Abstract
Objective:
To search for antibodies against neuronal cell surface proteins.
Methods:
Using immunoprecipitation from neuronal cultures and tandem mass spectrometry, we identified antibodies against the α1 subunit of the γ-aminobutyric acid A receptor (GABAAR) in a patient whose immunoglobulin G (IgG) antibodies bound to hippocampal neurons. We searched 2,548 sera for antibodies binding to GABAAR α, β, and γ subunits on live HEK293 cells and identified the class, subclass, and GABAAR subunit specificities of the positive samples.
Results:
GABAAR-Abs were identified in 40 of 2,046 (2%) referred sera previously found negative for neuronal antibodies, in 5/502 (1%) previously positive for other neuronal surface antibodies, but not in 92 healthy individuals. The antibodies in 40% bound to either the α1 (9/45, 20%) or the γ2 subunits (9/45, 20%) and were of IgG1 (94%) or IgG3 (6%) subclass. The remaining 60% had lower antibody titers (p = 0.0005), which were mainly immunoglobulin M (IgM) (p = 0.0025), and showed no defined subunit specificity. Incubation of primary hippocampal neurons with GABAAR IgG1 sera reduced surface GABAAR membrane expression. The clinical features of 15 patients (GABAAR α1 n = 6, γ2 n = 5, undefined n = 4) included seizures (47%), memory impairment (47%), hallucinations (33%), or anxiety (20%). Most patients had not been given immunotherapies, but one with new-onset treatment-resistant catatonia made substantial improvement after plasma exchange.
Conclusions:
The GABAAR α1 and γ2 are new targets for antibodies in autoimmune neurologic disease. The full spectrum of clinical features, treatment responses, correlation with antibody specificity, and in particular the role of the IgM antibodies will need to be assessed in future studies.
Antibodies directed against proteins expressed in the CNS have been identified in a number of neurologic disorders including various encephalopathies1–3 as well as subgroups of patients with epilepsy4,5 or psychiatric disease.6,7 The antibodies, usually immunoglobulin G (IgG), are directed against extracellular epitopes of proteins expressed on the surface of neuronal cells, including the NMDA receptor (NMDAR), leucine-rich, glioma-inactivated 1 (LGI1), and contactin-associated protein-like 2 (CASPR2), and less frequently against γ-aminobutyric acid B receptor (GABABR), α-amino-3-hydroxy-5-methyl-4-isoxazol-propionic acid receptor (AMPAR), or glycine receptors.8 The majority of the patients have a favorable response to immunotherapies9–11 and detection of the antibodies in patient sera and CSF have altered diagnosis and management.
Antibodies to the α1 and β3 subunits of GABAAR, 2 subunits of the heteropentameric ligand gated ion channel that mediates the majority of inhibitory neurotransmission in the brain, were recently reported in 18 patients.12 The 6 patients with high serum and CSF GABAAR-Abs presented mainly with seizures and refractory status epilepticus, whereas lower serum titers, without CSF antibodies, were observed in 12 patients with broader neurologic diagnoses including stiff-person syndrome and adult-onset opsoclonus myoclonus syndrome.
We independently identified the GABAA α1 subunit and the novel γ2 subunit as antibody targets and, using a live cell-based assay, detected them in 45 of a total of 2,548 sera referred for other CNS antibody tests. GABAAR-Abs fell into 2 broad groups defined by their subunit specificity, titer, and immunoglobulin class or subclass.
METHODS
Standard protocol approvals, registrations, and patient consents.
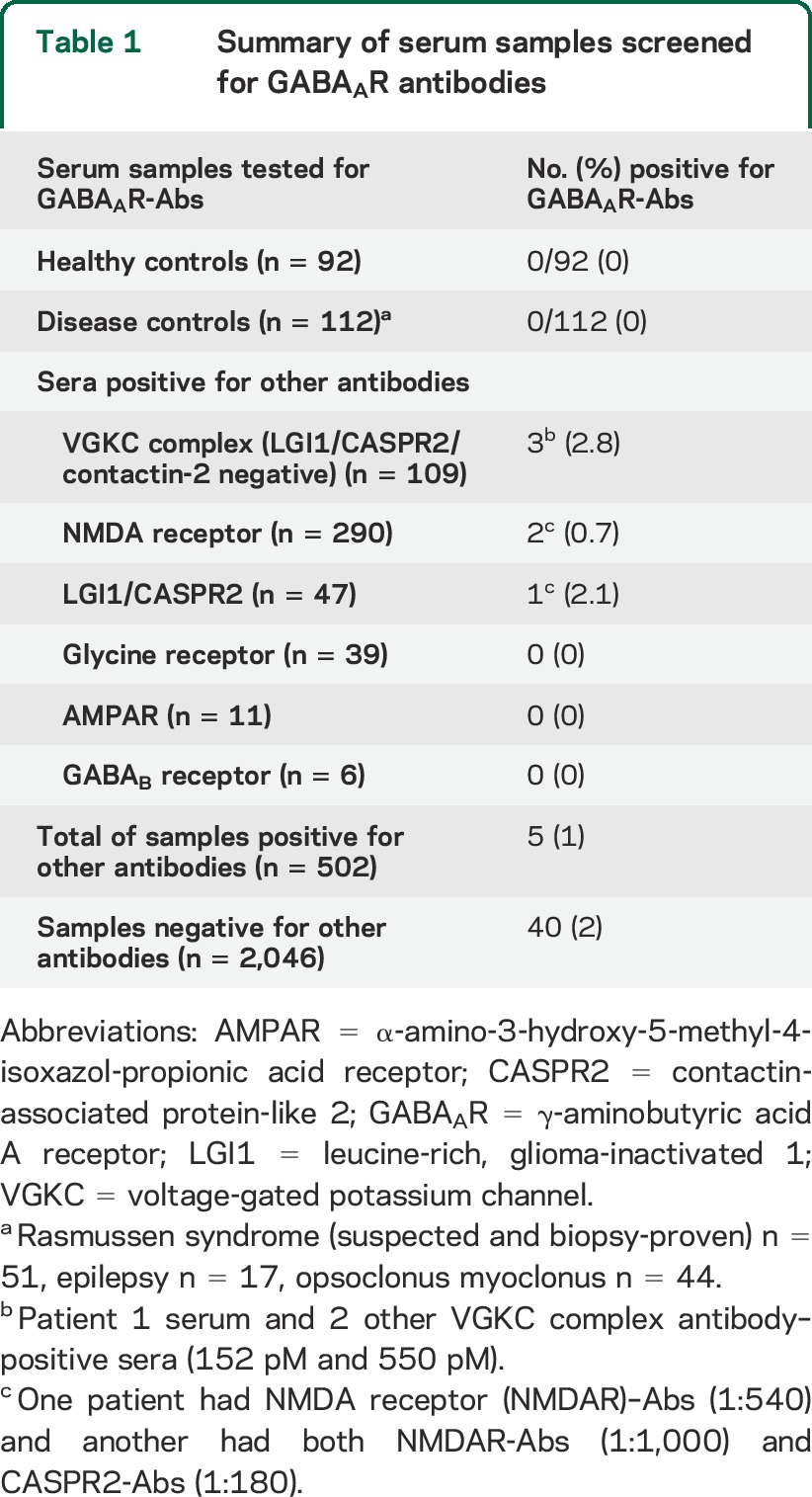
The research use of referred sera is approved by the Oxfordshire Research Ethics Committee A (07 Q160X/28). When GABAAR was identified, and a cell-based assay (CBA) established, 502 sera with voltage-gated potassium channel (VGKC) complex, NMDAR, or other antibodies, 92 healthy and 112 disease control sera (table 1), and a further 2,046 referred sera negative for the requested antibodies were tested for the presence of GABAAR-Abs. Brief clinical data were requested from referring neurologists of positive sera. Specific written consent was obtained from patient 2 for inclusion of his case report and videos.
Table 1.
Summary of serum samples screened for GABAAR antibodies
Immunoprecipitation from cortical neurons.
To identify new neuronal antibodies, sera were tested for binding to cultured primary rat hippocampal and cortical neurons. A serum with very strong binding was chosen for further study. The patient's IgG was bound to rat cortical neurons, and the immune complexes solubilized with 2% digitonin and captured using Protein G–Sepharose beads (Sigma, Dorset, UK). The immunoprecipitate was separated by gel electrophoresis and the GABAAR α1 subunit was identified as the target by mass spectroscopy from a sample of digested bands from the patient, but not healthy control, immunoprecipitate.
Expression of GABAAR in transfected human embryonic kidney cells.
As for other antibody tests,13–15 individual GABAAR subunits (α1, β2, β3, or γ2) were individually or coexpressed in human embryonic kidney 293 (HEK293) T cells and cell surface expression examined (e-Methods, tables e-1 and e-2 on the Neurology® Web site at Neurology.org). Antibody reactivity was initially assessed using HEK293 cells coexpressing α1β2γ2 GABAAR subunits and binding detected with Alexa Fluor 568 goat antihuman IgG (H + L) (1:750, A-21090, Invitrogen, Paisley, UK). All sera were scored (0: negative, 1: low positive, 2–4: positive) and colocalized with a commercial antibody against the α1 subunit of the GABAAR (1:500, clone N95/35, Antibodies Inc., Davis, CA). Endpoint dilution titers were established by determining the last dilution at which binding was scored as 1. To determine subunit specificities, all positive sera were tested by CBAs for binding to different GABAAR subunit combinations.
GABAAR IgG subclasses were determined by use of subclass-specific mouse antihuman IgG1, IgG2, IgG3, or IgG4 secondary antibodies (The Binding Site, Birmingham, UK), before washing, fixing, and visualization with Alexa Fluor 568 goat antimouse IgG (H + L) (1:750, A-11001, Invitrogen). Immunoglobulin M (IgM)–specific antibodies were determined using an Alexa Fluor 488 goat antihuman IgM antibody (1:750, A-2125, Invitrogen).
Effects of patient antibodies on GABAAR expression in vitro.
Primary P0 rat neuronal cultures (DIV 7) were incubated for 3 days with patient or healthy control serum (1:100; heated at 56°C for 30 minutes to inactivate complement). Subsequently, surface proteins were biotinylated, the cells lysed, and biotinylated surface proteins isolated on a NeutrAvidin agarose column (89881, Pierce Biotechnology, Rockford, IL). Isolated membrane proteins were eluted in sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) buffer (Invitrogen) containing 50 mM dithiothreitol; equal amounts of samples were then analyzed by SDS-PAGE and Western blot probing for the α1 and γ2 subunits of GABAAR. Antibody to the transferrin receptor (13-6800, Invitrogen) was used as a cell surface fraction loading control. Quantification of GABAAR receptor loss was determined by densitometric analysis of the Western blots using ImageJ software, and calculated as the ratio of α1:transferrin receptor and γ2:transferrin receptor.
RESULTS
Identification of GABAAR as a target of autoimmunity.
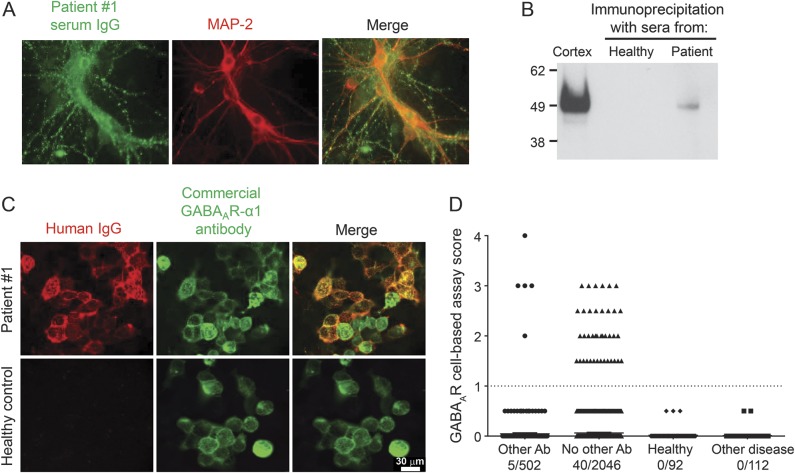
Patient 1 had a history of neuropsychological changes including elements of obsessive-compulsive disorder and increased anxiety but without psychosis. She was seen by a neurologist but there was no objective evidence of encephalitis and she returned to her care home. Subsequently, serum VGKC complex antibodies were reported (1938 pM), but all other antibody tests (antibodies to LGI1, CASPR2, NMDAR, AMPAR, GABABR, glycine R) were negative. However, her serum IgG bound intensely to the surface of both hippocampal and cortical neuronal cultures, indicating the presence of a potentially pathogenic antibody against a neuronal surface protein (figure 1A). Using immunoprecipitation and mass spectrometry (see Methods), her serum antibodies were found to bind to the α1 subunit of GABAAR (figure e-1A). GABAAR in the immunoprecipitate from the patient, but not from a healthy individual, was confirmed by Western blotting (figure 1B).
Figure 1. Identification of the GABAA receptor as an antibody target in CNS disease.
(A) The index patient sera showed antibody binding (green) to the surface of live hippocampal neurons that were identified postpermeabilization with the neuronal marker MAP-2 (red). (B) After identification of γ-aminobutyric acid A receptor (GABAAR) peptides by tandem mass spectroscopy of the immunoprecipitate from cultured neurons, its presence was confirmed by Western blotting using a commercial antibody against the α1 subunit of GABAAR (52 kDa); cortical brain homogenate (Cx) was used as positive control. (C) A cell-based assay was developed using human embryonic kidney 293 cells cotransfected the α1, β2, and γ2 subunits of GABAAR. Antibody binding to GABAAR was demonstrated with serum from patient 1 (red), which colocalized with commercial antibody against the α1 subunit (green; upper row). Immunoglobulin G (IgG) immunoreactivity to GABAAR was not observed with control serum (lower row). (D) GABAAR-Abs were identified in 5/502 sera with known antibodies (3 voltage-gated potassium channel complex, 2 NMDAR-Abs), and 40/2,046 sera previously found negative in other routine antibody tests. Samples scoring above 1 (dotted line) are considered positive. GABAAR-Abs were not present in healthy (n = 92) or disease (n = 112) control sera. Scale bars are 30 μm.
Detection of GABAAR-Abs in patient sera.
In vivo, GABAAR is composed of multiple subunits (α1-6, β1-3, γ1-3, π, ε, θ), which combine to form heteropentamers with a central pore; the α1β2γ2 is the most abundant neuronal GABAAR subtype.16 Individual homomeric GABAAR subunits and heteropentameric GABAARs (α1β2γ2 subunits) were expressed in HEK cells and their cell surface expression assessed. Immunostaining of permeabilized fixed cells showed intracellular pools of all of the GABAAR subunits (figure e-1B), but surface GABAAR expression was only found with cotransfection of all 3 GABAAR subunits, and we used α1β2γ2 to establish the CBA. Patient 1's antibody bound to the surface of live GABAAR-transfected cells, colocalizing with commercial GABAAR α1 subunit antibody (figure 1C).
GABAAR-Abs in patients and controls.
Sera from healthy and disease controls (table 1) did not bind to GABAAR-transfected cells (healthy control mean + 3 SD = 0.28, figure 1D). Only 2 of 108 (1.9%) additional sera positive for VGKC complex antibodies were positive for GABAAR-Abs, and adsorption of patient 1's serum showed that GABAAR was not a component of the VGKC complex (figure e-2). GABAAR α1β2γ2 antibodies were detected in only 2 of 393 (0.5%) sera positive for other known neuronal surface antibodies but were present in 40 of 2,046 (2%) sera previously found negative for NMDAR, AMPAR, or GABABR antibodies (table 1). Serum endpoint titers were between 1:80 and 1:4,860, and all 45 GABAAR-Abs-positive sera bound to live hippocampal neurons (as in figure 1A). There were no CSF samples available for testing from these patients.
Subunit specificities of GABAAR-Abs.
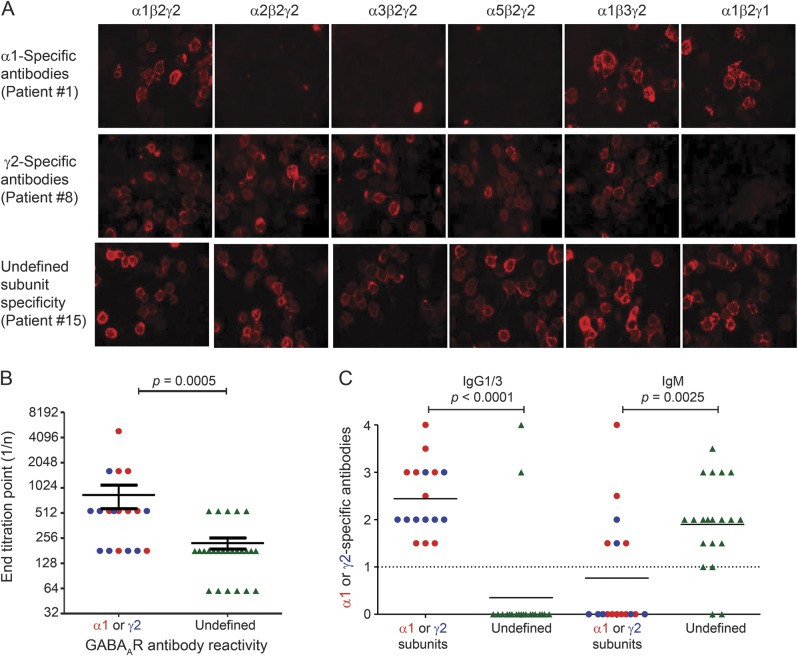
We tested reactivity to HEK293 cells after substitution of individual subunits (α1β2γ2, α2β2γ2, α3β2γ2, α5β2γ2, α1β2γ1, α1β3γ2, α3β3γ2) of the GABAAR heteropentamer (for examples, see figure 2A). Replacing the α1 subunit with the α2, α3, or α5 subunits abrogated the binding of serum antibody from 9 patients (20%, including patient 1), demonstrating specificity for the α1 subunit. Replacing γ2 with γ1 abolished binding in a further 9 sera (20%), indicating γ2 antibody specificity. However, neither these substitutions nor replacing β2 with β3 affected binding in the remaining 28 sera. Notably, patients with α1- or γ2-specific antibodies had higher antibody titers than patients lacking subunit specificity (Mann-Whitney p = 0.0005, figure 2B).
Figure 2. Specific GABAA receptor subunit reactivities and immunoglobulin classes.
(A) In the index patient 1, substitution of the α1 subunit with the α2, α3, or α5 subunit ablated binding to the γ-aminobutyric acid A receptor (GABAAR)–transfected cells, illustrating that the α1 subunit was the antigenic target. α1-Specific antibodies were observed in a further 8 patients (20% of total 45). Case 8 illustrates 1 of 9 sera (20%) that bound only to GABAARs containing the γ2 subunit, but not the γ1 subunit (second row). The third row shows sera from patient 15, which bound to all GABAARs without a defined subunit specificity. (B) Sera with subunit-specific GABAAR-Abs (α1 and γ2) had significantly higher antibody titers than sera without a distinct subunit reactivity (Mann-Whitney, p = 0.0005). (C) Sera with specific α1 (n = 9, red) or γ2 subunit (n = 8, blue) antibody reactivities had IgG1 (16) or IgG3 (1) antibodies, compared to only 2/20 sera without a defined GABAAR subunit (green) antibody reactivity (both IgG1), p < 0.0001, whose antibodies were predominantly immunoglobulin M (IgM), p = 0.025 (18/20). IgG = immunoglobulin G.
IgG class and subclass of GABAAR-Abs.
The antihuman IgG (H + L, A-21090, Invitrogen) is widely used for detection of human IgG binding but as it is directed against total IgG, including both heavy and light chains, it can crossreact with the light chains shared with other classes of antibody. Using anti-IgG or anti-IgM subclass-specific secondary antibodies only 19/37 (51.4%) available sera contained IgG1 (n = 18) or IgG3 (n = 1) GABAAR-Abs. Seventeen of the IgG antibodies were specific for GABAAR α1 or γ2 subunits; 6 of these also had IgM antibodies. By contrast, 18 of the 20 remaining sera, without a defined subunit specificity, were exclusively IgM-GABAAR-Abs (p = 0.0025, Mann-Whitney; figure 2C).
GABAAR-Abs reduce surface GABAAR expression on cultured neurons.
As patient sera bound to primary cortical neurons, we investigated the effects of 4 sera with high titers (endpoint dilutions 1:540–1:4,860) of IgG1 GABAAR α1 (n = 2, patients 1 and 2) or γ2 subunits (n = 2, patients 8 and 9) on GABAAR expression in vitro, as described by others.17 Neuronal cultures were exposed to the heat-inactivated sera (1:100) for 72 hours, and GABAAR expression assessed by Western blot. All 4 patient sera caused a reduction in the surface expression of both α1 and γ2 subunits when compared to neurons treated with 2 control sera (p = 0.0023 and p = 0.0067, respectively, figure 3, A and B).
Figure 3. Patient antibodies reduce GABAA receptor surface expression in neuronal cultures.
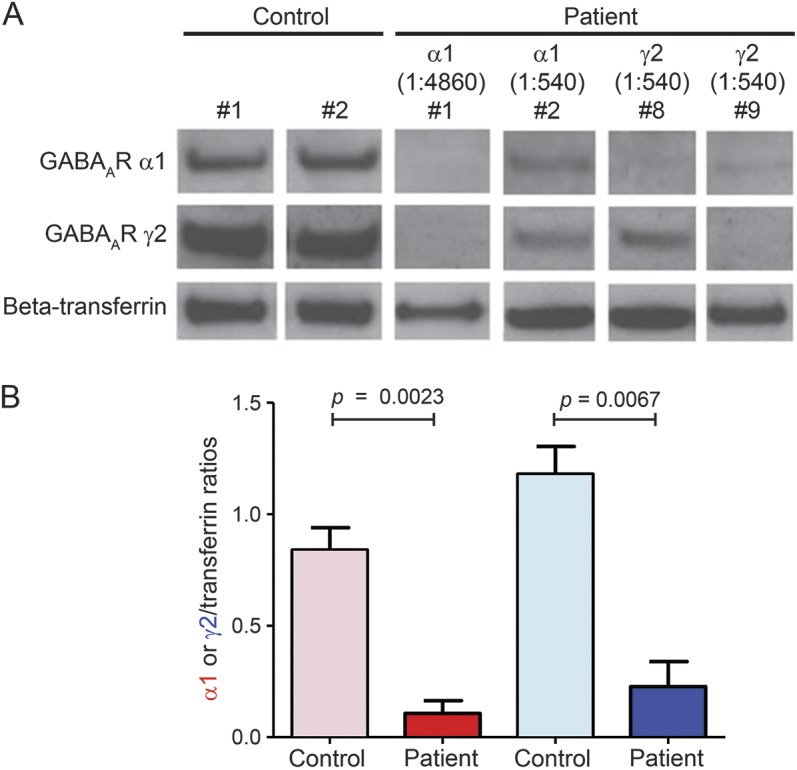
Primary cortical neuronal cultures (day 7 in vitro) were treated with serum (1:100) from γ-aminobutyric acid A receptor patients (n = 4) or healthy controls (n = 2) for 72 hours. (A) Western blot analysis showed that patient serum but not control decreased GABAAR levels (α1 [red] and γ2 [blue]) on the neuronal surface. β-Transferrin was used as a loading control. (B) Densitometry analysis showed that this loss was significant for both α1 and γ2 subunits treated with patient sera relative to healthy control (p = 0.0023 and p = 0.0067, respectively).
Clinical features of patients with GABAAR-Abs.
Overall, there were 22 male participants (age 2–76 years, median 51) and 23 female participants (age 13–80 years, median 54.5). Eight patients (3 M:5 F) were <20 years of age and 9 patients were young adults (21–30 years; 5 M:4 F). GABAAR-Abs subunit specificity did not correlate with sex (p = 0.7631, Fisher exact test) or age (p = 0.6444, Mann-Whitney test).
We subsequently obtained brief clinical data for 15 representative patients, 6 with α1 and 5 with γ2 GABAAR-Abs specificity, and 4 with undefined subunit specificity (table 2). The most common presenting features were seizures (n = 7, 47%), memory impairment (n = 7, 47%) with confusion or disorientation (n = 4, 27%), or psychiatric features (n = 5, 33%) with hallucinations (n = 2, 33%) or anxiety (n = 4, 27%). One 13-year-old girl had a dysembryoplastic neuroepithelial tumor resected earlier in life, with established neurodevelopment problems, but presented with unexplained onset of behavioral disturbance.
Table 2.
Summary of clinical data from 15 patients
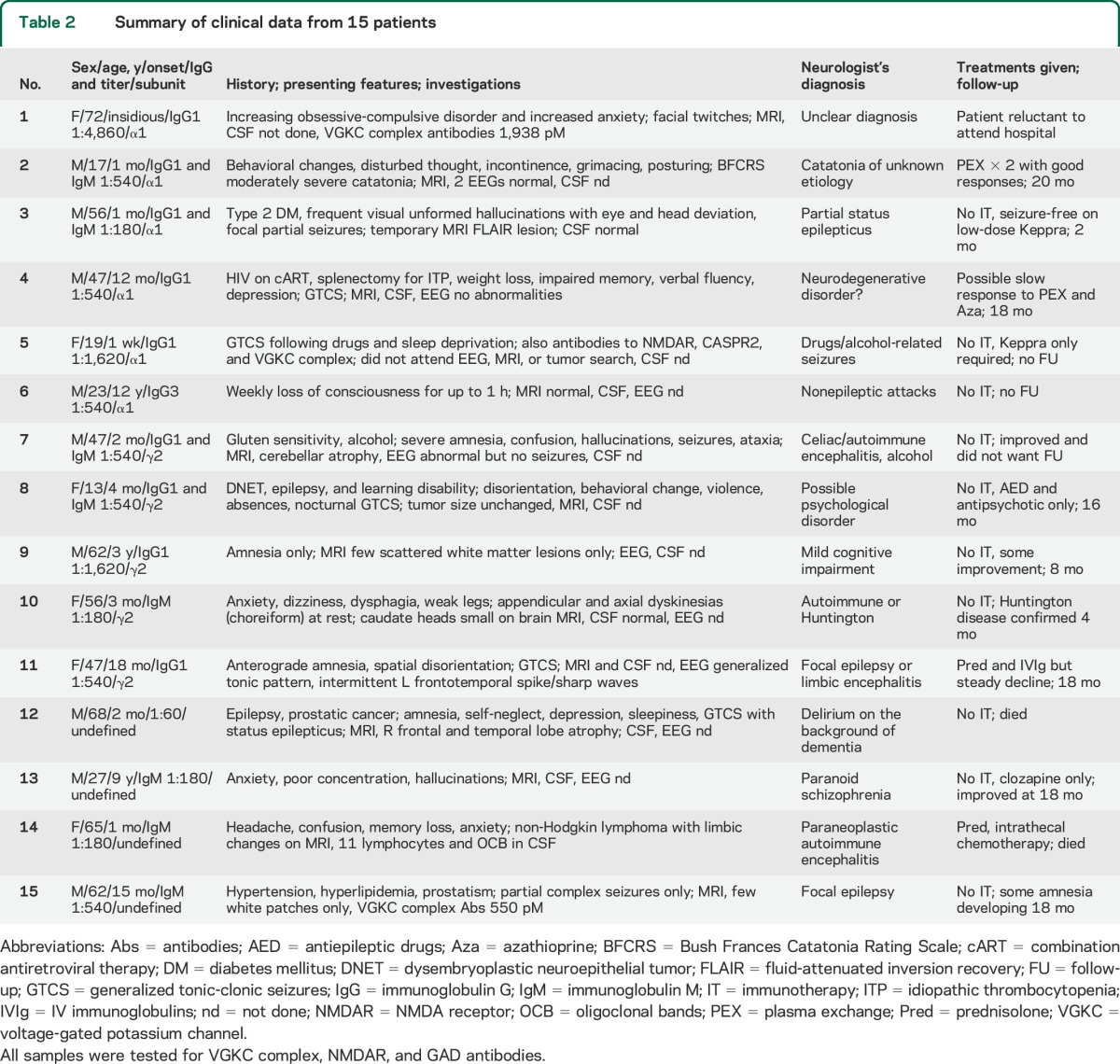
CSF had been examined at presentation in only 4/15 patients; 3/4 were normal. MRIs were normal in 4/9 or not performed (6/15). In the few with informative MRIs, patient 3 (α1-specific antibodies) had unilateral hippocampal high signal but this was thought at the time to be due to temporal lobe seizures rather than autoimmune encephalitis. Patient 10 (γ2-specific antibodies), with a small head of caudate on MRI, subsequently was diagnosed with Huntington disease. In 2 other patients there were nonspecific white matter lesions. Patient 14 (subunit undefined) was in remission from non-Hodgkin lymphoma after treatment when she presented with personality changes, memory loss, and confusion. CSF showed lymphocytic pleocytosis and oligoclonal bands, and there was temporal lobe high signal on MRI. The changes were consistent with a paraneoplastic limbic encephalitis rather than direct infiltration. However, there was mediastinal recurrence of the lymphoma and she failed to respond to intrathecal chemotherapy or immunotherapies, dying soon after. At further follow-up (either verbal or at clinic visit, up to 12 months after reporting the antibodies), 4 patients had done well on symptomatic treatment only, and a further 2 appeared to have improved spontaneously. Of the 3 patients who received some immunotherapy, one (patient 2, α1-specific antibodies) clearly improved (see below) and one showed modest improvement but one was declining after a short course. The others were either lost to follow-up or had died (see table 2).
Patient 2 presentation and clinical response to immunotherapy.
A 17-year-old boy presented to psychiatrists with a 1-month history of forgetfulness and behavioral changes (disturbed thoughts, including harming others, requesting a sex change, paranoid delusions, and attempts to self-harm). He had occasional tachycardia (up to 120 beats/minute) at rest, but neurologic examination and investigation with PET, CSF analysis, MRI, and 2 EEGs had normal results. He displayed intermittent drooling and long periods of staring and verbigeration; at other times he was verbally unresponsive, and sat abnormally still, with grimacing and posturing for more than 1 minute (video 1). He was given a diagnosis of catatonia of unknown etiology and admitted to a psychiatric unit, where he required continuous supervision; His Bush Frances Catatonia Rating Scale (BFCRS) score was 13 (moderate >9, normal 0). He was unresponsive to antidepressant (sertraline, fluoxetine), antipsychotic (olanzapine, haloperidol, quetiapine), and anxiolytic (lorazepam, clonazepam) treatment. He had a Frontal Assessment Battery (FAB) score of 6/18, indicating severe frontal dysfunction. Three months after presentation, GABAAR-Abs was detected in his serum (α1-subunit specific; 1:540, IgG1-isotype) and a possible autoimmune etiology was proposed. After consideration of the evidence implicating GABAAR in catatonia,18,19 the patient received 4 days of plasma exchange (PEX), after which antibodies were no longer detected in his serum and his frontal dysfunction and catatonia resolved within 2 weeks (BFCRS 0, FAB 18/18).
Six months after PEX, the patient relapsed with bizarre and unpredictable behavior (e.g., sudden onset intense handwashing for a few days followed by new-onset praying) and GABAAR-Abs were once again detected in his serum (1:180), but were undetectable in his CSF. There were subtle motor symptoms of catatonia and subtle frontal symptoms (BFCRS 5/FAB 11), and he received PEX again; symptoms of catatonia resolved within 2 weeks. He continued to have reduced verbal fluency, severe apathy, emotional and social withdrawal, blunted affect, and difficulty in abstract thinking (FAB 9/18). He received 5 days of methylprednisolone 1,000 mg IV and 5 days of immunoglobulins in January 2014 and was started on 1 mg/kg/OD prednisolone, which was weaned down to 10 mg/day in August 2014. His FAB returned to 17/18 in April 2014 (video 2). These symptoms improved slowly in the following months but did not disappear, perhaps due to the continued use of olanzapine and fluoxetine and an underlying diagnosis of mild Asperger syndrome made at age 11. His last GABAAR-Abs levels in April 2014 were undetectable. At this time his FAB was 17/18. His symptoms of catatonia and frontal dysfunction twice improved, strongly linked to disappearance of GABAAR-Abs with immunotherapy.
DISCUSSION
We identified a new antibody target, GABAAR, established a CBA using HEK cells expressing heteropentameric GABAARs, and identified a total of 45 patients with GABAAR-Abs. The antibodies in 40% of patients were IgG1 or IgG3, bound to GABAARs containing α1 or γ2 subunits, and all 4 sera tested were able to reduce GABAAR expression on live cortical neurons. In the remaining 60%, however, the titers were lower, the antibodies were mainly IgM, and they did not show subunit specificity, although the sera also bound to hippocampal neurons in culture. The clinical features of 15 representative patients included seizures, psychiatric and cognitive problems, and only one had a relevant malignancy. GABAAR-Abs are relatively common (up to 2% of referred sera compared with around 4% identified with NMDAR-Abs over the same time period), are potentially pathogenic, and associate with seizure and behavioral phenotypes. However, although the clinical features were variable and the paraclinical findings often normal, one boy with severe catatonia twice improved substantially following immunotherapy in parallel with normalization of his GABAAR-Abs.
GABAARs are ionotropic cell surface receptors that predominantly mediate the fast-inhibitory neurotransmission in the brain, and are usually assembled as heteropentamers. On activation, influx of chloride ions results in hyperpolarization and stabilization of the neuronal membrane potential. The GABAARs are the therapeutic target of many clinically important drugs, such as barbiturates, benzodiazepines, and topiramate, with anticonvulsant, anxiolytic, sedative, cognitive, and mood-altering properties (reviewed in reference 20). In 18 patients, we identified α1 or γ2 subunits as the main targets and showed that 4 of these were able to reduce GABAAR complexes from the neuronal surface in vitro, most likely through antibody cross-linking and internalization, as described for NMDAR and AMPAR antibodies,2,16 supporting the idea that these antibodies are pathogenic.
This study was initially designed to identify the target for antibodies in a patient with VGKC complex antibody of 1,938 pM. Despite preadsorption of GABAAR-Abs, the patient sera still bound to cultured neurons, indicating the presence of a second cell surface neuronal antibody. Thus VGKC complex antibodies that are negative for binding LGI1/CASPR2/Contactin-2 but bind cultured neurons require further study to identify their specific targets and to explore their pathogenicity.
The sera positive for GABAAR-Abs had all been sent for other CNS antibody tests. Although many of the patients had seizures, or cognitive or neuropsychiatric problems, they were given a range of tentative diagnoses (table 2). In most there was little to suggest a classical immune-mediated disease such as limbic encephalitis or NMDAR-Abs encephalitis, and in 2 patients a functional or psychogenic condition was suspected initially. Nevertheless, the large number of referrals for CNS autoantibodies (over 6,000 per year from the United Kingdom) and heterogeneity of the patients described here illustrates the increasing interest in identifying antibodies in patients with subacute onset of unexplained seizures or cognitive or psychiatric features.
GABAAR-Abs, binding the α1 or β3 subunits, were identified recently in 6 patients with refractory status epilepticus or epilepsia partialis continua and a change in cognition/behavior with extensive imaging abnormalities12 and in another 12 with a variety of phenotypes and lower titers. The authors did not report γ2 subunit specificity or examine the immunoglobulin classes and subclasses. IgG GABAA β3 antibodies were also recently reported in 2 patients with thymoma-associated encephalopathies.21 Both IgM and IgA NMDAR-Abs have previously been reported to be pathogenic in vitro, but their clinical relevance is not clear22,23; however, the serum GABAAR-IgM-Abs identified here, although low titers, were not observed in 92 healthy control sera, and they also bound to live hippocampal neurons. This suggests that they could be pathogenic in vivo if they are able to reach the brain parenchyma, or are synthesized intrathecally. However, these possibilities clearly need further study.
As this study was retrospective in design, there are several limitations, in particular the lack of available CSF samples and limited or no immunotherapy intervention in all but 2 of the patients. Nevertheless, this study, in suggesting that a potentially pathogenic antibody can associate with clinical features that are less characteristic of the well-known autoimmune encephalitis syndromes, could have implications for the field. Future prospective studies, detecting GABAAR-Abs at onset and testing CSF, with judicious use of immunotherapy, and in vitro and in vivo experiments comparing the effects of IgG and IgM antibodies, will be important in determining their clinical relevance.
GLOSSARY
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazol-propionic acid receptor
- BFCRS
Bush Frances Catatonia Rating Scale
- CASPR2
contactin-associated protein-like 2
- CBA
cell-based assay
- FAB
Frontal Assessment Battery
- GABAAR
γ-aminobutyric acid A receptor
- HEK293
human embryonic kidney 293
- IgG
immunoglobulin G
- IgM
immunoglobulin M
- LGI1
leucine-rich, glioma-inactivated 1
- NMDAR
NMDA receptor
- PEX
plasma exchange
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- VGKC
voltage-gated potassium channel
Footnotes
Editorial, page 1192
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Philippa Pettingill: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, contribution of vital reagents/tools/patients, acquisition of data, statistical analysis. Holger Kramer: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, contribution of vital reagents/tools/patients, acquisition of data. Jan Adriaan Coebergh: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data. Rosemary Pettingill: study concept or design, accepts responsibility for conduct of research and final approval, acquisition of data. Susan Maxwell: analysis or interpretation of data, accepts responsibility for conduct of research and final approval, contribution of vital reagents/tools/patients. Anjan Nibber: analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data. Andrea Malaspina: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and final approval. Anu Jacob: drafting/revising the manuscript, accepts responsibility for conduct of research and final approval, acquisition of data. Sarosh R. Irani: drafting/revising the manuscript, study concept or design, accepts responsibility for conduct of research and final approval, obtaining funding. Camilla Buckley: drafting/revising the manuscript, study concept or design, accepts responsibility for conduct of research and final approval, study supervision, obtaining funding. David Beeson: study concept or design, accepts responsibility for conduct of research and final approval, contribution of vital reagents/tools/patients, study supervision, obtaining funding. Bethan Lang: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, contribution of vital reagents/tools/patients, acquisition of data. Patrick Waters: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, contribution of vital reagents/tools/patients, acquisition of data. Angela Vincent: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, contribution of vital reagents/tools/patients, study supervision, obtaining funding.
STUDY FUNDING
Supported by the Medical Research Council UK (P.P., C.B., D.B.) and the Wellcome Trust OXION Ion Channels Initiative (H.B.K.). Partially supported by the Oxford NIHR Biomedical Research Centre and the National Health Service National Specialised Commissioning Group for Neuromyelitis Optica (P.W., A.V.), Epilepsy Research UK (B.L., S.R.I.), and the BMA Vera Down Grant (S.R.I.).
DISCLOSURE
P. Pettingill, H. Kramer, J. Coebergh, R. Pettingill, S. Maxwell, A. Nibber, and A. Malaspina report no disclosures relevant to the manuscript. A. Jacob has received honoraria as a speaker on neuromyelitis optica from Biogen Idec and Chugai and is on clinical trial advisory boards for Chugai and Alexion Pharmaceuticals. S. Irani is a coapplicant and receives royalties on patent application WO/2010/046716 titled “Neurological autoimmune disorders.” The patent has been licensed to Euroimmun AG for the development of assays for LGI1 and other VGKC complex antibodies. C. Buckley and D. Beeson report no disclosures relevant to the manuscript. P. Waters has received speaker honoraria from Biogen Idec and Euroimmun AG. A. Vincent and the Nuffield Department of Clinical Neurosciences in Oxford receive royalties and payments for antibody assays and A. Vincent is the named inventor on patent application WO/2010/046716 titled “Neurological autoimmune disorders.” The patent has been licensed to Euroimmun AG for the development of assays for LGI1 and other VGKC complex antibodies. S.R. Irani, P. Waters, and B. Lang are coinventors and have received royalties. A patent for the detection of GABAAγ2 receptor antibodies has been filed. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Dalmau J, Tüzün E, Wu H, Masjuan J. Paraneoplastic anti–N‐methyl‐D‐aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol 2007;61:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai M, Hughes EG, Peng X, et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol 2009;65:424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lancaster E, Lai M, Peng X, et al. Antibodies to the GABAB receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol 2010;9:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irani S, Michell A, Lang B, Pettingill P. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol 2011;69:1–9. [DOI] [PubMed] [Google Scholar]
- 5.Brenner T, Sills GJ, Hart Y, et al. Prevalence of neurologic autoantibodies in cohorts of patients with new and established epilepsy. Epilepsia 2013;54:1028–1035. [DOI] [PubMed] [Google Scholar]
- 6.Steiner J, Walter M, Glanz W, et al. Increased prevalence of diverse N-methyl-D-aspartate glutamate receptor antibodies in patients with an initial diagnosis of schizophrenia: specific relevance of IgG NR1a antibodies for distinction from N-methyl-D-aspartate glutamate receptor encephalitis. JAMA Psychiatry 2013;70:271–278. [DOI] [PubMed] [Google Scholar]
- 7.Tsutsui K, Kanbayashi T, Tanaka K, et al. Anti-NMDA-receptor antibody detected in encephalitis, schizophrenia, and narcolepsy with psychotic features. BMC Psychiatry 2012;12:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincent A, Bien CG, Irani SR, Waters P. Autoantibodies associated with diseases of the CNS: new developments and future challenges. Lancet Neurol 2011;10:759–772. [DOI] [PubMed] [Google Scholar]
- 9.Vincent A, Buckley C, Schott JM, et al. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain 2004;127:701–712. [DOI] [PubMed] [Google Scholar]
- 10.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008;7:1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013;12:157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petit-Pedrol M, Armangue T, Peng X, et al. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol 2014;13:276–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leite MI, Jacob S, Viegas S, et al. IgG1 antibodies to acetylcholine receptors in “seronegative” myasthenia gravis. Brain 2008;131:1940–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waters P, Jarius S, Littleton E, et al. Aquaporin-4 antibodies in neuromyelitis optica and longitudinally extensive transverse myelitis. Arch Neurol 2008;65:913–919. [DOI] [PubMed] [Google Scholar]
- 15.Irani SR, Alexander S, Waters P, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan's syndrome and acquired neuromyotonia. Brain 2010;133:2734–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 2000;101:815–850. [DOI] [PubMed] [Google Scholar]
- 17.Hughes EG, Peng X, Gleichman AJ, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci 2010;30:5866–5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Northoff G, Steinke R, Czcervenka C, et al. Decreased density of GABA-A receptors in the left sensorimotor cortex in akinetic catatonia: investigation of in vivo benzodiazepine receptor binding. J Neurol Neurosurg Psychiatry 1999;67:445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiebking C, Duncan NW, Qin P, et al. External awareness and GABA: a multimodal imaging study combining fMRI and [18F]flumazenil-PET. Hum Brain Mapp 2014;35:173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogawski MA, Löscher W. The neurobiology of antiepileptic drugs for the treatment of nonepileptic conditions. Nat Med 2004;10:685–692 Review. [DOI] [PubMed] [Google Scholar]
- 21.Ohkawa T, Satake S, Yokoi N, et al. Identification and characterization of GABA(A) receptor autoantibodies in autoimmune encephalitis. J Neurosci 2014;34:8151–8163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prüss H, Finke C, Höltje M, et al. N-methyl-D-aspartate receptor antibodies in herpes simplex encephalitis. Ann Neurol 2012;72:902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prüss H, Höltje M, Maier N, Gomez A, Buchert R. IgA NMDA receptor antibodies are markers of synaptic immunity in slow cognitive impairment. Neurology 2012;78:1743–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]