Abstract
Introduction
The prevalence of extensively drug resistant gram negative bacilli (XDR-GNB) is rapidly progressing; however in Egypt data are sparse. We conducted the present study to quantify the incidence, risk factors and outcome of patients harboring XDR-GNB.
Methods
A one year prospective study was done by collecting all the bacteriological reports for cultures sent from the surgical intensive care unit, Cairo university teaching hospital. XDR-GNB were defined as any gram negative bacilli resistant to three or more classes of antimicrobial agents. Patients with XDR-GNB compared with those sustaining non extensively drug-resistant infection. A multivariate logistic regression model was created to identify independent predictors of multi-resistance.
Results
During one-year study period, a total of 152 samples (65%) out of 234 gram negative bacilli samples developed extensively drug resistant infection. XDR strains were significantly higher in Acinetobacterspp (86%), followed by Pseudomonas (63%), then Proteus (61%), Klebsiella (52%), and E coli (47%). Fourth generation cephalosporine (Cefipime) had the lowest susceptibility (10%) followed by third generation cephalosporines (11%), Quinolones (31%), Amikacin (42%), Tazobactam (52%), Carbapinems (52%), and colistin (90%). Relaparotomy was the only significant risk factor for acquisition of XDR infection.
Conclusion
Extensively drug-resistant gram negative infections are frequent in our ICU. This is an alarming health care issue in Egypt which emphasizes the need to rigorously implement infection control practices.
Keywords: Extensive drug resistance, gram negative bacilli, critically ill patients
Introduction
Infection due to gram negative bacilli is a growing problem in clinical practice especially in tertiary medical centers. Patients in the intensive care unit (ICU) are highly vulnerable to infections because of impaired host defenses, administration of some drugs e.g. muscle relaxant, and the use of invasive devices that bypass the normal anatomical barriers. The growing emergence of resistant pathogens is considered an additional burden and a major threat to public health [1]. ICU is claimed to be the epicenter and the factory for rising, development and amplification of antimicrobial resistance. Although, there are currently no internationally accepted definition for the resistant microorganism, the term extensively-drug resistance (XDR) is used to denote isolates resistant to all but one or two classes of antimicrobial agents.
Surveillance studies are not only useful in detection of the prevalence of XDR organisms and taking infection control alarms, but also help determine the special pattern of antibiotic resistance to reach higher rates of initial appropriate therapy as well as saving last-line antibiotic agents [2]. Although some authors reported the prevalence and the burden of nosocomial infections in developing countries in terms of mortality, cost, and length of stay [3]; the data addressing prevalence of XDR is scarce in Egypt, we conducted a prospective cohort study to quantify the incidence, risk factors, and outcome of patients harboring XDR gram negative bacilli in our surgical intensive care unit.
Methods
A one year prospective study was conducted in the Surgical Intensive Care Unit (SICU), emergency department of general Surgery, Cairo University hospitals Cairo university teaching hospital is the largest tertiary hospital in Egypt with 5500 bed capacity. All Patients who had gram negative bacilli isolates recovered from clinical cultures within the first 48 h after ICU admission with clinical signs of sepsis which led to the use of antibiotics directed against the organism(s) isolated; otherwise the patient was considered to be colonized with the organism. Colonization cases, defined as any positive culture without clinical signs of infection, were excluded. Patients who did not sustain an infection also were excluded. Cultures showing more than 3 isolates were excluded. In addition, all infections that occurred before patients’ admission to the SICU, within the first 48 hr of SICU hospitalization, or after 48 h after their discharge were also excluded. 281 consecutive isolates recovered from clinical specimens, including blood, urine, wound/tissue, and respiratory specimens (one pathogen per cultured site per patient) of ICU patients. The 281 isolates obtained represented 230 patients, anaerobic and fungal organisms were excluded. Isolates were shipped to the reference laboratory (Health Sciences Centre, Winnipeg, Canada) on Amiescharcoal swabs, subcultured onto appropriate media.
Data collection
The following data were recorded from all patients with positive results; age, sex, primary diagnosis, APACHE II score, prior antimicrobial therapy within last 3 months, septic shock, repeated surgical intervention, mechanical ventilation, and ICU length of the stay, presence of central venous lines, parenteral nutrition, renal replacement therapy, blood transfusion, reoperation and hospital mortality. Antibiotic susceptibilities were determined by the disc diffusion method (Bio-Rad, Marnes la Coquettes, France) according to the recommendations of the Antibiogram Committee of the French Microbiology Society (CA-SFM). The bacteria were then classified as susceptible or resistant (resistant and intermediate). The susceptibility of aerobic Gram negative bacteria to 10 antibiotics (cefoperazone, cefotaxime, ceftazidime, cefepime, cefoperazone-sulbactam, imipenem, gentamicin, amikacin, colistin and ciprofloxacin) was reported. Extensive drug resistant (XDR) gram negative bacilli were defined as isolates resistant to three or more groups of antimicrobials. Prevalence of XDR isolates was reported among different isolate sites as well as different type of organism. Patients were further divided into XDR and non XDR groups, both groups were compared as regards possible risk factors as well as outcome.
Statistical analysis
Categorical variables were analyzed using the X2 or Fischer Exact test as appropriate. For continuous variables, the data are presented as median (range) and were analyzed with Mann-Whitney test. Parameters selected by univariate analysis to have P values < 0.10 were evaluated in the multivariate analysis. Risk factors independently associated with acquisition of XDR gram negative bacilli were identified by stepwise logistic regression analysis of variables selected by univariate analysis. The software SPSS v15.0 for Windows (SPSS, Inc, Chicago, Il, United States) was used for statistical analysis.
Results
Prevalence of XDR isolates
During one-year study period, a total of 152 samples (65%) out of 234 gram negative bacilli samples developed extensively drug resistant infection. XDR strains were significantly higher in Acinetobacterspp (86%), followed by Pseudomonas (63%), then Proteus (61%), Klebsiella (52%), and E coli (47%) (Table 1). As regards different sites of isolates, XDR strains were 75% of sputum cultures, 74% of blood cultures, 63% of urine cultures, 48% of surgical site cultures, and 100% of central line cultures (Figure 1).
Table 1.
Prevalence of XDR isolates among different organisms
| Isolate | Number of cultures (%) | XDR strains (%) |
|---|---|---|
| P. aeruginosa | 60 | 63% |
| K. pneumonia | 62 | 52% |
| A. baumannii | 54 | 86% |
| E. coli | 34 | 47% |
| P. mirabilis | 13 | 61% |
Figure 1.
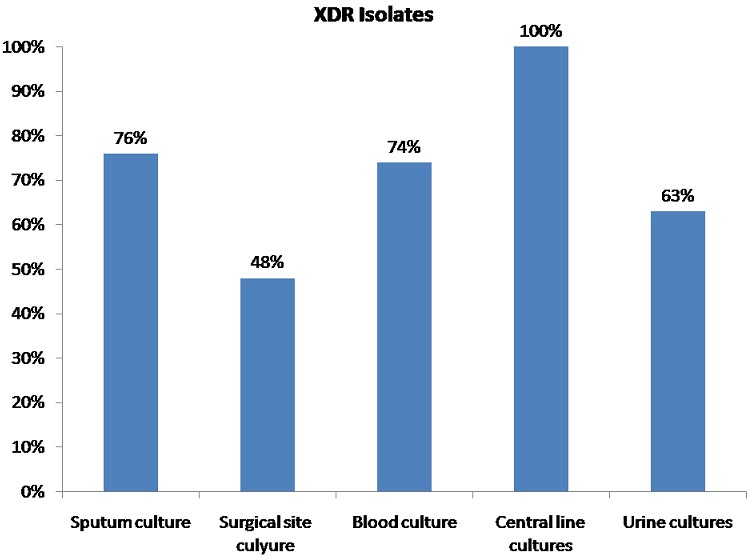
Prevalence of XDR strains among different sites of isolates. P value = 0.003
Antimicrobial susceptibility
The susceptibility rates for different antimicrobial agents in our critically ill patients is shown in Table 2 where the fourth generation cephalosporine (Cefipime) had the lowest susceptibility (10%) followed by third generation cephalosporines (11%), Quinolones (31%), Amikacin (42%), Tazobactam (52%), Carbapinems (52%), and colistin (90%).
Table 2.
Antibiotic susceptibility pattern of the most five abundant gram negative isolates
| C3 | C4 | Quinolones | Amikacin | Tazocin | Carbapinems | Colistin | |
|---|---|---|---|---|---|---|---|
| P. aeruginosa | 13% | 16% | 33.9% | 38.3% | 68.8% | 39% | 87.5% |
| A. baumannii | 3.7% | 2% | 18.5% | 17% | 20% | 24.5% | 100% |
| K. pneumonia | 11.3% | 10.5% | 45.9% | 45.8% | 35.7% | 88.7% | 100% |
| E. coli | 11.8% | 9.7% | 39.4% | 69.7% | 66.7% | 97.1% | 100% |
| P. mirabilis | 27.3% | 30% | 41.7% | 36.4% | 0% | 83.3% | 100% |
C3: 3rd generation cephalosporines, C4: 4th generation caphalosporines
Risk factors and outcome
Relaparotomy was the only risk factor reported to be associated with increased XDR infections by both univariate and multivariate analysis, all other risk factors included in the study were not significantly associated with acquisition of XDR (Table 3).
Table 3.
Demographic data, risk factors, and outcome of MDR and Non MDR groups of patients (Univariate analysis). Data are represented as mean (SD) and frequency
| XDR group (n = 152) | Non XDR group (n = 82) | P value | |
|---|---|---|---|
| Prevalence | 65% | 35% | |
| Age | 36(±31) | 30(±19) | |
| APACHE II | 19(±9) | 23(±10) | |
| Gender | 0.4 | ||
| Male | 62.9% | 67.9% | |
| Female | 37.1% | 32.1% | |
| Ventilation | 84.7% | 79% | 0.3 |
| Relaparotomy | 55% | 25% | 0.04* |
| Previous sepsis | 58.1% | 55.9% | 0.12 |
| Mortality | 57% | 42.9% | 0.07 |
Denotes statistical significance
Discussion
The main finding in our study was the high prevalence of XDR gram negative bacilli among all our isolates in all sites; this is similar to the findings of many studies all over the world [4–12]. Nosocomial infections carry a high burden in both developed and developing countries, this burden was reported in the terms of mortality, financial losses, and length of stay [1, 3]. although some studies reported the prevalence of XDR in Egypt [9–11], all of them were usually concerned with only one site of infection such as blood stream infections [9, 10] and lower respiratory tract infections [11], and only one study was concerned with critically ill patients in Egypt [9]. Our study described the prevalence of XDR gram negative bacilli in all sites of cultures among critically ill patients in a large teaching hospital.
Some studies reported lower prevalence of XDR than what we found [13, 14], this might be because they were conducted in the early 2000s [14] this is supported by the obvious increase of drug resistance year after year all over the world which was reported in few studies [15–18]. There was a unique exception reported in China by Qin Y et al [19] who described a decrease in XDR isolates, this decrease was explained by the adherence to the principles of antibiotic use and effective monitoring and preventive measures.
In our study the only significant risk factor for acquisition of XDR infection was relaparotomy while all other classic risk factors were not significantly associated with XDR infections. A similar finding was reported in a recent Belgian multicenter study which showed that some classic risk factors lost their predictive value as in 40% of infected patients with XDR microorganisms [20]. Seguin et al [21] reported a different finding where antimicrobial treatment in the 3 months preceding hospitalization, duration between first operation, and relaparotomy were shown to be independent risk factors for XDR in postoperative peritonitis, also Bayani el al [22] reported a correlation between the cause of hospitalization XDR Pseudomonas infection.
The main forces considered responsible for emergence and spread of XDR organisms are: 1) induction of resistant strains; 2) selection of resistant strains; 3) introduction of resistant strains; and 4) dissemination of resistant strains. These factors should be considered in the battle against the spread of antimicrobial resistance [23].
Prevalence of XDR isolates in our patients was higher in Pseudomonas and Acinetobacter species than other gram negative bacilli, this is similar to many studies that reported high antimicrobial resistance in these two organisms. Pseudomonas is intrinsically resistant to most antibiotics with multiple mechanisms that are responsible for Antimicrobial resistance such as hyperproduction of enzymes, such as beta-lactamases and DNA-gyrases, active efflux pumps, and permeability changes. Acinetobacter spp. are inheritably resistant to cephalosporins, penicillin's, and aminoglycosides, and especially cause opportunistic infections in critically ill patients. Some strains of A. baumannii have been detected that are resistant to all antibiotics [24, 25].
Having this high alarming prevalence of XDR infections needs aggressive strategies for infection control. The Center for Disease Control recommends four strategies for health care settings: 1) prevent infections; 2) diagnose and treat infections; 3) prudent and rational use of antimicrobials; and 4) prevent transmission [2, 26]. Another keystone in managing this problem is antibiotic stewardship to optimize the use of antimicrobials in ICU [27].
Conclusion
In conclusion, extensively drug-resistant gram negative infections are endemic in our ICU. This is an alarming health care issue in Egypt which emphasizes the need to rigorously implement infection control practices.
Competing interests
The authors declare no competing interest.
Authors’ contributions
All authors read and agreed to the final version of this manuscript and equally contributed to its content and to the management of the case.
References
- 1.Cevik MA, et al. Relationship between nosocomial infection and mortality in a neurology intensive care unit in Turkey. J Hosp Infect. 2005 Apr;59(4):324–30. doi: 10.1016/j.jhin.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Carlet J, Ben Ali A, Tabah A, Willems V, Philippart F, Chafine A, Garrouste-Orgeas M, Misset B. Multidrug resistant infections in the ICU: mechanisms, prevention and treatment. In: Kuhlen R, Moreno R, Ranieri VM, Rhodes A, editors. 25 Years of Progress and Innovation in Intensive Care Medicine. Berlin, Germany: Medizinisch Wissenschaftliche Verlagsgesellschaft; 2007. pp. 199–211. [Google Scholar]
- 3.Allegranzi B, et al. Burden of endemic health care-associated infection in developing countries: systematic review and meta-analysis. Lancet. 2011 Jan 15;377(9761):228–41. doi: 10.1016/S0140-6736(10)61458-4. [DOI] [PubMed] [Google Scholar]
- 4.Souli M, Galani I, Giamarellou H. Emergence of extensively drug-resistant and pandrug-resistant Gram-negative bacilli in Europe. Euro Surveill. 2008 Nov 20;13(47) [PubMed] [Google Scholar]
- 5.Wattal C, Raveendran R, Goel N, Oberoi JK, Rao BK. Ecology of blood stream infection and antibiotic resistance in intensive care unit at a tertiary care hospital in North India. Braz J Infect Dis. 2014 May-Jun;18(3):245–51. doi: 10.1016/j.bjid.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumari HB, Nagarathna S, Chandramuki A. Antimicrobial resistance pattern among aerobic gram-negative bacilli of lower respiratory tract specimensofintensive care unit patients in a neurocentre. Indian J Chest Dis Allied Sci. 2007 Jan-Mar;49(1):19–22. [PubMed] [Google Scholar]
- 7.Briceño DF, Correa A, Valencia C, Torres JA, Pacheco R, Montealegre MC, Ospina D, Villegas MV, Grupo de Resistencia Bacteriana Nosocomial de Colombia Antimicrobial resistance of Gram negative bacilli isolated from tertiary-care hospitals in Colombia. Biomedica. 2010 Jul-Sep;30(3):371–81. [PubMed] [Google Scholar]
- 8.Nele Brusselaers, Dirk Vogelaers, Stijn Blot. The rising problem of antimicrobial resistance in the intensive care unit. Ann Intensive Care. 2011 Nov 23;1:47. doi: 10.1186/2110-5820-1-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed SH, Daef EA, Badary MS, Mahmoud MA, Abd-Elsayed AA. Nosocomial blood stream infection in intensive care units at Assiut University Hospitals (Upper Egypt) with special reference to extended spectrum beta-lactamase producing organisms. BMC Res Notes. 2009 May 6;2:76. doi: 10.1186/1756-0500-2-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agmy G, Mohamed S, Gad Y, Farghally E, Mohammedin H, Rashed H. Bacterial profile, antibiotic sensitivity and resistance of lower respiratory tract infections in upper egypt. Mediterr J Hematol Infect Dis. 2013 Sep 2;5(1):e2013056. doi: 10.4084/MJHID.2013.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saied T, Elkholy A, Hafez SF, Basim H, Wasfy MO, El-Shoubary W, Samir A, Pimentel G, Talaat M. Antimicrobial resistance in pathogens causing nosocomial bloodstream infections in university hospitals in Egypt. Am J Infect Control. 2011 Nov;39(9):e61–5. doi: 10.1016/j.ajic.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Guervil DJ, Chau T. Trends in multidrug-resistant gram-negative bacilli and the role of prolonged β-lactam infusion in the intensive care unit. Crit Care Nurs Q. 2013 Oct-Dec;36(4):345–5. doi: 10.1097/CNQ.0b013e3182a10d2f. [DOI] [PubMed] [Google Scholar]
- 13.Zhanel GG, DeCorby M, Laing N, et al. Canadian antimicrobial resistance alliance (CARA) Hoban DJ; Antimicrobial resistant pathogens in intensive care units in Canada: results of the Canadian National Intensive Care Unit (CAN-ICU) study, 2005-2006. Antimicrob Agents Chemother. 2008 Apr;52(4):1430–7. doi: 10.1128/AAC.01538-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kader AA, Kumar AK. Prevalence of extended spectrum beta-lactamase among multidrug resistant gram-negative isolates from a general hospital in Saudi Arabiam. Saudi Med J. 2004 May;25(5):570–4. [PubMed] [Google Scholar]
- 15.Prabaker K, Weinstein RA. Trends in antimicrobial resistance in intensive care units in the United States. Curr Opin Crit Care. 2011 Oct;17(5):472–9. doi: 10.1097/MCC.0b013e32834a4b03. [DOI] [PubMed] [Google Scholar]
- 16.Rubio FG, Oliveira VD, Rangel RM, Nogueira MC, Almeida MT. Trends in bacterial resistance in a tertiary university hospital over one decade. Braz J Infect Dis. 2013 Jul-Aug;17(4):480–2. doi: 10.1016/j.bjid.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain S, Khety Z. Changing antimicrobial resistance pattern of isolates from an ICU over a 2 year period. J Assoc Physicians India. 2012 May;60:27–8, 33. [PubMed] [Google Scholar]
- 18.Gagneja D, Goel N, Aggarwal R, Chaudhary U. Changing trend of antimicrobial resistance among gram-negative bacilli isolated from lower respiratory tract of ICU patients: A 5-year study. Indian J Crit Care Med. 2011 Jul;15(3):164–7. doi: 10.4103/0972-5229.84900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin Y, Chen X, Huang D, Wei L. Distribution and drug resistance profiles of pathogenic bacteria isolated from patients with nosocomial infection in intensive care unit. Nan Fang Yi Ke Da Xue Xue Bao. 2012 Oct;32(10):1513–5. [PubMed] [Google Scholar]
- 20.Vogelaers D, De Bels D, Foret F, Cran S, Gilbert E, Schoonheydt K, Blot S. Patterns of antimicrobial therapy in severe nosocomial infections: empiric choices, proportion of appropriate therapy, and adaptation rates–a multicentre, observational survey in critically ill patients. Int J Antimicrob Agents. 2010;35:375–381. doi: 10.1016/j.ijantimicag.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Seguin P, Fédun Y, Laviolle B, Nesseler N, Donnio PY, Mallédant Y. Risk factors for multidrug-resistant bacteria in patients with post-operative peritonitis requiring intensive care. J Antimicrob Chemother. 2010 Feb;65(2):342–6. doi: 10.1093/jac/dkp439. [DOI] [PubMed] [Google Scholar]
- 22.Bayani M, Siadati S, Rajabnia R, Taher AA. Drug Resistance of Pseudomonas aeruginosa and Enterobacter cloacae Isolated from ICU, Babol, Northern Iran. Int J Mol Cell Med. 2013 Fall;2(4):204–9. [PMC free article] [PubMed] [Google Scholar]
- 23.Bonten MJ, Mascini EM. The hidden faces of the epidemiology of antibiotic resistance. Intensive Care Med. 2003 Jan;29(1):1–2. doi: 10.1007/s00134-002-1564-3. [DOI] [PubMed] [Google Scholar]
- 24.Clark NM, Patterson J, Lynch JP. Antimicrobial resistance among gram negative organisms in the intensive care unit. Curr Opin Crit Care. 2003 Oct;9(5):413–23. doi: 10.1097/00075198-200310000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Paterson DL. The epidemiological profile of infections with multidrug resistant Pseudomonas aeruginosa and Acinetobacter species. Clin Infect Dis. 2006;43(Suppl 2):S43–48. doi: 10.1086/504476. [DOI] [PubMed] [Google Scholar]
- 26.Salgado CD, O'Grady N, Farr BM. Prevention and control of antimicrobial resistant infections in intensive care patients. Crit Care Med. 2005 Oct;33(10):2373–82. doi: 10.1097/01.ccm.0000181727.04501.f3. [DOI] [PubMed] [Google Scholar]
- 27.Bal AM, Gould IM. Antibiotic stewardship: overcoming implementation barriers. Curr Opin Infect Dis. 2011 Aug;24(4):357–62. doi: 10.1097/QCO.0b013e3283483262. [DOI] [PubMed] [Google Scholar]


