Abstract
Vta1 and Vps60 are two ESCRT associated proteins, their direct interaction enhances Vps4 ATPase activity. The N-terminal domain of Vta1 (residues 1–167aa, named as Vta1NTD) contains two tandem MIT domains, which specifically recognize Vps60 and Did2 but not other ESCRT-III subunits. The fragment Vps60 (128–186aa) was reported to display full activity of Vps60, which stimulates Vps4 ATPase in a Vta1-dependent manner. To study the structural basis for the interaction between Vta1 and Vps60, as a first step, here, we report the resonance assignments of the sequential backbone atoms and the side chains of the residues in the two components of Vta1NTD/Vps60128–186 complex at pH 7.0 and 20 °C (BMRB No. 18521).
Keywords: Vta1, Vps60, ESCRT, Vps4, MIT domain
Biological context
The multivesicular body (MVB) sorting pathway plays critical roles in several eukaryotic cellular processes (Piper and Katzmann 2007). Proper function of the MVB sorting pathway requires reversible membrane association of the endosomal sorting complexes required for transport (ESCRT) proteins, a process catalyzed by Vps4 ATPase (Babst et al. 1998). Vta1 acts as a positive regulator of Vps4 by stimulating its assembly and ATPase activity (Azmi et al. 2006). Different from Vta1, Vps60 does not bind to Vps4 directly, and it only stimulates Vps4 in a Vta1-dependent manner (Azmi et al. 2008); however the mechanism of the allosteric regulation by Vps60 is still unclear.
The crystal structure of the N-terminal domain of Vta1 (Vta1NTD) in free form reveals that it has two previously unknown tandem microtubule interacting and transport (MIT) domains within the structure (Xiao et al. 2008). These two domains mediate the interaction between Vta1 and the ESCRT-III proteins Vps60 and Did2. It was reported that the N-terminal of Vta1 mainly interacts with the fragment of the residues 128–186 of Vps60 (Vps60128–186) (Azmi et al. 2008). However, the residues within the MIT domains of Vta1NTD perceived to be important for ligand binding based on their structural homology to the MIT domain of Vps4 are completely dispensable for the binding activity of Vta1 (Xiao et al. 2008). It thus appears that the interaction between the MIT domain of Vta1 and its binding partner utilizes a mechanism different from that of Vps4. To unravel this novel recognition mode of the MIT domain, here, we carried out our NMR experiments on the Vta1NTD/Vps60128–186 peptide complex.
Methods and experiments
The N-terminal domain of Vta1, amino acids 1–167 (Vta1NTD), and Vps60 fragment (amino acids 128–186) were amplified and cloned into a modified pET28b vector with a SUMO protein inserted between a His6-tag and the Vta1NTD or Vps60128–186 coding region via BamH I and Xho I restriction. The proteins were expressed in the Escherichia coli BL21 (DE3) strain grown in LB medium. For isotope Labeling, M9 Minimal medium was used supplemented with 15NH4Cl (Cambridge Isotope Laboratories) or 15NH4Cl and 13C-glucose (Cambridge Isotope Laboratories). Protein expression was induced (12 h, 20 °C) with 0.4 mM IPTG (OD600 = 0.8). Cells were harvested and resuspended in lysis buffer (50 mM Tris pH 8.0, 300 mM NaCl, 5 mM β-mercaptoethanol) and lysed with 10 μg/mL PMSF by sonication on ice. Lysates were clarified by centrifugation (30 min., 16,000 rpm), and the soluble proteins were purified by nickel affinity chromatography (G.E Inc) through using a linear gradient of 0–250 mM imidazole in the lysis buffer. Protein fractions were collected and dialyzed two times at 4°C into the lysis buffer. The His6-SUMO tag was removed by incubation with ULP1 protease followed by a second nickel column. The fractions containing Vta1NTD or Vps60128–186 were pooled together, exchanged with the buffer (20 mM Tris-HCl, 50 mM NaCl, 1 mM dithiothreitol, pH 8.0), followed by loading them into anion exchange Resource Q chromatography (G.E. Inc). The pure protein fractions were eluted by using a linear gradient of Tris-HCl buffer, and verified by running SDS-PAGE gel and electrospray mass spectrometry. The detailed purification steps will be published elsewhere.
Two NMR complex samples were prepared in NMR buffer (25 mM sodium phosphate pH 7.0, 100 mM NaCl, 5 mM dithiothreitol-d10 (DTT), 0.02% NaN3). One is 1.5 mM uniformly labeled 15N-/13C-labeled Vta1NTD mixed with 1.8 mM unlabeled Vps60128–186, the other is 1.5 mM uniformly labeled 15N-/13C-labeled Vps60 in complex with 1.8 mM unlabeled Vta1NTD. The mole ratio (1:1.2) used here was designed based on the binding affinity results from isothermal titration calorimetry (ITC) assay (which will be described elsewhere), and to keep 15N-/13C-labeled component in each complex in bound states. NMR experiments were conducted at 20 °C on a Varian Unity Inova 600 NMR spectrometer or on a Bruker Avance III-800 MHz NMR spectrometer, both equipped with cryoprobe, three or four channels and z-axis pulsed-field gradient. The standard suite of experiments for assigning the 1H, 13C and 15N backbone and side chain chemical shifts, and for the collection of NOE-based distance restraints were measured (Bax and Grzesiek 1993; Clore and Gronenborn 1998), which consists of the 2D 13C-edited HSQC in both aliphatic and aromatic regions, and 15N-edited HSQC; the 3D HNCA, HNCO, HN(CO)CA, HNCACB, CBCA(CO)NH, 15N-resolved HSQC-TOCSY, HCCH-TOCSY in both aliphatic and aromatic regions, 15N-resolved HSQC-NOESY, 13C-resolved HSQC-NOESY for both aliphatic and aromatic resonances, 2D (Hβ)Cβ(CγCδ)Hδ and (Hβ)Cβ(CγCδCε)Hε spectra for correlation of Cβ and Hδ or Hε in aromatic ring used in aromatic protons assignment (Yamazaki et al. 1993). All spectra were processed with NMRPipe (Delaglio et al. 1995) and analyzed with Sparky 3. The 1H chemical shifts of were referenced to 2,2-dimethylsilapentane-5-sulfonic acid (DSS), and the 13C- and 15N-resonances were indirectly referenced to DSS.
Assignments and data deposition
The sequential backbone and side chain resonance assignment had been deposited in the Biological Magnetic Resonance Data Bank (http://www.bmrb.wisc.edu) under the accession number 18521. The 1H-15N-TROSY-HSQC spectrum of the (U- 15N, 13C) Vta1NTD/Vps60128–186 complex sample is shown in Fig. 1, where the linewidth of each cross-peak seems to be narrow due to TROSY technique used in the experiment and slightly higher contour level of the spectrum. All the amide signals were observed with the exception of the amide signals of Met1, Glu141 and Leu161 of Vta1NTD. All the Cα were assigned, while about 96% and 97% of the Cβ and CO were assigned, respectively. 1H and 13C chemical shifts were obtained for approximately 98% of the CHn and aromatic side chains. In 3D HNCO, the cross peak at 15N 126.5 ppm, 1H 7.0 ppm and 13C 171 ppm was observed. However, only one cross peak (at 13C 43 ppm) was found in the vertical strip of 15N 126.5 ppm and 1H 7.0 ppm in 3D HNCA, and no cross-peak was obtained at the same strip in 3D HN(CO)CA. Thus, in Fig.1, the signal at 15N 126.5 ppm and 1H 7.0 ppm was suggested to be from NHε in Arg side-chain in Vta1NTD. In current study, its assignment could not be performed accurately.
Figure 1.
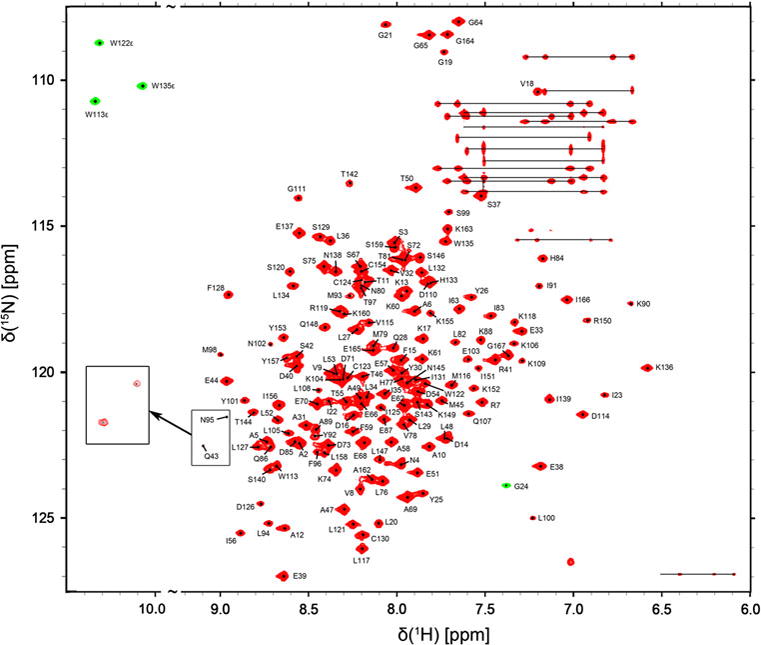
1H-15N-TROSY-HSQC spectrum of the (U-15N, 13C) Vta1NTD/Vps60128–186 complex measured at 20 °C at 800 MHz. Backbone resonance assignments are indicated in a one-letter amino acid code. Side-chain NH2 amide resonances of Asn and Gln, connected by horizontal lines. The Gly24 backbone and the Trp side-chain amide signals shown in green contours are folded under the experimental condition employed. Inset: the cross-peaks of amide signals belonging to residues N95 and Q43 were further displayed when the lowest contour level of the spectrum was decreased.
In Fig. 2, the 1H-15N-HSQC spectrum of the (U-15N, 13C) Vps60128–186/Vta1NTD complex is shown. The assignment of the labeled Vps60128–186 peptide in complex with Vta1NTD was completed to 89.4% of the backbone resonances (CA, CB, C, N, H, HA, HB). The amide signals of Asp135, Met136, Gln137, Asp138, Glu139 and Met140 were not observed, reflecting a high degree of conformational flexibility in this region. Except this flexible region (residues 135–139), the assignment of side-chain resonances is 93.0% complete. Caused by the cis-trans isomerization of Pro186 in the C-terminus, two minor peaks of Thr184 and Leu185 were observed which were labeled with T184′ and L185′, respectively (Fig. 2). The Pro186 adopts a major trans conformation as demonstrated by the differences between their Cβ and Cγ chemical shifts (Schubert et al. 2002)
Figure 2.
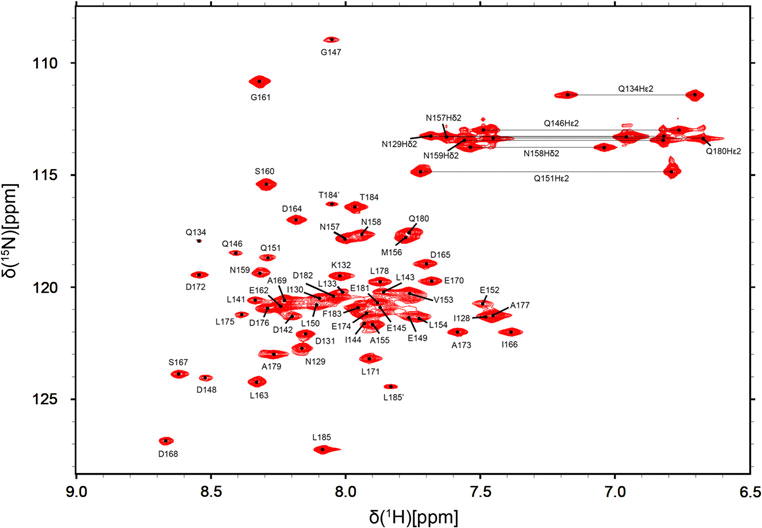
1H-15N-HSQC spectrum of the (U-15N, 13C) Vps60128–186/Vta1NTD complex measured at 20 °C at 600 MHz. Backbone resonance assignments are indicated in a one-letter amino acid code. Side-chain NH2 amide resonances of Asn and Gln, connected by horizontal lines.
Acknowledgments
This work was supported by funding from the National Basic Research Program of China under No. 2009CB918600 and 2011CB966300, by National Science Foundation of China No. 30970595 and 20921091, and by the International Cooperation Foundation from Science and Technology Commission of Shanghai Municipality under No. 09540703800, and by National New Drug Design Program from Ministry of Health of China under No. 2011ZX09506, and by the funding from State Key laboratory of Magnetic Resonance and Atomic and Molecular Physics, Wuhan Center for Magnetic Resonance, Wuhan Institute of Physics and Mathematics, CAS, under No. T151102.
References
- Azmi I, Davies B, Dimaano C, Payne J, Eckert D, et al. Recycling of ESCRTs by the AAA-ATPase Vps4 is regulated by a conserved VSL region in Vta1. J Cell Biol. 2006;172:705–717. doi: 10.1083/jcb.200508166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmi IF, Davies BA, Xiao J, Babst M, Xu Z, et al. ESCRT-III family members stimulate Vps4 ATPase activity directly or via Vta1. Dev Cell. 2008;14:50–61. doi: 10.1016/j.devcel.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Babst M, Wendland B, Estepa EJ, Emr SD. The Vps4p AAA ATPase regulates membrane association of a Vps protein complex required for normal endosome function. EMBO J. 1998;17:2982–2993. doi: 10.1093/emboj/17.11.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bax A, Grzesiek S. Methodological advances in protein NMR. Accounts of Chemical Research. 1993;26:131–138. [Google Scholar]
- Clore GM, Gronenborn AM. Determining the structures of large proteins and protein complexes by NMR. Trends Biotechnol. 1998;16:22–34. doi: 10.1016/S0167-7799(97)01135-9. [DOI] [PubMed] [Google Scholar]
- Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, et al. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- Piper RC, Katzmann DJ. Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol. 2007;23:519–547. doi: 10.1146/annurev.cellbio.23.090506.123319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M, Labudde D, Oschkinat H, Schmieder P. A software tool for the prediction of Xaa-Pro peptide bond conformations in proteins based on 13C chemical shift statistics. J Biomol NMR. 2002;24:149–154. doi: 10.1023/a:1020997118364. [DOI] [PubMed] [Google Scholar]
- Xiao J, Xia H, Zhou J, Azmi IF, Davies BA, et al. Structural basis of Vta1 function in the multivesicular body sorting pathway. Dev Cell. 2008;14:37–49. doi: 10.1016/j.devcel.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Forman-Kay JD, Kay LE. Two-dimensional NMR experiments for correlating carbon-13.beta. and proton.delta./.epsilon. chemical shifts of aromatic residues in 13C-labeled proteins via scalar couplings. Journal of the American Chemical Society. 1993;115:11054–11055. [Google Scholar]


