Abstract
Background
Patients with radioactive iodine (131I, RAI)-refractory locally advanced or metastatic differentiated thyroid cancer (DTC) have a poor prognosis due to the lack of effective treatment options.
Methods
This multicentre, randomized (1:1), double-blind, placebo-controlled, phase 3 study (DECISION; NCT00984282) investigated sorafenib (400 mg orally twice-daily) in patients with RAI-refractory locally advanced or metastatic DTC progressing within the past 14 months. The primary endpoint was progression-free survival (PFS) by central independent review. Patients receiving placebo could crossover to open-label sorafenib upon progression. Archival tumour tissue was examined for BRAF and RAS mutations. Serum thyroglobulin was measured at baseline and each visit.
Findings
A total of 417 patients were randomized to sorafenib (n=207) or placebo (n=210). Sorafenib treatment significantly improved PFS compared with placebo (hazard ratio, 0·59; 95% confidence interval, 0·45–0·76; P<0·0001; median 10·8 vs. 5·8 months, respectively). PFS improvement was seen in all pre-specified clinical and genetic biomarker subgroups irrespective of mutation status. There was no statistically significant difference in overall survival (hazard ratio, 0·80; 95% confidence interval, 0·54–1·19; P=0·14); median overall survival had not been reached and 150 (71%) patients receiving placebo crossed over to sorafenib upon progression. Response rates (all partial responses) were 12·2% (24/196; sorafenib) and 0·5% (1/201; placebo; p<0·0001). Median thyroglobulin levels increased in the placebo group, and decreased, then paralleled treatment responses in the sorafenib group. Most adverse events were grade 1 or 2. The most common treatment-emergent adverse events in the sorafenib arm were hand–foot skin reaction (76·3%), diarrhoea (68·6%), alopecia (67·1%), and rash/desquamation (50·2%).
Interpretation
Sorafenib significantly improved PFS compared with placebo in patients with progressive RAI-refractory DTC. Adverse events were consistent with the known sorafenib safety profile. These results suggest that sorafenib represents a new treatment option for patients with progressive RAI-refractory DTC.
INTRODUCTION
Differentiated thyroid cancer (DTC) constitutes approximately 95% of thyroid carcinomas. DTC arises from aberrant follicular cells and is classified histologically as either papillary, follicular (including Hürthle cell), or poorly differentiated.1,2 Generally DTC is effectively treated by surgery, radioactive iodine (RAI), and l-thyroxine therapy.1,2 However, 7–23% of patients develop distant metastases3, and two-thirds of patients with distant metastases become RAI-refractory.4 These patients have poor prognosis4, and lack of effective therapy (including chemotherapy) makes their clinical management difficult.5
Several genetic alterations have been identified in the molecular pathogenesis of thyroid cancer, most commonly RET/PTC translocations and BRAFV600E point mutations in papillary thyroid carcinoma, and RAS point mutations in follicular and poorly differentiated thyroid carcinoma.6 BRAFV600E has been associated with poor pathological features and poor clinical outcomes in papillary thyroid carcinoma, but not in all studies.7–10 Elevated expression of vascular endothelial growth factor (VEGF) and its receptors (VEGFR) may play a role in thyroid carcinoma.11 Antiangiogenic agents targeting the VEGF pathway have been assessed in phase 2 studies of RAI-refractory DTC.12–22 Sorafenib, an oral kinase inhibitor of VEGFR-1, -2, and -3, RET (including RET/PTC), RAF (including BRAFV600E), and platelet-derived growth factor receptor beta,23,24 has demonstrated median progression-free survival (PFS) longer than 1 year.12,16–18,20
We evaluated the efficacy and safety of sorafenib versus placebo in patients with locally advanced or metastatic progressive RAI-refractory DTC. Exploratory analyses were conducted to identify potential predictive, prognostic, or pharmacodynamic biomarkers.
METHODS
Study design and patients
DECISION was a multicentre, randomized, double-blind, placebo-controlled, phase 3 trial (NCT00984282;EudraCT 2009-012007-25;25 protocol available online). Key eligibility criteria included: age ≥18 years; locally advanced or metastatic RAI-refractory DTC (papillary, follicular [including Hürthle cell], and poorly differentiated) progressing within the past 14 months according to Response Evaluation Criteria in Solid Tumors (RECIST); at least one measurable lesion by computed tomography (CT) or magnetic resonance imaging (MRI) according to RECIST; Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0–1; adequate bone marrow, liver, and renal function; and serum thyroid-stimulating hormone (TSH)<0·5mIU/L. RAI-refractory DTC was defined as: (1) the presence of ≥onetarget lesion without iodine uptake; or (2) patients whose tumours had iodine uptake and (a) progressed after one RAI treatment within the past 16 months; (b) progressed after two RAI treatments within 16 months of each other, the last RAI treatment administered >16 months ago; or (c) received cumulative RAI activity ≥22·3 GBq (≥600 mCi). Patients who had received prior targeted therapy, thalidomide, or chemotherapy for thyroid cancer were excluded; low dose chemotherapy for radio sensitization was allowed. All patients provided written informed consent. An independent data monitoring committee (comprised of three oncologists, an endocrinologist, and a statistician) ensured patient safety and monitored study conduct.
Randomization and masking
Patients were randomized 1:1 via an interactive voice response system (IVRS) to either sorafenib 400 mg or matching placebo, both given orally twice-daily (taken 12 hours apart without food, ≥1 hour before or 2 hours after a meal). Patients, investigators, and sponsor were blinded to treatment assignment via unique drug pack numbers preprinted onto each bottle or package and assigned to the patient via IVRS. Further randomization details are in Supplementary Appendix B.
Procedures
Study drug dose interruption or sequential reduction (600 mg [divided doses: 400 and 200], 400 mg [divided 2 × 200], and 200 mg daily) and re-escalation were allowed based on specific criteria to manage adverse events (AEs; Supplementary Appendix B, Tables B1−B5). Treatment continued until progression, unacceptable toxicity, noncompliance, or withdrawal of consent. In the event of protocol-defined progression determined by the investigator, treatment could be unblinded and patients from both groups could begin open-label sorafenib and continue until lack of benefit based on investigator judgment.
The primary endpoint was PFS, assessed every 8 weeks by central independent blinded review using modified RECIST (endpoints fully defined in Supplementary Appendix C). Secondary endpoints included overall survival (OS), time to progression (TTP), objective response rate (ORR; complete or partial response [PR]), disease control rate (DCR; complete or PR and stable disease [SD] ≥4 weeks [or ≥6 months via post-hoc analysis]), and duration of response. Progression and objective response were confirmed by a repeat CT or MRI scan performed ≥4 weeks later. Safety was assessed according to National Cancer Institute Common Terminology Criteria for Adverse Events v3·0. Patients were followed up for safety for 30 days following the last study treatment, and then every 3 months for OS. Histologic diagnoses were assessed retrospectively by an independent pathology panel.
Statistical analysis
Assuming a one-sided alpha of 0·01, 90% power, and a 55·5% increase in median PFS, 267 PFS events were required from 420 randomized patients. PFS, TTP, and OS were assessed in all randomized patients by log-rank test using one-sided significance levels of 0·01 (PFS) and 0·025 (TTP and OS). Hazard ratios (HR) and confidence intervals (CI) were derived from a Cox proportional hazards model. ORR and DCR were assessed by Cochran–Mantel–Haenszel test (one-sided significance level: 0·025) in patients who received study medication and had a baseline and a post-baseline tumour evaluation. All tests were stratified by age (<60 versus ≥60 years) and geographical region (North America versus Europe versus Asia). Summary statistics were provided for safety outcomes during the double-blind period in all randomized patients who received ≥one dose of study medication.
Exploratory biomarker analyses
These were conducted to identify potential predictive, prognostic, or pharmacodynamic biomarker candidates. Archival formalin-fixed, paraffin-embedded biopsies from primary tumour or metastatic sites were collected for patients who gave consent. Extracted DNA was tested for BRAF and RAS (including NRAS, HRAS, and KRAS) mutations (listed in Supplementary Appendix Table D1) using OncoCarta™ Panel v1·0 (Sequenom Inc., San Diego, CA, USA). Serum thyroglobulin levels were measured at baseline and on day 1 of each treatment cycle (IMMULITE 2000 Thyroglobulin, Siemens Diagnostics, Tarrytown, NY, USA). Univariate and multivariate Cox proportional hazards models assessed the relationship between biomarkers and PFS, including a biomarker-treatment interaction term to assess potential differential treatment effects in biomarker-defined subgroups. Multivariate models included BRAF and RAS mutational status, sex, ethnicity, age, DTC histology, ECOG PS, and treatment group (for models including both treatment arms).
Role of the funding source
Study design, data collection, analysis, and interpretation of results were funded by Bayer HealthCare Pharmaceuticals and Onyx Pharmaceuticals Inc, an Amgen subsidiary. Employees of Bayer HealthCare Pharmaceuticals and Onyx Pharmaceuticals participated in the study design, data analysis, and interpretation. Data were obtained locally and the central study database was audited by Bayer HealthCare Pharmaceuticals and Onyx Pharmaceuticals. Emma Robinson (7.4 Limited, Oxford, UK) provided medical writing support funded by Bayer HealthCare Pharmaceuticals. The corresponding author had full access to all the study data and final responsibility for the decision to submit for publication.
RESULTS
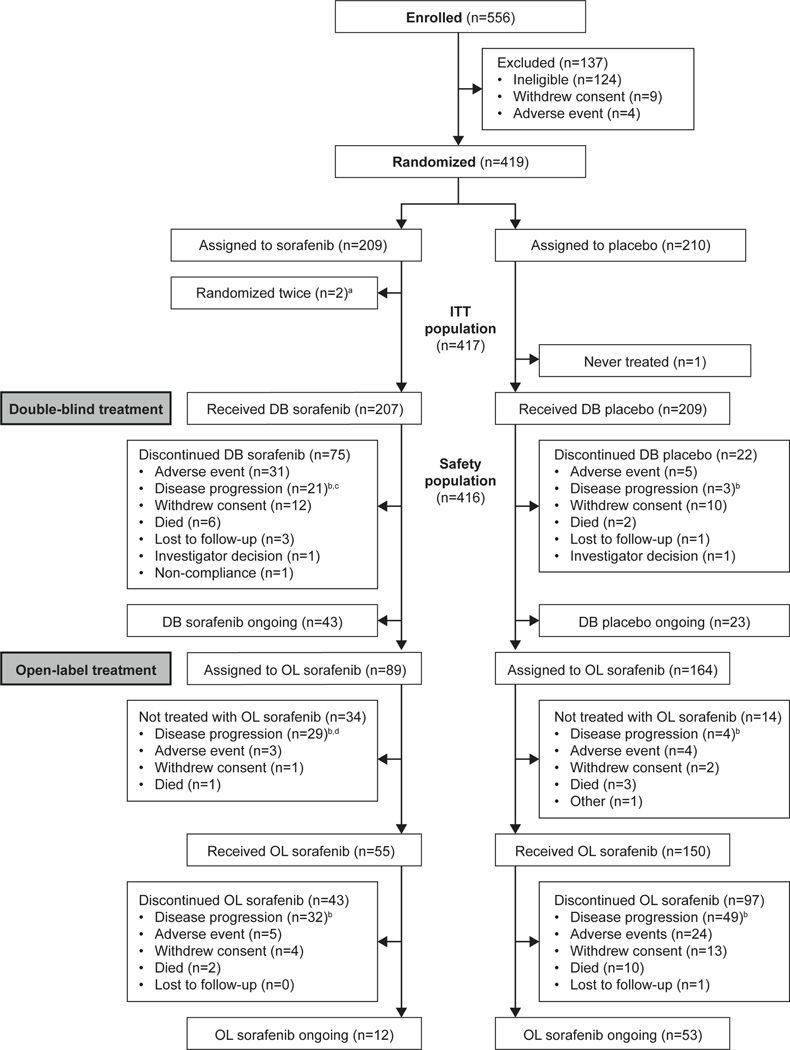
From October 2009 to August 2011, 417 patients from 77 centres in 18 countries were randomized to sorafenib (n=207) or placebo (n=210) (Fig. 1). Baseline demographic characteristics were well balanced (Table 1). In total, 96·4% (n=402/417) of patients had distant metastases, most commonly in lung (86·1%; n=359/417), lymph nodes (51·3%; n=214/417), and bone (27·1%; n=113/417). Over 75% of patients were positive for fluorodeoxyglucose (FDG) uptake on positron emission tomography scintigraphy.
Figure 1.
Patient disposition.
DB, double-blind; ITT, intention-to-treat; OL, open-label
aTwo patients were randomized twice by error and not included in the ITT population, therefore the total number of patients randomized to sorafenib was 207.
bDisease progression, recurrence, or relapse.
cFor one patient receiving double-blind sorafenib, disease progression was by clinical judgement.
dFor one patient assigned to open-label sorafenib, disease progression was by clinical judgement.
Table 1.
Demographic and clinical characteristics (intention-to-treat population)
| Sorafenib (n=207) |
Placebo (n=210) |
|
|---|---|---|
| Female, n (%) | 103 (49·8) | 115 (54·8) |
| Age (years) | ||
| Median (range) | 63 (24–82) | 63 (30–87) |
| ≥60 years, n (%) | 127 (61·4) | 129 (61·4) |
| Ethnicity, n (%) | ||
| White | 123 (59·4) | 128 (61·0) |
| Asian | 47 (22·7) | 52 (24·8) |
| Black | 6 (2·9) | 5 (2·4) |
| Hispanic | 2 (1·0) | 2 (1·0) |
| Not reported | 29 (14·0) | 23 (11·0) |
| Region, n (%) | ||
| Europe | 124 (59·9) | 125 (59·5) |
| North America | 36 (17·4) | 36 (17·1) |
| Asia | 47 (22·7) | 49 (23·3) |
| Metastases, n (%) | ||
| Locally advanced | 7 (3·4) | 8 (3·8) |
| Distant | 200 (96·6) | 202 (96·2) |
| Time from diagnosis, months | ||
| Median (range) | 66·2 (3·9–362·4) | 66·9 (6·6–401·8) |
| ECOG performance status, n (%) | ||
| 0 | 130 (62·8) | 129 (61·4) |
| 1 | 69 (33·3) | 74 (35·2) |
| 2 | 7 (3·4) | 6 (2·9) |
| Histology by central review,a n (%) | ||
| Papillary | 118 (57·0) | 119 (56·7) |
| Follicular | 50 (24·2) | 56 (26·7) |
| Poorly differentiated | 24 (11·6) | 16 (7·6) |
| Well differentiated | 2 (1·0) | 1 (0·5) |
| Nonthyroid | 0 | 1 (0·5) |
| Medullary | 0 | 1 (0·5) |
| Oncocytic carcinoma | 2 (1·0) | 0 |
| Carcinoma, not otherwise specified | 0 | 3 (1·4) |
| Missing/nondiagnostic | 13 (6·3) | 14 (6·7) |
| Most common metastatic lesion sites, n (%) | ||
| Lung | 178 (86·0) | 181 (86·2) |
| Lymph nodes | 113 (54·6) | 101 (48·1) |
| Bone | 57 (27·5) | 56 (26·7) |
| Pleura | 40 (19·3) | 24 (11·4) |
| Head and neck | 33 (15·9) | 34 (16·2) |
| Liver | 28 (13·5) | 30 (14·3) |
| Baseline FDG uptake | ||
| Positive | 161 (77·8) | 159 (75·7) |
| Negative | 14 (6·8) | 15 (7·1) |
| Missing | 32 (15·5) | 36 (17·1) |
| Prior therapy | ||
| Median cumulative radioiodine activity, mCi | 400 | 376 |
| Any prior systemic anticancer therapy, n (%) | 7 (3·4) | 6 (2·9) |
| Any prior radiotherapy, n (%) | 83 (40·1) | 91 (43·3) |
FDG, 2-[18F] fluoro-2-deoxy-D-glucose; ECOG, Eastern Cooperative Oncology Group
All patients had differentiated thyroid cancer as per investigator assessment.
Efficacy
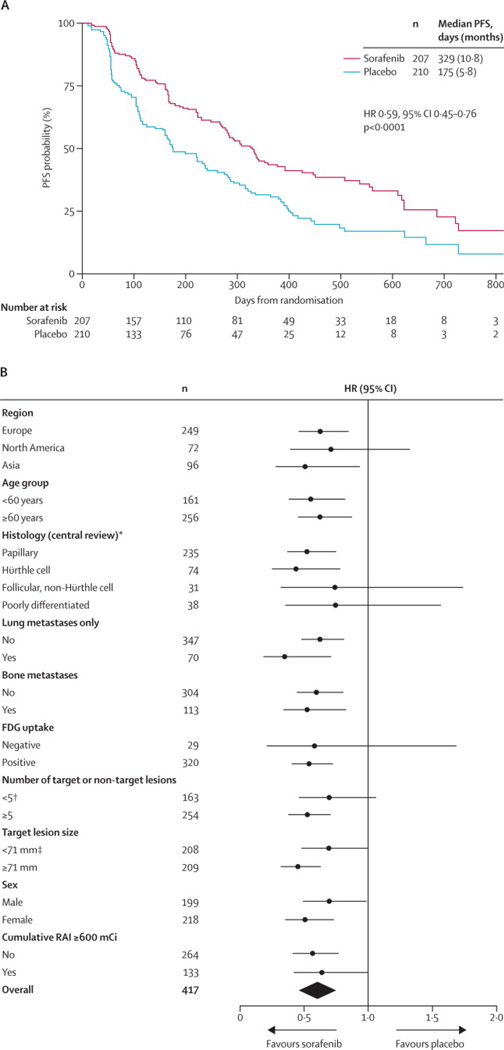
The study met its primary endpoint, showing significant improvement in PFS for sorafenib compared with placebo (HR, 0·59; 95%CI, 0·45–0·76; P<0·0001; median 10·8 vs 5·8 months, respectively [Fig. 2a]), with a 41% reduction in the risk of progression or death during the double-blind period. Investigator-assessed PFS closely matched the central review: HR, 0·49; 95%CI, 0·39–0·61; P<0·0001; median 10·8 (sorafenib) versus 5·4 (placebo) months.
Figure 2.
Progression-free survival by central review (intention-to-treat population) (a). Forest plot of progression-free survival in subgroups (central review) (b).
Exploratory subgroup analysis of PFS showed consistent improvement in all pre-specified subgroups (Fig. 2b). Median time from randomization until last known follow-up was 16.2 months (range, 0·03–33·2).
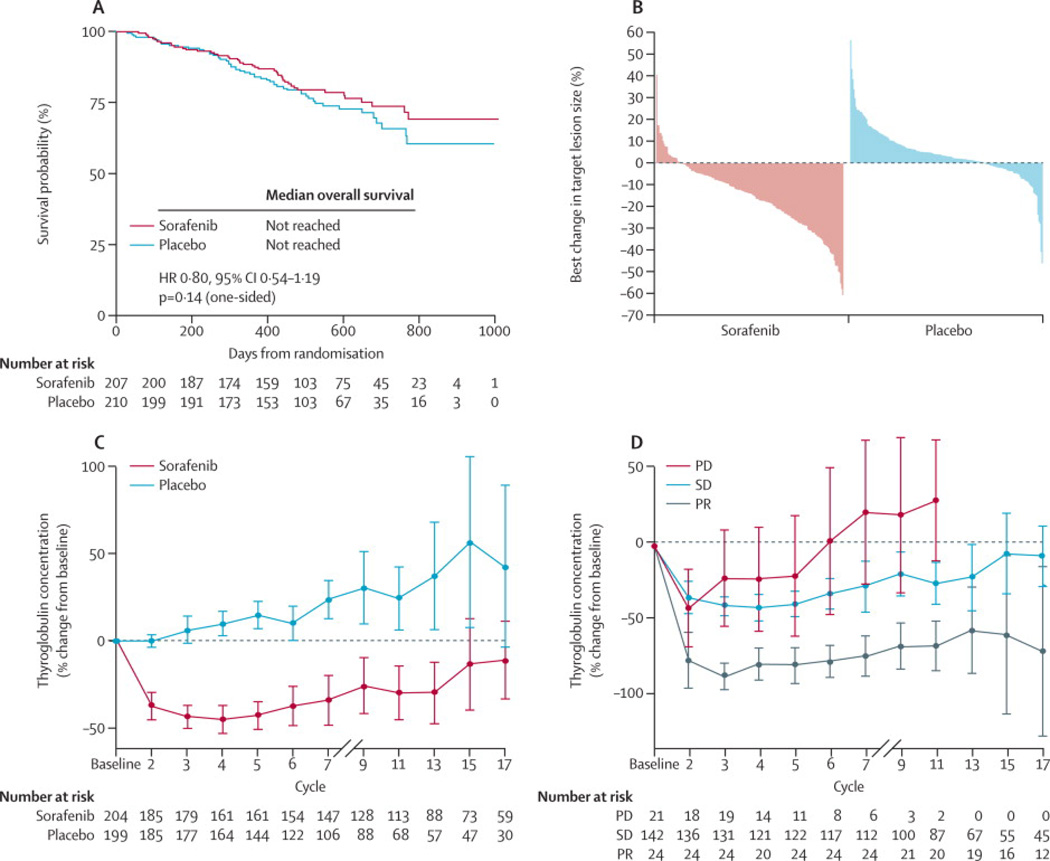
There was no statistically significant difference in OS (HR, 0·80; 95%CI, 0·54–1·19;P=0·14) (Fig. 3a). Median OS had not been reached at the time of primary analysis. A total of 150 (71·4%) patients receiving placebo crossed over to receive open-label sorafenib at progression (Fig. 1). Furthermore, 42 (20.3%) patients in the sorafenib arm and 18 (8.6%) patients in the placebo arm received subsequent anti-cancer therapy following the trial. ORR was 12·2% (n=24/196) versus 0·5% (n=1/201) with sorafenib versus placebo, respectively (P<0·0001), all PR. Median duration of response for patients with a PR to sorafenib was 10·2 months (95%CI, 7·4–16·6). Overall reduction in the sum of target lesions was greater with sorafenib (Fig. 3b). For patients without PR, SD for ≥4 weeks was observed in 74% (across both arms; n=294/397), and SD for ≥6 months (post-hoc analysis) in 41·8% (n=82/196; sorafenib) and 33·2% (n=67/202; placebo). DCR (PR plus SD ≥6 months; post-hoc analysis) was 54·1% (n=106/196) versus 33·8% (n=68/201) with sorafenib versus placebo, respectively (P<0·0001). Median TTP was 11·1 months (95%CI, 9·3–14·8) with sorafenib versus 5·7 months (95%CI, 5·3–7·8) with placebo (HR, 0·56; 95%CI, 0·43–0·72; P<0·0001).
Figure 3.
Overall survival (a). Waterfall plot showing maximum reduction in target lesion size (central review) (b). Thyroglobulin levels according to treatment arm (c). Thyroglobulin levels according to tumour response (d).
Safety
Median treatment duration was 10·6 months (range, 0·07–31·1) with sorafenib, and 6·5 months (range, 0·4–30·4) with placebo. Mean (standard deviation) daily dose was 651 (159) mg with sorafenib and 793 (26) mg with placebo. AEs occurred in 204 (98·6%) patients receiving sorafenib during the double-blind period and in 183 (87·6%) patients receiving placebo. AEs were predominantly grades 1 or 2 (Table 2) and tended to occur early in treatment. The most common AE sin the sorafenib arm were: hand–foot skin reaction (HFSR), diarrhoea, alopecia, rash/desquamation, fatigue, weight loss, and hypertension (Table 2). Increase in serum TSH level >0·5mIU/L was reported in 33 ·3% (n=69/207) of patients, and hypocalcaemia in18·8% (n=39/207) of patients in the sorafenib arm (Table 2).
Table 2.
Treatment-emergent adverse events occurring in ≥10% of patients in either arm during the double-blind period (safety population).
| Sorafenib (n=207) | Placebo (n=209) | |||||
|---|---|---|---|---|---|---|
| Adverse event, n (%) | Any grade | Grade 3 | Grade 4 | Any grade | Grade 3 | Grade 4 |
| Hand–foot skin reaction | 158 (76·3) | 42 (20·3) | – | 20 (9·6) | 0 | – |
| Diarrhoea | 142 (68·6) | 11 (5·3) | 1 (0·5) | 32 (15·3) | 2 (1·0) | 0 |
| Alopecia | 139 (67·1) | – | – | 16 (7·7) | – | – |
| Rash/desquamation | 104 (50·2) | 10 (4·8) | 0 | 24 (11·5) | 0 | 0 |
| Fatigue | 103 (49·8) | 11 (5·3) | 1 (0·5) | 53 (25·4) | 3 (1·4) | 0 |
| Weight loss | 97 (46·9) | 12 (5·8) | – | 29 (13·9) | 2 (1·0) | – |
| Hypertension | 84 (40·6) | 20 (9·7) | 0 | 26 (12·4) | 5 (2·4) | 0 |
| Anorexia | 66 (31·9) | 5 (2·4) | 0 | 10 (4·8) | 0 | 0 |
| Oral mucositis (functional/symptomatic) | 48 (23·2) | 1 (0·5) | 1 (0·5) | 7 (3·3) | 0 | 0 |
| Pruritus | 44 (21·3) | 2 (1·0) | – | 22 (10·5) | 0 | – |
| Nausea | 43 (20·8) | 0 | 0 | 24 (11·5) | 0 | 0 |
| Headache | 37 (17·9) | 0 | 0 | 15 (7·2) | 0 | 0 |
| Cough | 32 (15·5) | 0 | – | 32 (15·3) | 0 | - |
| Constipation | 31 (15·0) | 0 | 0 | 17 (8·1) | 1 (0·5) | 0 |
| Dyspnea | 30 (14.5) | 10 (4.8) | 0 | 28 (13.4) | 4 (1.9) | 2 (1.0) |
| Neuropathy: sensory | 30 (14.5) | 2 (1.0) | 0 | 13 (6.2) | 0 | 0 |
| Abdominal pain - not otherwise specified | 29 (14.0) | 3 (1.4) | 0 | 8 (3.8) | 1 (0.5) | 0 |
| Pain, extremity – limb | 28(13.5) | 1 (0.5) | 0 | 18 (8.6) | 1 (0.5) | 0 |
| Dermatology - Other | 27 (13.0) | 2 (1.0) | 0 | 5 (2.4) | 0 | 0 |
| Voice changes | 25 (12.1) | 1 (0.5) | 0 | 6 (2.9) | 0 | 0 |
| Fever | 23 (11.1) | 2 (1.0) | 1 (0.5) | 10 (4.8) | 0 | 0 |
| Vomiting | 23 (11.1) | 1 (0.5) | 0 | 12 (5.7) | 0 | 0 |
| Back pain | 22 (10.6) | 2 (1.0) | 0 | 22 (10.5) | 2 (1.0) | 1 (0.5) |
| Pain, other | 22 (10.6) | 1 (0.5) | 0 | 16 (7.7) | 1 (0.5) | 0 |
| Pain, throat/pharynx/larynx | 21 (10.1) | 0 | 0 | 8 (3.8) | 0 | 0 |
| Laboratory | ||||||
| Metabolic/laboratory – othera | 74 (35·7) | 0 | 0 | 35 (16·7) | 0 | 0 |
| Serum TSH increase (MedDRA) | 69 (33·3) | 0 | 0 | 28 (13·4) | 0 | 0 |
| Hypocalcaemia | 39 (18·8) | 12 (5·8) | 7 (3·4) | 10 (4·8) | 1 (0·5) | 2 (1·0) |
| ALT | 26 (12.6) | 5 (2.4) | 1 (0.5) | 9 (4.3) | 0 | 0 |
| AST | 23 (11.1) | 2 (1.0) | 0 | 5 (2.4) | 0 | 0 |
TSH, thyroid-stimulating hormone; MedDRA, Medical Dictionary for Regulatory Activities; NCI CTCAE, National Cancer Institute-Common Terminology Criteria for Adverse Events
TSH levels >0·5 mIU/L are included within this NCI CTCAE term and blood TSH increase (MedDRA v15·1 term) is also reported.
Adverse events reported using NCI CTCAE v3·0.
Dose interruptions, reductions, or withdrawals due to AEs occurred in 66·2% (n=137/207), 64·3% (n=133/207), and 18·8% (n=39/207) of patients, respectively, receiving sorafenib, and in 25·8% (n=54/209), 9·1% (n=19/209), and 3·8% (n=8/209) of patients, respectively, receiving placebo. HFSR was the most common reason for sorafenib dose interruptions, reductions, and withdrawals (26·6% [n=55/207], 33·8% [n=70/207], and 5·3% [n=11/207], respectively).
Serious AEs occurred in 77 (37·2%) patients receiving sorafenib and 55 (26·3%) patients receiving placebo. Serious AEs occurring in ≥2% of patients receiving sorafenib were secondary malignancy (4·3% [n=9/207]), dyspnoea (3·4% [n=7/207]), and pleural effusion (2·9% [n=6/207]); corresponding rates with placebo were 1·9% [n=4/209], 2·9% [n=6/209], and 1·9% [n=4/209], respectively. In the sorafenib group, secondary malignancies occurred in nine patients, including seven with squamous cell carcinomas (SCC) of the skin (one patient also had melanoma) and one each with acute myeloid leukaemia and bladder cancer. In the placebo group, there were single cases of bladder cancer, colon carcinoma, pulmonary carcinoid, and gastric cancer. There were 12 deaths by the end of the 30-day safety follow-up period in the sorafenib group and six in the placebo group; sorafenib: seven deaths due to underlying disease, two to unknown causes, and one each to lung infection, chronic obstructive lung disease, and myocardial infarction; placebo: four due to underlying disease and one each for pulmonary embolism and subdural haematoma. One death in each arm was attributed to study drug; myocardial infarction (sorafenib) and subdural haematoma (placebo).
Biomarker analyses
Tumour mutation data were available for 256 (61·4%) patients: 126 sorafenib and 130 placebo. The genetic subpopulation was similar to the overall population except for a lower percentage of patients from Asia (11·3% [n=29/256] vs 23·7% [n=99/417]) (Supplementary Appendix D, Table D2). BRAF mutations were present in 27·0% (n=34/126; sorafenib) and 33·1%(n=43/130; placebo) of tumour samples, and RAS mutations in 19·0% (n=24/126; sorafenib) and 20·0%(n=26/130; placebo). BRAF mutation frequency was highest in papillary thyroid carcinoma (46·2%; n=72/156); RAS mutations were the next highest at 17·9%(n=28/156). RAS mutation frequency was highest in poorly differentiated histology (32·3%; n=10/31]).
Median PFS was longer in patients with BRAF mutations treated with sorafenib compared to placebo (20·5 vs 9·4 months; HR, 0·46; 95%CI, 0·24–0·90; P=0·02; Supplementary Appendix D, Fig. D1). Sorafenib treatment also doubled median PFS in the wild-type BRAF subgroup (8·9 vs 3·8 months; HR, 0·55; 95%CI, 0·38–0·79; P<0·001). Similarly, both RAS mutation and wild-type subgroups benefited from sorafenib versus placebo; median PFS was 5·5 versus 3·5 months, respectively, in the RAS mutation subgroup (HR, 0·49; 95%CI, 0·24–1·00; P=0·045), and 10·8 vs 5·8 months, respectively (HR, 0·60; 95%CI, 0·42–0·85; P=0·004) in the RAS wild-type subgroup. While BRAF and RAS mutations seemed to associate with prognosis, indicated by the difference in median PFS for patients with and without mutations in the placebo arm, neither BRAF nor RAS mutation status was predictive of sorafenib benefit for PFS, evidenced by the similar sorafenib/placebo HRs in each mutation subgroup (BRAF-PFS interaction P=0·653; RAS-PFS interaction P=0·422; Supplementary Appendix D, Fig. D1). Likewise, multivariate analysis indicated that only histology (papillary vs poorly differentiated), age, and sorafenib treatment, but not BRAF or RAS mutation status, were independently prognostic for PFS benefit (Appendix D, Table D3). Similarly, mutation status was not independently prognostic for PFS when multivariate analysis was restricted to papillary patients (Table D3).
Sorafenib significantly improved median PFS irrespective of high or low baseline thyroglobulin (subgroups split according to median values of 449·4 ng/mL; interaction P=0·992; Supplementary Appendix D, Fig. D1e–f). Median serum thyroglobulin increased from baseline over treatment in the placebo arm, but initially dropped and then paralleled treatment responses in the sorafenib arm (Fig. 3c): rising in patients with progressive disease, remaining below baseline in patients with SD, and decreasing further in patients with PR (Fig. 3c–d).
DISCUSSION
This is the first phase 3 study in RAI-refractory DTC to be reported. While DTC is generally considered an indolent disease, patients in the DECISION trial had progressing disease refractory to standard treatment with RAI. Furthermore, a median PFS of 5·8 months and the high incidence of serious AEs (one-quarter of patients) and dose modifications due to AEs (one-third of patients) in patients receiving placebo together argue that the entry criteria accurately identified a population of RAI-refractory DTC patients with high disease burden and aggressive disease.
The study met its primary endpoint with a significant and clinically relevant 5-month improvement in median PFS with sorafenib versus placebo. The PFS benefit was observed in all pre-specified subgroups, including age, sex, geographical region, histology, sites of metastases, and tumour burden. While the ORR was modest in the sorafenib arm (12·2%; n=24/196), shrinkage of target lesions was seen in a majority of sorafenib-treated patients. Likewise, sorafenib increased DCR and prolonged TTP. Median OS was not reached in either arm and there was no statistically significant difference in OS at data cut-off. OS results may be confounded by post-progression crossover from placebo to open-label sorafenib by the majority of placebo patients.
Elucidation of prognostic or predictive biomarkers has potential value in the management of RAI-refractory DTC. BRAF and RAS mutations have been associated with poor outcomes in DTC patients,6–10 but less is known about the prognostic or predictive value of these mutations in patients with RAI-refractory DTC. The exploratory analyses conducted here suggest that the patient subset with BRAF mutations fared better on sorafenib than those with wild-type BRAF, with a median PFS >20 months. However, this appears to be related to the higher predominance of BRAF mutations in patients with papillary histology and the overall better outcome of those with papillary thyroid carcinoma compared to other histologies. Similarly, although patients with RAS mutations tended to do worse than those with wild-type, RAS mutations were not independently prognostic for PFS. Indeed, sorafenib improved PFS regardless of BRAF or RAS mutation status as evidenced by the similar HRs. Thus, although limited by sample size, these results suggest that BRAF and RAS mutations are neither independently prognostic nor predictive of sorafenib benefit with regards to PFS prolongation. It is important to note that the biomarker analysis subset constituted only 61.4% of the study population (patients who provided genetic consent from whom tumour samples could be obtained); therefore these results may be affected by selection bias and imbalances of unknown factors.
The role of monitoring thyroglobulin in patients with advanced DTC during treatment with antiangiogenic agents is not well established. In the present study, median thyroglobulin levels gradually increased in patients treated with placebo, and initially decreased in patients in the sorafenib arm, suggesting that changes may reflect disease progression. This is underlined by the dynamic changes in median thyroglobulin in patients in the sorafenib arm based on their radiologic progression. Patients with a PR had the greatest drop in median thyroglobulin levels, whereas levels remained nearer to baseline for patients with SD and initially dropped and then rose in the group of patients with radiologic progression. Decreases13,15,17,21,26 or no change19 in thyroglobulin levels have been reported with antiangiogenic agents, including sorafenib, in patients with advanced thyroid cancer, but to what extent serum thyroglobulin determination can be used on an individual basis to monitor treatment remains to be determined.
AEs were generally consistent with the known sorafenib safety profile. Certain expected side effects, such as HFSR, alopecia, diarrhoea, hypertension, SCC of the skin, and hypocalcaemia, were more common, however, than previously reported in renal cell carcinoma and hepatocellular carcinoma phase 3 pivotal trials with sorafenib.27–29 The reason for the higher incidence of these AEs is not clear, but could include longer reporting periods for sorafenib or the different dose reduction schema used in this trial compared to the previous trials (Supplementary Appendix B, Table B1). HFSR was the most common AE in the sorafenib arm in DECISION, occurring in 76·3% [n=158/207] of patients, but only 5·3% [n=11/207] of patients discontinued treatment due to HFSR. Nevertheless, the dermatologic AEs highlight the importance of monitoring the skin during sorafenib treatment. The higher incidence of hypocalcaemia was likely related to postsurgical hypoparathyroidism. Increases in TSH of more than 0·5mlU/L were reported in a third of sorafenib-treated patients, suggesting that serum TSH levels should be monitored frequently and elevations controlled with adjustments in l-thyroxine dose to maintain adequate TSH suppression.
The number of deaths in the double-blind part of the study was low in both sorafenib and placebo groups (12 and 6, respectively), with the majority of causes being related to underlying disease and only one death in each arm attributed to study drug.
In conclusion, these results support sorafenib as a new treatment option for patients with RAI-refractory DTC, a setting in which there is currently no standard therapy. AEs were generally consistent with the known sorafenib safety profile. BRAF and RAS mutations are neither prognostic biomarkers for PFS nor predictive biomarkers for RAI-refractory DTC treated with sorafenib. Thyroglobulin levels are not predictive for sorafenib benefit, but may be a pharmacodynamic biomarker.
PANEL: RESEARCH IN CONTEXT
Systematic review
Two literature reviews have assessed studies in advanced thyroid cancer30 and RAI-refractory DTC.22 We also did a PubMed literature search on 19 December 2013, using the terms "clinical trial, phase ii" [Publication Type] AND "thyroid neoplasms" [MeSH Terms] (no date restriction). This yielded 50 reports, of which only ten reported phase 2 studies of antiangiogenic agents in DTC. A similar search for phase 3 studies ("clinical trial, phase iii" [Publication Type]) yielded no results in DTC except for the present study design.25
Interpretation
Previously, only phase 2 studies of antiangiogenic agents have been reported in RAI-refractory DTC: axitinib,15 motesanib,21 pazopanib,13 sunitinib,14 vandetanib,19 and sorafenib.12,16–18,20 Therefore, data in this setting are limited, and the present phase 3 randomized study demonstrating significantly improved PFS with sorafenib versus placebo provides valuable clinical evidence. These results suggest that sorafenib represents a new treatment option for patients with progressive RAI-refractory DTC.
Supplementary Material
Acknowledgements
The authors thank the patients, their caregivers, and the investigators who participated in this study; the principal investigators are listed in Supplementary Appendix A. We also thank Emma Robinson (7.4 Limited, Oxford, UK), who provided medical writing support funded by Bayer HealthCare Pharmaceuticals. This study was supported by Bayer HealthCare Pharmaceuticals and Onyx Pharmaceuticals Inc., an Amgen subsidiary.
Funding
This work was funded by Bayer HealthCare Pharmaceuticals and Onyx Pharmaceuticals Inc., an Amgen subsidiary.
Conflicts of interest
MSB has received consultancy fees/honorarium and research support from Bayer HealthCare Pharmaceuticals; consultancy fees and research support from Exelixis; consultancy fees from Onyx Pharmaceuticals; and research support from Eisai, Novartis, and Roche/Genentech.
CMN, RP, and YKS have received research support from Bayer HealthCare Pharmaceuticals.
BJ has received honorarium and research support from Bayer HealthCare Pharmaceuticals; consultancy fees/honorarium from AstraZeneca and Sobi; and honorarium from Eisai, Ipsen, Novartis, OxiGene, Pfizer, Roche, and Sanofi.
RE has received consultancy fees/honorarium and research support from Bayer HealthCare Pharmaceuticals; and consultancy fees/honorarium from AstraZeneca and Genzyme.
SS has received consultancy fees and research support from Bayer HealthCare Pharmaceuticals; and consultancy fees from Amgen, Celgene, Genomic Health, Roche, and Sanofi Aventis.
LB has received consultancy fees and research support from Bayer HealthCare Pharmaceuticals; and consultancy fees from AstraZeneca.
CF has received consultancy fees/honorarium and research support from Bayer HealthCare Pharmaceuticals; consultancy fees from AstraZeneca, Sanofi-Aventis, and Sobi; and a grant from Roche.
FP has received honorarium and research support from Bayer HealthCare Pharmaceuticals.
SIS has received research support from Bayer HealthCare Pharmaceuticals; consultancy fees/honorarium and research support from Amgen; consultancy fees/honorarium from AstraZeneca, Eisai, Exelixis, Lilly, NovoNordisk, and Veracyte; research support from Genzyme and Pfizer; and consultancy fees/honorarium from Onyx and Roche.
JWAS has received honorarium and research support from Bayer HealthCare Pharmaceuticals.
JC, CP, and IM are employees of Bayer HealthCare Pharmaceuticals. CP owns stock in Bayer AG. CK is an employee of Bayer Pharma AG.
MJS has received consultancy fees and research support from Bayer HealthCare Pharmaceuticals and Eisai; consultancy fees/honorarium and research support from AstraZeneca and Genzyme-Sanofi; consultancy fees from Exelixis; and consultancy fees/honorarium from Sobi.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
All authors were involved in writing the manuscript and approved the final draft. MSB, CMN, BJ, RE, SS, LB, CF, FP, RP, YKS, SIS, JWAS, and MJS were involved in data collection. MSB, CMN, YKS, SIS, JWAS, JC, CP, IM, CK, and MJS were involved in study design, data analysis, and interpretation.
References
- 1.Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Thyroid Carcinoma v2. [Accessed 1 April 2013];2013 NCCN.org. [Google Scholar]
- 3.Shoup M, Stojadinovic A, Nissan A, et al. Prognostic indicators of outcomes in patients with distant metastases from differentiated thyroid carcinoma. J Am Coll Surg. 2003;197:191–197. doi: 10.1016/S1072-7515(03)00332-6. [DOI] [PubMed] [Google Scholar]
- 4.Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892–2899. doi: 10.1210/jc.2005-2838. [DOI] [PubMed] [Google Scholar]
- 5.Schlumberger M, Brose M, Elisei R, et al. Definition and management of radioactive iodine-refractory differentiated thyroid cancer. The Lancet Diabetes and Endocrinology. 2014 doi: 10.1016/S2213-8587(13)70215-8. Early Online Publication: 30 January 2014. [DOI] [PubMed] [Google Scholar]
- 6.Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13:184–199. doi: 10.1038/nrc3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elisei R, Ugolini C, Viola D, et al. BRAFV600E mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab. 2008;93:3943–3949. doi: 10.1210/jc.2008-0607. [DOI] [PubMed] [Google Scholar]
- 8.Xing M. Prognostic utility of BRAF mutation in papillary thyroid cancer. Mol Cell Endocrinol. 2010;321:86–93. doi: 10.1016/j.mce.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gouveia C, Can NT, Bostrom A, Grenert JP, van ZA, Orloff LA. Lack of association of BRAF mutation with negative prognostic indicators in papillary thyroid carcinoma: the University of California, San Francisco, experience. JAMA Otolaryngol Head Neck Surg. 2013;139:1164–1170. doi: 10.1001/jamaoto.2013.4501. [DOI] [PubMed] [Google Scholar]
- 10.Xing M, Haugen BR, Schlumberger M. Progress in molecular-based management of differentiated thyroid cancer. Lancet. 2013;381:1058–1069. doi: 10.1016/S0140-6736(13)60109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein M, Vignaud J-M, Hennequin V, et al. Increased expression of the vascular endothelial growth factor is a pejorative prognosis marker in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2001;86:656–658. doi: 10.1210/jcem.86.2.7226. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed M, Barbachano Y, Riddell A, et al. Analysis of the efficacy and toxicity of sorafenib in thyroid cancer - a phase II study in a UK based population. Eur J Endocrinol. 2011;165:315–322. doi: 10.1530/EJE-11-0129. [DOI] [PubMed] [Google Scholar]
- 13.Bible KC, Suman VJ, Molina JR, et al. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study. Lancet Oncol. 2010;11:962–972. doi: 10.1016/S1470-2045(10)70203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carr LL, Mankoff DA, Goulart BH, et al. Phase II study of daily sunitinib in FDG-PET-positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin Cancer Res. 2010;16:5260–5268. doi: 10.1158/1078-0432.CCR-10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen EE, Rosen LS, Vokes EE, et al. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol. 2008;26:4708–4713. doi: 10.1200/JCO.2007.15.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta-Abramson V, Troxel AB, Nellore A, et al. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol. 2008;26:4714–4719. doi: 10.1200/JCO.2008.16.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoftijzer H, Heemstra KA, Morreau H, et al. Beneficial effects of sorafenib on tumor progression, but not on radioiodine uptake, in patients with differentiated thyroid carcinoma. Eur J Endocrinol. 2009;161:923–931. doi: 10.1530/EJE-09-0702. [DOI] [PubMed] [Google Scholar]
- 18.Kloos RT, Ringel MD, Knopp MV, et al. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol. 2009;27:1675–1684. doi: 10.1200/JCO.2008.18.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leboulleux S, Bastholt L, Krause T, et al. Vandetanib in locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 2 trial. Lancet Oncol. 2012;13:897–905. doi: 10.1016/S1470-2045(12)70335-2. [DOI] [PubMed] [Google Scholar]
- 20.Schneider TC, Abdulrahman RM, Corssmit EP, Morreau H, Smit JW, Kapiteijn E. Long-term analysis of the efficacy and tolerability of sorafenib in advanced radio-iodine refractory differentiated thyroid carcinoma: final results of a phase II trial. Eur J Endocrinol. 2012;167:643–650. doi: 10.1530/EJE-12-0405. [DOI] [PubMed] [Google Scholar]
- 21.Sherman SI, Wirth LJ, Droz J-P, et al. Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med. 2008;359:31–42. doi: 10.1056/NEJMoa075853. [DOI] [PubMed] [Google Scholar]
- 22.Anderson RT, Linnehan JE, Tongbram V, Keating K, Wirth LJ. Clinical, safety, and economic evidence in radioactive iodine–refractory differentiated thyroid cancer: a systematic literature review. Thyroid. 2013;23:392–407. doi: 10.1089/thy.2012.0520. [DOI] [PubMed] [Google Scholar]
- 23.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 24.Carlomagno F, Anaganti S, Guida T, et al. BAY 43-9006 inhibition of oncogenic RET mutants. J Natl Cancer Inst. 2006;98:326–334. doi: 10.1093/jnci/djj069. [DOI] [PubMed] [Google Scholar]
- 25.Brose MS, Nutting CM, Sherman SI, et al. Rationale and design of DECISION: a double-blind, randomized, placebo-controlled phase III trial evaluating the efficacy and safety of sorafenib in patients with locally advanced or metastatic radioactive iodine (RAI)-refractory, differentiated thyroid cancer. BMC Cancer. 2011;11:349. doi: 10.1186/1471-2407-11-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marotta V, Ramundo V, Camera L, et al. Sorafenib in advanced iodine-refractory differentiated thyroid cancer: efficacy, safety and exploratory analysis of role of serum thyroglobulin and FDG-PET. Clin Endocrinol (Oxf) 2013;78:760–767. doi: 10.1111/cen.12057. [DOI] [PubMed] [Google Scholar]
- 27.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 28.Escudier B, Eisen T, Stadler WM, et al. Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol. 2009;27:3312–3318. doi: 10.1200/JCO.2008.19.5511. [DOI] [PubMed] [Google Scholar]
- 29.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 30.Kapiteijn E, Schneider TC, Morreau H, Gelderblom H, Nortier JW, Smit JW. New treatment modalities in advanced thyroid cancer. Ann Oncol. 2012;23:10–18. doi: 10.1093/annonc/mdr117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.