Abstract
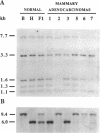
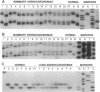
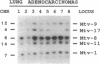
To identify the potential involvement of tumor-suppressor gene inactivation during neoplastic development in B6C3F1 mice, genetic losses were determined from allelotypes of butadiene-induced lung and mammary adenocarcinomas. By using length polymorphisms in restriction fragments and simple sequence repeats, or "microsatellites," markers on each autosome were analyzed for allele losses in tumor DNAs. Losses of heterozygosity on chromosome 11 were observed at several loci surrounding the p53 tumor-suppressor gene (Trp53) in 12 of 17 mammary tumors and 2 of 8 lung tumors. Although most of these alterations appeared to result from nondisjunction, at least two examples of somatic recombination or deletion were also observed. Southern analysis revealed a homozygous deletion of the remaining Trp53 allele of one of these mammary tumors. Losses of heterozygosity were also detected at the Rb-1 tumor-suppressor gene in 7 of 17 mammary tumors and 1 lung tumor. Finally, frequent allele losses were observed on chromosome 4 in lung tumors. Analysis of nine chromosome 4 loci defined an interstitial deletion containing the Ifa gene cluster in one of the lung tumors. A tumor-suppressor gene was previously mapped to this region of chromosome 4 in studies with somatic cell hybrids. In addition, homozygous deletions have been reported in a homologous region of human chromosome 9p for acute lymphocytic leukemias, glioblastomas, melanomas, and lung carcinomas. These findings suggest that the inactivation of tumor-suppressor genes including Trp53, Rb-1, and an unidentified gene on chromosome 4 plays a significant role during carcinogenesis in mice.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott C. M., Blank R., Eppig J. T., Friedman J. M., Huppi K. E., Jackson I., Mock B. A., Stoye J., Wiseman R. Mouse chromosome 4. Mamm Genome. 1992;3(Spec No):S55–S64. doi: 10.1007/BF00648422. [DOI] [PubMed] [Google Scholar]
- Balmain A., Brown K. Oncogene activation in chemical carcinogenesis. Adv Cancer Res. 1988;51:147–182. doi: 10.1016/s0065-230x(08)60222-5. [DOI] [PubMed] [Google Scholar]
- Bianchi A. B., Navone N. M., Aldaz C. M., Conti C. J. Overlapping loss of heterozygosity by mitotic recombination on mouse chromosome 7F1-ter in skin carcinogenesis. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7590–7594. doi: 10.1073/pnas.88.17.7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein D., White R. L., Skolnick M., Davis R. W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet. 1980 May;32(3):314–331. [PMC free article] [PubMed] [Google Scholar]
- Bremner R., Balmain A. Genetic changes in skin tumor progression: correlation between presence of a mutant ras gene and loss of heterozygosity on mouse chromosome 7. Cell. 1990 May 4;61(3):407–417. doi: 10.1016/0092-8674(90)90523-h. [DOI] [PubMed] [Google Scholar]
- Buchberg A. M., Buckwalter M. S., Camper S. A. Mouse chromosome 11. Mamm Genome. 1992;3(Spec No):S162–S181. doi: 10.1007/BF00648429. [DOI] [PubMed] [Google Scholar]
- Buonanno A., Mudd J., Merlie J. P. Isolation and characterization of the beta and epsilon subunit genes of mouse muscle acetylcholine receptor. J Biol Chem. 1989 May 5;264(13):7611–7616. [PubMed] [Google Scholar]
- Burns P. A., Kemp C. J., Gannon J. V., Lane D. P., Bremner R., Balmain A. Loss of heterozygosity and mutational alterations of the p53 gene in skin tumours of interspecific hybrid mice. Oncogene. 1991 Dec;6(12):2363–2369. [PubMed] [Google Scholar]
- Cannon-Albright L. A., Goldgar D. E., Meyer L. J., Lewis C. M., Anderson D. E., Fountain J. W., Hegi M. E., Wiseman R. W., Petty E. M., Bale A. E. Assignment of a locus for familial melanoma, MLM, to chromosome 9p13-p22. Science. 1992 Nov 13;258(5085):1148–1152. doi: 10.1126/science.1439824. [DOI] [PubMed] [Google Scholar]
- Copeland N. G., Jenkins N. A., Gilbert D. J., Eppig J. T., Maltais L. J., Miller J. C., Dietrich W. F., Weaver A., Lincoln S. E., Steen R. G. A genetic linkage map of the mouse: current applications and future prospects. Science. 1993 Oct 1;262(5130):57–66. doi: 10.1126/science.8211130. [DOI] [PubMed] [Google Scholar]
- Dietrich W., Katz H., Lincoln S. E., Shin H. S., Friedman J., Dracopoli N. C., Lander E. S. A genetic map of the mouse suitable for typing intraspecific crosses. Genetics. 1992 Jun;131(2):423–447. doi: 10.1093/genetics/131.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower L. A., Harvey M., Slagle B. L., McArthur M. J., Montgomery C. A., Jr, Butel J. S., Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992 Mar 19;356(6366):215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Fountain J. W., Karayiorgou M., Ernstoff M. S., Kirkwood J. M., Vlock D. R., Titus-Ernstoff L., Bouchard B., Vijayasaradhi S., Houghton A. N., Lahti J. Homozygous deletions within human chromosome band 9p21 in melanoma. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10557–10561. doi: 10.1073/pnas.89.21.10557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futreal P. A., Söderkvist P., Marks J. R., Iglehart J. D., Cochran C., Barrett J. C., Wiseman R. W. Detection of frequent allelic loss on proximal chromosome 17q in sporadic breast carcinoma using microsatellite length polymorphisms. Cancer Res. 1992 May 1;52(9):2624–2627. [PubMed] [Google Scholar]
- Goodrow T. L., Storer R. D., Leander K. R., Prahalada S. R., van Zwieten M. J., Bradley M. O. Murine p53 intron sequences 5-8 and their use in polymerase chain reaction/direct sequencing analysis of p53 mutations in CD-1 mouse liver and lung tumors. Mol Carcinog. 1992;5(1):9–15. doi: 10.1002/mc.2940050105. [DOI] [PubMed] [Google Scholar]
- Goodrow T., Reynolds S., Maronpot R., Anderson M. Activation of K-ras by codon 13 mutations in C57BL/6 X C3H F1 mouse tumors induced by exposure to 1,3-butadiene. Cancer Res. 1990 Aug 1;50(15):4818–4823. [PubMed] [Google Scholar]
- Groot P. C., Moen C. J., Dietrich W., Stoye J. P., Lander E. S., Demant P. The recombinant congenic strains for analysis of multigenic traits: genetic composition. FASEB J. 1992 Jul;6(10):2826–2835. doi: 10.1096/fasebj.6.10.1634045. [DOI] [PubMed] [Google Scholar]
- Hall J. M., Friedman L., Guenther C., Lee M. K., Weber J. L., Black D. M., King M. C. Closing in on a breast cancer gene on chromosome 17q. Am J Hum Genet. 1992 Jun;50(6):1235–1242. [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Dissecting multistep tumorigenesis in transgenic mice. Annu Rev Genet. 1988;22:479–519. doi: 10.1146/annurev.ge.22.120188.002403. [DOI] [PubMed] [Google Scholar]
- Harris H. The analysis of malignancy by cell fusion: the position in 1988. Cancer Res. 1988 Jun 15;48(12):3302–3306. [PubMed] [Google Scholar]
- Hearne C. M., McAleer M. A., Love J. M., Aitman T. J., Cornall R. J., Ghosh S., Knight A. M., Prins J. B., Todd J. A. Additional microsatellite markers for mouse genome mapping. Mamm Genome. 1991;1(4):273–282. doi: 10.1007/BF00352339. [DOI] [PubMed] [Google Scholar]
- Hegi M. E., Söderkvist P., Foley J. F., Schoonhoven R., Swenberg J. A., Kari F., Maronpot R., Anderson M. W., Wiseman R. W. Characterization of p53 mutations in methylene chloride-induced lung tumors from B6C3F1 mice. Carcinogenesis. 1993 May;14(5):803–810. doi: 10.1093/carcin/14.5.803. [DOI] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Idzerda R. L., Behringer R. R., Theisen M., Huggenvik J. I., McKnight G. S., Brinster R. L. Expression from the transferrin gene promoter in transgenic mice. Mol Cell Biol. 1989 Nov;9(11):5154–5162. doi: 10.1128/mcb.9.11.5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Bedigian H. G., Lee B. K. Ecotropic murine leukemia virus DNA content of normal and lymphomatous tissues of BXH-2 recombinant inbred mice. J Virol. 1982 May;42(2):379–388. doi: 10.1128/jvi.42.2.379-388.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley K. A., Kozak C. A., Dandoy F., Sor F., Skup D., Windass J. D., DeMaeyer-Guignard J., Pitha P. M., DeMaeyer E. Mapping of murine interferon-alpha genes to chromosome 4. Gene. 1983 Dec;26(2-3):181–188. doi: 10.1016/0378-1119(83)90188-9. [DOI] [PubMed] [Google Scholar]
- Kingsley D. M. Mouse chromosome 9. Mamm Genome. 1992;3(Spec No):S136–S152. doi: 10.1007/BF00648427. [DOI] [PubMed] [Google Scholar]
- Lee B. K., Eicher E. M. Segregation patterns of endogenous mouse mammary tumor viruses in five recombinant inbred strain sets. J Virol. 1990 Sep;64(9):4568–4572. doi: 10.1128/jvi.64.9.4568-4572.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love J. M., Knight A. M., McAleer M. A., Todd J. A. Towards construction of a high resolution map of the mouse genome using PCR-analysed microsatellites. Nucleic Acids Res. 1990 Jul 25;18(14):4123–4130. doi: 10.1093/nar/18.14.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick R. L., Huff J., Chou B. J., Miller R. A. Carcinogenicity of 1,3-butadiene in C57BL/6 x C3H F1 mice at low exposure concentrations. Cancer Res. 1990 Oct 15;50(20):6592–6599. [PubMed] [Google Scholar]
- Mullins J. J., Zeng Q., Gross K. W. Mapping of the mouse atrial natriuretic factor gene. Evidence for tight linkage to the Fv-1 locus. Hypertension. 1987 May;9(5):518–521. doi: 10.1161/01.hyp.9.5.518. [DOI] [PubMed] [Google Scholar]
- Nadeau J. H., Berger F. G., Cox D. R., Crosby J. L., Davisson M. T., Ferrara D., Fuchs E., Hart C., Hunihan L., Lalley P. A. A family of type I keratin genes and the homeobox-2 gene complex are closely linked to the rex locus on mouse chromosome 11. Genomics. 1989 Oct;5(3):454–462. doi: 10.1016/0888-7543(89)90009-8. [DOI] [PubMed] [Google Scholar]
- Nadeau J. H., Cox R. Mouse chromosome 14. Mamm Genome. 1992;3(Spec No):S206–S219. doi: 10.1007/BF00648432. [DOI] [PubMed] [Google Scholar]
- Narod S. A., Feunteun J., Lynch H. T., Watson P., Conway T., Lynch J., Lenoir G. M. Familial breast-ovarian cancer locus on chromosome 17q12-q23. Lancet. 1991 Jul 13;338(8759):82–83. doi: 10.1016/0140-6736(91)90076-2. [DOI] [PubMed] [Google Scholar]
- Nigro J. M., Baker S. J., Preisinger A. C., Jessup J. M., Hostetter R., Cleary K., Bigner S. H., Davidson N., Baylin S., Devilee P. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989 Dec 7;342(6250):705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- Olopade O. I., Bohlander S. K., Pomykala H., Maltepe E., Van Melle E., Le Beau M. M., Diaz M. O. Mapping of the shortest region of overlap of deletions of the short arm of chromosome 9 associated with human neoplasia. Genomics. 1992 Oct;14(2):437–443. doi: 10.1016/s0888-7543(05)80238-1. [DOI] [PubMed] [Google Scholar]
- Olopade O. I., Buchhagen D. L., Malik K., Sherman J., Nobori T., Bader S., Nau M. M., Gazdar A. F., Minna J. D., Diaz M. O. Homozygous loss of the interferon genes defines the critical region on 9p that is deleted in lung cancers. Cancer Res. 1993 May 15;53(10 Suppl):2410–2415. [PubMed] [Google Scholar]
- Propst F., Vande Woude G. F., Jenkins N. A., Copeland N. G., Lee B. K., Hunt P. A., Eicher E. M. The Mos proto-oncogene maps near the centromere on mouse chromosome 4. Genomics. 1989 Jul;5(1):118–123. doi: 10.1016/0888-7543(89)90094-3. [DOI] [PubMed] [Google Scholar]
- Ruggeri B., Caamano J., Goodrow T., DiRado M., Bianchi A., Trono D., Conti C. J., Klein-Szanto A. J. Alterations of the p53 tumor suppressor gene during mouse skin tumor progression. Cancer Res. 1991 Dec 15;51(24):6615–6621. [PubMed] [Google Scholar]
- Sato T., Akiyama F., Sakamoto G., Kasumi F., Nakamura Y. Accumulation of genetic alterations and progression of primary breast cancer. Cancer Res. 1991 Nov 1;51(21):5794–5799. [PubMed] [Google Scholar]
- Sato T., Saito H., Morita R., Koi S., Lee J. H., Nakamura Y. Allelotype of human ovarian cancer. Cancer Res. 1991 Oct 1;51(19):5118–5122. [PubMed] [Google Scholar]
- Sato T., Tanigami A., Yamakawa K., Akiyama F., Kasumi F., Sakamoto G., Nakamura Y. Allelotype of breast cancer: cumulative allele losses promote tumor progression in primary breast cancer. Cancer Res. 1990 Nov 15;50(22):7184–7189. [PubMed] [Google Scholar]
- Scrable H. J., Sapienza C., Cavenee W. K. Genetic and epigenetic losses of heterozygosity in cancer predisposition and progression. Adv Cancer Res. 1990;54:25–62. doi: 10.1016/s0065-230x(08)60807-6. [DOI] [PubMed] [Google Scholar]
- Stone J. C., Crosby J. L., Kozak C. A., Schievella A. R., Bernards R., Nadeau J. H. The murine retinoblastoma homolog maps to chromosome 14 near Es-10. Genomics. 1989 Jul;5(1):70–75. doi: 10.1016/0888-7543(89)90088-8. [DOI] [PubMed] [Google Scholar]
- Tan T. H., Wallis J., Levine A. J. Identification of the p53 protein domain involved in formation of the simian virus 40 large T-antigen-p53 protein complex. J Virol. 1986 Sep;59(3):574–583. doi: 10.1128/jvi.59.3.574-583.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd J. A., Aitman T. J., Cornall R. J., Ghosh S., Hall J. R., Hearne C. M., Knight A. M., Love J. M., McAleer M. A., Prins J. B. Genetic analysis of autoimmune type 1 diabetes mellitus in mice. Nature. 1991 Jun 13;351(6327):542–547. doi: 10.1038/351542a0. [DOI] [PubMed] [Google Scholar]
- Tsuchiya E., Nakamura Y., Weng S. Y., Nakagawa K., Tsuchiya S., Sugano H., Kitagawa T. Allelotype of non-small cell lung carcinoma--comparison between loss of heterozygosity in squamous cell carcinoma and adenocarcinoma. Cancer Res. 1992 May 1;52(9):2478–2481. [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Kern S. E., Hamilton S. R., Preisinger A. C., Nakamura Y., White R. Allelotype of colorectal carcinomas. Science. 1989 Apr 14;244(4901):207–211. doi: 10.1126/science.2565047. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. Tumor suppressor genes. Science. 1991 Nov 22;254(5035):1138–1146. doi: 10.1126/science.1659741. [DOI] [PubMed] [Google Scholar]