Abstract
The Allium genus includes garlic, onions, shallots, leeks, and chives. These vegetables are popular in cuisines worldwide and are valued for their potential medicinal properties. Epidemiological studies, while limited in their abilities to assess Allium consumption, indicate some associations of Allium vegetable consumption with decreased risk of cancer, particularly cancers of the gastrointestinal tract. Limited intervention studies have been conducted to support these associations. The majority of supportive evidence on Allium vegetables cancer preventive effects comes from mechanistic studies. These studies highlight potential mechanisms of individual sulfur-containing compounds and of various preparations and extracts of these vegetables, including decreased bioactivation of carcinogens, antimicrobial activities, and redox modification. Allium vegetables and their components have effects at each stage of carcinogenesis and affect many biological processes that modify cancer risk. This review discusses the cancer preventive effects of Allium vegetables, particularly garlic and onions, and their bioactive sulfur compounds and highlights research gaps.
Keywords: garlic, onion, cancer prevention, Allium
Increasingly governmental entities and other organizations are proposing a wide range of food policies to promote health. These stem from the belief that essential and non-essential food components allow one to achieve his/her genetic potential, increase physical and cognitive performance, and reduce the risk of diseases. Multiple foods have been championed for their medicinal properties with varying degrees of evidence for and against their benefits. Knowledge about foods and their components offers exciting opportunities for modifying agricultural and production approaches to improve health.
Altering dietary habits may be a practical and cost-effective means of reducing cancer risk and modifying tumor behavior. Approximately 30–40% of cancers are preventable by appropriate food and nutrition, physical activity, and maintenance of healthy body weight (1). This means choosing foods that help to maintain a healthy body weight, reducing consumption of foods such as red or processed meats that may increase cancer risk, and increasing consumption of foods that may decrease cancer risk, including foods of plant origin (1). There is an increasing public health demand to identify those dietary patterns, bioactive foods, and components that may decrease cancer risk. One particular group of foods that has raised considerable interest for their putative cancer preventive properties is the Allium genus.
Allium is the Latin word for garlic. It is part of a monocot genus of flowering plants frequently referred to as the onion genus. The genus includes approximately 500 species (2), including edible onions (A. cepa), garlics (A. sativum), shallots (A. ascalonicum), chives (A. schoenoprasum), and leeks (A. porrum). Garlic and onions are originally native to central Asia and are among the oldest cultivated plants in the world (2). Garlic’s edible bulbs are an important culinary spice and constituent of traditional Chinese medicine. The bulbs and leaves of onions have a wide variety of flavors and textures, and therefore many culinary uses. Shallots, which are closely related to onions, are characterized by their less pungent onion flavor and are commonly used in cooked dishes or are pickled. Chives are distinguished by their edible green scapes with mild onion flavor. Leeks have edible leaf sheaths with mild onion flavor. All of these vegetables have been valued in many cultures for their pungent flavors and culinary uses and for their health benefits for over 4000 years (3, 4). Ancient medical texts from Egypt, Greece, Rome, China, and India cite therapeutic applications for Allium vegetables (2). An Egyptian medical papyrus, The Codex Ebers (~1550 B.C.), lists 22 preparations in which garlic was added. Hippocrates advocated garlic as a laxative and a diuretic, and Aristophanes and Galenal suggested garlic for the treatment of uterine tumors. Furthermore, several medicinal uses for both garlic and onion were cited by the Roman naturalist Pliny the Elder in his Historia naturalis.
Intakes
Consumption patterns vary widely, but onions are typically consumed in larger quantities than garlic, chives, shallots, or leeks (5). Obtaining precise estimates of usual intake of Allium vegetables is a complex problem. Garlic, onions, leeks, and shallots are typically used in mixed dishes in varying amounts, and shallots and chives may be used in small amounts or as garnishes. Further, these vegetables, particularly leeks, are frequently used to add flavor to stocks and then removed before consumption. When dehydrated onions or garlic are added to processed or prepared foods, the consumer may not be aware of such additions and therefore may not report consumption of onions or garlic. For all of these reasons, self-report of Allium vegetable use may be unreliable and precise estimates of intake are difficult to obtain.
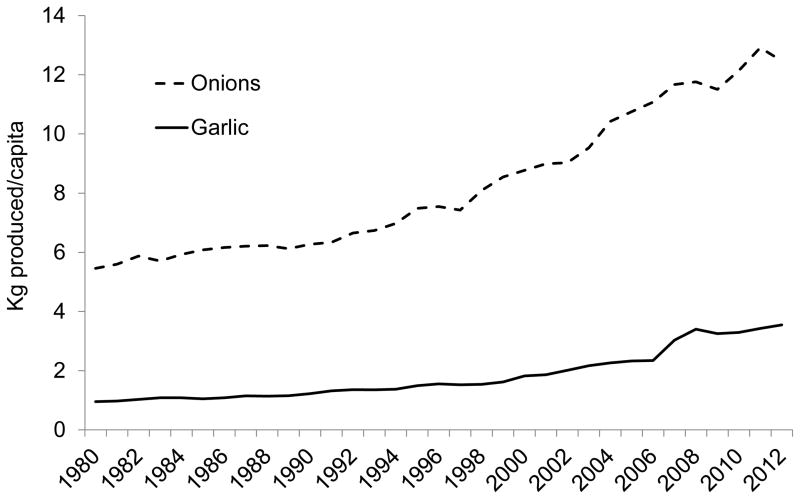
However, data from the Food and Agriculture Organization of the United Nations show that global per capita production of garlic and onions has been increasing steadily since 1980 (Figure 1) (6). The most pungent yellow and white onions have typically been used worldwide for cooking; however, consumption of sweeter onions, chives, and shallots for uncooked use in the U.S. is increasing (7).
Figure 1.
Global per capita production of garlic and onions, 1980–2012 (96). Onions refers to the sum of onions, shallots, green onions, and dried onions.
Bioactive compounds
Allium Vegetables contain similar quantities of many nutrients, particularly macronutrients, though garlic is a richer source of many minerals, including selenium. Onions, because they are consumed in larger quantities than other Allium vegetables, are a more significant dietary source of carbohydrates, fiber, potassium, iron, and vitamin C (Table 1) (8). Allium vegetables contain a variety of bioactive compounds, including flavonoids, oligosaccharides, arginine, and selenium (9); however, much of Allium’s health benefits and the majority of studies on them focus on their sulfur-containing components (9, 10). Thus, this review will focus on the cancer preventive effects of Allium vegetables as a group, of individual Allium vegetables, and of Allium’s bioactive sulfur compounds. A majority of studies focus on garlic and/or onions over the other Allium vegetables; this review will reflect that.
Table 1.
Content of selected nutrients in raw Allium vegetables (8)
| Component | Garlic Amount/100g | Onions Amount/100g | Shallots Amount/100g | Chives Amount/100g | Leeks Amount/100g |
|---|---|---|---|---|---|
| Energy, kcal | 149 | 40 | 72 | 30 | 61 |
| Protein, g | 6.4 | 1.1 | 2.5 | 3.3 | 1.5 |
| Total lipid, g | 0.5 | 0.1 | 0.1 | 0.7 | 0.3 |
| Carbohydrate, g | 33.1 | 9.3 | 16.8 | 4.4 | 14.2 |
| Fiber, total dietary, g | 2.1 | 1.7 | 3.2 | 2.5 | 1.8 |
| Sugars, total, g | 1.0 | 4.2 | 7.9 | 1.9 | 3.9 |
| Calcium, mg | 181 | 23 | 37 | 92 | 59 |
| Iron, mg | 1.70 | 0.21 | 1.20 | 1.60 | 2.10 |
| Magnesium, mg | 25 | 10 | 21 | 42 | 28 |
| Phosphorus, mg | 153 | 29 | 60 | 58 | 35 |
| Potassium, mg | 401 | 146 | 334 | 296 | 180 |
| Selenium, mcg | 14.2 | 0.5 | 1.0 | 0.9 | 1.0 |
| Vitamin C, mg | 31.2 | 7.4 | 8.0 | 58.1 | 12.0 |
| Folate, mcg | 3 | 19 | 34 | 105 | 64 |
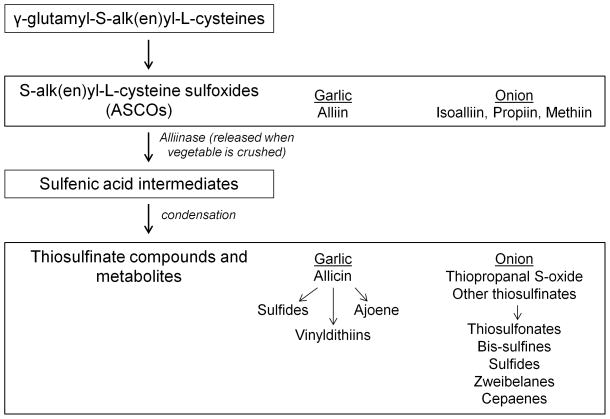
The characteristic flavors and odors of Allium vegetables arise from their sulfur-containing compounds. In fact, sulfur comprises approximately 1% of the dry weight of garlic (11) and up to 0.5% of the dry weight of onions (12). Sulfur-containing compounds in garlic and onions are largely derived from the precursors γ-glutamyl-S-alk(en)yl-L-cysteines and S-alk(en)yl-L-cysteine sulfoxides (ASCOs) (13). Alliin (S-allylcysteine sulfoxide) is the major ASCO found in garlic and isoalliin (trans-(+)-S-(propen-1-yl)-L-cysteine sulfoxide) is the predominant ASCO in onions (5, 13) (Figure 2). Propiin ((+)-S-propyl-L-cysteine) and methiin ((+)-S-methyl-L-cysteine sulfoxide) also contribute to onion’s ASCO content. Upon damage or crushing of the vegetable bulbs, the enzyme alliinase is released from the vacuoles of cells and catalyzes the cleavage of ASCOs to sulfenic acid intermediates (14). The intermediates are highly reactive and rapidly produce thiosulfinate compounds via condensation reactions. The major garlic thiosulfinate produced is allicin (thio-2-propene-1-sulfinic acid S-allyl ester). Allicin and its oil-soluble metabolites are largely responsible for garlic’s odor. Allicin is unstable and breaks down further to ajoene, vinyldithiins, and sulfides including diallyl sulfide (DAS), diallyl disulfide (DADS), and diallyl trisulfide (DATS) (10, 15–17). In onions, cleavage of isoalliin and other precursor compounds and the subsequent condensation of the sulfenic acid intermediates results in the formation of lachrymatory factor (thiopropanal S-oxide), and in thiosulfonates, bis-sulfines, sulfides including DAS, DADS, and DATS; zweiebelanes, and cepaenes, all of which contribute to the flavor of onions (5, 10, 13).
Figure 2.
Bioactive sulfur compounds in Allium vegetables. S-alk(en)yl-L-cysteine sulfoxides (ASCOs) are the precursors to the biologically active compounds in Allium vegetables. ASCOs are formed form γ-glutamyl-S-alk(en)yl-L-cysteines in the vegetables cells. When the vegetables are crushed, chopped, or chewed, the enzyme alliinase is released from vacuoles and catalyzes the conversion of ASCOs to highly reactive sulfenic acid intermediates. Thiosulfinate compounds are formed from the condensation of these intermediates. Thiosulfinate compounds and their metabolites are responsible for the characteristic flavors and odors of Allium vegetables. Predominant compounds of ASCOs and thiosulfinate compounds/metabolites in garlic and onions are listed. See text for citations of references.
Garlic and onions are available in multiple fresh and dehydrated preparations, and are used in a wide variety of dishes. Temperature, pH, time, processing, and the food matrix can influence the activity of alliinase and the stability of the bioactive compounds. Heating results in denaturation of alliinase, which leads to decreased allicin metabolites in garlic. This decrease in sulfur compounds is associated with a decrease in garlic odor and in a reduction of garlic’s anticancer and antimicrobial potential (18). While multiple sulfur compounds may have potential preventive activities, the compounds must first be generated from the parent compounds in the vegetables. Storage of garlic over time results in an increase in alliin due to increased transformation of γ-glutamylcysteines (9). Investigators characterized crushed fresh garlic, enteric-coated dried fresh garlic, and dried aged garlic extract for use in a clinical trial (19). They found that S-allylcysteine (SAC) was stable for 12 months at ambient temperature. In addition, crushing fresh garlic had minimal impact on the stability of allyl thiosulfinates after 3 days when the garlic was added to various condiments, including creamy horseradish, salsa, and fat-free mayonnaise, sour cream, and yogurt.
In a study of the effect of processing or cooking on onion thiosulfinate content, cooking by convection oven reduced the total thiosulfinate content of whole onions by >5-fold after 20–30 minutes and microwave heating similarly reduced thiosulfinate content within 0.75 seconds/g onion. Crushed onions retained the same levels of total thiosulfinate compounds as raw onions, even after convection oven or microwave heating (20). With alliinase destroyed from cooking, onions lost the enzymatic ability to form bioactive components, while many thiosulfinates formed prior to cooking remained stable.
Information on the bioavailability and pharmacokinetics of Allium-derived sulfur compounds in humans is limited. By measuring breath acetone and breath allyl methyl sulfide (AMS) in humans following consumption of various garlic preparations or individual sulfur compounds, Lawson and Wang concluded that allicin and its metabolites are rapidly converted to AMS after consumption (21). By measuring breath AMS for 32 h following consumption of crushed raw garlic and a commercially available garlic powder, Lawson and Wang found that the bioavailability of allyl thiosulfinates in human subjects was found to be similar for the two preparations (19). However, peak breath allyl methyl sulfide concentrations were reached 3.1 hours sooner in subjects consuming raw garlic (2.9 h vs. 6.0 h for raw garlic and garlic powder, respectively). At 32 h, the breath concentrations of AMS concentrations were less than 1% of the maximum concentration for both preparations.
Lachmann and colleagues reported the distribution of constituents from garlic oil macerate feeding to rats (22). 65% of Allicin was absorbed and 73% of vinyldithiins were absorbed, as suggested by urinary excretions. Blood concentrations of 35S-labeled alliin, allicin, and vinyldithiins peaked at 10 minutes, 30–60 minutes, and 120 minutes, respectively, following exposure. Allicin and vinyldithiins were still present in the blood when the study concluded at 72 h. In another study, allicin was rapidly transformed in rat liver to DADS and allyl mercaptan (AM) (23). DADS was also found to be transformed to AM, AMS, allyl methyl sulfoxide, and allyl methyl sulphone in stomach, plasma, liver and urine of rats (24).
The intriguing chemistry and metabolism of Allium vegetables and their sulfur-containing compounds has helped to stimulate the study of these vegetables, especially garlic and onions, in health and disease, including cancer prevention.
Epidemiological studies on Allium vegetables and cancer risk
While epidemiological studies support some association between increased intake of onions and/or garlic and decreased risk of certain cancers, the data are limited and sometimes conflicting. Additionally, Allium vegetables are frequently grouped together for epidemiological analysis, which prohibits separation of effects. The strongest epidemiological evidence points to protective effects of garlic and/or onions against cancers of the digestive tract. These observations from recent systematic reviews and meta analyses, as well as more recent epidemiological studies that have not yet been included in such reviews, are summarized below.
Stomach cancer
A recent meta-analysis of 19 case-control and 2 cohort studies showed that consumption of large amounts of total Allium vegetables reduced risk of gastric cancer when comparing the highest and lowest consumption groups (odds ratio (OR): 0.54; 95% CI 0.43–0.65) (25). Results were similar for individual Allium vegetables, including garlic, onion, leeks, Chinese chives, scallions, and garlic stalks, but not onion leaves. The summary OR for decreased risk of gastric cancer with an increment of 20 g/day of total Allium vegetables, (the average weight of one garlic bulb), was 0.91 (95% CI 0.88–0.94).
The World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) expert panel also conducted a meta-analysis using 14 case-control studies that investigated Allium vegetables and stomach cancer and 5 case-control studies that investigated garlic and stomach cancer. Their summary OR was 0.59 (95% CI 0.47–0.74) per 100 g per day for total Allium vegetable intake with high heterogeneity, and 0.41 (95% CI 0.23–0.73) per serving of garlic per day (1). They also conducted meta analyses of two cohort studies that investigated total Allium vegetables and cancer, which produced a summary effect estimate of 0.55 (95% CI 035–0.87) per 100 g per day with no heterogeneity. They concluded that due to consistent evidence, dose-response relationship, and plausible mechanisms, a cancer preventive relationship between Allium vegetables and vegetables was probable (1).
Investigators with the European Prospective Investigation into Cancer and Nutrition (EPIC) study were unable to corroborate the findings of the above meta-analyses. After 6.5 years of follow-up and 330 cases of gastric cancer, Allium vegetables (garlic, young garlic, onion, and shallot) collectively were associated with a weak, non-significant inverse association with gastric adenocarcinoma (26). However, after 11 years of follow-up and 683 cases, even this weak association was no longer evident for total gastric adenocarcinoma. Allium vegetables were also not associated with decreased risk of cardia, noncardia, intestinal, and diffuse subtypes of gastric cancer (27). The EPIC cohort, which included men and women aged 35–70 recruited from 1992–1998, had relatively high fruit and vegetable consumption even in the lowest quintile of intake, thus making it difficult to compare high to low or no intake.
Colorectal cancer
A meta-analysis of 8 different prospective cohort studies on total Allium vegetable intake and colorectal cancer risk showed no decrease in risk with increased Allium consumption (28). The WCRF/AICR panel found no statistically significantly decreased risk of colorectal cancer with highest garlic intake in two prospective cohorts, while three case-control studies showed significantly decreased risk of colorectal cancer for those with the highest garlic intake and three other case-control studies did not (1).
Other case-control evidence has been suggestive of benefit. In a network of Italian and Swiss case-control studies that included 1037 cases and 2020 controls, intake of onions and garlic was assessed separately via food frequency questionnaire (29). The investigators found that both onions and garlic were protective against cancers of the large bowel. All levels of onion intake were associated with decreased risk (p-trend <0.0001) compared to non-consumers of onion. The highest of 4 categories of onion consumption (≥7 servings per week) was associated with a decreased risk of colorectal cancer with an OR of 0.44 (95% CI 0.28–0.67). Intermediate and high garlic use were associated with decreased risk of colorectal cancer with ORs of 0.88 (95% CI 0.78–0.98) and 0.74 (95%CI 0.63–8.86), respectively, compared to low or no use (p-trend <0.001). In this study, subjects were classified into no, low, intermediate, and high usage categories for both onions and garlic based on 2 questions included in a food frequency questionnaire. No information of preparation or cooking of the vegetables was available, nor was information on whether subjects were taking garlic supplements.
The association of onion and garlic intake on colorectal adenoma was also assessed using 562 cases and 5932 controls from subjects in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (30). Investigators assessed onion and garlic intake collectively for the 12 months prior to screening using a food frequency questionnaire. After adjustment for multiple non-dietary colorectal cancer risk factors, the fifth quintile of garlic/onion intake was associated with an OR of colorectal adenoma of 0.87 (95% CI 0.77–0.99) compared to the lowest quintile.
Esophageal cancer
Chen et al (31) found that consumption of raw garlic/onion at least one time per week was associated with an adjusted OR of esophageal cancer of 0.2 (95% CI 0.1–0.5) in Taiwanese men. Cooked garlic or onions were not addressed in this study. The Italian and Swiss case-control study also reported that consumption of ≥7 portions of onions per week was protective against esophageal cancer (OR = 0.12; 95% CI 0.02–0.58) and that increasing garlic intake was protective (p-trend <0.0001) with the highest intake associated with an OR of 0.74 (0.64–0.86) (29).
The WCRF/AICR report reviewed one cohort and eight case-control studies that assessed the effect of garlic, onions, or Allium vegetables collectively on risk of esophageal cancer (1). Only one of the case-control studies showed a statistically significant decrease in risk.
Prostate cancer
In a population-based case-control study in Shanghai, Hsing et. al. (32) found that individuals in the highest of 3 intake categories of total Allium vegetables had a 53% decreased OR of prostate cancer compared to those with the lowest intake (OR = 0.47; 95% CI 0.34–0.76; p-trend <0.001). Both garlic (OR = 0.47; 95%CI 0.31–0.71, p-trend <0.001) and scallion (OR = 0.30; 95% CI 0.18–0.51; p-trend<0.001) alone were also associated with decreased ORs, while Chinese chives, leeks, and onions were not.
The Italian and Swiss case-control networks study showed that those with the highest intake of both onions and garlic did not have significantly decreased OR of prostate cancer compared to those with the lowest intake (OR onions = 0.29; 95% CI 0.07–1.03; OR garlic = 0.81; 95% CI 0.64–1.00), although there was evidence of a trend of decreased odds with increasing intake (p-trend for both onions and garlic = 0.05). A third study, using data from the Vitamins And Lifestyle cohort, analyzed the association of garlic supplements on prostate cancer risk and found that garlic supplement use was not associated with a decreased risk of prostate cancer among men in western Washington state (HR = 1.00; 95% CI 0.85–1.17) (33).
Other cancer sites
In addition to the above in colorectal, esophageal, and prostate cancer, the investigators of the Italian and Swiss case-control studies investigated other possible associations between onions or garlic and cancers of the oral cavity/pharynx, larynx, renal cells, breast, ovary, and endometrium (29, 34). Statistically significant inverse associations were observed for those who ate onions ≥7 times/week for oral cavity/pharyngeal cancers (OR = 0.16; 95% CI 0.06–0.46; p-trend=0.02), laryngeal cancer (OR = 0.17; 95% CI 0.02–0.58; p-trend<0.0001), and ovarian cancer (OR = 0.27; 95% CI 0.08–0.85; p-trend=0.0005) compared to those who did not eat onions. Additionally, 1–7 servings of onions/week was associated with significantly decreased odds of laryngeal (OR = 0.44; 95% CI 0.30–0.63) and ovarian cancers (OR = 0.57; 95% CI 0.43–0.75) compared to nonusers, and >2 compared to 0 servings/week was associated with decreased risk of endometrial cancer (OR = 0.40; 95% CI 0.22–0.72; p-trend = 0.01). High garlic intake was also associated with decreased odds of oral cavity/pharyngeal cancer (OR = 0.43; 95% CI 0.28–0.67; p-trend = 0.002), laryngeal cancer (OR = 0.56; 95% CI 0.38–0.82; p-trend = 0.005), ovarian cancer (OR = 0.78; 95% CI 0.62–0.98; p-trend = 0.10), renal cell carcinoma (OR = 0.69; 95% CI 0.53–0.92; p-trend = 0.003), and endometrial cancer (OR = 0.62; 95% CI 0.42–0.92; p-trend = 0.02) compared to low or no garlic intake. No significant associations were observed for onions or garlic and breast cancer or for onions and renal cell carcinoma.
A study of cases from the Hawaii Tumor Registry and controls randomly selected from a list of Oahu residents found that onions (OR = 0.5; 95% CI 0.3–0.9; p-trend = 0.001), but not garlic (OR = 0.7; 95% CI 0.4–1.1; p-trend = 0.12), were associated with decreased risk of lung cancer in those in the highest quartile of intake compared to the lowest (35). When lung cancer subtypes were considered, onions were more strongly associated with decreased risk of squamous cell carcinoma (OR = 0.1; 95% CI 0.0–0.6; p-trend = 0.003) than with adenocarcinoma (OR = 0.6; 95% CI 0.3–1.2; p-trend = 0.24). The association with squamous cell carcinoma was modified by CYP1A1 genotype, as onions were more protective in those with the homozygous wild-type *1/*1 genotype compared to those with *1/*2 or *2/*2 genotypes.
Further studies on the VITAL cohort revealed that high use of garlic supplements over 10 years prior to baseline was associated with 45% decreased odds (95% CI 0.34–0.87; p-trend = 0.028) of hematological malignancies compared no or low use (36). Additional analyses of data from the EPIC cohort found that after 9 years of follow-up, garlic and onions combined had no effect on cervical carcinoma in situ or on invasive squamous cervical cancer (27).
Summary of epidemiological studies
Epidemiological studies provide variable evidence for cancer preventive activities of garlic, onions, and related Allium vegetables, with stronger evidence for prevention of cancers of the gastrointestinal tract, including gastric, colorectal, and to some extent esophageal cancers. However, not all individuals respond to increased consumption, based on a host of factors including genetics, preparation of the vegetables, or other dietary components. Body composition, previous history of cancer or precancerous conditions, or other cancer risk factors likely also contribute to determining those who will respond the most. In addition, other factors including epigenetic modifications and variability in the gut microbiome, currently largely unexplored in studies on associations between Allium intake and cancer risk, may also influence responses.
Epidemiological studies are powerful tools for determining associations with cancer risk in large populations. However, these studies have limitations, including multiple testing and potential for false discovery. In addition, studying associations with Allium vegetables proves to be particularly challenging due to the difficulty in assessment of intake levels. The common use of quantiles in epidemiological studies makes it difficult to compare results across studies, as many cohorts have different ranges of Allium or total vegetable intake. Improved methods for assessment of intake of garlic and onions may help to further clarify the relationship between these vegetables and cancer risk.
Intervention studies
Few intervention studies have examined the efficacy of dietary or supplemental Allium vegetables in cancer prevention. One double-blind randomized controlled trial in Japanese patients with colorectal adenomas revealed that a higher dose of aged garlic extract reduced the risk of new colorectal adenomas by 50% compared to a lower dose garlic extract (37). Synthetic DATS (200 mg/day) plus selenium (100 μg every other day) was assessed in a randomized placebo-controlled double-blind clinical trial in Shandong, China (38). Incidence of neither total nor gastric cancer decreased significantly in the overall study population after five years, though the combined intervention resulted in a relative risk for all tumors of 0.51 (95% CI 0.30–0.85) and for gastric cancer of 0.36 (95% CI 0.14–0.92) compared to controls in males only. Another randomized multi-intervention trial in Shandong, China tested the efficacy of 800 mg garlic extract plus 4 mg steam-distilled garlic oil daily to inhibit progression of precancerous gastric lesions (39). After 7.3 years of follow up, the garlic treatment did not decrease the prevalence of precancerous gastric lesions, nor did it significantly affect gastric cancer incidence (OR 0.80, 95% CI 0.53–120) or mortality (OR 0.92, 95% CI 0.75–1.12) (40). Further intervention studies are warranted to overcome the confounding issues of epidemiological analysis of Allium vegetables and cancer risk, including accurate assessment of intake and separation of effects of garlic and onions.
Possible mechanisms
Mechanistic studies provide compelling evidence that garlic, onions, and their sulfur components alter the biological behavior of tumors, tumor microenvironments, or precancerous cells, and decrease cancer risk. No animal studies have examined whole Allium vegetables, though multiple garlic preparations and compounds have demonstrated efficacy against carcinogenesis in preclinical rodent models. DATS, DADS, DAS, SAC, ajoene, garlic powder, and garlic extracts (reviewed in (41) and (42)) all inhibited cancers in multiple sites in various rodent xenograft, spontaneous, or chemically-induced models of carcinogenesis. Cancer protection by Allium vegetables may arise from several mechanisms, detailed below. It is probable that several of these cellular events are modified simultaneously.
A. Cancer Initiation
Inhibition of nitrosamines and heterocyclic amines
Nitrosamines and heterocyclic amines (HCA) are potential dietary carcinogens which are not normally present in foods but may arise during preservation or cooking (43). Evidence points to the ability of allyl sulfur compounds to suppress the spontaneous and bacterial mediated formation of nitrosamines (44), although not all allyl sulfur compounds prevent nitrosamine formation equally. SAC and its analog S-propyl cysteine, but not DADS, dipropyl disulfide, or DAS, retarded nitrosamine formation, suggesting a critical role for a cysteine residue in inhibition (45). Further, water extracts of garlic, deodorized garlic powder, and onion, but not leeks, decreased nitrosamine formation (45). The reduction in nitrosamine formation may be a result of increased formation of nitrosothiols, as sulfur compounds might reduce nitrite availability for nitrosamine formation (46).
In humans, ingesting 5 g of garlic per day completely blocked the enhanced urinary excretion of nitrosoproline, an indicator for the synthesis of potentially carcinogenic nitrosamines, that occurred as a result of ingesting supplemental nitrate and proline (47). More recent evidence suggests that as little as 1 g of garlic may be sufficient to suppress nitrosoproline formation (48).
Allium allyl sulfur compounds are also effective in blocking DNA alkylation, an early step in nitrosamine carcinogenesis (49). Consistent with this reduction in bioactivation, Dion et. al. found that both water-soluble SAC and lipid-soluble DADS blocked nitrosomorpholine mutagenicity in Salmonella typhimurium TA100 (45). A block in nitrosamine bioactivation may arise from inactivation of cytochrome P450 2E1 (CYP2E1) (50, 51). Inhibition of CYP2E1 by an autocatalytic destruction mechanism may account for some of the chemoprotective effects of DAS and potentially other allyl sulfur compounds (52). Fluctuations in the content and overall activity of CYP2E1 may be an important variable in determining the magnitude of the protection provided by garlic and associated allyl sulfur components.
HCAs arise from surfaces of well-done meats (43). Addition of onion powder to hamburger meat prior to cooking decreased the HCAs 2-amino-3,8-dimethylimidazo (4,5- f) quinoxaline (MeIQx) and 2-amino-1-methyl-6-phenylimidazo(4,5-b)pyridine (PhIP) by 73% and 94.3%, respectively (53). Garlic powder reduced levels of MeIQx and PhIP by 66.2 and 85.0%, respectively. A separate study showed that addition of 2 g of fresh cut onion to beef patties fried at 230 °C for 8 min on each side inhibited the formation of MeIQx by 88% and PhIP by 79%, respectively (54), suggesting that a variety of onion preparations could be effective. In a study testing the ability of oil marinades containing various levels of onion, garlic, and lemon juice to inhibit HCA formation, increasing garlic and onion, but not lemon juice, in the marinades significantly decreased the formation of MeIQx. The concentrations of onion, garlic, and lemon that led to the maximal MeIQx reduction were 31.2, 28.6, and 14.6%, respectively (55).
Once ingested, HCAs are bioactivated by N-oxidation via cytochrome P4501A2, N-acetyltransferases (NAT), and sulfotransferases, to electrophilic species that are much more reactive with DNA than HCAs are. Yu et. al. demonstrated that a suppression in NAT mRNA expression accounts for the majority of the reduction in the activity of garlic (56). Platt et. al. demonstrated that onion juice inhibited the genotoxicity of the HCA 2-amino-3-methylimidazo{4,5-f}quinoline in genetically engineered metabolically active Chinese hamster lung fibroblasts with IC50 of 0.54% (v/v) via inhibition of human NAT activity (57).
Bioactivation and detoxification
Allium vegetables and several of their allyl sulfur compounds can also effectively block the bioactivation and carcinogenicity of non-nitrosamines/HCAs. This protection involves a diverse array of compounds and several target tissue sites, suggestive of multiple mechanisms and/or a widespread biological effect.
Garlic extracts and compounds reduced the incidence of tumors resulting from the carcinogen N-nitroso-N-methylurea (MNU) (58). SAC and DADS in the diet decreased MNU-induced O6-methylguanine adducts bound to mammary cell DNA in rats (59). Dried onion at 5% in the rat diet reduced the number and size of aberrant crypt foci induced by azoxymethane or MNU (60). Aqueous garlic extracts reduced the mutagenicity of ionizing radiation, peroxides, Adriamycin, and N-methyl-N′-nitrosoguanidine (61). DAS diminished the DNA hypermethylation of esophagus, liver, and nasal mucosa that arose from treatment with N-nitrosomethylbenzylamine (62). However, Cohen et. al. reported that SAC did not protect against MNU-induced mammary tumors (63). The reason for this discrepancy is unknown but may be reflective of the proportion of lipid in the diet or the dose of carcinogen given. If garlic and onion compounds are indeed effective blockers of carcinogenicity of these compounds, the mechanism(s) remain unresolved. The role of organosulfur compounds in biotransformation may be substrate-specific.
The observed protection from garlic and onions may also involve other enzymes involved in the bioactivation or removal of carcinogenic metabolites. Singh et. al. provided evidence that the efficacy of various organosulfides to suppress benzo(a)pyrene tumorigenesis was correlated with their ability to induce NAD(P)H:quinone oxidoreductase (NQO1), an enzyme involved with the removal of quinones associated with this carcinogen (64). This inductive effect appears to be mediated by the antioxidant response element enhancer sequence bound by the nuclear factor E2-related factor 2 (Nrf2) in the NQO1 and the heme oxygenase 1 (HO1) gene promoters. DAS, DADS, and DATS all differentially mediated the transcriptional levels of NQO1 and HO1, with DATS having the strongest effect (65).
Phase II detoxification, specifically changes in glutathione concentration and the activity of specific glutathione-S-transferases (GST), may also be important in the protection provided by garlic and onions. Both DADS and DATS increased the activity of GST in a variety of rat tissues (66). Garlic also protected against aflatoxin carcinogenesis in rats via induction of GST A5 levels (67).
B. Cancer Promotion
Garlic and onion constituents, including DADS, DATS, S-allylmercaptocysteine, and ajoene, have the ability to suppress proliferation of a wide variety of cancer cells by retarding cell cycle progression and/or inducing apoptosis (42, 68, 69). Several complex and potentially coordinated mechanisms have been cited for the effect of garlic and onion constituents on cell cycle arrest, including reduced Cdk1/cyclin B kinase activity or activation of ERK1/2 (68, 70). For example, DADS suppressed Cdk1 activity during cell cycle arrest in G2/M that was associated with a temporal and dose-dependent increase in cyclin B1 protein level, a reduction in the level of Cdk1-cyclin B1 complex formation, an inactivation of Cdk1 by hyperphosphorylation, and a decrease in Cdc25C protein level (71). Further gene expression analysis suggested that alterations in DNA repair and cellular adhesion factors may also be involved in the G2/M block following DADS exposure (70).
Current knowledge of the mechanisms by which these compounds cause apoptosis indicates that they target various apoptotic signaling molecules from initiation to execution. Molecules affected include the map kinases JNK, ERK1/2, and p38; p53, NF-κB, bcl-2/bax family constituents, and caspases, but not all of the signaling molecules were influenced by each Allium compound (68). However, in many studies, the apoptotic effects of sulfur compounds were triggered by increased intracellular production of reactive oxygen species (ROS), suggesting the importance of the intracellular redox environment for induction of apoptosis. For example, DATS increased hydrogen peroxide formation, lowered thiol levels, and induced caspase 3 activity in HepG2 cells (72).
C. Antimicrobial properties
Substantial evidence indicates that garlic extracts can inhibit a range of Gram-negative and Gram-positive bacteria, and serve as antifungal agents (73). Various sulfur compounds including allicin, DAS, DADS, and ajoene derivatives may contribute to garlic’s antimicrobial properties (74). Garlic’s inhibitory effect against the bacteria Helicobacter pylori is of note as H. pylori colonization of the gastric mucosa is linked with gastritis and a greater propensity to develop gastric cancer. Cellini et al. (75) demonstrated that aqueous garlic extracts (2–5 mg/ml) inhibited H. pylori proliferation. Both DAS and DADS elicited a dose dependent depression in H. pylori proliferation in culture (76). Raw garlic extracts and three commercially available garlic tablets varied in their efficacy against H. pylori, as indicated by minimum inhibitory concentrations in the range between 10 to 17.5 μg dry weight/ml (77).
The ability of garlic to reduce H. pylori infection in humans is inconclusive. While an epidemiological study suggested an association between increased garlic consumption and reduced H. pylori infection (78), two clinical studies that tested different garlic preparations in H. pylori infected subjects did not show efficacy (79, 80). Neither of these interventions resulted in the elimination of the organism, change in the severity of gastritis, or a significant change in symptom scores. Both studies were not randomized and had small sample sizes, suggesting that a well-designed clinical trial is still needed to determine the efficacy of garlic consumption in reducing H. pylori infection and symptoms.
The primary antimicrobial effect of garlic may reflect chemical reactions with selected thiol groups of enzymes and/or a change in the overall redox state of the organism. Specifically, the antimicrobial action of allicin and its breakdown products has been suggested to be due to its rapid interaction with SH-containing molecules, including amino acids and cellular proteins within microbial organisms (81). Changes in thiol status have been suggested as one possible mechanism by which garlic and related sulfur compounds might also suppress tumor proliferation.
D. Redox modification
Some, but not all, Allium organosulfur compounds have reported antioxidant properties. Alliin and allicin possessed antioxidant properties in a Fenton oxygen-radical generating system (82). DADS, but not DAS, dipropyl sulfide or dipropyl disulfide, inhibited liver microsomal lipid peroxidation induced by NADPH, ascorbate and doxorubicin (83). The presence of both the allyl and sulfur groups appears to magnify the antioxidant capabilities of the molecule. Both the number of sulfur atoms and the oxidation state of sulfur atoms can influence the overall antioxidant potential (84).
Garlic oil was also effective antioxidant against oxidative damage caused by various agents (85), indicating that lipid-soluble organosulfur compounds can also be effective antioxidants. While some ether extracted garlic oil preparations in the marketplace may contain about nine times as much vinyldithiins and four times as much ajoene as crushed fresh garlic, these preparations had no free radical scavenging properties, again indicating not all organosulfur constituents have antioxidant properties (16).
Allium vegetables also contain a wide variety of other antioxidant compounds including flavonoids, glutathione, selenium compounds, and vitamins E and C. Nuutila et. al. found that the scavenging activities of onion and garlic extracts correlated with the total phenolic content. Onions had higher radical scavenging activities than garlic, red onions had higher activities than yellow onions, and outer portions of onion bulbs had higher activities than inner portions (86).
E. Inflammation
While limited, there is some evidence that Allium-associated sulfur components can inhibit cyclooxygenase (COX) and lipoxygenase activities (87, 88). DAS, DADS, and, to a lesser extent, AMS differentially regulated nitric oxide, prostaglandin E2 (PGE2), and cytokine production in mouse macrophages stimulated by lipopolysaccharide (LPS) (89). Similar to several nonsteroidal anti-inflammatory drugs, ajoene inhibited the release of PGE2 from LPS-activated RAW 264.7 cells, which was associated with a dose-dependent inhibition of COX-2 enzyme activity (90).
F. Immune modulation
Garlic, onions, and their extracts and compounds may also have immunomodulatory effects. Both an aqueous and ethanolic extract of garlic powder significantly stimulated proliferation of rat spleen lymphocytes in culture. This correlated with the up regulation of interleukin 2 (IL2) receptor alpha expression and an increase in IL2 production (91). The effect of the extracts on lymphocyte proliferation in vitro differed depending on the specific stimulator of cell proliferation. Garlic powder extracts also modulated proliferation of rat thymocytes and splenocytes in response to concanavalin A in vitro. At higher concentrations, the extracts had an inhibitory effect on T cell proliferation but lower concentrations of extracts resulted in an increase in T cell proliferation. Additionally, DAS treatment of BALB/c mice blocked the suppression of the antibody response caused by N-nitrosodimethylamine to T cell-dependent antigens, and the lymphoproliferative response to T cell and the B cell mitogens (92).
Conclusion
For centuries, Allium vegetables have been used in a wide variety of cuisines worldwide and are valued for their potential medicinal properties. During the first Olympic Games in Greece, garlic was consumed as a stimulant and in Roman times, soldiers chewed garlic before battle for strength. Presently, these vegetables continue to hold their fascination for their unique flavor, chemistry, and biological properties. Epidemiological studies indicate some protective associations of Allium vegetable consumption against cancers, particularly cancers of the gastrointestinal tract. However, difficulties in assessing Allium consumption hamper efforts to further define these effects. If garlic consumption does reduce the risk of cancer, the amount needed to lower risk remains unknown. Intervention studies have potential to overcome challenges present in epidemiological studies. Mechanistic studies indicate potential mechanisms of the anticancer activity of various Allium vegetable extracts and preparations, and highlight the activities of the sulfur-containing compounds. These compounds have effects at each stage of carcinogenesis and affect many physiological processes that modify cancer risk.
Several research gaps exist. Further research is warranted to improve methods for assessment of consumption of Allium vegetables for epidemiological studies. Randomized, controlled trials on the effects of garlic/onion consumption will help to address the issues with intake assessment and with possible confounding factors. Furthermore, the effect of Allium vegetables on cancer processes cannot be considered in isolation; rather, they are likely dependent on several environmental and dietary variables. Dietary factors that have shown effect modification include total fat, selenium, methionine and vitamin A (69). Other factors that may influence the relationship of Allium vegetables and cancer prevention are the interactions of these foods and their constituents with the oral and gut microbiota (93, 94). Additionally, the contribution of Allium vegetables to thiol signaling is an emerging area in redox biology that requires further study (95). Finally, determining those who will respond most to increased consumption and determining the optimal amount(s) and preparation(s) of Allium vegetables for cancer prevention will aid the scientific community in making public health recommendations for garlic and onion consumption.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.World Cancer Research Fund / American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington DC: AICR; 2007. [Google Scholar]
- 2.Guercio V, Galeone C, Turati F, La Vecchia C. Gastric cancer and allium vegetable intake: a critical review of the experimental and epidemiologic evidence. Nutrition and cancer. 2014;66:757–73. doi: 10.1080/01635581.2014.904911. [DOI] [PubMed] [Google Scholar]
- 3.Milner JA. Garlic: its anticarcinogenic and antitumorigenic properties. Nutrition reviews. 1996;54:S82–6. doi: 10.1111/j.1753-4887.1996.tb03823.x. [DOI] [PubMed] [Google Scholar]
- 4.Sengupta A, Ghosh S, Das S. Modulatory influence of garlic and tomato on cyclooxygenase-2 activity, cell proliferation and apoptosis during azoxymethane induced colon carcinogenesis in rat. Cancer letters. 2004;208:127–36. doi: 10.1016/j.canlet.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 5.Griffiths G, Trueman L, Crowther T, Thomas B, Smith B. Onions--a global benefit to health. Phytotherapy research : PTR. 2002;16:603–15. doi: 10.1002/ptr.1222. [DOI] [PubMed] [Google Scholar]
- 6.FAOSTAT. Food and Agriculture Organization of the United Nations Statistics Division. 2014 faostat.fao.org.
- 7.Lucier G. Onions: The Sweet Smell of Success. 1998. Report No.: AO-255. [Google Scholar]
- 8.U.S. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 24. Nutrient Data Laboratory Home Page. 2011 http://www.ars.usda.gov/ba/bhnrc/ndl.
- 9.Milner JA. Preclinical perspectives on garlic and cancer. The Journal of nutrition. 2006;136:827S–31S. doi: 10.1093/jn/136.3.727S. [DOI] [PubMed] [Google Scholar]
- 10.Block E. The chemistry of garlic and onions. Scientific American. 1985;252:114–9. doi: 10.1038/scientificamerican0385-114. [DOI] [PubMed] [Google Scholar]
- 11.Fenwick GR, Hanley AB. The genus Allium--Part 3. Critical reviews in food science and nutrition. 1985;23:1–73. doi: 10.1080/10408398509527419. [DOI] [PubMed] [Google Scholar]
- 12.Lee EJ, Yoo KS, Jifon J, Patil BS. Characterization of shortday onion cultivars of 3 pungency levels with flavor precursor, free amino acid, sulfur, and sugar contents. Journal of food science. 2009;74:C475–80. doi: 10.1111/j.1750-3841.2009.01243.x. [DOI] [PubMed] [Google Scholar]
- 13.Kubec R, Svobodova M, Velisek J. Gas chromatographic determination of S-alk(en)ylcysteine sulfoxides. Journal of chromatography A. 1999;862:85–94. doi: 10.1016/s0021-9673(99)00902-4. [DOI] [PubMed] [Google Scholar]
- 14.Lanzotti V. The analysis of onion and garlic. Journal of chromatography A. 2006;1112:3–22. doi: 10.1016/j.chroma.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Lawson LD, Wang ZJ, Hughes BG. Identification and HPLC quantitation of the sulfides and dialk(en)yl thiosulfinates in commercial garlic products. Planta medica. 1991;57:363–70. doi: 10.1055/s-2006-960119. [DOI] [PubMed] [Google Scholar]
- 16.Lawson LD, Wood SG, Hughes BG. HPLC analysis of allicin and other thiosulfinates in garlic clove homogenates. Planta medica. 1991;57:263–70. doi: 10.1055/s-2006-960087. [DOI] [PubMed] [Google Scholar]
- 17.Tamaki T, Sonoki S. Volatile sulfur compounds in human expiration after eating raw or heat-treated garlic. Journal of nutritional science and vitaminology. 1999;45:213–22. doi: 10.3177/jnsv.45.213. [DOI] [PubMed] [Google Scholar]
- 18.Song K, Milner JA. The influence of heating on the anticancer properties of garlic. The Journal of nutrition. 2001;131:1054S–7S. doi: 10.1093/jn/131.3.1054S. [DOI] [PubMed] [Google Scholar]
- 19.Lawson LD, Gardner CD. Composition, stability, and bioavailability of garlic products used in a clinical trial. Journal of agricultural and food chemistry. 2005;53:6254–61. doi: 10.1021/jf050536+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cavagnaro PF, Galmarini CR. Effect of processing and cooking conditions on onion (Allium cepa L.) induced antiplatelet activity and thiosulfinate content. Journal of agricultural and food chemistry. 2012;60:8731–7. doi: 10.1021/jf301793b. [DOI] [PubMed] [Google Scholar]
- 21.Lawson LD, Wang ZJ. Allicin and allicin-derived garlic compounds increase breath acetone through allyl methyl sulfide: use in measuring allicin bioavailability. Journal of agricultural and food chemistry. 2005;53:1974–83. doi: 10.1021/jf048323s. [DOI] [PubMed] [Google Scholar]
- 22.Lachmann G, Lorenz D, Radeck W, Steiper M. The pharmacokinetics of the S35 labeled labeled garlic constituents alliin, allicin and vinyldithiine. Arzneimittel-Forschung. 1994;44:734–43. [PubMed] [Google Scholar]
- 23.Egen-Schwind C, Eckard R, Kemper FH. Metabolism of garlic constituents in the isolated perfused rat liver. Planta medica. 1992;58:301–5. doi: 10.1055/s-2006-961471. [DOI] [PubMed] [Google Scholar]
- 24.Germain E, Auger J, Ginies C, Siess MH, Teyssier C. In vivo metabolism of diallyl disulphide in the rat: identification of two new metabolites. Xenobiotica; the fate of foreign compounds in biological systems. 2002;32:1127–38. doi: 10.1080/0049825021000017902. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Y, Zhuang W, Hu W, Liu GJ, Wu TX, Wu XT. Consumption of large amounts of Allium vegetables reduces risk for gastric cancer in a meta-analysis. Gastroenterology. 2011;141:80–9. doi: 10.1053/j.gastro.2011.03.057. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez CA, Pera G, Agudo A, Bueno-de-Mesquita HB, Ceroti M, Boeing H, et al. Fruit and vegetable intake and the risk of stomach and oesophagus adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) International journal of cancer Journal international du cancer. 2006;118:2559–66. doi: 10.1002/ijc.21678. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez CA, Travier N, Lujan-Barroso L, Castellsague X, Bosch FX, Roura E, et al. Dietary factors and in situ and invasive cervical cancer risk in the European prospective investigation into cancer and nutrition study. International journal of cancer Journal international du cancer. 2011;129:449–59. doi: 10.1002/ijc.25679. [DOI] [PubMed] [Google Scholar]
- 28.Zhu B, Zou L, Qi L, Zhong R, Miao X. Allium Vegetables and Garlic Supplements Do Not Reduce Risk of Colorectal Cancer, Based on Meta-analysis of Prospective Studies. Clin Gastroenterol Hepatol. 2014 doi: 10.1016/j.cgh.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 29.Galeone C, Pelucchi C, Levi F, Negri E, Franceschi S, Talamini R, et al. Onion and garlic use and human cancer. The American journal of clinical nutrition. 2006;84:1027–32. doi: 10.1093/ajcn/84.5.1027. [DOI] [PubMed] [Google Scholar]
- 30.Millen AE, Subar AF, Graubard BI, Peters U, Hayes RB, Weissfeld JL, et al. Fruit and vegetable intake and prevalence of colorectal adenoma in a cancer screening trial. The American journal of clinical nutrition. 2007;86:1754–64. doi: 10.1093/ajcn/86.5.1754. [DOI] [PubMed] [Google Scholar]
- 31.Chen YK, Lee CH, Wu IC, Liu JS, Wu DC, Lee JM, et al. Food intake and the occurrence of squamous cell carcinoma in different sections of the esophagus in Taiwanese men. Nutrition. 2009;25:753–61. doi: 10.1016/j.nut.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Hsing AW, Chokkalingam AP, Gao YT, Madigan MP, Deng J, Gridley G, et al. Allium vegetables and risk of prostate cancer: a population-based study. Journal of the National Cancer Institute. 2002;94:1648–51. doi: 10.1093/jnci/94.21.1648. [DOI] [PubMed] [Google Scholar]
- 33.Brasky TM, Kristal AR, Navarro SL, Lampe JW, Peters U, Patterson RE, et al. Specialty supplements and prostate cancer risk in the VITamins and Lifestyle (VITAL) cohort. Nutrition and cancer. 2011;63:573–82. doi: 10.1080/01635581.2011.553022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galeone C, Pelucchi C, Dal Maso L, Negri E, Montella M, Zucchetto A, et al. Allium vegetables intake and endometrial cancer risk. Public health nutrition. 2009;12:1576–9. doi: 10.1017/S1368980008003820. [DOI] [PubMed] [Google Scholar]
- 35.Le Marchand L, Murphy SP, Hankin JH, Wilkens LR, Kolonel LN. Intake of flavonoids and lung cancer. Journal of the National Cancer Institute. 2000;92:154–60. doi: 10.1093/jnci/92.2.154. [DOI] [PubMed] [Google Scholar]
- 36.Walter RB, Brasky TM, Milano F, White E. Vitamin, mineral, and specialty supplements and risk of hematologic malignancies in the prospective VITamins And Lifestyle (VITAL) study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20:2298–308. doi: 10.1158/1055-9965.EPI-11-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka S, Haruma K, Kunihiro M, Nagata S, Kitadai Y, Manabe N, et al. Effects of aged garlic extract (AGE) on colorectal adenomas: a double-blinded study. Hiroshima journal of medical sciences. 2004;53:39–45. [PubMed] [Google Scholar]
- 38.Li H, Li H, Wang Y, Xu HX, Fan W, Wang M, et al. An intervention study to prevent gastric cancer by micro-selenium and large dose of allitridum. Chin Med J. 2004;117:1155–60. [PubMed] [Google Scholar]
- 39.Gail MH, You WC. A factorial trial including garlic supplements assesses effect in reducing precancerous gastric lesions. The Journal of nutrition. 2006;136:813S–5S. doi: 10.1093/jn/136.3.813S. [DOI] [PubMed] [Google Scholar]
- 40.Ma JL, Zhang L, Brown LM, Li JY, Shen L, Pan KF, et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. Journal of the National Cancer Institute. 2012;104:488–92. doi: 10.1093/jnci/djs003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shukla Y, Kalra N. Cancer chemoprevention with garlic and its constituents. Cancer letters. 2007;247:167–81. doi: 10.1016/j.canlet.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 42.Antony ML, Singh SV. Molecular mechanisms and targets of cancer chemoprevention by garlic-derived bioactive compound diallyl trisulfide. Indian journal of experimental biology. 2011;49:805–16. [PMC free article] [PubMed] [Google Scholar]
- 43.Jakszyn P, Agudo A, Ibanez R, Garcia-Closas R, Pera G, Amiano P, et al. Development of a food database of nitrosamines, heterocyclic amines, and polycyclic aromatic hydrocarbons. The Journal of nutrition. 2004;134:2011–4. doi: 10.1093/jn/134.8.2011. [DOI] [PubMed] [Google Scholar]
- 44.Milner JA. Mechanisms by which garlic and allyl sulfur compounds suppress carcinogen bioactivation. Garlic and carcinogenesis. Advances in experimental medicine and biology. 2001;492:69–81. doi: 10.1007/978-1-4615-1283-7_7. [DOI] [PubMed] [Google Scholar]
- 45.Dion ME, Agler M, Milner JA. S-allyl cysteine inhibits nitrosomorpholine formation and bioactivation. Nutrition and cancer. 1997;28:1–6. doi: 10.1080/01635589709514545. [DOI] [PubMed] [Google Scholar]
- 46.Williams DLH. Nitrosation Mechanisms. Adv Phys Org Chem. 1983;19:381–428. [Google Scholar]
- 47.Mei XLJ, Liu XY, Song PJ, Hu JF. The blocking effect of garlic on the formation of N-nitrosoproline in humans. Acta Nutrimenta Sinica. 1989;11:141–5. [Google Scholar]
- 48.Cope K, Seifried H, Seifried R, Milner J, Kris-Etherton P, Harrison EH. A gas chromatography-mass spectrometry method for the quantitation of N-nitrosoproline and N-acetyl-S-allylcysteine in human urine: application to a study of the effects of garlic consumption on nitrosation. Analytical biochemistry. 2009;394:243–8. doi: 10.1016/j.ab.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang CS, Hong JY, Wang ZY. Inhibition of Chemical Toxicity and Carcinogenesis by Garlic Components. Abstr Pap Am Chem S. 1992;204:33. -Agfd. [Google Scholar]
- 50.Wargovich MJ. Diallylsulfide and allylmethylsulfide are uniquely effective among organosulfur compounds in inhibiting CYP2E1 protein in animal models. The Journal of nutrition. 2006;136:832S–4S. doi: 10.1093/jn/136.3.832S. [DOI] [PubMed] [Google Scholar]
- 51.Yang CS, Chhabra SK, Hong JY, Smith TJ. Mechanisms of inhibition of chemical toxicity and carcinogenesis by diallyl sulfide (DAS) and related compounds from garlic. The Journal of nutrition. 2001;131:1041S–5S. doi: 10.1093/jn/131.3.1041S. [DOI] [PubMed] [Google Scholar]
- 52.Jin L, Baillie TA. Metabolism of the chemoprotective agent diallyl sulfide to glutathione conjugates in rats. Chemical research in toxicology. 1997;10:318–27. doi: 10.1021/tx9601768. [DOI] [PubMed] [Google Scholar]
- 53.Rounds L, Havens CM, Feinstein Y, Friedman M, Ravishankar S. Plant extracts, spices, and essential oils inactivate Escherichia coli O157:H7 and reduce formation of potentially carcinogenic heterocyclic amines in cooked beef patties. Journal of agricultural and food chemistry. 2012;60:3792–9. doi: 10.1021/jf204062p. [DOI] [PubMed] [Google Scholar]
- 54.Dong A, Lee J, Shin HS. Influence of natural food ingredients on the formation of heterocyclic amines in fried beef patties and chicken breasts. Food Sci Biotechnol. 2011;20:359–65. [Google Scholar]
- 55.Gibis M. Effect of oil marinades with garlic, onion, and lemon juice on the formation of heterocyclic aromatic amines in fried beef patties. Journal of agricultural and food chemistry. 2007;55:10240–7. doi: 10.1021/jf071720t. [DOI] [PubMed] [Google Scholar]
- 56.Yu FS, Yu CS, Lin JP, Chen SC, Lai WW, Chung JG. Diallyl disulfide inhibits N-acetyltransferase activity and gene expression in human esophagus epidermoid carcinoma CE 81T/VGH cells. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2005;43:1029–36. doi: 10.1016/j.fct.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 57.Platt KL, Edenharder R, Aderhold S, Muckel E, Glatt H. Fruits and vegetables protect against the genotoxicity of heterocyclic aromatic amines activated by human xenobiotic-metabolizing enzymes expressed in immortal mammalian cells. Mutation research. 2010;703:90–8. doi: 10.1016/j.mrgentox.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 58.Lin XY, Liu JZ, Milner JA. Dietary garlic suppresses DNA adducts caused by N-nitroso compounds. Carcinogenesis. 1994;15:349–52. doi: 10.1093/carcin/15.2.349. [DOI] [PubMed] [Google Scholar]
- 59.Schaffer EM, Liu JZ, Green J, Dangler CA, Milner JA. Garlic and associated allyl sulfur components inhibit N-methyl-N-nitrosourea induced rat mammary carcinogenesis. Cancer letters. 1996;102:199–204. doi: 10.1016/0304-3835(96)04160-2. [DOI] [PubMed] [Google Scholar]
- 60.Tache S, Ladam A, Corpet DE. Chemoprevention of aberrant crypt foci in the colon of rats by dietary onion. Eur J Cancer. 2007;43:454–8. doi: 10.1016/j.ejca.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 61.Knasmuller S, de Martin R, Domjan G, Szakmary A. Studies on the antimutagenic activities of garlic extract. Environmental and molecular mutagenesis. 1989;13:357–65. doi: 10.1002/em.2850130413. [DOI] [PubMed] [Google Scholar]
- 62.Ludeke BI, Domine F, Ohgaki H, Kleihues P. Modulation of N-nitrosomethylbenzylamine bioactivation by diallyl sulfide in vivo. Carcinogenesis. 1992;13:2467–70. doi: 10.1093/carcin/13.12.2467. [DOI] [PubMed] [Google Scholar]
- 63.Cohen LA, Zhao Z, Pittman B, Lubet R. S-allylcysteine, a garlic constituent, fails to inhibit N-methylnitrosourea-induced rat mammary tumorigenesis. Nutrition and cancer. 1999;35:58–63. doi: 10.1207/S1532791458-63. [DOI] [PubMed] [Google Scholar]
- 64.Singh SV, Pan SS, Srivastava SK, Xia H, Hu X, Zaren HA, et al. Differential induction of NAD(P)H:quinone oxidoreductase by anti-carcinogenic organosulfides from garlic. Biochemical and biophysical research communications. 1998;244:917–20. doi: 10.1006/bbrc.1998.8352. [DOI] [PubMed] [Google Scholar]
- 65.Chen C, Pung D, Leong V, Hebbar V, Shen G, Nair S, et al. Induction of detoxifying enzymes by garlic organosulfur compounds through transcription factor Nrf2: effect of chemical structure and stress signals. Free radical biology & medicine. 2004;37:1578–90. doi: 10.1016/j.freeradbiomed.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 66.Munday R, Munday CM. Relative activities of organosulfur compounds derived from onions and garlic in increasing tissue activities of quinone reductase and glutathione transferase in rat tissues. Nutrition and cancer. 2001;40:205–10. doi: 10.1207/S15327914NC402_18. [DOI] [PubMed] [Google Scholar]
- 67.Berges R, Siess MH, Arnault I, Auger J, Kahane R, Pinnert MF, et al. Comparison of the chemopreventive efficacies of garlic powders with different alliin contents against aflatoxin B1 carcinogenicity in rats. Carcinogenesis. 2004;25:1953–9. doi: 10.1093/carcin/bgh200. [DOI] [PubMed] [Google Scholar]
- 68.Herman-Antosiewicz A, Singh SV. Signal transduction pathways leading to cell cycle arrest and apoptosis induction in cancer cells by Allium vegetable-derived organosulfur compounds: a review. Mutation research. 2004;555:121–31. doi: 10.1016/j.mrfmmm.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 69.Milner JA. A historical perspective on garlic and cancer. The Journal of nutrition. 2001;131:1027S–31S. doi: 10.1093/jn/131.3.1027S. [DOI] [PubMed] [Google Scholar]
- 70.Knowles LM, Milner JA. Possible mechanism by which allyl sulfides suppress neoplastic cell proliferation. The Journal of nutrition. 2001;131:1061S–6S. doi: 10.1093/jn/131.3.1061S. [DOI] [PubMed] [Google Scholar]
- 71.Knowles LM, Milner JA. Diallyl disulfide inhibits p34(cdc2) kinase activity through changes in complex formation and phosphorylation. Carcinogenesis. 2000;21:1129–34. [PubMed] [Google Scholar]
- 72.Iciek M, Kwiecien I, Chwatko G, Sokolowska-Jezewicz M, Kowalczyk-Pachel D, Rokita H. The effects of garlic-derived sulfur compounds on cell proliferation, caspase 3 activity, thiol levels and anaerobic sulfur metabolism in human hepatoblastoma HepG2 cells. Cell biochemistry and function. 2012;30:198–204. doi: 10.1002/cbf.1835. [DOI] [PubMed] [Google Scholar]
- 73.Adetumbi MA, Lau BH. Allium sativum (garlic)--a natural antibiotic. Medical hypotheses. 1983;12:227–37. doi: 10.1016/0306-9877(83)90040-3. [DOI] [PubMed] [Google Scholar]
- 74.Tsao SM, Yin MC. In-vitro antimicrobial activity of four diallyl sulphides occurring naturally in garlic and Chinese leek oils. Journal of medical microbiology. 2001;50:646–9. doi: 10.1099/0022-1317-50-7-646. [DOI] [PubMed] [Google Scholar]
- 75.Cellini L, Di Campli E, Masulli M, Di Bartolomeo S, Allocati N. Inhibition of Helicobacter pylori by garlic extract (Allium sativum) FEMS immunology and medical microbiology. 1996;13:273–7. doi: 10.1111/j.1574-695X.1996.tb00251.x. [DOI] [PubMed] [Google Scholar]
- 76.Chung JG, Chen GW, Wu LT, Chang HL, Lin JG, Yeh CC, et al. Effects of garlic compounds diallyl sulfide and diallyl disulfide on arylamine N-acetyltransferase activity in strains of Helicobacter pylori from peptic ulcer patients. The American journal of Chinese medicine. 1998;26:353–64. doi: 10.1142/S0192415X98000397. [DOI] [PubMed] [Google Scholar]
- 77.Jonkers D, van den Broek E, van Dooren I, Thijs C, Dorant E, Hageman G, et al. Antibacterial effect of garlic and omeprazole on Helicobacter pylori. The Journal of antimicrobial chemotherapy. 1999;43:837–9. doi: 10.1093/jac/43.6.837. [DOI] [PubMed] [Google Scholar]
- 78.You WC, Zhang L, Gail MH, Ma JL, Chang YS, Blot WJ, et al. Helicobacter pylori infection, garlic intake and precancerous lesions in a Chinese population at low risk of gastric cancer. International journal of epidemiology. 1998;27:941–4. doi: 10.1093/ije/27.6.941. [DOI] [PubMed] [Google Scholar]
- 79.Aydin A, Ersoz G, Tekesin O, Akcicek E, Tuncyurek M. Garlic oil and Helicobacter pylori infection. The American journal of gastroenterology. 2000;95:563–4. doi: 10.1111/j.1572-0241.2000.t01-1-01812.x. [DOI] [PubMed] [Google Scholar]
- 80.Graham DY, Anderson SY, Lang T. Garlic or jalapeno peppers for treatment of Helicobacter pylori infection. The American journal of gastroenterology. 1999;94:1200–2. doi: 10.1111/j.1572-0241.1999.01066.x. [DOI] [PubMed] [Google Scholar]
- 81.Davis SR. An overview of the antifungal properties of allicin and its breakdown products--the possibility of a safe and effective antifungal prophylactic. Mycoses. 2005;48:95–100. doi: 10.1111/j.1439-0507.2004.01076.x. [DOI] [PubMed] [Google Scholar]
- 82.Rabinkov A, Miron T, Mirelman D, Wilchek M, Glozman S, Yavin E, et al. S-Allylmercaptoglutathione: the reaction product of allicin with glutathione possesses SH-modifying and antioxidant properties. Biochimica et biophysica acta. 2000;1499:144–53. doi: 10.1016/s0167-4889(00)00119-1. [DOI] [PubMed] [Google Scholar]
- 83.Dwivedi C, John LM, Schmidt DS, Engineer FN. Effects of oil-soluble organosulfur compounds from garlic on doxorubicin-induced lipid peroxidation. Anti-cancer drugs. 1998;9:291–4. doi: 10.1097/00001813-199803000-00013. [DOI] [PubMed] [Google Scholar]
- 84.Atmaca G. Antioxidant effects of sulfur-containing amino acids. Yonsei medical journal. 2004;45:776–88. doi: 10.3349/ymj.2004.45.5.776. [DOI] [PubMed] [Google Scholar]
- 85.Agarwal MK, Iqbal M, Athar M. Garlic oil ameliorates ferric nitrilotriacetate (Fe-NTA)-induced damage and tumor promotion: implications for cancer prevention. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2007;45:1634–40. doi: 10.1016/j.fct.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 86.Nuutila AM, Puupponen-Pimia R, Aarni M, Oksman-Caldentey KM. Comparison of antioxidant activities of onion and garlic extracts by inhibition of lipid peroxidation and radical scavenging activity. Food Chem. 2003;81:485–93. [Google Scholar]
- 87.Ali M. Mechanism by which garlic (Allium sativum) inhibits cyclooxygenase activity. Effect of raw versus boiled garlic extract on the synthesis of prostanoids. Prostaglandins, leukotrienes, and essential fatty acids. 1995;53:397–400. doi: 10.1016/0952-3278(95)90102-7. [DOI] [PubMed] [Google Scholar]
- 88.Belman S, Solomon J, Segal A, Block E, Barany G. Inhibition of soybean lipoxygenase and mouse skin tumor promotion by onion and garlic components. Journal of biochemical toxicology. 1989;4:151–60. doi: 10.1002/jbt.2570040303. [DOI] [PubMed] [Google Scholar]
- 89.Chang HP, Huang SY, Chen YH. Modulation of cytokine secretion by garlic oil derivatives is associated with suppressed nitric oxide production in stimulated macrophages. Journal of agricultural and food chemistry. 2005;53:2530–4. doi: 10.1021/jf048601n. [DOI] [PubMed] [Google Scholar]
- 90.Dirsch VM, Vollmar AM. Ajoene, a natural product with non-steroidal anti-inflammatory drug (NSAID)-like properties? Biochemical pharmacology. 2001;61:587–93. doi: 10.1016/s0006-2952(00)00580-3. [DOI] [PubMed] [Google Scholar]
- 91.Colic M, Savic M. Garlic extracts stimulate proliferation of rat lymphocytes in vitro by increasing IL-2 and IL-4 production. Immunopharmacology and immunotoxicology. 2000;22:163–81. doi: 10.3109/08923970009016413. [DOI] [PubMed] [Google Scholar]
- 92.Jeong HG, Lee YW. Protective effects of diallyl sulfide on N-nitrosodimethylamine-induced immunosuppression in mice. Cancer letters. 1998;134:73–9. doi: 10.1016/s0304-3835(98)00246-8. [DOI] [PubMed] [Google Scholar]
- 93.Houshmand B, Mahjour F, Dianat O. Antibacterial effect of different concentrations of garlic (Allium sativum) extract on dental plaque bacteria. Indian journal of dental research : official publication of Indian Society for Dental Research. 2013;24:71–5. doi: 10.4103/0970-9290.114957. [DOI] [PubMed] [Google Scholar]
- 94.Filocamo A, Nueno-Palop C, Bisignano C, Mandalari G, Narbad A. Effect of garlic powder on the growth of commensal bacteria from the gastrointestinal tract. Phytomedicine : international journal of phytotherapy and phytopharmacology. 2012;19:707–11. doi: 10.1016/j.phymed.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 95.Zhao Y, Biggs TD, Xian M. Hydrogen sulfide (H2S) releasing agents: chemistry and biological applications. Chem Commun (Camb) 2014;50:11788–805. doi: 10.1039/c4cc00968a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wells HF, Thornsbury S, Bond JK. Vegetables and Pulses Outlook: March 2013. VGS-353. United States Department of Agriculture; 2013. p. 45. [Google Scholar]