Highlights
-
•
First FISH study of ovine FR to examine pathogen spatial distribution and load.
-
•
D. nodosus cell counts are significantly associated with both ID and SFR.
-
•
F. necrophorum cell counts are significantly associated with SFR.
-
•
Highlights FISH as a useful tool for studying microbial populations in situ.
Keywords: Dichelobacter nodosus, Fusobacterium necrophorum, Footrot, Fluorescence in situ hybridisation, Confocal microscopy
Abstract
Analysis of bacterial populations in situ provides insights into pathogen population dynamics and potential reservoirs for disease. Here we report a culture-independent study of ovine footrot (FR); a debilitating bacterial disease that has significant economic impact on sheep farming worldwide. Disease begins as an interdigital dermatitis (ID), which may then progress to separation of the hoof horn from the underlying epidermis causing severe footrot (SFR). Dichelobacter nodosus is the causative agent of ovine FR, however, the role of Fusobacterium necrophorum and other bacteria present in the environment and on the feet of sheep is less clear. The objective of this study was to use fluorescence in situ hybridisation (FISH) to detect, localise and quantify D. nodosus, F. necrophorum and the domain Bacteria from interdigital skin biopsies of healthy, ID- and SFR-affected feet. D. nodosus and F. necrophorum populations were restricted primarily to the epidermis, but both were detected more frequently in feet with ID or SFR than in healthy feet. D. nodosus cell counts were significantly higher in feet with ID and SFR (p < 0.05) than healthy feet, whereas F. necrophorum cell counts were significantly higher only in feet with SFR (p < 0.05) than healthy feet. These results, together with other published data, indicate that D. nodosus likely drives pathogenesis of footrot from initiation of ID to SFR; with D. nodosus cell counts increasing prior to onset of ID and SFR. In contrast, F. necrophorum cell counts increase after SFR onset, which may suggest an accessory role in disease pathogenesis, possibly contributing to the severity and duration of SFR.
1. Introduction
Ovine footrot (FR) is an infectious bacterial disease that causes lameness and affects sheep flocks worldwide (Kaler and Green, 2008; Hussain et al., 2009; König et al., 2010). Damage to the interdigital skin is thought to be required for disease to occur (Beveridge, 1941). Early stages of FR present as an inflammation of the interdigital skin (interdigital dermatitis (ID)) and later stages present with sloughing of necrotic epithelium (severe footrot (SFR)) (Beveridge, 1941; Egerton et al., 1969). In some sheep this is followed by inflammation of the epidermis below the horn that causes the horn capsule to separate from the epithelium leading to severe footrot (SFR) (Beveridge, 1941). The primary causative agent of ovine FR is Dichelobacter nodosus (Beveridge, 1941; Kennan et al., 2001, 2010). Fusobacterium necrophorum is commonly detected in FR lesions and is reported to be essential for disease initiation (Egerton et al., 1969; Roberts and Egerton, 1969). However, changes in D. nodosus and F. necrophorum populations detected by qPCR indicate that F. necrophorum load increases after SFR has occurred suggesting that it is an opportunistic, secondary pathogen (Witcomb et al., 2014). This is supported by a plethora of studies investigating D. nodosus, ranging from molecular infection trials (Kennan et al., 2001, 2010) to molecular genetic epidemiological studies correlating genotype with clinical presentation (Kennan et al., 2014; Stäuble et al., 2014).
D. nodosus and F. necrophorum have been detected using a range of culture-dependent and -independent techniques from both swabs and biopsies collected from the interdigital skin of sheep with ID and SFR (Bennett et al., 2009; Calvo-Bado et al., 2011; Witcomb et al., 2014). The advantages and disadvantages of culture-dependent and -independent methods have been discussed elsewhere (Rogers et al., 2009), but due to the fastidious nature of both anaerobes, PCR is more sensitive than culturing methods (Moore et al., 2005). Additionally problematic is the marked pleomorphism of F. necrophorum and other bacteria and the limited morphologies presented within a genus; making reliable identification of F. necrophorum and D. nodosus using morphology alone prone to error (Hofstad, 2006; Young, 2007). In contrast, fluorescence in situ hybridisation (FISH) can be used to detect bacteria in their natural environment, providing information regarding bacterial load and localisation (Baumgart et al., 2007; Amann and Fuchs, 2008). The use of specific fluorescently tagged oligonucleotide probes provides a sensitive and specific improvement on conventional light microscopy, which relies on phenotypic recognition of bacterial species. FISH has recently been used to detect D. nodosus and F. necrophorum in cases of ovine and bovine foot disease (Rasmussen et al., 2012; Klitgaard et al., 2013; Knappe-Poindecker et al., 2014) but no study to date has used FISH to investigate bacterial load and localisation patterns for cases of ID and SFR in sheep.
The aim of this study was to use FISH to investigate the spatial distribution and load of the domain Bacteria, D. nodosus and F. necrophorum populations from all four feet of six sheep (n = 24 biopsies) with a range of non-experimentally induced disease states (healthy (H), ID and SFR). Results demonstrate that changes in D. nodosus and F. necrophorum cell counts correlate with changes in disease state.
2. Methods
2.1. Collection of interdigital skin biopsies and swabs
A flock of 99 ewes with 146 lambs were monitored for 10 months (Smith et al., 2014). At the end of the 10-month study, six sheep were selected based on disease history (healthy n = 2, ID n = 2, SFR n = 2) and for those with disease, the disease severity was recorded (Foddai et al., 2012) at the time of slaughter. Interdigital swabs (n = 24) were collected from feet prior to biopsy punch, and chromosomal DNA extracted as previously described (Moore et al., 2005). F. necrophorum (rpoB) and D. nodosus (rpoD) amplicons were then detected and quantified by qPCR as done elsewhere (Witcomb et al., 2014).
Interdigital skin punch biopsies (n = 24) were collected from all four feet of these six sheep using disposable sterile Biopsy Punches (8 mm diameter) (Stiefel Laboratories, UK) immediately post mortem at the EU-licensed red meat abattoir at Bristol Veterinary School. There were n = 12 biopsies from healthy feet, n = 6 biopsies from feet with ID and n = 6 biopsies from feet with SFR. A total of 4/12 healthy foot biopsies came from sheep with other feet affected by ID and/or SFR, the remaining samples belonged to sheep with all feet being classified as healthy. Biopsies were fixed immediately in 3.8–4.0% (w/v) neutral buffered formalin (NBF) overnight and embedded and sectioned at Bristol Pathology Laboratory (Bristol Veterinary School, University of Bristol, Langford, UK). Collection of ovine clinical material was approved by the University of Bristol local ethical committee.
2.2. Fluorescence in situ hybridisation (FISH) probes and protocol
The D. nodosus probe (5′-TCGGTACCGAGTATTTCTAC-3′) was modified from the Cc primer sequence (La Fontaine et al., 1993) targeting the 16S rRNA gene sequence positions 821–840 (Dewhirst et al., 1990). The F. necrophorum_183 probe (Boye et al., 2006), the EUB338 probe set (-I, -II, -III) consisting of three probes (Alm et al., 1996; Daims et al., 1999) and the EUK1195 probe (Giovannoni et al., 1988) were also used for this study. The EUB338-I probe covers 90% of the domain Bacteria, and EUB338-II and -III were included to extend coverage (Alm et al., 1996; Daims et al., 1999). The EUK1195 probe was used to provide definition to eukaryotic cell junctions (Supplementary Fig. 1). Bacterial probes and the EUK1195 probe were labelled at the 5′-end with Cy3 and FITC, respectively.
Supplementary Fig. 1 related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vetmic.2015.01.022.
Supplementary Fig. 1.
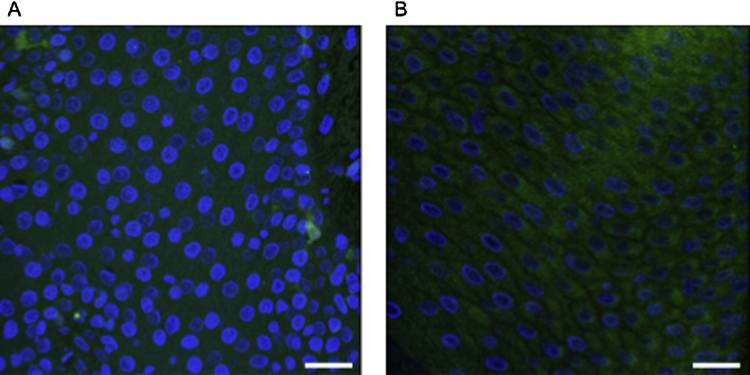
DAPI stained ovine interdigital biopsy section (A) with fluorescence in situ hybridisation reaction using EUK1195 probe (B). Cell junctions can be seen with EUK1195 staining. Scale bars: 5 μm.
The FISH procedure was performed as described previously (Amann et al., 1996; Peters et al., 2011). Briefly, after dehydration steps in an ethanol series, slides were incubated with hybridisation buffers for 4 h at 46 °C. Hybridisation reactions contained 50–60 ng μl−1 FISH probe (D. nodosus, F. necrophorum or EUB338 probe set in conjunction with EUK1195); an equimolar mixture of the EUB338-I, -II, -III probes was used. After washing, the slides were dried using pressurised air canisters and mounted in VectaShield Mounting Medium containing 4′,6′-diamidino-2-phenylindole (DAPI) (H-1200). All tissue sections were pre-treated with proteinase K (5 μg ml−1) for 10 min at room temperature (20–22 °C) (Peters et al., 2011). Tissue sections to be screened with the EUB338 probe set were pre-treated with lysozyme (10 mg ml−1). Lysozyme pre-treatment was not required for the D. nodosus or F. necrophorum screens.
2.3. FISH optimisation
The in silico specificity of the D. nodosus probe was determined using probeCheck (http://www.microbial-ecology.net/probecheck) (Loy et al., 2008). A series of positive and negative control microorganisms (n = 14) were then screened using the D. nodosus oligonucleotide to assess the specificity of the probe. The microorganisms screened were selected because they had previously been detected on the ovine foot by pyro-sequencing (Calvo-Bado et al., 2011) or isolation (Nicky Buller, personal communication) or they represented environmentally relevant non-target microorganisms (Table 1). Empirical optimisations were carried out for each probe/probe set to determine specificity and optimal formamide concentrations (0–35% [v/v]) to adjust the stringency of the hybridisation. Biopsies from healthy feet were subcutaneously inoculated using sterile 25 gauge (G) needles (BD Microlance™ 3, BD, Drogheda, Ireland) with 100 μl of either F. necrophorum (BS-1) or D. nodosus (VCS1703A) at several sites to distribute the inocula evenly and incubated at 37 °C under anaerobic conditions for 1 h and 24 h to act as spiked positive controls (data not shown). Tissue controls were then screened using the relevant bacterial probe and the EUB338 probe set.
Table 1.
Specificity of D. nodosus oligonucleotide probe (modified from the Cc forward primer) (La Fontaine et al., 1993). Binding conditions altered by increasing formamide within the hybridisation buffer.
| D. nodosus strain (positive controls) | Result | Negative controls | Result |
|---|---|---|---|
| VCS1703A | + | Aeromonas hydrophilaa | − |
| BS-1 | + | Aeromonas mediaa | − |
| BS-6 | + | Arcanobacterium pyogenes (DS7M 20–630) | − |
| A198 | + | Bacillus circulans (WL-12)b | − |
| Serogroup A | + | Citrobacter freundiia | − |
| Escherichia coli (K12) | − | ||
| Fusobacterium necrophorum (BS-1) | − | ||
| Klebsiella pneumoniaea | − | ||
| Macrococcus caseolyticusc | − |
Environmental isolate (river water), United Kingdom.
Environmental isolate (soil), United Kingdom.
Bovine isolate (milk), United Kingdom.
2.4. Confocal microscopy and image analysis
Images were obtained using a scanning confocal Leica TCS SP5 microscope (Leica Microsystems Ltd., Milton Keynes, UK) equipped with Blue Diode (405 nm), Argon gas (488 nm), Ti sapphire (561 nm) and Orange HeNe (596 nm) lasers. Pre-set narrow bandwidth settings were used to analyse the DAPI, FITC and Cy3 signals. Image processing was performed using the Leica LAS image analysis software and open source ImageJ software (http://rsb.info.nih.gov.ij) (Abramoff et al., 2004). Multiple images/fields of view (FOV) (n = 9–12) were taken from each foot biopsy for localisation purposes. Bacterial counts and localisation patterns were recorded per image and each image standardised to 156 μm2 (Davenport and Curtis, 2004; Baumgart et al., 2007); this FOV size was used to accurately identify individual bacterial cells for quantification. Bacterial counts were then +1 log10 transformed for downstream analysis and data expressed as log10 bacterial count/FOV.
2.5. Statistical analysis and multinomial modelling
Bacterial counts were +1 log10 transformed (to reduce skew) and then averaged for each biopsy. A mixed effect continuous outcome model was used to estimate log10 mean load of the bacteria by disease state adjusted for clustering of feet within sheep. An unordered multinomial mixed effects model accounting for clustered feet within sheep in MLwiN 2.21 software, Bristol, UK (Rasbash et al., 2005) was used to examine the associations between D. nodosus and F. necrophorum log10 load by disease status. The outcome variable had three categories; healthy, ID- and SFR-affected feet. The explanatory variables were D. nodosus and F. necrophorum log10 load. The model was built using a forward stepwise approach. The equation took the form:
where log(π1jk/pi0jk) = the probability of ID versus healthy and log(π2jk/pi0jk) = the probability of SFR versus healthy, β0k and β1k are constants for ID and SFR, β0x and β1x are vectors of fixed effects for ID and SFR varying at level 1 and 2, where level 1(j) = feet and level 2(k) = sheep, where v0k and v1k are level 2 residual variances and level 1 is assumed to take a binomial error distribution. The model was developed using RIGLS (Restricted Iterative Generalised Least Squares) and then MCMC (Markov Chain Monte Carlo) was used to adjust for the possibility of overinflated standard errors. A burn in of 5,000 followed by 50,000 iterations was done. Significance was determined using the Wald's statistic, where 95% confidence intervals (CI) did not include unity. The model fit was tested by outputting the predictions from the model and comparing sum ranked fitted quintile estimates against the summed observations for the number of cases of ID and SFR combined each week using the Hosmer Lemeshow test (Doohoo et al., 2003).
3. Results
3.1. In vitro and ex vivo optimisation of FISH protocol
The D. nodosus oligonucleotide probe was determined to be specific and produce the highest signal-to-noise ratio with 25% formamide in the hybridisation buffer (data not shown), with no binding to non-specific microorganisms observed (Table 1). In addition, the FISH protocols were tested empirically both in vitro and on tissue biopsies spiked with D. nodosus or F. necrophorum cells (data not shown).
3.2. Tissue observations and detection of bacterial populations from interdigital swabs and biopsies
For healthy feet, 1/12 and 6/12 swabs were positive for F. necrophorum (rpoB) and D. nodosus (rpoD), respectively. Samples that were positive were on the limit of detection (∼103 copies swab−1), which is consistent with earlier findings (Calvo-Bado et al., 2011; Witcomb et al., 2014). A total of 5/6 and 6/6 swabs from ID feet were positive for F. necrophorum (rpoB) and D. nodosus (rpoD), respectively. Similarly, 100% of swabs were positive for both bacterial species from feet with SFR. Significant necrosis of the stratum corneum was present in tissue from feet with ID and SFR and bacterial cells were observed in the sloughed necrotic tissue, sometimes in large numbers (Fig. 1). In addition, the infiltration of erythrocytes was associated with both stages of disease and absent in healthy feet (Fig. 2). Erythrocytes appeared as auto-fluorescent cells under all three channels.
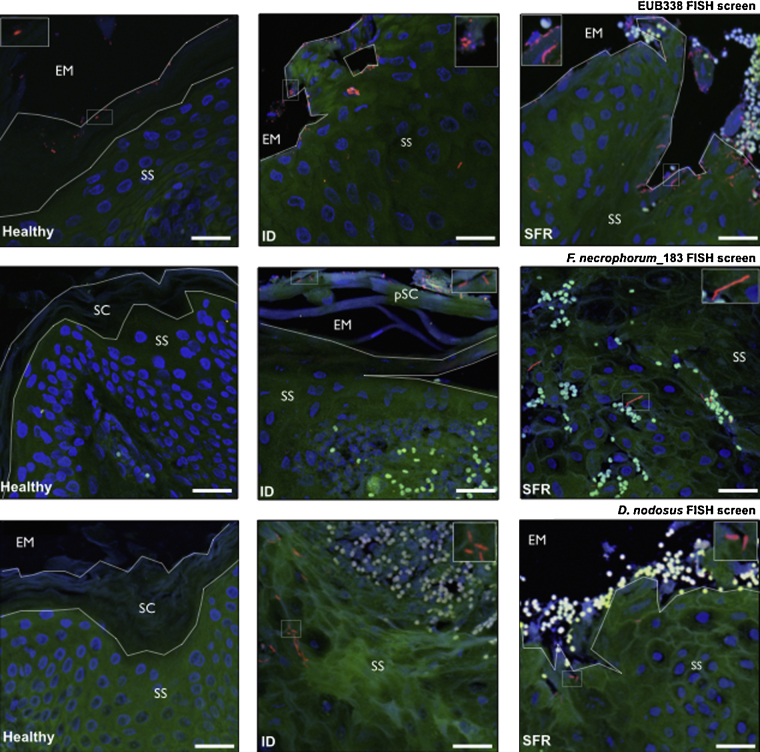
Fig. 1.
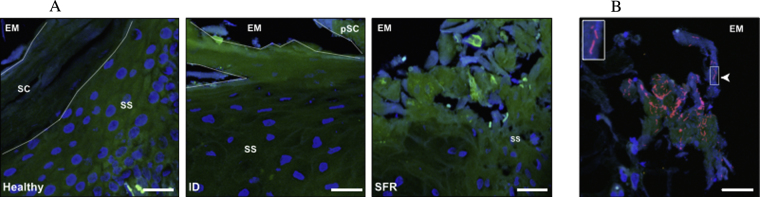
Tissue morphology of biopsy sections by disease state (healthy, ID and SFR) (A), and evidence of sloughing of necrotic tissue carrying bacterial cells (B). Bacterial cells (red), epithelial cells (green), epithelial cell nuclei (blue) and erythrocytes (white – autofluorescence). Stratum corneum (SC), partial stratum corneum (pSC), stratum spinosum (SS) and extracellular milieu (EM) are shown (red channel images removed from (A) for tissue morphology to be observed). Scale bars: 25 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
Representative FISH images from biopsy sections by disease state (healthy, ID and SFR). Bacterial cells (red), epithelial cells (green), epithelial cell nuclei (blue) and erythrocytes (white – autofluorescence). Stratum corneum (SC), partial stratum corneum (pSC), stratum spinosum (SS) and extracellular milieu (EM) are shown. Scale bars: 25 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
EUB338-I, -II, -III labelled cells were detected on the surface of or within the epidermis of 100% of biopsies (24/24), acting as a positive FISH control (Moter and Göbel, 2000). F. necrophorum cells were detected in 1/12, 4/6 and 5/6 of H, ID and SFR interdigital skin biopsies, respectively. In contrast, D. nodosus cells were not detected in biopsies from healthy feet, whether other feet from the sheep were diseased or not (n = 0/12), but were detected in 50% of biopsies from feet with ID (n = 3/6) and SFR (n = 3/6). The vast majority of microorganisms detected by FISH were located in the epidermis, which is consistent with other work (Egerton et al., 1969; Rasmussen et al., 2012). However, one F. necrophorum cell and one D. nodosus cell were detected in the dermis of two different biopsies (Supplementary Fig. 2).
Supplementary Fig. 2 related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vetmic.2015.01.022.
Supplementary Fig. 2.
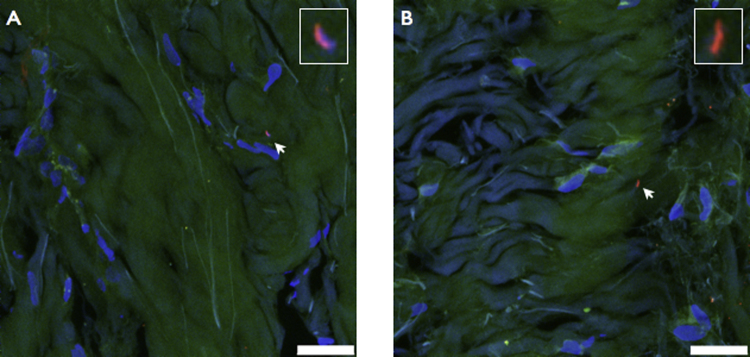
Detection of D. nodosus (A) and F. necrophorum (B) within dermal layers of interdigital skin biopsies. Scale bars: 5 μm.
Fluorescently tagged bacterial cells were counted in individual FOVs for the epidermis (n = 3–4) from each biopsy per oligonucleotide probe. Quantification was limited to images of the epidermis, as D. nodosus and F. necrophorum populations were primarily restricted to this region. Bacterial cell counts from images were +1 log10 transformed and compared by disease state (H, ID and SFR) using a mixed effect model accounting for clustering of feet by ewe (Table 2). Representative images are shown in Fig. 2. Mean cell counts for the domain Bacteria were not significantly different (p > 0.05) between disease states. In contrast, D. nodosus cell counts were significantly higher in both feet with ID and SFR than healthy feet (p < 0.05) and F. necrophorum cell counts were only significantly higher in feet with SFR than healthy feet (p < 0.05).
Table 2.
Three mixed effects regression models providing log10 mean cell counts/FOV for the domain Bacteria (EUB338), D. nodosus (Dn) and F. necrophorum (Fn) in feet with ID (n = 6) and SFR (n = 6) compared with a baseline of healthy feet (n = 12).a
| Log10 mean | s.e. | Lower 95% CI | Upper 95% CI | ||
|---|---|---|---|---|---|
| EUB338 | Healthy/baseline | 1.074 | 0.144 | 0.792 | 1.356 |
| ID | 0.305 | 0.250 | −0.185 | 0.795 | |
| SFR | 0.283 | 0.251 | −0.209 | 0.775 | |
| Dn | Healthy/baseline | −0.023 | 0.142 | −0.301 | 0.255 |
| ID | 0.412 | 0.187 | 0.045 | 0.779 | |
| SFR | 0.469 | 0.215 | 0.0476 | 0.8904 | |
| Fn | Healthy/baseline | 0.006 | 0.113 | −0.215 | 0.227 |
| ID | 0.321 | 0.195 | −0.061 | 0.703 | |
| SFR | 0.714 | 0.195 | 0.332 | 1.096 | |
ID: interdigital dermatitis, SFR: severe footrot, s.e.: standard error, CI: confidence interval, bold: p < 0.05.
Adjusted for repeated measures within feet and sheep.
4. Discussion
This study used FISH to detect, quantify and localise D. nodosus and F. necrophorum populations from biopsies collected from healthy, ID- and SFR-affected ovine interdigital skin.
The key novel result was that whilst the general bacterial population remained relatively stable by disease state, D. nodosus and F. necrophorum populations changed with clinical presentation. F. necrophorum cell counts were significantly higher in cases of SFR (p < 0.05), whilst D. nodosus cell counts were significantly higher in cases of ID and SFR than in healthy feet (p < 0.05); the model accounted for dependencies between feet within sheep (Table 2). These findings suggest a shift in the D. nodosus and F. necrophorum populations within the interdigital skin before ID and between ID and SFR and these data are consistent with Calvo-Bado et al. (2011) and Witcomb et al. (2014) who both reported that D. nodosus (rpoD) load was highest before and during an episode of ID and before SFR by qPCR and that F. necrophorum (rpoB) load instead increased after SFR (Witcomb et al., 2014). The findings from the current study therefore provide further evidence supporting Witcomb et al. (2014) who proposed that an increase in D. nodosus population numbers drives pathogenesis of footrot from healthy to ID to SFR whilst F. necrophorum population increased only after SFR developed. The causal role of D. nodosus is further supported by a number of genomic and epidemiological studies, which correlate D. nodosus genotype with clinical presentation (Kennan et al., 2001, 2010, 2014; Stäuble et al., 2014).
The bacterial populations in the ovine interdigital biopsies were primarily restricted to the superficial epidermal layers, consistent with previous reports (Egerton et al., 1969; Rasmussen et al., 2012). The presence of D. nodosus by FISH (50% ID and SFR biopsies) was lower than that detected by qPCR studies, where D. nodosus DNA (rpoD) was detected in 86% and 71% of swabs from feet with ID and SFR, respectively (Witcomb et al., 2014). The FISH data are more in-line with isolation results; with D. nodosus detected in 67.9% and 55.8% swabs from feet with ID and SFR, respectively, and not detectable in samples from healthy feet (Moore et al., 2005). We postulate that this is due to the higher sensitivity of the qPCR assays compared with FISH, which is supported by the increased detection frequency of rpoB/rpoD amplicons from the interdigital skin. The swab samples gather superficial bacteria from a wide area of the interdigital skin, whilst the biopsy only samples an 8 mm region of the epidermis. D. nodosus may not have been present in the samples collected, however, we feel this is unlikely considering the qPCR data and the strong association with disease. Whilst F. necrophorum was detected in most biopsies of ID and SFR, load was not significantly associated with ID, consistent with findings elsewhere (Witcomb et al., 2014). In addition, a larger bacterial population (as represented by load or cell count) may indicate a longer established community, suggesting D. nodosus preceded F. necrophorum in cases of ID. Other studies that have reported determining the presence/absence of F. necrophorum have also reported an increased detection frequency of F. necrophorum in FR (Bennett et al., 2009), and concluded that this organism therefore likely contributes to the pathogenesis of FR. In contrast, Witcomb et al. (2014) found that both F. necrophorum and D. nodosus are frequently present, even in healthy feet, and therefore examining bacterial load is more informative than the presence/absence in studies of causality.
Erythrocytes were present in diseased tissue sections, indicative of damage to local capillaries and seepage of blood out from leaky capillary walls. There was also evidence of sloughing of epidermal tissue in sections from diseased biopsies and the tissue included bacterial cells; which might indicate a route of transfer of infection between diseased and susceptible feet and sheep (Beveridge, 1941). D. nodosus and F. necrophorum cells were detected within the dermal layers in two separate biopsies. It is possible that these were artefacts produced during processing of tissue samples, however, once the tissues are fixed, their architecture is maintained (Hopwood, 1991) and so the formation of artefacts affecting bacterial localisation seems unlikely. If this observation is a true finding, it would suggest that these anaerobes are able to penetrate the deeper dermal layers on occasion, which may act as a potential reservoir for chronic infection. Egerton et al. (1969) also reported that F. necrophorum was present in the dermis of ovine feet in cases of FR.
The small sample size is a limitation of this study, which may have implications for interpretation of results, however, the data are consistent with a number of other studies using a variety of culture-dependent and -independent methodologies (Moore et al., 2005; Calvo-Bado et al., 2011; Witcomb et al., 2014). Ideally longitudinal sampling would also have been performed to follow disease progression over time in the same individual, however punch biopsies are invasive and taken over time would likely predispose sheep feet to infection and change the natural progression of disease.
In conclusion, this study describes in detail the detection, spatial distribution and quantification of D. nodosus, F. necrophorum and the domain Bacteria within ovine interdigital skin biopsies and their association with healthy, ID and SFR using FISH. We also present evidence that bacterial cell counts change with clinical presentation, with D. nodosus counts significantly higher in ID and SFR and F. necrophorum cell counts increasing only after progression to SFR. This is consistent with previous work (Calvo-Bado et al., 2011; Witcomb et al., 2014) indicating that D. nodosus initiates ID and is present before SFR develops, whilst F. necrophorum cell counts only increase when SFR is present. Finally, this study supports FISH as an invaluable tool that can be used to examine the microbial community associated with ovine FR.
Funding
L.A. Witcomb was a NERC CASE studentship with Pfizer Animal Health as the industrial partner. All other authors were supported by Combating Endemic Diseases of Farmed Animals for Sustainability (CEDFAS) initiative, Grant No. BBE01870X1 from the Biotechnology and Biological Sciences Research Council (BBSRC).
References
- Abramoff M.D., Magalhaes P.J., Ram S.J. Image processing with ImageJ. Biophotonics Int. 2004;11(7):36–42. [Google Scholar]
- Alm E.W., Oerther D.B., Larsen N., Stahl D.A., Raskin L. The oligonucleotide probe database. Appl. Environ. Microbiol. 1996;62(10):3557–3559. doi: 10.1128/aem.62.10.3557-3559.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann R., Fuchs B.M. Single-cell identification in microbial communities by improved fluorescence in situ hybridization techniques. Nat. Rev. Microbiol. 2008;6:339–348. doi: 10.1038/nrmicro1888. [DOI] [PubMed] [Google Scholar]
- Amann R., Snaidr J., Wagner M., Ludwig W., Shleifer K.H. In situ visualization of high genetic diversity in a natural microbial community. J. Bacteriol. 1996;178(12):3496–3500. doi: 10.1128/jb.178.12.3496-3500.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart M., Dogan B., Rishniw M., Weitzman G., Bosworth B., Yantiss R B. Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn's disease involving the ileum. ISME J. 2007;1:403–418. doi: 10.1038/ismej.2007.52. [DOI] [PubMed] [Google Scholar]
- Bennett G., Hickford J., Sedcole R., Zhou H. Dichelobacter nodosus, Fusobacterium necrophorum and the epidemiology of footrot. Anaerobe. 2009;15(4):173–176. doi: 10.1016/j.anaerobe.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Beveridge W.I.B. Foot-rot in sheep: a transmissable disease due to infection with Fusiformis nodosus (n. sp.): studies on its cause, epidemiology and control. CSIRO Aust. Bull. 1941;140:1–56. [Google Scholar]
- Boye M., Aalbaek B., Agerholm J.S. Fusobacterium necrophorum determined as abortifacient in sheep by laser capture microdissection and fluorescence in situ hybridization. Mol. Cell. Probes. 2006;20(6):330–336. doi: 10.1016/j.mcp.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Calvo-Bado L.A., Oakley B.B., Dowd S.E., Green L.E., Medley G.F., Ul-Hassan A. Ovine pedomics: the first study of the ovine foot 16S rRNA-based microbiome. ISME J. 2011;5(9):1426–1437. doi: 10.1038/ismej.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daims H., Brühel A., Amann R., Schleifer K.-H., Wagner M. The domain-specific probe EUB338 is insufficient for the detection of all bacteria: development and evaluation of a more comprehensive probe set. Syst. Appl. Microbiol. 1999;22:434–444. doi: 10.1016/S0723-2020(99)80053-8. [DOI] [PubMed] [Google Scholar]
- Davenport R.J., Curtis T.P. Quantitative fluorescence in situ hybridisation (FISH): statistical methods for valid cell counting. In: Kowalchuk G.A., de Bruijn F.J., Head I.M., Akkermans A.D., van Elsas J.D., editors. vol. 1. Springer; New York: 2004. pp. 1487–1516. (Molecular Microbial Ecology Manual). [Google Scholar]
- Dewhirst F.E., Paster B.J., La Fontaine S., Rood J.I. Transfer of Kingella indologenes (Snell and Lapage, 1976) to the genus Suttonella gen. nov. as Suttonella indologenes comb. nov.; transfer of Bacteroides nodosus (Beveridge, 1941) to the genus Dichelobacter gen. nov. as Dichelobacter nodosus comb. nov.; and assignment of the genera Cardiobacterium, Dichelobacter, and Suttonella to Cardiobacteriaceae fam. nov. in the gamma division of Proteobacteria based on 16S ribosomal ribonucleic acid sequence comparisons. Int. J. Bacteriol. 1990;40:426–433. doi: 10.1099/00207713-40-4-426. [DOI] [PubMed] [Google Scholar]
- Doohoo I., Martin W., Stryhn H. AVC Incorporated/Atlantic Veterinary College; Charlottetown, Prince Edward Island/Canada: 2003. Veterinary Epidemiologic Research; pp. 499–520. [Google Scholar]
- Egerton J.R., Roberts D.S., Parsonson I.M. The aetiology and pathogenesis of ovine foot-rot. I. A histological study of the bacterial invasion. J. Comp. Pathol. 1969;79:207–217. doi: 10.1016/0021-9975(69)90007-3. [DOI] [PubMed] [Google Scholar]
- Foddai A., Green L.E., Mason S.A., Kaler J. Evaluating observer agreement of scoring systems for foot integrity and footrot lesions in sheep. BMC Vet. Res. 2012;8:65. doi: 10.1186/1746-6148-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni S.J., DeLong E.F., Olsen G.J., Pace N.R. Phylogenetic group-specific oligodeoxynucleotide probes for identification of single microbial cells. J. Bacteriol. 1988;170(2):720–726. doi: 10.1128/jb.170.2.720-726.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstad T. The genus Fusobacterium. In: Dworkin M., Falkow S., Rosenberg E., Schleifer K.-H., Stackebrandt E., editors. The Prokaryotes. Volume 7: Proteobacteria: Delta, Epsilon Subclass. Springer; New York: 2006. pp. 1016–1027. [Google Scholar]
- Hopwood D. Fixation of tissue for histochemistry in histochemical and immunohistochemical techniques. In: Bach P.H., Baker J., editors. Histochemical and Immunohistochemical Techniques. Springer; Netherlands: 1991. pp. 147–148. [Google Scholar]
- Hussain I., Wani S.A., Qureshi S.D., Farooq S. Serological diversity and virulence determination of Dichelobacter nodosus from footrot in India. Mol. Cell. Probes. 2009;2:112–114. doi: 10.1016/j.mcp.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Kaler J., Green L.E. Naming and recognition of six foot lesions of sheep using written and pictorial information: a study of 809 English sheep farmers. Prev. Vet. Med. 2008;83(1):52–64. doi: 10.1016/j.prevetmed.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Kennan R.M., Dhungyel O.P., Whittington R.J., Egerton J.R., Rood J.I. The type IV fimbrial subunit gene (fimA) of Dichelobacter nodosus is essential for virulence, protease secretion and natural competence. J. Bacteriol. 2001;183(15):4451–4458. doi: 10.1128/JB.183.15.4451-4458.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennan R.M., Wong W., Dhungyel O.P., Han X., Wong D., Parker D. The subtilisin-like protease AprV2 is required for virulence and uses a novel disulphide-tethered exosite to bind substrates. PLoS Pathog. 2010;6(11):e1001210. doi: 10.1371/journal.ppat.1001210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennan R.M., Gilhuus M., Frosth S., Seemann T., Dhungyel O.P., Whittington R.J., Boyce J.D., Powell D.R., Aspán A., Jørgensen H.J., Bulach D.M., Rood J.I. Genomic evidence for a globally distributed, bimodal population in the ovine footot pathogen Dichelobacter nodosus. mBio. 2014;5(5):e01821–e1914. doi: 10.1128/mBio.01821-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klitgaard K., Bretó A.F., Boye M., Jensen T.K. Targeting the treponemal microbiome of digital dermatitis infections by high-resolution phylogenetic analyses and comparison with fluorescence in situ hybridization. J. Clin. Microbiol. 2013;51(7):2212–2219. doi: 10.1128/JCM.00320-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knappe-Poindecker M., Gilhuus M., Jensen T.K., Vatn S., Jørgensen H.J., Fjeldaas T. Cross-infection of virulent Dichelobacter nodosus between sheep and co-grazing cattle. Vet. Microbiol. 2014;170(3–4):375–382. doi: 10.1016/j.vetmic.2014.02.044. [DOI] [PubMed] [Google Scholar]
- König U., Nyman A.-K.J., de Verdier K. Prevalence of footrot in Swedish slaughter lambs. Acta Vet. Scand. 2010;53:27. doi: 10.1186/1751-0147-53-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Fontaine S., Egerton J.R., Rood J.I. Detection of Dichelobacter nodosus using species-specific oligonucleotides as PCR primers. Vet. Microbiol. 1993;35(1–2):101–117. doi: 10.1016/0378-1135(93)90119-r. [DOI] [PubMed] [Google Scholar]
- Loy A., Arnold R., Tischler P., Rattei T., Wagner M., Horn M. probeCheck – a central resource for evaluating oligonucleotide probe coverage and specificity. Environ. Microbiol. 2008;10(10):2894–2898. doi: 10.1111/j.1462-2920.2008.01706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore L.J., Wassink G.J., Green L.E., Grogono-Thomas R. The detection and characterisation of Dichelobacter nodosus from cases of ovine footrot in England and Wales. Vet. Microbiol. 2005;108:57–67. doi: 10.1016/j.vetmic.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Moter A., Göbel U.B. Fluorescence in situ hybridization (FISH) for direct visualization of microorganisms. J. Microbiol. Methods. 2000;41:85–112. doi: 10.1016/s0167-7012(00)00152-4. [DOI] [PubMed] [Google Scholar]
- Peters I.R., Helps C.R., Willi B., Hofmann-Lehmann R., Gruffydd-Jones T.J., Day M.J., Tasker S. Detection of feline haemoplasma species in experimental infection by in-situ hybridisation. Microb. Pathog. 2011;50(2):94–99. doi: 10.1016/j.micpath.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasbash J., Carlton C., Browne W.J., Healy M., Cameron B. Centre for Multilevel Modelling, University of Bristol; 2005. MLwiN Version 2.02. [Google Scholar]
- Rasmussen M., Capion N., Klitgaard K., Rogdo T., Fjeldaas T., Boye M., Jensen T.K. Bovine digital dermatitis: possible pathogenic consortium consisting of Dichelobacter nodosus and multiple Treponema species. Vet. Microbiol. 2012;160:151–161. doi: 10.1016/j.vetmic.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Roberts D.S., Egerton J.R. The aetiology and pathogenesis of ovine foot-rot. II. The pathogenic association of Fusiformis nodosusand F. necrophorus. J. Comp. Pathol. 1969;79:217–227. doi: 10.1016/0021-9975(69)90008-5. [DOI] [PubMed] [Google Scholar]
- Rogers G.B., Carroll M.P., Bruce K.D. Studying bacterial infections through culture-independent approaches. J. Med. Microbiol. 2009;58:1401–1418. doi: 10.1099/jmm.0.013334-0. [DOI] [PubMed] [Google Scholar]
- Smith E.M., Green O.D., Calvo-Bado L.A., Witcomb L.A., Grogono-Thomas R., Russell C.L., Brown J.C., Medley G.F., KilBride A.L., Wellington E.M.H., Green L.E. Dynamics and impact of footrot and climate on hoof horn length in 50 ewes from one farm over a period of 10 months. Vet. J. 2014;201(3):295–301. doi: 10.1016/j.tvjl.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stäuble A., Steiner A., Normand L., Kuhnert P., Frey J. Molecular genetic analysis of Dichelobacter nodosus proteases AprV2/B2, AprV5/B5 and BprV/B in clinical material from European sheep flocks. Vet. Microbiol. 2014;168:177–184. doi: 10.1016/j.vetmic.2013.11.013. [DOI] [PubMed] [Google Scholar]
- Witcomb L.A., Green L.E., Kaler J., Ul-Hassan A., Calvo-Bado L.A., Medley G.F. A longitudinal study of the role of Dichelobacter nodosus and Fusobacterium necrophorum load in initiation and severity of footrot in sheep. Prev. Vet. Med. 2014;115(1–2):48–55. doi: 10.1016/j.prevetmed.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K.D. Bacterial morphology: why have different shapes? Curr. Opin. Microbiol. 2007;10(6):596–600. doi: 10.1016/j.mib.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]