculated an annual cost related to genital warts of around £31 million [74].
5.0 RESPIRATORY PAPILLOMATOSIS
Recurrent respiratory papillomatosis (RRP) is a consequence of a mucosal infection of the airway caused by HPV-6 and 11 and rarely by HPV-16 (less than 5%) [86]. Few studies have explored the HPV DNA type distribution in RPP. Table 4 shows that HPV- 6 and 11 account for virtually all cases of RRP [Table 5: d1–d17].
Table 4.
HPV DNA type prevalence in subjects with recurrent respiratory papillomatosis.
| Study [Reference code] (see Table 5) |
Country | HPV detection method | N infections |
HPV prevalence N (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any HPV | Multiple infection |
HPV-6 | HPV-11 | HPV-16 | HPV-18 | HPV-31 | HPV-33 | HPV-35 | HPV-39 | ||||
| Gomez MA et al. 1995 [Table 5: d1] |
Argentina | PCR MY09-11/nested GP5-6 | 12 | 12 (100%) | - | 7 | - | - | - | - | - | - | |
| 58% | |||||||||||||
| Levi JE et al. 1989 [Table 5: d2] |
Brazil | PCR MY09-11 | 19 | 19 (100%) | 1 (5%) | 14 (74%) | 3 (16%) | - | - | - | - | - | - |
| Draganov P et al. 2006 [Table 5: d3] |
Bulgaria | PCR MY09-11/TS primers 6,11,16,18,31,33 |
23 | 21 (91%) | 3 (13%) | 8 (35%) | 16 (70%) | 0 | 0 | 0 | 0 | - | - |
| Fu M et al. 2000 [Table 5: d4] |
China | PCR (HPV-L1 region primers) | 13 | 13(100%) | 0 | 8 (62%) | 4 (31%) | 0 | 1 (8%) | 0 | - | - | - |
| Sun AK 1993 [Table 5: d5] |
China | In situ hybridization | 45 | 36 (80%) | - | 17 (38%) | 19 (42%) | 0 | - | - | - | - | - |
| Lindeberg J et al. 1990 [Table 5: d6] |
Denmark | Not given | 20 | 19 (95%) | - | 19 | 0 | 0 | - | - | - | - | |
| 95% | |||||||||||||
| Dickens P et al. 1991 [Table 5: d7] |
Hong Kong | PCR (primer not given) | 27 | 16 (59%) | 3 (5%) | 3 (5%) | 16 (22%) | 3 (5%) | 0 | - | - | - | - |
| Velyvyte D et al. 2002 [Table 5: d8] |
Lithuania | PCR (4 different primers) | 36 | 35 (97%) | 17 (47%)a | 32 | 18 (50%) | 3 (8%) | 0 | 0 | 0 | 0 | |
| 89% | |||||||||||||
| Peñaloza-Plasencia M et al. 2000 [Table 5: d9] |
Mexico | PCR CPI/CPIIG (E1) | 47 | 47 (100%) | 35 (74%) | 15 (32%) | 18 (38%) | 39 (83%) | 0 | 2 (4%) | 26 (55%) | 13 (28%) | 7 (15%) |
| Obchinnikov IuM et al. 2004 [Table 5: d10] |
Russia | PCR (primers not reported) | 26 | 26 (100%) | 4 (15%) | 11 (42%) | 19 (73%) | 0 | 0 | - | - | - | - |
| Padayachee A et al. 1993 [Table 5: d11] |
South Africa | Not given | 20 | 20 (100%) | 0 | 5 (25%) | 15(75%) | 0 | 0 | - | - | - | - |
| Szeps M et al. 2005 [Table 5: d12] |
Sweden and Finland | PCR GP5+/6+, TS primer 6/11/16/18/33 | 25 | 20 (80%) | - | 12 (48%) | 6 (24%) | 0 | 0 | - | 0 | - | - |
| Ushikai M et al. 1994 [Table 5: d13] |
Thailand | Dot blot analysis/ PCR | 25 | 22 (88%) | - | 1 (4%) | 21(84%) | 0 | 0 | - | - | - | - |
| Maloney EM et al. 2006 [Table 5: d14] |
USA | PCR TS primers HPV 6,11, 16, 18 | 15 | 15 (100%) | 7 (47%) | 11(73%) | 11(73%) | 0 | 0 | - | - | - | - |
| Rabah R et al. 2001 [Table 5: d15] |
USA | PCR TS primers HPV 6,11, 16, 18 | 61 | 61 (100%) | 0 | 29 (48%) | 32 (52%) | 0 | 0 | - | - | - | - |
| Pou AM et al. 1995 [Table 5: d16] |
USA | PCR TS primer 6,11,16,18, 31,33 | 24 of 29 typed |
24 (100%) | - | 21 (88%) | 2 (8%) | 1 (4%) | 0 | 0 | 0 | - | - |
| Bello de Alford M et al. 2001 [Table 5: d17] |
Venezuela | PCR (primers not reported) | 15 | 8 (53%) | - | 4 | 0 | 0 | 0 | 0 | 0 | - | |
| 27% | |||||||||||||
HPV-6/11 considered as single infection when counting at multiple infections.
N Infections: Number of subjects with HPV DNA typed from the recurrent respiratory papillomatosis except for Lindeberg J et al. [Table 5: d6], where 16 subjects had 20 papillomas investigated.
GP: General primer; PCR: Polymerase chain reaction; TS: Type-specific primer.
Sources of data: [Table 5: d1–d17].
RRPs appear to follow a bimodal age distribution with a peak in childhood presenting high aggressiveness and equal incidence in both genders. Vertical HPV transmission (from mother to child during birth) is the proposed mechanism of transmission. A more clinically benign second peak in incidence is observed in adulthood with a slight male preference most likely due to oral-genital contacts or the reactivation of silent infections.
The incidence per year of RRP is uncertain but is estimated to be 4.3 in children and 1.8 per 100,000 adults in the USA [87], whereas in Denmark it was reported to be 3.6 and 3.9 per 100,000, respectively [88]. In spite of the rarity of the disease, since it affects very young children, spontaneous regression is unlikely and multiple surgical procedures are necessary to constrain the progression and growth of these lesion, RRP is a condition with very high social and economic costs [89].
HPV-6 and 11 in the respiratory tract provide an example of a latent HPV infection in clinically normal tissue (i.e., the vocal cord) in which HPV DNA can be isolated from biopsy material in the absence of any clinical manifestations even several years following treatment [90]. Risk factors for reactivation are largely unknown. Inherited susceptibility to HPV and tissue-specific susceptibility to reactivation (i.e., less frequently in the tracheal as compared to the vocal cord) are factors described in relation to the complex natural history of RRP. Malignant transformation can occur, albeit rarely (3 – 7% of the cases, [91]) with the risk suspected to be higher for HPV-11 related papillomatosis [1].
6.0 CONCLUSIONS
HPV infection in the external genital tract has been detected in a range of 0 to 73% of healthy men. The wide incidence range may be explained by variability in the sampling methods used and the number of different anatomic sites and samples included. The coronal gland and the penile shaft appear to be the most important anatomic sites to be sampled in men.
Viral persistence of oncogenic infections in men, compared to women, appear to be shorter with approximately 75% of HPV DNA clearance at one year. Age does not appear to be as strongly associated with HPV incidence or duration of infection in men as it is typically in women.
The incidence of invasive vulvar and vaginal cancer has remained stable over time signaling the limited impact of cervical cancer screening efforts on other genital cancers. In some countries there has been a dramatic increase in the incidence of VIN 2/3 and VAIN among women ages less than 50. The HPV DNA prevalence in pre-invasive lesions has been reported as 72 to 100% for VIN3; 82 to 100% for VAIN 3; 27.3 to 100% for vulvar cancer and 64 to 91% for vaginal cancer. HPV-16 is consistently the most common type accounting for approximately 65 – 93% in VIN3, 71% in vulvar cancer and 70 – 88% in vaginal cancer followed by HPV- 18, the second most common type identified. Lifetime numbers of sexual partners, smoking, and HSV-2 seropositivity have been independently associated with both vulvar and vaginal carcinoma. Low socio-economic status, early age first sexual intercourse and current smoking are additional risk factors for vaginal cancer.
Anal cancer follows a similar epidemiology and risk factor profile as cervical cancer. Trends indicate an increase in both genders over time and especially among immunocompromised individuals. HPV accounts for over 80% of cancerous lesions, with an increasing prevalence according to the severity of the lesion (75% AIN1, 86% AIN2, and 94% AIN3). HPV-16 is the most common type (up to 87% among HPV-positive tumors) and HPV-18 is the second most commonly detected type (9%).
The summary prevalence of HPV DNA in head and neck cancers is currently in the 25–30% range, with marked differences according anatomic site. The importance of HPV as risk factor is clearly seen in 15 to 20% of cases without exposure to alcohol or tobacco. The HPV prevalence in oropharyngeal cancer appears to be higher than previously observed, with reports of up to 72% HPV positivity and a clear relationship with oral sex practices.
Genital warts are a clinical manifestation of HPV infections largely caused by HPV-6 and 11 in both sexually active men and women. Genital warts are a common sexually-transmitted condition with an estimated prevalence of 1% of sexually active individuals. Although genital warts are benign conditions, treatment often faces recurrences and implies significant public health and emotional costs. Juvenile RRP is a rare disease occurring in newborns and children of HPV-positive mothers. Adult onset respiratory papillomatosis has been related to oral sex. Both entities are largely caused by HPV- 6 and -11 and, although only anecdothically undergo malignant transformation, the treatment of airway obstruction often requires multiple surgeries at very high social and emotional costs.
ACKNOWLEDGEMENTS
Anna R. Giuliano’s work is supported by grants from the US National Institutes Health, National Cancer Institute, The Centers for Disease Control and Prevention, and the Arizona Disease Control Research Commission (ADCRC) provided partial support for the work presented here. Digene kindly provided STM collection media free of charge and Roche kindly provided reagents for HPV detection free of charge to some of the studies presented. F. Xavier Bosch’s, Silvia de Sanjose’s and Elena Ferrer’s work was partially supported by Spanish public grants from the Instituto de Salud Carlos III (grants RCESP C03/09, RTICESP C03/10 and RTIC RD06/0020 /0095 and CIBERESP) and from the Agència de Gestió d’Ajuts Universitaris i de Recerca (AGAUR 2005SGR 00695). Ann N. Burchell’s work is supported by the Canadian Cancer Society through a research studentship award from the National Cancer Institute of Canada.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosed potential conflicts of interest
ARG: Advisory Board (Merck and Co., Inc); Research Grants (Merck and Co., Inc); Speakers Bureau (Merck and Co., Inc).
GT-L: Speakers Bureau (Merck Sharp and Dohme).
EF: Speakers bureau (GlakoSmithKline).
SS: Research Grants (Merck & Co. Inc., Sanofi Pasteur MSD).
SKK: Advisory Board (Sanofi Pasteur MSD); Research Grants (Merck and Co., Inc, Sanofi Pasteur MSD); Steering Committee (Merck and Co., Inc); Travel Grants (Merck and Co., Inc, Sanofi-Pasteur MSD).
NM: Advisory Board (Merck and Co., Inc, Sanofi Pasteur MSD); Speakers Bureau (Sanofi Pasteur MSD); Steering Committee (Merck and Co., Inc).
FXB: Advisory Board (GlaxoSmithKline, Merck Sharp & Dohme, Sanofi Pasteur MSD); Speakers Bureau (GlaxoSmithKline); Research Grants (Merck Sharp & Dohme, Sanofi Pasteur MSD).
Reference List
- 1.IARC. IARC Monographs on the Evaluation of carcinogenic risk to humans. Vol. 90. Lyon: IARC Press; 2007. Human Papillomavirus. [Google Scholar]
- 2.Haga T, Kim SH, Jensen RH, Darragh T, Palefsky JM. Detection of genetic changes in anal intraepithelial neoplasia (AIN) of HIV-positive and HIV-negative men. J Acquir Immune Defic Syndr. 2001;26(3):256–262. doi: 10.1097/00042560-200103010-00007. [DOI] [PubMed] [Google Scholar]
- 3.Palefsky JM. Human papillomavirus infection and anogenital neoplasia in human immunodeficiency virus-positive men and women. J Natl Cancer Inst Monogr. 1998;(23):15–20. doi: 10.1093/oxfordjournals.jncimonographs.a024166. [DOI] [PubMed] [Google Scholar]
- 4.Palefsky JM, Holly EA, Ralston ML, Da Costa M, Greenblatt RM. Prevalence and risk factors for anal human papillomavirus infection in human immunodeficiency virus (HIV)-positive and high-risk HIV-negative women. J Infect Dis. 2001;183(3):383–391. doi: 10.1086/318071. [DOI] [PubMed] [Google Scholar]
- 5.Xi LF, Critchlow CW, Wheeler CM, Koutsky LA, Galloway DA, Kuypers J, et al. Risk of anal carcinoma in situ in relation to human papillomavirus type 16 variants. Cancer Res. 1998;58(17):3839–3844. [PubMed] [Google Scholar]
- 6.Critchlow CW, Hawes SE, Kuypers JM, Goldbaum GM, Holmes KK, Surawicz CM, et al. Effect of HIV infection on the natural history of anal human papillomavirus infection. AIDS. 1998;12(10):1177–1184. doi: 10.1097/00002030-199810000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Munoz N, Castellsague X, Bosch FX, Tafur L, de Sanjose S, Aristizabal N, et al. Difficulty in elucidating the male role in cervical cancer in Colombia, a high-risk area for the disease. J Natl Cancer Inst. 1996;88(15):1068–1075. doi: 10.1093/jnci/88.15.1068. [DOI] [PubMed] [Google Scholar]
- 8.Castellsague X, Bosch FX, Munoz N, Meijer CJ, Shah KV, de Sanjose S, et al. Male circumcision, penile human papillomavirus infection, and cervical cancer in female partners. N Engl J Med. 2002;346(15):1105–1112. doi: 10.1056/NEJMoa011688. [DOI] [PubMed] [Google Scholar]
- 9.Castellsague X, Ghaffari A, Daniel RW, Bosch FX, Munoz N, Shah KV. Prevalence of penile human papillomavirus DNA in husbands of women with and without cervical neoplasia: a study in Spain and Colombia. J Infect Dis. 1997;176(2):353–361. doi: 10.1086/514052. [DOI] [PubMed] [Google Scholar]
- 10.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14(2):467–475. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 11.Giuliano AR, Nielson CM, Flores R, Dunne EF, Abrahamsen M, Papenfuss MR, et al. The optimal anatomic sites for sampling heterosexual men for human papillomavirus (HPV) detection: the HPV detection in men study. J Infect Dis. 2007;196(8):1146–1152. doi: 10.1086/521629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunne EF, Nielson CM, Stone KM, Markowitz LE, Giuliano AR. Prevalence of HPV infection among men: A systematic review of the literature. J Infect Dis. 2006;194(8):1044–1057. doi: 10.1086/507432. [DOI] [PubMed] [Google Scholar]
- 13.Nielson CM, Flores R, Harris RB, Abrahamsen M, Papenfuss MR, Dunne EF, et al. Human papillomavirus prevalence and type distribution in male anogenital sites and semen. Cancer Epidemiol Biomarkers Prev. 2007;16(6):1107–1114. doi: 10.1158/1055-9965.EPI-06-0997. [DOI] [PubMed] [Google Scholar]
- 14.Weaver BA, Feng Q, Holmes KK, Kiviat N, Lee SK, Meyer C, et al. Evaluation of genital sites and sampling techniques for detection of human papillomavirus DNA in men. J Infect Dis. 2004;189(4):677–685. doi: 10.1086/381395. [DOI] [PubMed] [Google Scholar]
- 15.Flores R, Abalos AT, Nielson CM, Abrahamsen M, Harris RB, Giuliano AR. Reliability of sample collection and laboratory testing for HPV Detection in Men. J Virol Methods. 2008;149(1):136–143. doi: 10.1016/j.jviromet.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Giuliano AR, Lu B, Nielson C, et al. Age Specific Prevalence, Incidence, and Duration of Human Papillomavirus Infections Among a Cohort of 290 US Men. J Infect Dis. 2008 doi: 10.1086/591095. in press. [DOI] [PubMed] [Google Scholar]
- 17.Nielson CM, Dunne EF, Abrahamsen M, Harris R, Giuliano AR. Associations between male anogenital HPV infection and circumcision vary by anatomic site sample. Abstract PS8-44. 24th International Papillomavirus Conference and Clinical Workshop; November 2007; Beijing, China. (Abstracts book). [Google Scholar]
- 18.Giuliano AR, Lazcano E, Villa LL, et al. Factors associated with HPV detection among men internationally: The HIM Study. Abstract 8B-04. 24th International Papillomavirus Conference and Clinical Workshop; November 2007; Beijing, China. (Abstracts book) [Google Scholar]
- 19.Giuliano AR, Lazcano E, Villa LL, et al. The Human Papillomavirus Infection in Men (HIM) Study: HPV Prevalence and Type-Distribution among Men Residing in Brazil, Mexico, and the US. Cancer Epidemiol Biomarkers Prev. 2008 doi: 10.1158/1055-9965.EPI-08-0151. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kyo S, Inoue M, Koyama M, Fujita M, Tanizawa O, Hakura A. Detection of high-risk human papillomavirus in the cervix and semen of sex partners. J Infect Dis. 1994;170(3):682–685. doi: 10.1093/infdis/170.3.682. [DOI] [PubMed] [Google Scholar]
- 21.Campion MJ, Singer A, Clarkson PK, McCance DJ. Increased risk of cervical neoplasia in consorts of men with penile condylomata acuminata. Lancet. 1985;1(8435):943–946. doi: 10.1016/s0140-6736(85)91724-6. [DOI] [PubMed] [Google Scholar]
- 22.Partridge JM, Hughes JP, Feng Q, Winer RL, Weaver BA, Xi LF, et al. Genital human papillomavirus infection in men: incidence and risk factors in a cohort of university students. J Infect Dis. 2007;196(8):1128–1136. doi: 10.1086/521192. [DOI] [PubMed] [Google Scholar]
- 23.Giuliano AR, Harris R, Sedjo RL, Baldwin S, Roe D, Papenfuss MR, et al. Incidence, prevalence, and clearance of type-specific human papillomavirus infections: The Young Women's Health Study. J Infect Dis. 2002;186(4):462–469. doi: 10.1086/341782. [DOI] [PubMed] [Google Scholar]
- 24.Van Doornum GJ, Prins M, Juffermans LH, Hooykaas C, van den Hoek JA, Coutinho RA, et al. Regional distribution and incidence of human papillomavirus infections among heterosexual men and women with multiple sexual partners: a prospective study. Genitourin Med. 1994;70(4):240–246. doi: 10.1136/sti.70.4.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Svare EI, Kjaer SK, Nonnenmacher B, Worm AM, Moi H, Christensen RB, et al. Seroreactivity to human papillomavirus type 16 virus-like particles is lower in high-risk men than in high-risk women. J Infect Dis. 1997;176(4):876–883. doi: 10.1086/516505. [DOI] [PubMed] [Google Scholar]
- 26.Slavinsky J, III, Kissinger P, Burger L, Boley A, DiCarlo RP, Hagensee ME. Seroepidemiology of low and high oncogenic risk types of human papillomavirus in a predominantly male cohort of STD clinic patients. Int J STD AIDS. 2001;12(8):516–523. doi: 10.1258/0956462011923615. [DOI] [PubMed] [Google Scholar]
- 27.Hagensee ME, Koutsky LA, Lee SK, Grubert T, Kuypers J, Kiviat NB, et al. Detection of cervical antibodies to human papillomavirus type 16 (HPV-16) capsid antigens in relation to detection of HPV-16 DNA and cervical lesions. J Infect Dis. 2000;181(4):1234–1239. doi: 10.1086/315364. [DOI] [PubMed] [Google Scholar]
- 28.Joura EA, Leodolter S, Hernandez-Avila M, Wheeler CM, Perez G, Koutsky LA, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369(9574):1693–1702. doi: 10.1016/S0140-6736(07)60777-6. [DOI] [PubMed] [Google Scholar]
- 29.Sankaranarayanan R, Ferlay J. Worldwide burden of gynaecological cancer: the size of the problem. Best Pract Res Clin Obstet Gynaecol. 2006;20(2):207–225. doi: 10.1016/j.bpobgyn.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Rotmensch J, Yamada SD. Ne oplasms of the Vulva and Vagina. In: Kufe DW, Pollock RE, Weichselbaum RR, Bast JrR, Gansler TSHJFea, editors. Holland-Frei Cancer Medicine. 6th ed. Hamilton, Ontario: B.C. Decker, Inc; 2003. [Google Scholar]
- 31.SEER Cancer Statistics Review, 1975–2004. Bethesda, MD: National Cancer Institute; 2003. [Google Scholar]
- 32.Van Seters M, Van Beurden M, de Craen AJ. Is the assumed natural history of vulvar intraepithelial neoplasia III based on enough evidence? A systematic review of 3322 published patients. Gynecol Oncol. 2005;97(2):645–651. doi: 10.1016/j.ygyno.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Jones RW, Rowan DM, Stewart AW. Vulvar intraepithelial neoplasia: aspects of the natural history and outcome in 405 women. Obstet Gynecol. 2005;106(6):1319–1326. doi: 10.1097/01.AOG.0000187301.76283.7f. [DOI] [PubMed] [Google Scholar]
- 34.Srodon M, Stoler MH, Baber GB, Kurman RJ. The distribution of low and high-risk HPV types in vulvar and vaginal intraepithelial neoplasia (VIN and VaIN) Am J Surg Pathol. 2006;30(12):1513–1518. doi: 10.1097/01.pas.0000213291.96401.48. [DOI] [PubMed] [Google Scholar]
- 35.Sideri M, Jones RW, Heller DS, Haefner H, Neill S, Preti M, et al. The distribution of low and high-risk HPV types in vulvar and vaginal intraepithelial neoplasia (VIN and VaIN) Am J Surg Pathol. 2006;30:1513–1518. doi: 10.1097/01.pas.0000213291.96401.48. Comment on the Article: Srodon M, Stoler MH, Baber GB, et al. Am J Surg Pathol 2007;31(9):1452–4. [DOI] [PubMed] [Google Scholar]
- 36.Sideri M, Jones RW, Wilkinson EJ, Preti M, Heller DS, Scurry J, et al. Squamous vulvar intraepithelial neoplasia: 2004 modified terminology, ISSVD Vulvar Oncology Subcommittee. J Reprod Med. 2005;50(11):807–810. [PubMed] [Google Scholar]
- 37.Scurry J, Campion M, Scurry B, Kim SN, Hacker N. Pathologic audit of 164 consecutive cases of vulvar intraepithelial neoplasia. Int J Gynecol Pathol. 2006;25(2):176–181. doi: 10.1097/01.pgp.0000189238.19027.df. [DOI] [PubMed] [Google Scholar]
- 38.Jones RW. Vulval intraepithelial neoplasia: current perspectives. Eur J Gynaecol Oncol. 2001;22(6):393–402. [PubMed] [Google Scholar]
- 39.Skapa P, Zamecnik J, Hamsikova E, Salakova M, Smahelova J, Jandova K, et al. Human papillomavirus (HPV) profiles of vulvar lesions: possible implications for the classification of vulvar squamous cell carcinoma precursors and for the efficacy of prophylactic HPV vaccination. Am J Surg Pathol. 2007;31(12):1834–1843. doi: 10.1097/PAS.0b013e3180686d10. [DOI] [PubMed] [Google Scholar]
- 40.Judson PL, Habermann EB, Baxter NN, Durham SB, Virnig BA. Trends in the incidence of invasive and in situ vulvar carcinoma. Obstet Gynecol. 2006;107(5):1018–1022. doi: 10.1097/01.AOG.0000210268.57527.a1. [DOI] [PubMed] [Google Scholar]
- 41.Madeleine MM, Daling JR, Tamimi HK. Vulva and Vagina. In: Franco EL, Rohan TE, editors. Cancer Precursors: Epidemiology, Detection, and Prevention. New York: Springer-Verlag; 2002. pp. 321–332. [Google Scholar]
- 42.Howe HL, Wingo PA, Thun MJ, Ries LA, Rosenberg HM, Feigal EG, et al. Annual report to the nation on the status of cancer (1973 through 1998), featuring cancers with recent increasing trends. J Natl Cancer Inst. 2001;93(11):824–842. doi: 10.1093/jnci/93.11.824. [DOI] [PubMed] [Google Scholar]
- 43.Cancer Research UK. www.cancerresearchuk.org. [last accessed: April 2008]; Available at: www.cancerresearchuk.org. [Google Scholar]
- 44.Kosary CL. Cancer of the Vulva. In: Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M-J, editors. SEER survival monograph: Cancer survival among adults: US SEER Program 1998–2001, Patient and Tumor Characteristics. Bethesda, MD: National Cancer Institute, NIH; 2007. [Google Scholar]
- 45.Kurman RJ, Toki T, Schiffman MH. Basaloid and warty carcinomas of the vulva. Distinctive types of squamous cell carcinoma frequently associated with human papillomaviruses. Am J Surg Pathol. 1993;17(2):133–145. doi: 10.1097/00000478-199302000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Trimble CL, Hildesheim A, Brinton LA, Shah KV, Kurman RJ. Heterogeneous etiology of squamous carcinoma of the vulva. Obstet Gynecol. 1996;87(1):59–64. doi: 10.1016/0029-7844(95)00351-7. [DOI] [PubMed] [Google Scholar]
- 47.Leibowitch M, Neill S, Pelisse M, Moyal-Baracco M. The epithelial changes associated with squamous cell carcinoma of the vulva: a review of the clinical, histological and viral findings in 78 women. Br J Obstet Gynaecol. 1990;97(12):1135–1139. doi: 10.1111/j.1471-0528.1990.tb02502.x. [DOI] [PubMed] [Google Scholar]
- 48.Nascimento C, Clifford G, DeVuyst H, Franceschi S. HPV prevalence and type distribution in carcinomas and intraepithelial neoplasia lesions of the vulva and vagina. The first in a series of meta-analysis in anogenital sites other than cervix. Abstract 4B-01; 24th International Papillomavirus Conference and Clinical Workshop; November 2007; Beijing, China. (Abstracts book). [Google Scholar]
- 49.Bosch FX, Vallès X, Muñoz N, Alejo M, Klaustermeier J, Lloveras B, et al. HPV contribution and genotype distribution in invasive cancer of the vulva and the vagina in 14 countries. Abstract PA2-06. 24th International Papillomavirus Conference and Clinical Workshop; November 2007; Beijing, China. (Abstracts book). [Google Scholar]
- 50.Hildesheim A, Han CL, Brinton LA, Kurman RJ, Schiller JT. Human papillomavirus type-16 risk of preinvasive and invasive vulvar cancer: results from a seroepidemiological case-control study. Obstet Gynecol. 1997;90(5):748–754. doi: 10.1016/S0029-7844(97)00467-5. [DOI] [PubMed] [Google Scholar]
- 51.Madeleine MM, Daling JR, Carter JJ, Wipf GC, Schwartz SM, McKnight B, et al. Cofactors with human papillomavirus in a population-based study of vulvar cancer. J Natl Cancer Inst. 1997;89(20):1516–1523. doi: 10.1093/jnci/89.20.1516. [DOI] [PubMed] [Google Scholar]
- 52.Parkin DM, Whelan SLFJ, Teppo L, Thomas DB. Cancer Incidence in Five Continents. VIII. Lyon: IARC Scientific Publications No. 155; 2003. [Google Scholar]
- 53.Levi F, Randimbison L, La Vecchia C. Descriptive epidemiology of vulvar and vaginal cancers in Vaud, Switzerland, 1974–1994. Ann Oncol. 1998;9(11):1229–1232. doi: 10.1023/a:1008433817832. [DOI] [PubMed] [Google Scholar]
- 54.Kosary CL. Cancer of the Vagina. In: Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M-J, editors. SEER survival monograph: Cancer survival among adults: US SEER Program 1998–2001, Patient and Tumor Characteristics. Bethesda, MD: National Cancer Institute, NIH; 2007. [Google Scholar]
- 55.Daling JR, Sherman KJ, Hislop TG, Maden C, Mandelson MT, Beckmann AM, et al. Cigarette smoking and the risk of anogenital cancer. Am J Epidemiol. 1992;135(2):180–189. doi: 10.1093/oxfordjournals.aje.a116270. [DOI] [PubMed] [Google Scholar]
- 56.Daling JR, Madeleine MM, Schwartz SM, Shera KA, Carter JJ, McKnight B, et al. A population-based study of squamous cell vaginal cancer: HPV and cofactors. Gynecol Oncol. 2002;84(2):263–270. doi: 10.1006/gyno.2001.6502. [DOI] [PubMed] [Google Scholar]
- 57.Brinton LA, Reeves WC, Brenes MM, Herrero R, de Britton RC, Gaitan E, et al. Oral contraceptive use and risk of invasive cervical cancer. Int J Epidemiol. 1990;19(1):4–11. doi: 10.1093/ije/19.1.4. [DOI] [PubMed] [Google Scholar]
- 58.Carter JJ, Madeleine MM, Shera K, Schwartz SM, Cushing-Haugen KL, Wipf GC, et al. Human papillomavirus 16 and 18 L1 serology compared across anogenital cancer sites. Cancer Res. 2001;61(5):1934–1940. [PubMed] [Google Scholar]
- 59.Brinton LA, Nasca PC, Mallin K, Schairer C, Rosenthal J, Rothenberg R, et al. Case-control study of in situ and invasive carcinoma of the vagina. Gynecol Oncol. 1990;38(1):49–54. doi: 10.1016/0090-8258(90)90010-i. [DOI] [PubMed] [Google Scholar]
- 60.Daling JR, Sherman KJ. Cancer of the vulva and vagina. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer Epidemiology and Prevention. New York: Oxford University Press; 1996. pp. 1117–1129. [Google Scholar]
- 61.Daling JR, Madeleine MM, Johnson LG, Schwartz SM, Shera KA, Wurscher MA, et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer. 2004;101(2):270–280. doi: 10.1002/cncr.20365. [DOI] [PubMed] [Google Scholar]
- 62.Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006;21(24) Suppl. 3:S11–S25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]
- 63.Varnai AD, Bollmann M, Griefingholt H, Speich N, Schmitt C, Bollmann R, et al. HPV in anal squamous cell carcinoma and anal intraepithelial neoplasia (AIN). Impact of HPV analysis of anal lesions on diagnosis and prognosis. Int J Colorectal Dis. 2006;21(2):135–142. doi: 10.1007/s00384-005-0777-7. [DOI] [PubMed] [Google Scholar]
- 64.IARC. Cancer Incidence in Five Continents. IX. Lyon: IARC; 2007. IARC Scientific Publications No. 160. [Google Scholar]
- 65.Micali G, Nasca MR, Innocenzi D, Schwartz RA. Penile cancer. J Am Acad Dermatol. 2006;54(3):369–391. doi: 10.1016/j.jaad.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 66.Hoffman HT, Karnell LH, Funk GF, Robinson RA, Menck HR. The National Cancer Data Base report on cancer of the head and neck. Arch Otolaryngol Head Neck Surg. 1998;124(9):951–962. doi: 10.1001/archotol.124.9.951. [DOI] [PubMed] [Google Scholar]
- 67.Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers? Cancer. 2007;110(7):1429–1435. doi: 10.1002/cncr.22963. [DOI] [PubMed] [Google Scholar]
- 68.Soares RC, Oliveira MC, Souza LB, Costa AL, Medeiros SR, Pinto LP. Human papillomavirus in oral squamous cells carcinoma in a population of 75 Brazilian patients. Am J Otolaryngol. 2007;28(6):397–400. doi: 10.1016/j.amjoto.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 69.Pintos J, Black MJ, Sadeghi N, Ghadirian P, Zeitouni AG, Viscidi RP, et al. Human papillomavirus infection and oral cancer: A case-control study in Montreal, Canada. Oral Oncol. 2008;44(3):242–250. doi: 10.1016/j.oraloncology.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 70.D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 71.Herrero R, Castellsague X, Pawlita M, Lissowska J, Kee F, Balaram P, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95(23):1772–1783. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 72.Maw RD, Reitano M, Roy M. An international survey of patients with genital warts: perceptions regarding treatment and impact on lifestyle. Int J STD AIDS. 1998;9(10):571–578. doi: 10.1258/0956462981921143. [DOI] [PubMed] [Google Scholar]
- 73.Friis S, Kjaer SK, Frisch M, Mellemkjaer L, Olsen JH. Cervical intraepithelial neoplasia, anogenital cancer, and other cancer types in women after hospitalization for condylomata acuminata. J Infect Dis. 1997;175(4):743–748. doi: 10.1086/513966. [DOI] [PubMed] [Google Scholar]
- 74.Lacey CJ, Lowndes CM, Shah KV. Chapter 4: Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine. 2006 Aug 21;24(Suppl 3):S35–S41. doi: 10.1016/j.vaccine.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 75.Fenton KA, Korovessis C, Johnson AM, McCadden A, McManus S, Wellings K, et al. Sexual behaviour in Britain: reported sexually transmitted infections and prevalent genital Chlamydia trachomatis infection. Lancet. 2001;358(9296):1851–1854. doi: 10.1016/S0140-6736(01)06886-6. [DOI] [PubMed] [Google Scholar]
- 76.Grulich AE, de Visser RO, Smith AM, Rissel CE, Richters J. Sex in Australia: knowledge about sexually transmissible infections and blood-borne viruses in a representative sample of adults. Aust N Z J Public Health. 2003;27(2):230–233. doi: 10.1111/j.1467-842x.2003.tb00813.x. [DOI] [PubMed] [Google Scholar]
- 77.Dinh TH, Sternberg M, Dunne EF, Markowitz LE. Genital warts among 18- to 59-year-olds in the United States, national health and nutrition examination survey, 1999–2004. Sex Transm Dis. 2008 Apr;35(4):357–360. doi: 10.1097/OLQ.0b013e3181632d61. [DOI] [PubMed] [Google Scholar]
- 78.Kjaer SK, Tran TN, Sparen P, Tryggvadottir L, Munk C, Dasbach E, et al. The burden of genital warts: a study of nearly 70,000 women from the general female population in the 4 Nordic countries. J Infect Dis. 2007;196(10):1447–1454. doi: 10.1086/522863. [DOI] [PubMed] [Google Scholar]
- 79.Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 80.Gall SA. Female genital warts: global trends and treatments. Infect Dis Obstet Gynecol. 2001;9(3):149–154. doi: 10.1155/S1064744901000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Centers for Disease Control and Prevention. Centers for Disease Control and Prevention; 2008. [last accessed: April 2008]. Available at: http://www.cdc.gov/ [Google Scholar]
- 82.Health Protection Agency. UK: Health Protection Agency; 2008. Apr, [last accessed: April 2008]. Available at: http://www.hpa.org.uk/ [Google Scholar]
- 83.Insinga RP, Dasbach EJ, Myers ER. The health and economic burden of genital warts in a set of private health plans in the United States. Clin Infect Dis. 2003;36(11):1397–1403. doi: 10.1086/375074. [DOI] [PubMed] [Google Scholar]
- 84.Insinga RP, Dasbach EJ, Elbasha EH. Assessing the annual economic burden of preventing and treating anogenital human papillomavirus-related disease in the US: analytic framework and review of the literature. Pharmacoeconomics. 2005;23(11):1107–1122. doi: 10.2165/00019053-200523110-00004. [DOI] [PubMed] [Google Scholar]
- 85.Brown RE, Breugelmans JG, Theodoratou D, Benard S. Costs of detection and treatment of cervical cancer, cervical dysplasia and genital warts in the UK. Curr Med Res Opin. 2006;22(4):663–670. doi: 10.1185/030079906X99972. [DOI] [PubMed] [Google Scholar]
- 86.Dickens P, Srivastava G, Loke SL, Larkin S. Human papillomavirus 6, 11, and 16 in laryngeal papillomas. J Pathol. 1991;165(3):243–246. doi: 10.1002/path.1711650308. [DOI] [PubMed] [Google Scholar]
- 87.Armstrong LR, Preston EJ, Reichert M, Phillips DL, Nisenbaum R, Todd NW, et al. Incidence and prevalence of recurrent respiratory papillomatosis among children in Atlanta and Seattle. Clin Infect Dis. 2000;31(1):107–109. doi: 10.1086/313914. [DOI] [PubMed] [Google Scholar]
- 88.Lindeberg H. Laryngeal papillomas: histomorphometric evaluation of multiple and solitary lesions. Clin Otolaryngol Allied Sci. 1991;16(3):257–260. doi: 10.1111/j.1365-2273.1991.tb00925.x. [DOI] [PubMed] [Google Scholar]
- 89.Derkay CS, Darrow DH. Recurrent respiratory papillomatosis. Ann Otol Rhinol Laryngol. 2006;115(1):1–11. doi: 10.1177/000348940611500101. [DOI] [PubMed] [Google Scholar]
- 90.Abramson AL, Nouri M, Mullooly V, Fisch G, Steinberg BM. Latent Human Papillomavirus infection is comparable in the larynx and trachea. J Med Virol. 2004;72(3):473–477. doi: 10.1002/jmv.20013. [DOI] [PubMed] [Google Scholar]
- 91.Hobbs CG, Birchall MA. Human papillomavirus infection in the etiology of laryngeal carcinoma. Curr Opin Otolaryngol Head Neck Surg. 2004;12(2):88–92. doi: 10.1097/00020840-200404000-00006. [DOI] [PubMed] [Google Scholar]