Abstract
The first reports of antibiotic pathogens occurred a few short years after the introduction of these powerful new agents, heralding a new kind of war between medicine and pathogens. Although originally described in Staphylococcus aureus, resistance among bacteria has now become a grim race to determine which classes of bacteria will become more resistant, pitting the Gram positive staphylococci, enterococci, and streptococci against the increasingly resistant Gram negative pathogens, e. g., carbapenemase-resistant enterobacteriaceae. In addition, the availability of antibacterial agents has allowed the development of whole new kinds of diseases caused by non-bacterial pathogens, related largely to fungi that are inherently resistant to antibacterials. All of these organisms are becoming more prevalent and, ultimately, more clinically relevant for surgeons.
It is ironic that despite their ubiquity in our communities, there is seldom a second thought given to viral infections in patients with surgical illness. The extent of most surgeon’s interest in viral infections ends with hepatitis and HIV, no doubt related to transmissibility as well as the implications that these viruses might have in a patient’s hepatic or immune functions. There are chapters and even textbooks written about these viruses so these will not be considered here. Instead, we will present the growing body of knowledge of the herpes family viruses and their occurrence and consequences in patients with concomitant surgical disease or critical illness. We have also chosen to focus this chapter on previously immune competent patients, as the impact of herpes family viruses in immunosuppressed patients such as transplant or AIDS patients has received thorough treatment elsewhere.
Keywords: Surgery, Resistance, Bacteria, Fungi, Viruses
Resistant pathogens and fungi
Introduction
Infections of all kinds are an unfortunate and common condition among the surgical population. Management of these infections often requires multiple treatment modalities but usually involves some form of antimicrobial therapy. Over the years, antimicrobial resistance has become increasingly common across a wide range of pathogens. In this section, we discuss some of the more common resistant pathogens that the surgeon is likely to encounter.
HA-MRSA: Hospital-associated Methicillin-resistant Staphylococcus aureus
Staphylococcus aureus is the most commonly isolated bacterial pathogen (1). It is therefore not surprising that MRSA (both community-acquired [CA-MRSA] and hospital-acquired [HA-MRSA]) is one of the most common resistant pathogens encountered by the surgeon. Since its discovery in 1960, the incidence of nosocomial infection caused by MRSA has increased steadily. Today, S. aureus is the predominate isolate in intensive care units in the United States (2). Resistance to methicillin, and other beta lactams, results from the acquisition of the mecA gene cassette, which modifies the penicillin binding protein in the cell wall (3).
Risk Factors for HA-MRSA: (1, 4)
-
Any of the following within the last year:
Hospitalization
Surgery
Intubation
Dialysis
Residence in long-term care facility
Indwelling catheter or other percutaneous device
Prior exposure to antibiotics
Prior history of MRSA infection
Pressure ulcer
Colonization
The clinical spectrum of all MRSA infections ranges from asymptomatic colonization to severe invasive disease. Compared to CA-MRSA, HA-MRSA is less likely to cause skin and soft tissue infections (SSTI). SSTI accounts for only 37% of HA-MRSA infections (4). Uncomplicated abscesses 5 cm or less in diameter may be managed with incision and drainage alone. However, systemic signs or evidence of invasive disease such as cellulitis, pneumonia, endocarditis, bone or joint infection require systemic antibiotics. Vancomycin is the empiric treatment of choice where MRSA is suspected. Other agents such as linezolid, daptomycin, quinpristin/dalfopristin, and tigecycline may be considered as well (4). Local susceptibility patterns should be reviewed when choosing the appropriate antibiotic. A recent study surprisingly demonstrated no difference in outcomes between hospital and community-associated MRSA infections (5).
While the prevalence of MRSA colonization varies widely in the literature, it is an important risk factor for subsequent clinical MRSA infection. (6–9). In a recent study, 40% of all patients with a clinical MRSA infection were known to be previously colonized within the prior one-year period (9). Efforts to decolonize patients of MRSA, particularly upon admission or before surgery, have met with some success (10–15). These strategies typically consist of chlorhexidine bathing or intra-nasal mupirocin either alone or in combination. A recent multicenter randomized trial demonstrated a marked decline in clinical MRSA infections after the implementation of a universal decolonization strategy for ICU patients (15).
CA-MRSA: Community-associated Methicillin-resistant Staphylococcus aureus
In the early 1990’s, reports began to emerge of infections caused by MRSA in populations that did not exhibit traditional risk factors for MRSA colonization or infection. Typically, these patients were younger and healthier than the usual population susceptible to MRSA (1). Eventually, these infections were identified as a new strain of MRSA dubbed community-associated MRSA (CA-MRSA). CA-MRSA varies molecularly from HA-MRSA by having a smaller mec chromosomal cassette (Type IV or V compared to I, II, or III for HA-MRSA) (1, 16, 17). In the United States, USA300 and USA400 are the dominant clonal isolates, with USA300 being the most common (18). Despite its name, CA-MRSA is often encountered in the hospital setting. One recent study demonstrated that 52% of all MRSA isolates from the intensive care unit were CA-MRSA (19).
CA-MRSA has become an increasingly common pathogen, however, evaluating its true epidemiology is difficult given inconsistencies in the definition (20). The CDC definition underestimates the proportion of CA-MRSA in the population. Genetic testing is not routinely performed and is therefore not practical in defining CA-MRSA for the average physician. Others advocate a practical definition based on either temporal patterns or antimicrobial susceptibility (1). We suggest using all of these factors in evaluating patients for potential CA-MRSA infection.
Various Definitions of CA-MRSA: (1)
CDC Definition
Outpatient diagnosis
Diagnosis within 48 hours of admission if no other risk factors for HA-MRSA (see HA-MRSA)
Temporal Definition
Outpatient diagnosis
Diagnosis within 48 hours of admission
Antimicrobial Susceptibility Definition
No or limited resistance to non-beta lactam antimicrobials (particularly clindamycin)
Risk Factors for CA-MRSA Infection: (1, 4, 9)
Children (Neonates in particular)
Adults age 65 or older
Women (pregnant and post-partum)
Athletes
Household contacts of MRSA SSTI patients
Emergency department patients
Urban and/or low socioeconomic status
Indigenous populations
Populations living in close proximity (military, jail or prison)
Cystic fibrosis patients
Men who have sex with men (MSM)
HIV patients
Veterinarians, livestock handlers, and pet owners
History of endocarditis
Antibiotic exposure within the last year
Chronic skin disorder
Tobacco use
Tattoo recipients
SSTI represent 90% of CA-MRSA infections (21). Typically, these infections present as a superficial abscess often mistaken for a spider bite. The presence of an abscess with surrounding erythema with a central black eschar is 94% predictive of some form of MRSA isolate. Unfortunately, CA-MRSA cannot be distinguished from HA-MRSA, MSSA, or other causes of SSTI on physical characteristics alone (1). However, one study of 137 patients presenting with cellulitis identified the presence of abscesses (OR 2.7; 95% CI, 1.3–5.8) and a body mass index (BMI) greater than 30 (OR 2.3; 95% CI, 1.1–5.0) to be independently associated with the presence of CA-MRSA.
Uncomplicated SSTI, presenting as an abscess, (without systemic signs) may be managed with incision and drainage alone (1, 17). If antibiotics are indicated (cellulitis), clindamycin or trimethoprim-sulfamethoxazole (TMP-SMX) are the empiric antibiotics of choice, since USA300 is typically sensitive to these antimicrobials (17, 18). However, as clindamycin use has increased, so has clindamycin resistance (17). Doxycycline and minocycline may also be considered. Linezolid is also an effective choice but is limited by its high cost (1). Resistance to fluouroquinolones (particularly ciprofloxacin) may be high in certain populations (MSM) (4, 18). Caution should be urged when choosing antimicrobials for cellulitis as doxycycline and TMP-SMX may not be effective against group A streptococci (GAS) (17). Local susceptibility patterns should always be considered when selecting appropriate antimicrobials.
Invasive infections, particularly pneumonia, can be rapidly fatal (mortality 50–63%). Patients with necrotizing CA-MRSA pneumonia present with hemoptysis, leukopenia, high fever and cavitary lung lesions (22). These patients have an odds ratio of dying of 11.3 (95% CI, 5.6 to 23) compared to other severe CA-MRSA infections (1, 23). One study reported a 56% mortality rate for CA-MRSA pneumonia with a median age of 14.5 (1, 24).
Regardless of the site of infection, vancomycin remains the mainstay of severe MRSA related infections. Linezolid may be considered in cases of a high vancomycin MIC’s, while clindamycin may be considered (for CA-MRSA only) as an adjunctive antimicrobial to reduce toxin production. Linezolid may also be considered in necrotizing CA-MRSA pneumonia given the relatively low lung penetration of vancomycin (1).
VRE: Vancomycin-resistant Enterococcus spp
Enterococcus faecalis and Enterococcus faecium are normal part of human intestinal flora. Combined, these account for the majority of Enterococcus infections in humans. These pathogens are notoriously difficult to treat, as they are intrinsically resistant to most penicillins, cephalosporins, and TMP-SMX. Furthermore, they easily acquire resistance to many other antibiotic classes. While some strains of E. faecalis may be susceptible to some penicillins, cephalosporins and fluoroquinolones, this is not the case for E. faecium. Resistance to vancomycin is mediated through the acquisition of a group of genes collectively known as the van gene complex. These genes encode an alteration in the cell wall with reduced affinity for vancomycin (25).
Risk factors for VRE Infection: (25, 26)
VRE colonization – generally required for infection
Advanced age
Severe underlying illness
Inter-hospital transfer
Resident of long-term care facility
Nutritional support (TPN)
Central venous catheterization
Hematologic malignancies
Surgery for inflammatory bowel disease
Biliary tract or liver pathology
Transplant patients
Hemodialysis
-
Previous antibiotic exposure. Particularly:
Vancomycin
3rd generation cephalosporins
Anti-anaerobic antibiotics (Metronidazole)
Antibiotic combinations
Long-term antibiotic use
Colonized patients develop VRE infections that are similar in scope to vancomycin-susceptible isolates: intra-abdominal, skin and soft tissue, urinary tract, bloodstream and endocarditis. VRE pneumonia or CNS infections are rare (25). Approximately 8% of colonized patients develop a VRE infection either during or shortly after hospital admission (26). The associated mortality for these infections remains high (13–46%) (27).
Linezolid or daptomycin are the drugs of choice for most VRE infections. Daptomycin’s rapid bactericidal activity makes it the preferred agent in bloodstream infections and endocarditis. Some strains of E. faecalis may be susceptible to ampicillin or piperacillin, but this is becoming uncommon. Quinpristin/dalfopristin has some use against E. faecium only (25, 28). Resistance to linezolid has been reported and is an emerging problem (28).
Susceptible patients while undergoing medical care easily acquire VRE. A recent study found that 12.3% of patients who were initially VRE negative were colonized before leaving the intensive care unit (29). VRE transmission often occurs via healthcare workers and once acquired may be life-long. Therefore methods to reduce transmission such as active screening and isolation have been instituted in some locations. Screening and isolation for VRE-positive patients, however, remains controversial with no consensus criteria for the removal of patients from isolation (25).
ESBL: Extended-spectrum Beta-lactamase Producing Bacteria
Many organisms have acquired, either through point mutation or plasmid acquisition, the ability to produce a group of enzymes collectively known as extended-spectrum beta-lactamases. Carapenem-resistant Enterobacteriaceae (discussed below) actually represent a special case of this phenomenon. These enzymes were originally described as a point mutation in the classic TEM and SHV beta-lactamases, thereby conferring expanded activity. Soon other plasmid mediated ESBL enzymes were also discovered. Unlike CRE’s, ESBL enzymes are not limited to the Enterobacteriaceae family and are commonly found in Pseudomonas species. In 2007, a survey identified that up to 17% of K. pneumoniae and 10% of E. coli demonstrated ESBL activity. Most of these (65%) produce members of the CTX-M class of beta-lactamases (30).
ESBL transmission between pathogens generally occurs via a large plasmid, which often encodes resistance to other antibiotic classes, particularly fluoroquinolones, aminoglycosides and TMP-SMX. The high likelihood of concomitant resistance among ESBL producing pathogens limits therapeutic options. For this reason cephalosporins, fluoroquinolones, and TMP-SMX are not appropriate options for treating most ESBL infections. Unless CRE is suspected, carbapenems remain the antibiotic class of choice for treating these infections (30, 31). Although the data is limited, beta-lactam/beta-lactamase inhibitor combinations, such as piperacillin/tazobactam, have also demonstrated efficacy in treating these infections, particularly in the urinary tract (30–32). Among the beta-lactamase inhibitors, tazobactam is the most potent and is active against many ESBL classes (TEM, SHV, and CTX-M) (30). Tigecycline, colistin, and fosfomycin are additional therapeutic options (30, 32). The wide range of ESBL activities, along with associated resistances, highlight the need to perform appropriate antibiotic sensitivity assays, as well as consult local antibiotic susceptibility patterns when choosing the appropriate therapeutic agent.
CRE: Carbapenem-resistant Enterobacteriaceae
Enterobacteriaceae is a large family of gram-negative rods that contains many common human pathogens, including: Escherischia coli, Klebsiella species, Enterobacter species, and over 70 other genera. Resistance to many broad-spectrum antibiotics is common among members of this family and until recently physicians could depend on the carbapenem antimicrobial class to reliably treat these pathogens. However, since 2000, carbapenem resistance has been growing. While still uncommon, the percentage of Enterobacteriaceae in the United States that were CRE increased from 1.2% in 2001 to 4.2% in 2011. The largest increase was among Klebsiella species, particularly K. pneumoniae (33). Another report describes carbapenem resistance in E. coli at 4.0% and at 10.8% among K. pneumoniae (34).
CRE produce ESBL with the largest spectrum of activity. Unlike MRSA or VRE, carbapenem resistance is not mediated by a single set of genes within a single species. Instead, CRE is mediated through multiple plasmid-encoded enzymes across an entire family of organisms (33, 35). The most common resistance gene is the highly transmissible Klebsiella pneumoniae carbapenemase (kpc), so named because of its initial discovery in that species (33). The metallo-beta-lactamases (MBLs) VIM (Verona integrin-encoded MBL) and IMPs (active on imipenem) are also common (34).
Risk Factors for CRE: (35, 36)
Advanced age
Intensive care unit stay in previous 3 months
Central venous catheterization
Receipt of antibiotics (particularly fluoroquinolones) in previous 3 months
Diabetes mellitus
Recent invasive procedure in the last 6 months
Isolation of resistant bacteria in previous 6 months
Dependent functional status
Permanent residency in institution
Charlson comorbidity index greater or equal to 3
CRE infection and colonization have been managed with strict cohorting and isolation (37). A recent study suggests that, unlike VRE, asymptomatic colonization may not be life-long. This study found that at one year following the index admission with CRE, only 39% of patients remained positive. A lack of hospitalization during this time increased the likelihood of becoming CRE negative (38).
CRE infections carry a high mortality rate between 40–50%, which is increased to 72% in CRE bloodstream infections (33, 39). The current mainstays of treatment include colistin, tigecycline, and aminoglycosides. Fosfomycin and polymixin are additional options. Some authors advocate prolonged profusion carbapenem or double carbapenem therapy for CRE with an MIC less than or equal to 4 mg/L (39).
Candida
Candida species are the most common invasive fungal pathogens in humans. It is the third most common cause of infection overall and is the second most common pathogen in North American ICU’s (40). While Candida albicans remains the most common individual isolate in the United States and Canada, non-albicans species make up 57.1% of all cases of candidemia (40). When all forms of candidiasis are considered, including hair, skin, and nail infections, C. albicans is the causative pathogen in 80% of cases (41). The incidence of invasive candidiasis is increasing. Between 2003 and 2005, the incidence of candidemia increased from 3.65 to 5.56 per 100,000 people (42).
Risk Factors for Invasive Candidiasis: (40–42)
Colonization of several body sites
Extremes of age
Exposure to broad spectrum antibiotics
-
Immunocompromised
Cytotoxic chemotherapy
Corticosteroids
Transplant
Neutropenia
HIV
Disruption of the physiological barriers of the GI tract
Major abdominal surgery
Other surgery during hospitalization
Surgery on the urinary system in the setting of candiduria
Major trauma (ISS > 20)
APACHE II score > 20
Candiduria > 105 cfu/ml
Diabetes
Hemodialysis
Mechanical ventilation
Central venous catheterization
Enterocutaneous fistula
Total parenteral nutrition
ICU stay > 7 days
Multiple transfusions
Colonization by Candida species is a key risk factor for invasive candidiasis. Alteration of normal host flora via broad-spectrum antibiotic exposure allows for fungal overgrowth. Increasing burden of Candida as demonstrated by semiquantitative cultures from multiple sites at multiple time points has been associated with the development of invasive candidiasis. Some view the identification of Candida from more than two body sites as a justification for antifungal therapy (42). Eggimann, et al. describes criteria for “pre-emptive” antifungal therapy as substantial colonization in the presence of multiple risk factors. Likewise, “prophylactic” antifungal therapy is justified for certain subgroups at particularly high risk for infection: organ-transplant recipients or immunocompromised patients with expected or long-term neutropenia (43).
Selective pressure from the frequent use of prophylactic fluconazole has contributed to the increase in azole-resistant Candida species, particularly C. glabrata and C. krusei (40, 42, 44). C. glabrata is the most common of the non-albicans species and tends to occur in patients with prior antifungal therapy, older patients, and transplant recipients (both solid organ and hematopoietic stem cell transplants). It is uncommon in younger patients and neonates (40, 41). Risk factors for C. parapsilosis infection include recent surgery, younger age, transplant patients, and those receiving TPN. It is also a frequent NICU pathogen (40, 41). C. Tropicalis is common in patients with hematologic malignancies and neutropenia (40, 41). Most patients with C. krusei candidemia have had prior antifungal exposure, neutropenic or received a hematopoietic stem cell transplant (40).
Overall, mortality following fungal infection remains high but varies somewhat based upon the individual pathogen. Ninety-day mortality following candidemia ranged from 30% for C. parapsilosis to 46.4% for C. krusei (40). Overall mortality for invasive candidiasis ranges between 40–60% but can be as high as 80% in selected populations (immunocompromised) (42, 43)
Azole class antifungals, particularly fluconazole, are the most commonly prescribed antifungals in the surgical population. This usage is probably appropriate in many cases, particularly for proven C. albicans infection or empirically in an otherwise low-risk patient. However, in critically ill patients or those with previous azole exposure, echinocandins (caspofungin, micafungin, anidulafungin) should be considered first-line agents. Amphotericin B is another option for azole-resistant strains but comes with a significant side effect profile. Lipid formulations have lowered but not eliminated the risks of nephrotoxicity and infusion-related reactions. Therefore, amphotericin B should be reserved for salvage therapy (45).
Viruses
Human Herpes Viruses in Surgical Patients
Introduction of human herpes viruses
There are 8 human herpes viruses (HHV), including herpes simplex virus – 1 (HSV-1/HHV-1), herpes simplex – 2 (HSV-2/HHV-2), varicella-zoster virus (VZV/HHV-3), Epstein-Barr virus (EBV/HHV-4), cytomegalovirus (CMV/HHV-5), Roseola virus (HHV-6 and HHV-7), and Kaposi Sarcoma associated virus (KSHV/HHV-8). As summarized in the table below, these viruses are highly prevalent in humans (46–51). These viruses share a similar life cycle of primary infection, often inducing mononucleosis or flu-like symptoms, followed by control and disease resolution in immune competent hosts. Following resolution, these viruses then enter a state of latency, characterized as relative dormancy with little if any appreciable viral replication during most of the host’s life. Although these viruses are known to reactivate in immunosuppressed individuals, sometimes causing devastating disease, until recently little attention has been given to these viruses in immune competent hosts. During the last three decades, there has been increasing awareness of reactivation of latent herpes viruses in immune competent hosts with surgical disease.
Overview and Epidemiology of Herpes Viruses (Table 1)
Table 1.
Overview and Epidemiology of Herpes Viruses
| Common Name | Formal Name | Prevalence | Primary Targets | Transmission | Sites of Latency | Primary Disease |
|---|---|---|---|---|---|---|
| Herpes Simplex Virus-1 | HHV1 | 50–80% | mucoepithelia | oropharyngeal contact | Sensory and cranial nerve ganglia | cold sores |
| Herpes Simplex Virus- 2 | HHV 2 | 20–25% | mucoepithelia | sexual contact, congenital | Sensory nerve ganglia | genital lesions |
| Varicella Zoster Virus | HHV 3 | >90% (prevaccine) | mucoepithelia | airborne | dorsal root ganglia | chicken pox |
| Epstein-Barr Virus | HHV 4 | 95% | Epithelial, oral lymphoid cells | saliva | B lymphocytes | infectious mononucleosis |
| Cytomegalovirus | HHV 5 | 50–80% | Monocytes, lymphocytes, and epithelia | saliva, sexual contact, congenital, blood transfusions, transplant | Monocytes, Lymphocytes | asymptomatic, mono-like symptoms |
| Roseola Virus | HHV 6, 7 | 100% | T cells | saliva | various leukocytes | asymptomatic (90%), roseola infantum (10%) |
| Kaposi Sarcoma associated Virus | HHV 8 | varies | Lymphocytes and epithelia | sexual contact, saliva | B cells | asymptomatic, oncogenic in immune-suppressed |
Latency and reactivation
To better understand reactivation, it is important to first define latency. In general, herpes viruses become quiescent after infection, with viral DNA present in host cells with very little (if any) transcriptional activity. Such latency seems to be maintained by a combination of host immunity and epigenetic regulation. Following primary infections, the host mounts concomitant innate and adaptive immune responses to control viral spread, but control typically occurs well after full dissemination of virus to its target cells/tissues. Thus, in addition to inducing non-specific innate anti-viral responses, all HHV induce epitope specific immunoglobulin and CD4/CD8 T-cell responses. It is important to note that most of what we know about latency and reactivation mechanisms comes from animal models of CMV and HSV, with less known about VZV, EBV, or HHV6-8 because of a lack of good animal models for these infections.
The importance of epigenetics in herpesvirus latency is becoming increasingly clear. Herpesvirus DNA are not typically integrated into host DNA, but are maintained as episomes in infected cells. Like other eukaryotic DNA, HHV-DNA become wrapped around histone proteins in a repeating nucleosome pattern (reviewed in (52)). This leaves most of the viral genomes inaccessible to transcription and replication. It is clear that most viral genomes in host tissues are thereby epigenetically regulated and quiescent at any given time (53–55){Liu, 2008 #1661}. This “chromatinization” of viral DNA occurs very rapidly after infection, thereby contributing to development of latency (56).
Most likely as a viral survival advantage, this epigenetic regulation can be interrupted, leading to localized reactivation events (57). Such reactivation events likely lead to transient viral replication and shedding, thus allowing perpetuation of virus within communities. In immune competent hosts, however, these reactivation episodes are quickly controlled by memory T and B cell responses, leading to resumption of latency. In contrast, hosts with impaired immunity often have reactivation episodes that progress to viral disease, with shedding and transmission of live virus. Unlike HHV with cutaneous manifestations, such as HSV-1/2 and VZV, whose reactivation episodes are obvious, it is unknown how frequently the other HHV may reactivate in immune competent hosts. It does seem clear, however, that transient compromise in host immunity will allow transcriptional reactivation of these viruses (58, 59). For the purposes of this article, we will define immune competent hosts as those not undergoing canonical immune suppression or have disease related immune compromise (such as AIDS), understanding that following surgery, trauma or critical illness there may very well be transient compromise in host immunity (60).
Diagnosis of HHV infection/reactivation
One of the major obstacles in our understanding of HHV infection and reactivation is that with the exception of HSV and VZV, there are no cutaneous manifestations of reactivation. For most, it is hard not to notice a perioral cold sore, or the painful outbreak of VZV/HHV-3 in the form of herpes zoster, making diagnosis of reactivation episodes for these viruses a simple matter. The other herpes viruses do not typically have cutaneous manifestations, thus requiring serologic or tissue diagnosis. Prior to introduction of the “monospot test”, Paul and Bunnels testing for EBV infection was the method used to detect EBV associated infectious mononucleosis (61). Although immune globulin monitoring remains one of the best ways to diagnose acute or previous HHV infection, for monitoring/diagnosing reactivation it has far less accuracy and utility. Immune globulin monitoring has therefore been mostly replaced by DNA based molecular methods to diagnose HHV reactivation in immune competent hosts.
Triggers of HHV reactivation
There are myriad triggers for reactivation of latent HHV, and one of the best studied in healthy hosts is stress. Glaser et al. were among the first to show that the social stress during academic examinations can induce reactivation of HHV in healthy medical students (62). These findings were supported further by an animal model of HSV-1/HHV-1 reactivation following social stress (63). Certainly among the most healthy immune competent hosts are astronauts, and there are numerous studies that show the preflight stress as well as the stress of space flight can stimulate reactivation of CMV, HSV, VZV and EBV (64–67). It should therefore come as no surprise in the sections that follow that more aggressive stressors, such as surgical disease or infections that induce critical illness are also triggers for viral reactivation.
HHV following Cardiac Surgery
Herpesviruses were first associated with surgical disease in immune competent patients in the late 50’s. Battele and Hewlett, and subsequently others, described a peculiar viral illness that befell 4–30% of patients undergoing cardiac surgery with extracorporeal bypass (68–73). These cases usually occurred 3–6 weeks after surgery, had symptoms consistent with viral mononucleosis, but most were EBV-negative (by Paul Bunnell testing). Ultimately, Paloheimo et al made the first connection between these febrile illnesses and CMV, and it was later concluded that many cases were likely a consequence of blood transfusion practices during extracorporeal circulation in that era (74). Because of limitations in diagnostics at that time, it remained unclear whether post-pump CMV was a primary infection or reactivation of latent virus until later work suggested that most were reactivations (75).
The consequences of CMV reactivation in cardiac surgery patients remain unclear. Although the early observations of CMV activity were not linked with worsened outcomes, later work in patients with complications after cardiac surgery suggested otherwise (76). It was observed that patients with CMV infection concomitant to bacterial infections often had far worse outcomes (77). For example, in patients with mediastinitis following cardiac surgery, CMV viremia or viruria was associated with higher mortality and impaired clearance of local infection (76). This was hypothesized to be related to impaired neutrophil function (78). Likewise, Rand observed that mice given primary CMV plus bacterial infection had experienced 80–100% mortality, compared to none in control groups (79).
Less is known about EBV and HSV associated disease in cardiac surgery. VZV has been reported to reactivate as herpes zoster following cardiac surgeries (80–82). Prompt diagnosis and treatment is important as misdiagnosis can lead to short-term and long-term pain control issues or other related sequelae.
HHV have also been associated with other cardiac diseases. CMV viremia correlates with disease severity in patients admitted with acute heart failure (p < 0.001) and this is thought to be secondary to the pro-inflammatory state associated with heart failure progression. (83). HHV have also been implicated in atherosclerosis, and biopsy of the major arteries in young trauma patients identified HSV and CMV in atherosclerotic lesions and foam cells of the intimal layer (84, 85). Acute infection with CMV in rats causes endothelial injury (86) and some have postulated that this contributes to hypertension (87). Later studies evaluating mechanisms indicate that presence of lifelong, latent herpesviruses in atherosclerotic plaque may exert pathogenic effects by penetrating the arterial wall, modifying lipid metabolism, and stimulating the production of pro-inflammatory cytokines and growth factors (88).
HHV after trauma/burn injury
Although HHVs have likely been reactivating in humans since their acquaintance hundreds of thousands of years ago (89), description of reactivation events didn’t become common until the 20th century. It is perhaps not surprising that the first described trauma associated HHV reactivation was a case of VZV/HHV-3 herpes zoster, although its association with a lightning strike makes it a bit remarkable (90). The association between herpes zoster and trauma has been subsequently elaborated in case reports and series, and recently confirmed in a large case control study of Medicare patients (91). The first report of CMV/HHV-5 related to trauma followed some years after recognition of CMV reactivation in cardiac surgery patients, and occurred in a man who died of disseminated CMV disease following severe facial fractures (92). Soon after this, the first case of HSV/HHV-1 was reported after oral trauma (93). It was several years later that the first confirmed case of EBV/HHV-4 was reported in a patient that had suffered trauma requiring splenectomy and transfusions (94).
HHVs also have a long-standing association with burn injury, beginning with the observations of systemic CMV (95) and herpes simplex activity in burn wounds (95). Some years later other reports emerged describing CMV infections in patients with burn injuries (96–98). Subsequent studies suggested high rates of reactivation (>50%) as well as primary infections (12–15%) following burn injury (99, 100). Major burns have subsequently been shown to be one of the strongest CMV reactivation stimuli in humans with incidence as high as ~70% (101, 102). There are only case reports of VZV in adult burn patients (103), and pediatric burn patients with primary VZV have higher likelihoods for pneumonitis (104). It seems that the editors of the British Medical Journal were prescient when they observed that “…there seems to be at least as much for virologists to do in the surgical wards as in the medical ones.” (105). Unfortunately, at least for burns, little attention is being paid in the US to this entity (106).
This lack of attention may be because it is unclear what impact HHV may have on outcomes in burns. Reactivation of CMV in burn patients has not been associated with worsened mortality (98, 99, 107), although it has been associated with longer ICU stays and duration of mechanical ventilation (101). Primary infection in burn patients is also of concern, as CMV can be detected in cadaveric skin grafts (108) and transmission by seropositive skin grafts can cause severe disease (109). Cutaneous HSV reactivation, seen in up to 25% of burns, presents 1–3 weeks post-burn as a cluster of vesicles around the margins of a healing burn (110). Areas of active epidermal regeneration appear to be the most commonly affected (100), but progression to visceral HSV involvement has been described, manifesting as necrotizing adrenal and hepatic lesions (95). HSV reactivation of the respiratory tract is seen in up to 50% of burn patients (110, 111). The impact of pulmonary HSV reactivation on mortality has been mixed, with larger prospective studies still needed (107, 112, 113)).
HHV in Critical Care/Sepsis
Similar to trauma patients, there have been numerous reports of HHV eruptions/cutaneous manifestations during critical illness, but those HHVs without obvious outward signs have taken much longer to recognize as an entity. During the mid to late nineties, several different investigators began reporting herpesvirus infections/reactivations in previously immune competent patients during critical illness. These first reports included both HSV and CMV, and since then more limited work has evaluated the other herpes family viruses during critical illness. There is a growing body of knowledge in this area that includes both medical and surgical patients, and the use of animal models has contributed significantly to our understanding of mechanisms and consequences of such reactivation events. Although it has not been confirmed for all HHV, because of the similarities in infection, control and development of latency between the HHVs, it seems likely that each HHV follows a pattern of reactivation similar to CMV. In the sections that follow, we will review each of the herpes family viruses and their associations with critical illness in previously immune competent patients.
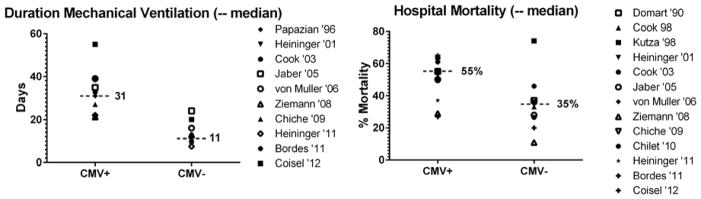
It has become clear that CMV can reactivate during critical illness, with sensitive PCR based methods showing that approximately one-third of latently infected patients have CMV reactivation during critical illness (114). These reactivation events can lead to live virus shed in the blood and pulmonary secretions of affected hosts (115, 116). It seems likely that these reactivation events are consequent to inflammatory insults that trigger release of cytokines, epigenetic deregulation of viral genomes, and possibly immune compromise (58, 67, 117–119). It remains unclear, however, whether CMV is a true pathogen or merely an indicator of severity of illness. When considering all of the currently available studies (76, 101, 115, 116, 120–129), reactivation is associated with roughly doubled risks of hospital mortality and duration of mechanical ventilation (Figure 1). When one considers the potential pathogenic mechanisms associated with CMV, including pulmonary injury (117) and immune modulation (130), the possibility that CMV actually contributes to poor outcomes in immune competent patients is intriguing.
Figure 1.
HSV-1/HHV1 is the second best studied HHV that reactivates during critical illness. In mechanically ventilated patients, HSV-1 can be detected in tracheal aspirates and in lower respiratory tract secretions of intensive care unit patients in 22–54% and 16–32% of cases, respectively (131). This is confused however by the observation that asymptomatic shedding of HSV can occur in up to 5–10% of healthy individuals (132, 133). Furthermore, HSV haplotypes isolated from lower respiratory tracts of intubated patients have been shown to be identical to those isolated from the oropharynx, suggesting possible spread down the tracheobronchial tree through secretions (134). The lack of association of HSV reactivation with worsened outcomes in numerous studies casts further doubt on its importance as a possible pathogen (116, 128, 135–138). Nonetheless there are reports that link HSV reactivation with prolonged mechanical ventilation (136, 138), prolonged ICU stays (136), and even increased mortality (131, 139, 140). One possible explanation is that viral load is important as suggested by worsened mortality in patients with high viral loads (141). For now, however, the preponderance of data suggests that HSV activity during critical illness is simply an indicator of disease severity.
To date there are very few data for the other HHV in critically ill patients. EBV has been thought to reactivate during times of stress as detected by elevated antibody levels, which has since been confirmed by data showing EBV reactivation in 61% of ICU patients (142, 143). The lack of in-vivo models has impeded progress in understanding EBV reactivation, and therefore there are no published data on mechanisms or consequences of EBV reactivation in immune competent hosts. Similarly there are very few data for VZV/HHV3 and HHV6-8 during critical illness. VZV has been reported following spinal surgery but otherwise remains understudied in critically ill patients (81, 144–147). As with other HHV, aberrations in cell-mediated immunity are thought to be one of the causes of VZV reactivation (148, 149). HHV6 and HHV7 have been shown to reactivate during critical illness, but the consequence of this is unknown (150). Finally, like CMV, KSHV/HHV8 has been associated with lung disease (idiopathic pulmonary fibrosis), but has not yet been reported in critically ill immune competent hosts (151).
HHV in Gastrointestinal Disease
HHV can cause a variety of gastrointestinal diseases, many of which have surgical implications. One example is the relatively long-standing relationship between HHV and gastrointestinal ulcerative diseases in immune suppressed HIV and transplant patients. It is now recognized that immune competent trauma, critical care and postoperative patients can also suffer from HHV-related intestinal ulceration. CMV for example can cause colonic mucosal ulceration sometimes leading to perforation in immune competent hosts (152–154). Because this presentation can mimic other infectious colitides, CMV colitis should be considered when an infectious etiology is suspected but cannot be identified. Severe inflammation and ulcerative lesions have primarily been noted in areas of predominant endothelial distribution of CMV inclusion bodies (155), and ischemia from narrowing of the capillary circulation has been postulated in ulcer/perforation pathogenesis. CMV enteritis has been suggested to have higher mortality in immune competent patients than immunocompromised populations, possibly due to lower index of suspicion (156, 157). Although CMV is a common cause of upper GI ulceration in transplant and AIDS patients, it does not appear to play a meaningful role in immune competent patients (158). Diagnosis of HHV associated ulceration can be made with endoscopic or surgical biopsy, but is often made in surgical specimens post-hoc. Whether patients with perforation and subsequent diagnoses made by pathologic evaluation will benefit from antiviral treatment is unclear and will require further study.
Another common disease recently associated with HHV is appendicitis. There has been a long standing suspicion that non-HHV viral infections are associated with appendicitis, given it’s bimodal and seasonal occurrence (158). The first described association of HHV with appendicitis was in an AIDS patient (159), but a recent study of childhood appendicitis has shown periodic reactivation (21%) of CMV in lymphoid tissue of the appendix (160). In this study, HHV-6 was also identified in about 8% of specimens. It is unclear whether this is a cause or simply a consequence of the appendicitis, given that sepsis can cause HHV reactivation (161).
There is also a long-standing relationship between HHV/infectious mononucleosis and splenic disease. HHV have been known for many years to cause splenomegaly, which on occasion can lead to splenic rupture (162). Spontaneous splenic rupture is less common in acute CMV infection than EBV infection, despite one-third of acute CMV infections demonstrating splenomegaly (163–165). In contrast, HHV infection after splenectomy likely represents a distinct clinicopathologic syndrome (166). Acute CMV infection was first identified in post-splenectomy trauma patients in 1982 (167). Review of case reports shows that these infections typically occur within 2 to 4 weeks after splenectomy (166–169). The syndrome likely results from poor control of early viremia because of the lack of both splenic function and the typical brisk IgM response. Although it is difficult to determine acute versus reactivated CMV in these cases one study using anti-CMV IgG maturation indices supports acute infection in this syndrome (170). These cases of widely disseminated post-splenectomy CMV can sometimes be fatal (153).
Treatment of HHV reactivation/infection
There are few data to support or refute treatment of HHV reactivation in surgical patients. While there are scattered reports of treatment in immune competent patients, these all follow reactivation, likely suffer from selection bias and perhaps not surprisingly show no benefit. The best available data come from animal models, showing that CMV reactivation events induced by sepsis can be prevented with antiviral therapy (117, 171). Unfortunately antiviral treatment works best if administered prophylactically, which would require treating all patients, many of whom would never develop reactivation (172). For now there are no good data to support treatment of critically ill patients with HHV reactivation outside of a clinical trial (173). Fortunately there is a randomized control trial evaluating ganciclovir for prevention of CMV reactivation and acute lung injury in immune competent hosts (ClinicalTrials.gov #NCT01335932).
There are also no strong data for treatment of other HHV reactivations in critical care populations. A randomized control trial showed acyclovir treatment can prevent HSV reactivation, but appears to have no effect on survival or duration of mechanical ventilation (174, 175). Clinically severe or symptomatic cutaneous HSV or herpes zoster reactivations are usually treated with acyclovir once diagnosis is confirmed (176). There are some concerns that delay in diagnosis and treatment of HSV may lead to systemic dissemination causing necrotizing hepatic and adrenal lesions, bacterial superinfection, and even death, although data supporting this are lacking (97, 177). There are no data to support or refute treatment of HHV 2, 4, 6, 7 or 8 during critical illness in immune competent hosts.
Conclusion
The complexity of patients managed by surgeons continues to increase. With this complexity comes the unique host susceptibility to infections with microbes that were unknown pathogens even 50 years ago, including antimicrobial-resistant bacteria, fungi, and viruses. Although most surgeons will not primarily manage these organisms, it will be important for them to maintain a working knowledge of them to be able to provide optimum care for their most vulnerable patients.
Key Points.
The complexity of patients managed by surgeons continues to increase. With this complexity comes the unique host susceptibility to infections with microbes that were unknown pathogens even 50 years ago, including antimicrobial-resistant bacteria, fungi, and viruses.
Although most surgeons will not primarily manage these organisms, it will be important for them to maintain a working knowledge of them to be able to provide optimum care for their most vulnerable patients.
Acknowledgments
Funding: The National Institutes of Health Awards T32-AI078875, T32-CA090223 & RO1- GM 066115 supported research reported in this publication.
Footnotes
Disclosure Statement: The authors have no conflicts of interest to report.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23:616–687. doi: 10.1128/CMR.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boucher HW, Corey GR. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2008;46 (Suppl 5):S344–349. doi: 10.1086/533590. [DOI] [PubMed] [Google Scholar]
- 3.Goyal N, Miller A, Tripathi M, et al. Methicillin-resistant Staphylococcus aureus (MRSA): colonisation and pre-operative screening. Bone Joint J. 2013;95-B:4–9. doi: 10.1302/0301-620X.95B1.27973. [DOI] [PubMed] [Google Scholar]
- 4.Hansra NK, Shinkai K. Cutaneous community-acquired and hospital-acquired methicillin-resistant Staphylococcus aureus. Dermatol Ther. 2011;24:263–272. doi: 10.1111/j.1529-8019.2011.01402.x. [DOI] [PubMed] [Google Scholar]
- 5.Eells SJ, McKinnell JA, Wang AA, et al. A comparison of clinical outcomes between healthcare-associated infections due to community-associated methicillin-resistant Staphylococcus aureus strains and healthcare-associated methicillin-resistant S. aureus strains. Epidemiol Infect. 2013;141:2140–2148. doi: 10.1017/S0950268812002634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med. 2011;364:1419–1430. doi: 10.1056/NEJMoa1007474. [DOI] [PubMed] [Google Scholar]
- 7.McKinnell JA, Huang SS, Eells SJ, et al. Quantifying the impact of extranasal testing of body sites for methicillin-resistant Staphylococcus aureus colonization at the time of hospital or intensive care unit admission. Infect Control Hosp Epidemiol. 2013;34:161–170. doi: 10.1086/669095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edgeworth JD. Has decolonization played a central role in the decline in UK methicillin-resistant Staphylococcus aureus transmission? A focus on evidence from intensive care. J Antimicrob Chemother. 2011;66(Suppl 2):ii41–47. doi: 10.1093/jac/dkq325. [DOI] [PubMed] [Google Scholar]
- 9.Otter JA, Herdman MT, Williams B, et al. Low prevalence of meticillin-resistant Staphylococcus aureus carriage at hospital admission: implications for risk-factor-based vs universal screening. J Hosp Infect. 2013;83:114–121. doi: 10.1016/j.jhin.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Kassakian SZ, Mermel LA, Jefferson JA, et al. Impact of chlorhexidine bathing on hospital-acquired infections among general medical patients. Infect Control Hosp Epidemiol. 2011;32:238–243. doi: 10.1086/658334. [DOI] [PubMed] [Google Scholar]
- 11.Rupp ME, Cavalieri RJ, Lyden E, et al. Effect of hospital-wide chlorhexidine patient bathing on healthcare-associated infections. Infect Control Hosp Epidemiol. 2012;33:1094–1100. doi: 10.1086/668024. [DOI] [PubMed] [Google Scholar]
- 12.Climo MW, Yokoe DS, Warren DK, et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med. 2013;368:533–542. doi: 10.1056/NEJMoa1113849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans HL, Dellit TH, Chan J, et al. Effect of chlorhexidine whole-body bathing on hospital-acquired infections among trauma patients. Arch Surg. 2010;145:240–246. doi: 10.1001/archsurg.2010.5. [DOI] [PubMed] [Google Scholar]
- 14.Ridenour G, Lampen R, Federspiel J, et al. Selective use of intranasal mupirocin and chlorhexidine bathing and the incidence of methicillin-resistant Staphylococcus aureus colonization and infection among intensive care unit patients. Infect Control Hosp Epidemiol. 2007;28:1155–1161. doi: 10.1086/520102. [DOI] [PubMed] [Google Scholar]
- 15.Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013;368:2255–2265. doi: 10.1056/NEJMoa1207290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maree CL, Daum RS, Boyle-Vavra S, et al. Community-associated methicillin-resistant Staphylococcus aureus isolates causing healthcare-associated infections. Emerg Infect Dis. 2007;13:236–242. doi: 10.3201/eid1302.060781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Odell CA. Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) skin infections. Curr Opin Pediatr. 2010;22:273–277. doi: 10.1097/MOP.0b013e328339421b. [DOI] [PubMed] [Google Scholar]
- 18.Chua K, Laurent F, Coombs G, et al. Antimicrobial resistance: Not community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA)! A clinician’s guide to community MRSA - its evolving antimicrobial resistance and implications for therapy. Clin Infect Dis. 2011;52:99–114. doi: 10.1093/cid/ciq067. [DOI] [PubMed] [Google Scholar]
- 19.Wang JT, Liao CH, Fang CT, et al. Incidence of and risk factors for community-associated methicillin-resistant Staphylococcus aureus acquired infection or colonization in intensive-care-unit patients. J Clin Microbiol. 2010;48:4439–4444. doi: 10.1128/JCM.00784-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otter JA, French GL. Community-associated meticillin-resistant Staphylococcus aureus: the case for a genotypic definition. J Hosp Infect. 2012;81:143–148. doi: 10.1016/j.jhin.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Skov R, Christiansen K, Dancer SJ, et al. Update on the prevention and control of community-acquired meticillin-resistant Staphylococcus aureus (CA-MRSA) Int J Antimicrob Agents. 2012;39:193–200. doi: 10.1016/j.ijantimicag.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 22.Karampela I, Poulakou G, Dimopoulos G. Community acquired methicillin resistant Staphylococcus aureus pneumonia: an update for the emergency and intensive care physician. Minerva Anestesiol. 2012;78:930–940. [PubMed] [Google Scholar]
- 23.Wiersma P, Tobin D’Angelo M, Daley WR, et al. Surveillance for severe community-associated methicillin-resistant Staphylococcus aureus infection. Epidemiol Infect. 2009;137:1674–1678. doi: 10.1017/S0950268809002490. [DOI] [PubMed] [Google Scholar]
- 24.Gillet Y, Vanhems P, Lina G, et al. Factors predicting mortality in necrotizing community-acquired pneumonia caused by Staphylococcus aureus containing Panton-Valentine leukocidin. Clin Infect Dis. 2007;45:315–321. doi: 10.1086/519263. [DOI] [PubMed] [Google Scholar]
- 25.Mazuski JE. Vancomycin-resistant enterococcus: risk factors, surveillance, infections, and treatment. Surg Infect (Larchmt) 2008;9:567–571. doi: 10.1089/sur.2008.9955. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen GC, Leung W, Weizman AV. Increased risk of vancomycin-resistant enterococcus (VRE) infection among patients hospitalized for inflammatory bowel disease in the United States. Inflamm Bowel Dis. 2011;17:1338–1342. doi: 10.1002/ibd.21519. [DOI] [PubMed] [Google Scholar]
- 27.Whang DW, Miller LG, Partain NM, et al. Systematic review and meta-analysis of linezolid and daptomycin for treatment of vancomycin-resistant enterococcal bloodstream infections. Antimicrob Agents Chemother. 2013;57:5013–5018. doi: 10.1128/AAC.00714-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Napolitano LM. Emerging issues in the diagnosis and management of infections caused by multi-drug-resistant, gram-positive cocci. Surg Infect (Larchmt) 2005;6(Suppl 2):S-5–22. [PubMed] [Google Scholar]
- 29.Kim YJ, Kim SI, Kim YR, et al. Risk factors for vancomycin-resistant enterococci infection and mortality in colonized patients on intensive care unit admission. Am J Infect Control. 2012;40:1018–1019. doi: 10.1016/j.ajic.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Kanj SS, Kanafani ZA. Current concepts in antimicrobial therapy against resistant gram-negative organisms: extended-spectrum beta-lactamase-producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and multidrug-resistant Pseudomonas aeruginosa. Mayo Clin Proc. 2011;86:250–259. doi: 10.4065/mcp.2010.0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vardakas KZ, Tansarli GS, Rafailidis PI, et al. Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum beta-lactamases: a systematic review and meta-analysis. J Antimicrob Chemother. 2012;67:2793–2803. doi: 10.1093/jac/dks301. [DOI] [PubMed] [Google Scholar]
- 32.Pitout JD. Infections with extended-spectrum beta-lactamase-producing enterobacteriaceae: changing epidemiology and drug treatment choices. Drugs. 2010;70:313–333. doi: 10.2165/11533040-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 33.Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morb Mortal Wkly Rep. 2013;62:165–170. [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta N, Limbago BM, Patel JB, et al. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis. 2011;53:60–67. doi: 10.1093/cid/cir202. [DOI] [PubMed] [Google Scholar]
- 35.Schechner V, Kotlovsky T, Kazma M, et al. Asymptomatic rectal carriage of blaKPC producing carbapenem-resistant Enterobacteriaceae: who is prone to become clinically infected? Clin Microbiol Infect. 2013;19:451–456. doi: 10.1111/j.1469-0691.2012.03888.x. [DOI] [PubMed] [Google Scholar]
- 36.Marchaim D, Chopra T, Bhargava A, et al. Recent exposure to antimicrobials and carbapenem-resistant Enterobacteriaceae: the role of antimicrobial stewardship. Infect Control Hosp Epidemiol. 2012;33:817–830. doi: 10.1086/666642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenberger LH, Hranjec T, Politano AD, et al. Effective cohorting and “superisolation” in a single intensive care unit in response to an outbreak of diverse multi-drug-resistant organisms. Surg Infect (Larchmt) 2011;12:345–350. doi: 10.1089/sur.2010.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zimmerman FS, Assous MV, Bdolah-Abram T, et al. Duration of carriage of carbapenem-resistant Enterobacteriaceae following hospital discharge. Am J Infect Control. 2013;41:190–194. doi: 10.1016/j.ajic.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 39.van Duin D, Kaye KS, Neuner EA, et al. Carbapenem-resistant Enterobacteriaceae: a review of treatment and outcomes. Diagn Microbiol Infect Dis. 2013;75:115–120. doi: 10.1016/j.diagmicrobio.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pfaller M, Neofytos D, Diekema D, et al. Epidemiology and outcomes of candidemia in 3648 patients: data from the Prospective Antifungal Therapy (PATH Alliance(R)) registry, 2004–2008. Diagn Microbiol Infect Dis. 2012;74:323–331. doi: 10.1016/j.diagmicrobio.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Silva S, Negri M, Henriques M, et al. Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol Rev. 2012;36:288–305. doi: 10.1111/j.1574-6976.2011.00278.x. [DOI] [PubMed] [Google Scholar]
- 42.Eggimann P, Bille J, Marchetti O. Diagnosis of invasive candidiasis in the ICU. Ann Intensive Care. 2011;1:37. doi: 10.1186/2110-5820-1-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eggimann P, Garbino J, Pittet D. Management of Candida species infections in critically ill patients. Lancet Infect Dis. 2003;3:772–785. doi: 10.1016/s1473-3099(03)00831-4. [DOI] [PubMed] [Google Scholar]
- 44.Gleason TG, May AK, Caparelli D, et al. Emerging evidence of selection of fluconazole-tolerant fungi in surgical intensive care units. Arch Surg. 1997;132:1197–1201. doi: 10.1001/archsurg.1997.01430350047008. [DOI] [PubMed] [Google Scholar]
- 45.Playford EG, Eggimann P, Calandra T. Antifungals in the ICU. Curr Opin Infect Dis. 2008;21:610–619. doi: 10.1097/QCO.0b013e3283177967. [DOI] [PubMed] [Google Scholar]
- 46.Whitley RJ, Roizman B. Herpes simplex virus infections. Lancet. 2001;357:1513–1518. doi: 10.1016/S0140-6736(00)04638-9. [DOI] [PubMed] [Google Scholar]
- 47.Stone RC, Micali GA, Schwartz RA. Roseola infantum and its causal human herpesviruses. Int J Dermatol. 2014;53:397–403. doi: 10.1111/ijd.12310. [DOI] [PubMed] [Google Scholar]
- 48.Butterly A, Schmidt U, Wiener-Kronish J. Methicillin-resistant Staphylococcus aureus colonization, its relationship to nosocomial infection, and efficacy of control methods. Anesthesiology. 2010;113:1453–1459. doi: 10.1097/ALN.0b013e3181fcf671. [DOI] [PubMed] [Google Scholar]
- 49.Mesri EA, Cesarman E, Boshoff C. Kaposi’s sarcoma and its associated herpesvirus. Nat Rev Cancer. 2010;10:707–719. doi: 10.1038/nrc2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jenson HB. Epstein-Barr virus. Pediatr Rev. 2011;32:375–383. doi: 10.1542/pir.32-9-375. quiz 384. [DOI] [PubMed] [Google Scholar]
- 51.Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010;20:202–213. doi: 10.1002/rmv.655. [DOI] [PubMed] [Google Scholar]
- 52.Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217:833–842. e831–833. doi: 10.1016/j.jamcollsurg.2013.07.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sinclair J. Human cytomegalovirus: Latency and reactivation in the myeloid lineage. Journal of Clinical Virology. 2008;41:180–185. doi: 10.1016/j.jcv.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 54.Duan S, Yang YC, Xiang LF, et al. Incidence and risk factors of HIV infection among sero-negative spouses of HIV patients in Dehong prefecture of Yunnan province. Zhonghua Liu Xing Bing Xue Za Zhi. 2010;31:997–1000. [PubMed] [Google Scholar]
- 55.Lieberman PM. Keeping it quiet: chromatin control of gammaherpesvirus latency. Nat Rev Microbiol. 2013;11:863–875. doi: 10.1038/nrmicro3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nitzsche A, Paulus C, Nevels M. Temporal dynamics of cytomegalovirus chromatin assembly in productively infected human cells. J Virol. 2008;82:11167–11180. doi: 10.1128/JVI.01218-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hummel M, Yan S, Li Z, et al. Transcriptional reactivation of murine cytomegalovirus ie gene expression by 5-aza-2′-deoxycytidine and trichostatin A in latently infected cells despite lack of methylation of the major immediate-early promoter. J Gen Virol. 2007;88:1097–1102. doi: 10.1099/vir.0.82696-0. [DOI] [PubMed] [Google Scholar]
- 58.Campbell J, Trgovcich J, Kincaid M, et al. Transient CD8-memory contraction: a potential contributor to latent cytomegalovirus reactivation. Journal of Leukocyte Biology. 2012;92:933–937. doi: 10.1189/jlb.1211635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ogata M, Satou T, Kawano R, et al. High incidence of cytomegalovirus, human herpesvirus-6, and Epstein-Barr virus reactivation in patients receiving cytotoxic chemotherapy for adult T cell leukemia. J Med Virol. 2011;83:702–709. doi: 10.1002/jmv.22013. [DOI] [PubMed] [Google Scholar]
- 60.Wang H, Liu XX, Liu YX, et al. Incidence and risk factors of non-fatal injuries in Chinese children aged 0–6 years: a case-control study. Injury. 2011;42:521–524. doi: 10.1016/j.injury.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 61.Seitanidis B. A comparison of the Monospot with the Paul-Bunnell test in infectious mononucleosis and other diseases. J Clin Pathol. 1969;22:321–323. doi: 10.1136/jcp.22.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Glaser R, Kiecolt-Glaser JK, Speicher CE, et al. Stress, loneliness, and changes in herpesvirus latency. J Behav Med. 1985;8:249–260. doi: 10.1007/BF00870312. [DOI] [PubMed] [Google Scholar]
- 63.Padgett DA, Sheridan JF, Dorne J, et al. Social stress and the reactivation of latent herpes simplex virus type 1. [erratum appears in Proc Natl Acad Sci U S A 1998 Sep 29;95(20):12070.] Proceedings of the National Academy of Sciences of the United States of America. 1998;95:7231–7235. doi: 10.1073/pnas.95.12.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mehta SK, Cohrs RJ, Forghani B, et al. Stress-induced subclinical reactivation of varicella zoster virus in astronauts. J Med Virol. 2004;72:174–179. doi: 10.1002/jmv.10555. [DOI] [PubMed] [Google Scholar]
- 65.Bhamidipati CM, LaPar DJ, Mehta GS, et al. Albumin is a better predictor of outcomes than body mass index following coronary artery bypass grafting. Surgery. 2011;150:626–634. doi: 10.1016/j.surg.2011.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pierson DL, Stowe RP, Phillips TM, et al. Epstein-Barr virus shedding by astronauts during space flight. Brain Behav Immun. 2005;19:235–242. doi: 10.1016/j.bbi.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 67.Mehta SK, Crucian BE, Stowe RP, et al. Reactivation of latent viruses is associated with increased plasma cytokines in astronauts. Cytokine. 2013;61:205–209. doi: 10.1016/j.cyto.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 68.Battle JD, Jr, Hewlett JS. Hematologic changes observed after extracorporeal circulation during open-heart operations. Cleveland Clinic Quarterly. 1958;25:112–115. doi: 10.3949/ccjm.25.2.112. [DOI] [PubMed] [Google Scholar]
- 69.Holswade GR, Engle MA, Redo SF, et al. Development of Viral Diseases and a Viral Disease-Like Syndrome After Extracorporeal Circulation. Circulation. 1963;27:812–815. [Google Scholar]
- 70.Seaman AJ, Starr A. Febrile postcardiotomy lymphocytic splenomegaly: a new entity. Ann Surg. 1962;156:956–960. doi: 10.1097/00000658-196212000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ross BA. Pyrexia after Heart Surgery Due to Virus Infection Transmitted by Blood Transfusion. Thorax. 1964;19:159–161. doi: 10.1136/thx.19.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith DR. A Syndrome Resembling Infectious Mononucleosis after Open-Heart Surgery. Br Med J. 1964;1:945–948. doi: 10.1136/bmj.1.5388.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perillie PE, Glenn WW. Fever, splenomegaly, lymphocytosis and eosinophilia. Yale J Biol Med. 1962;34:625–628. [PMC free article] [PubMed] [Google Scholar]
- 74.Kääriäinen L, Klemola E, Paloheimo J. Rise of Cytomegalovirus Antibodies in an Infectious-mononucleosis-like Syndrome after Transfusion. BMJ. 1966;1:1270–1272. doi: 10.1136/bmj.1.5498.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Adler SP, Baggett J, McVoy M. Transfusion-associated cytomegalovirus infections in seropositive cardiac surgery patients. Lancet. 1985;326:743–746. doi: 10.1016/s0140-6736(85)90628-2. [DOI] [PubMed] [Google Scholar]
- 76.Domart Y, Trouillet JL, Fagon JY, et al. Incidence and morbidity of cytomegaloviral infection in patients with mediastinitis following cardiac surgery [see comments] Chest. 1990;97:18–22. doi: 10.1378/chest.97.1.18. [DOI] [PubMed] [Google Scholar]
- 77.Abramson JS, Mills EL. Depression of neutrophil function induced by viruses and its role in secondary microbial infections. Rev Infect Dis. 1988;10:326–341. doi: 10.1093/clinids/10.2.326. [DOI] [PubMed] [Google Scholar]
- 78.Hamilton JR, Overall JC, Glasgow LA. Synergistic effect on mortality in mice with murine cytomegalovirus and Pseudomonas aeruginosa, Staphylococcus aureus, or Candida albicans infections. Infection & Immunity. 1976;14:982–989. doi: 10.1128/iai.14.4.982-989.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rand KH, Merigan TC. Cytomegalovirus: a not so innocent bystander. JAMA. 1978;240:2470–2471. [PubMed] [Google Scholar]
- 80.Dirbas FM, Swain JA. Disseminated cutaneous herpes zoster following cardiac surgery. J Cardiovasc Surg (Torino) 1990;31:531–532. [PubMed] [Google Scholar]
- 81.Godfrey EK, Brown C, Stambough JL. Herpes zoster--varicella complicating anterior thoracic surgery: 2 case reports. J Spinal Disord Tech. 2006;19:299–301. doi: 10.1097/01.bsd.0000204499.76600.a5. [DOI] [PubMed] [Google Scholar]
- 82.Sachdeva S, Prasher P. Herpes zoster following saphenous venectomy for coronary bypass surgery. J Card Surg. 2010;25:28–29. doi: 10.1111/j.1540-8191.2009.00946.x. [DOI] [PubMed] [Google Scholar]
- 83.Nunez J, Chilet M, Sanchis J, et al. Prevalence and prognostic implications of active cytomegalovirus infection in patients with acute heart failure. Clinical Science. 2010;119:443–452. doi: 10.1042/CS20100162. [DOI] [PubMed] [Google Scholar]
- 84.Yamashiroya HM, Ghosh L, Yang R, et al. Herpesviridae in the coronary arteries and aorta of young trauma victims. American Journal of Pathology. 1988;130:71–79. [PMC free article] [PubMed] [Google Scholar]
- 85.Fabricant CG, Fabricant J, Litrenta MM, et al. Virus-induced atherosclerosis. J Exp Med. 1978;148:335–340. doi: 10.1084/jem.148.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Span AH, Van Boven CP, Bruggeman CA. The effect of cytomegalovirus infection on the adherence of polymorphonuclear leucocytes to endothelial cells. Eur J Clin Invest. 1989;19:542–548. doi: 10.1111/j.1365-2362.1989.tb00272.x. [DOI] [PubMed] [Google Scholar]
- 87.Aung AK, Skinner MJ, Lee FJ, et al. Changing epidemiology of bloodstream infection pathogens over time in adult non-specialty patients at an Australian tertiary hospital. Commun Dis Intell. 2012;36:E333–341. doi: 10.33321/cdi.2012.36.28. [DOI] [PubMed] [Google Scholar]
- 88.Epstein SE, Zhu J, Najafi AH, et al. Insights into the role of infection in atherogenesis and in plaque rupture. Circulation. 2009;119:3133–3141. doi: 10.1161/CIRCULATIONAHA.109.849455. [DOI] [PubMed] [Google Scholar]
- 89.Mukherjee S, Allen RM, Lukacs NW, et al. STAT3-mediated IL-17 production by postseptic T cells exacerbates viral immunopathology of the lung. Shock. 2012;38:515–523. doi: 10.1097/SHK.0b013e31826f862c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Parfitt DN. Herpes Zoster as a Sequel to Lightning Trauma. Br Med J. 1936;1:111. doi: 10.1136/bmj.1.3915.111-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu XF, Wang X, Yan S, et al. Epigenetic control of cytomegalovirus latency and reactivation. Viruses. 2013;5:1325–1345. doi: 10.3390/v5051325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Constant E, Davis DG, Maldonado WE. Disseminated cytomegalovirus infection associated with death in a patient with severe facial injuries. Case report. Plast Reconstr Surg. 1973;51:336–339. doi: 10.1097/00006534-197303000-00022. [DOI] [PubMed] [Google Scholar]
- 93.Guggenheimer J, Fletcher RD. Traumatic induction of an intraoral reinfection with herpes simplex virus: Report of a case. Oral Surgery, Oral Medicine, Oral Pathology. 1974;38:546–549. doi: 10.1016/0030-4220(74)90085-1. [DOI] [PubMed] [Google Scholar]
- 94.Purtilo DT, Paquin LA, Sakamoto K, et al. Persistent transfusion-associated infectious mononucleosis with transient acquired immunodeficiency. The American Journal of Medicine. 1980;68:437–440. doi: 10.1016/0002-9343(80)90116-3. [DOI] [PubMed] [Google Scholar]
- 95.Foley FD, Greenawald KA, Nash G, et al. Herpesvirus infection in burned patients. New England Journal of Medicine. 1970;282:652–656. doi: 10.1056/NEJM197003192821205. [DOI] [PubMed] [Google Scholar]
- 96.Deepe GS, Jr, MacMillan BG, Linnemann CC., Jr Unexplained fever in burn patients due to cytomegalovirus infection. JAMA. 1982;248:2299–2301. [PubMed] [Google Scholar]
- 97.Kagan RJ, Naraqi S, Matsuda T, et al. Herpes simplex virus and cytomegalovirus infections in burned patients. Journal of Trauma-Injury Infection & Critical Care. 1985;25:40–45. doi: 10.1097/00005373-198501000-00007. [DOI] [PubMed] [Google Scholar]
- 98.Kealey GP, Bale JF, Strauss RG, et al. Cytomegalovirus infection in burn patients. Journal of Burn Care & Rehabilitation. 1987;8:543–545. [PubMed] [Google Scholar]
- 99.Bale JF, Jr, Kealey GP, Massanari RM, et al. The epidemiology of cytomegalovirus infection among patients with burns. Infection Control & Hospital Epidemiology. 1990;11:17–22. doi: 10.1086/646073. [DOI] [PubMed] [Google Scholar]
- 100.Hayden FG, Himel HN, Heggers JP. Herpesvirus infections in burn patients. Chest. 1994;106:15S–21S. doi: 10.1378/chest.106.1_supplement.15s. discussion 34S–35S. [DOI] [PubMed] [Google Scholar]
- 101.Bordes J, Gaillard T, Maslin J, et al. Cytomegalovirus infection monitored by quantitative real-time PCR in critically ill patients. Critical Care. 2011;15:412. doi: 10.1186/cc10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Limaye AP, Kirby KA, Rubenfeld GD, et al. Cytomegalovirus Reactivation in Critically Ill Immunocompetent Patients. JAMA. 2008;300:413–422. doi: 10.1001/jama.300.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schroeder JE, Tessone A, Angel M, et al. Disseminated Varicella infection in an adult burn victim--a transfused disease? Burns. 2009;35:297–299. doi: 10.1016/j.burns.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 104.Sheridan RL, Weber JM, Pasternak MM, et al. A 15-year experience with varicella infections in a pediatric burn unit. Burns. 1999;25:353–356. doi: 10.1016/s0305-4179(99)00003-0. [DOI] [PubMed] [Google Scholar]
- 105.Herpesvirus Infection in Burned Patients. BMJ. 1970;2:618–619. Editorial. [PMC free article] [PubMed] [Google Scholar]
- 106.Tenenhaus M, Rennekampff HO, Pfau M, et al. Cytomegalovirus and burns: current perceptions, awareness, diagnosis, and management strategies in the United States and Germany. Journal of Burn Care & Research. 2006;27:281–288. doi: 10.1097/01.BCR.0000216727.89220.24. [DOI] [PubMed] [Google Scholar]
- 107.D’Avignon LC, Hogan BK, Murray CK, et al. Contribution of bacterial and viral infections to attributable mortality in patients with severe burns: an autopsy series. Burns. 2010;36:773–779. doi: 10.1016/j.burns.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 108.Kobayashi H, Kobayashi M, McCauley RL, et al. Cadaveric skin allograft-associated cytomegalovirus transmission in a mouse model of thermal injury. Clinical Immunology. 1999;92:181–187. doi: 10.1006/clim.1999.4735. [DOI] [PubMed] [Google Scholar]
- 109.Kealey GP, Aguiar J, Lewis RW, 2nd, et al. Cadaver skin allografts and transmission of human cytomegalovirus to burn patients. Journal of the American College of Surgeons. 1996;182:201–205. [PubMed] [Google Scholar]
- 110.Fidler PE, Mackool BT, Schoenfeld DA, et al. Incidence, outcome, and long-term consequences of herpes simplex virus type 1 reactivation presenting as a facial rash in intubated adult burn patients treated with acyclovir. J Trauma. 2002;53:86–89. doi: 10.1097/00005373-200207000-00017. [DOI] [PubMed] [Google Scholar]
- 111.Nash G, Asch MJ, Foley FD, et al. Disseminated cytomegalic inclusion disease in a burned adult. JAMA. 1970;214:587–588. [PubMed] [Google Scholar]
- 112.Tuxen DV, Cade JF, McDonald MI, et al. Herpes simplex virus from the lower respiratory tract in adult respiratory distress syndrome. Am Rev Respir Dis. 1982;126:416–419. doi: 10.1164/arrd.1982.126.3.416. [DOI] [PubMed] [Google Scholar]
- 113.Byers RJ, Hasleton PS, Quigley A, et al. Pulmonary herpes simplex in burns patients. Eur Respir J. 1996;9:2313–2317. doi: 10.1183/09031936.96.09112313. [DOI] [PubMed] [Google Scholar]
- 114.Kalil AC, Florescu DF. Prevalence and mortality associated with cytomegalovirus infections in non-immunosuppressed ICU patients. Critical Care Medicine. 2009;37:2350–2358. doi: 10.1097/CCM.0b013e3181a3aa43. [DOI] [PubMed] [Google Scholar]
- 115.Cook CH, Yenchar JK, Kraner TO, et al. Occult herpes family viruses may increase mortality in critically ill surgical patients. American Journal of Surgery. 1998;176:357–360. doi: 10.1016/s0002-9610(98)00205-0. [DOI] [PubMed] [Google Scholar]
- 116.Cook CH, Martin LC, Yenchar JK, et al. Occult herpes family viral infections are endemic in critically ill surgical patients. Critical Care Medicine. 2003;31:1923–1929. doi: 10.1097/01.CCM.0000070222.11325.C4. [DOI] [PubMed] [Google Scholar]
- 117.Aarts MA, Granton J, Cook DJ, et al. Empiric antimicrobial therapy in critical illness: results of a surgical infection society survey. Surg Infect (Larchmt) 2007;8:329–336. doi: 10.1089/sur.2006.072. [DOI] [PubMed] [Google Scholar]
- 118.Docke WD, Prosch S, Fietze E, et al. Cytomegalovirus reactivation and tumour necrosis factor. Lancet. 1994;343:268–269. doi: 10.1016/s0140-6736(94)91116-9. [DOI] [PubMed] [Google Scholar]
- 119.Seckert CK, Griessl M, Buttner JK, et al. Immune Surveillance of Cytomegalovirus Latency and Reactivation in Murine Models: Link to Memory Inflation. In: Reddehase MJ, editor. Cytomegaloviruses. Norfolk, UK: Caister Academic Press; 2013. pp. 374–416. [Google Scholar]
- 120.Kutza AS, Muhl E, Hackstein H, et al. High incidence of active cytomegalovirus infection among septic patients. Clinical Infectious Diseases. 1998;26:1076–1082. doi: 10.1086/520307. [DOI] [PubMed] [Google Scholar]
- 121.Heininger A, Jahn G, Engel C, et al. Human cytomegalovirus infections in nonimmunosuppressed critically ill patients. Critical Care Medicine. 2001;29:541–547. doi: 10.1097/00003246-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 122.Jaber S, Chanques G, Borry J, et al. Cytomegalovirus Infection in Critically Ill Patients: Associated Factors and Consequences. Chest. 2005;127:233–241. doi: 10.1378/chest.127.1.233. [DOI] [PubMed] [Google Scholar]
- 123.von Muller L, Klemm A, Weiss M, et al. Active cytomegalovirus infection in patients with septic shock. Emerging Infectious Diseases. 2006;12:1517–1522. doi: 10.3201/eid1210.060411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ziemann M, Sedemund-Adib B, Reiland P, et al. Increased mortality in long-term intensive care patients with active cytomegalovirus infection. Critical Care Medicine. 2008;36:3145–3150. doi: 10.1097/CCM.0b013e31818f3fc4. [DOI] [PubMed] [Google Scholar]
- 125.Chiche L, Forel JM, Roch A, et al. Active Cytomegalovirus infection is common in mechanically ventilated medical intensive care unit patients. Critical Care Medicine. 2009;37:1850–1857. doi: 10.1097/CCM.0b013e31819ffea6. [DOI] [PubMed] [Google Scholar]
- 126.Chilet M, Aguilar G, Benet I, et al. Virological and immunological features of active cytomegalovirus infection in nonimmunosuppressed patients in a surgical and trauma intensive care unit. Journal of Medical Virology. 2010;82:1384–1391. doi: 10.1002/jmv.21825. [DOI] [PubMed] [Google Scholar]
- 127.Heininger A, Haeberle H, Fischer I, et al. Cytomegalovirus reactivation and associated outcome of critically ill patients with severe sepsis. Critical care. 2011;15:R77. doi: 10.1186/cc10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Coisel Y, Bousbia S, Forel J-M, et al. Cytomegalovirus and Herpes Simplex Virus Effect on the Prognosis of Mechanically Ventilated Patients Suspected to Have Ventilator-Associated Pneumonia. PLoS ONE [Electronic Resource] 2012;7:e51340. doi: 10.1371/journal.pone.0051340. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 129.Papazian L, Fraisse A, Garbe L, et al. Cytomegalovirus. An unexpected cause of ventilator-associated pneumonia. Anesthesiology. 1996;84:280–287. doi: 10.1097/00000542-199602000-00005. [DOI] [PubMed] [Google Scholar]
- 130.Chatterjee SN, Fiala M, Weiner J, et al. Primary cytomegalovirus and opportunistic infections. Incidence in renal transplant recipients. JAMA. 1978;240:2446–2449. [PubMed] [Google Scholar]
- 131.Bouza E, Giannella M, Torres MV, et al. Herpes simplex virus: a marker of severity in bacterial ventilator-associated pneumonia. J Crit Care. 2011;26:432, e431–436. doi: 10.1016/j.jcrc.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 132.Schuller D. Lower respiratory tract reactivation of herpes simplex virus. Comparison of immunocompromised and immunocompetent hosts. Chest. 1994;106:3S–7S. doi: 10.1378/chest.106.1_supplement.3s. discussion 34S–35S. [DOI] [PubMed] [Google Scholar]
- 133.Vital signs: central line-associated blood stream infections--United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011;60:243–248. [PubMed] [Google Scholar]
- 134.Deback C, Luyt CE, Lespinats S, et al. Microsatellite analysis of HSV-1 isolates: from oropharynx reactivation toward lung infection in patients undergoing mechanical ventilation. J Clin Virol. 2010;47:313–320. doi: 10.1016/j.jcv.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 135.Simoons-Smit AM, Kraan EM, Beishuizen A, et al. Herpes simplex virus type 1 and respiratory disease in critically-ill patients: Real pathogen or innocent bystander? Clinical Microbiology & Infection. 2006;12:1050–1059. doi: 10.1111/j.1469-0691.2006.01475.x. [DOI] [PubMed] [Google Scholar]
- 136.Luyt C-E, Combes A, Deback C, et al. Herpes Simplex Virus Lung Infection in Patients Undergoing Prolonged Mechanical Ventilation. Am J Respir Crit Care Med. 2007;175:935–942. doi: 10.1164/rccm.200609-1322OC. [DOI] [PubMed] [Google Scholar]
- 137.van den Brink J-W, Simoons-Smit AM, Beishuizen A, et al. Respiratory herpes simplex virus type 1 infection/colonisation in the critically ill: marker or mediator? Journal of Clinical Virology. 2004;30:68–72. doi: 10.1016/j.jcv.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 138.Scheithauer S, Manemann AK, Kruger S, et al. Impact of herpes simplex virus detection in respiratory specimens of patients with suspected viral pneumonia. Infection. 2010;38:401–405. doi: 10.1007/s15010-010-0036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Engelmann I, Gottlieb J, Meier A, et al. Clinical relevance of and risk factors for HSV-related tracheobronchitis or pneumonia: results of an outbreak investigation. Critical Care. 2007;11:R119. doi: 10.1186/cc6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Apisarnthanarak A, Holzmann-Pazgal G, Hamvas A, et al. Antimicrobial use and the influence of inadequate empiric antimicrobial therapy on the outcomes of nosocomial bloodstream infections in a neonatal intensive care unit. Infect Control Hosp Epidemiol. 2004;25:735–741. doi: 10.1086/502469. [DOI] [PubMed] [Google Scholar]
- 141.Linssen CFM, Jacobs JA, Stelma FF, et al. Herpes simplex virus load in bronchoalveolar lavage fluid is related to poor outcome in critically ill patients. Intensive Care Medicine. 2008;34:2202–2209. doi: 10.1007/s00134-008-1231-4. [DOI] [PubMed] [Google Scholar]
- 142.Glaser R, Pearson GR, Jones JF, et al. Stress-related activation of Epstein-Barr virus. Brain, Behavior, and Immunity. 1991;5:219–232. doi: 10.1016/0889-1591(91)90018-6. [DOI] [PubMed] [Google Scholar]
- 143.Honda H, Krauss MJ, Coopersmith CM, et al. Staphylococcus aureus nasal colonization and subsequent infection in intensive care unit patients: does methicillin resistance matter? Infect Control Hosp Epidemiol. 2010;31:584–591. doi: 10.1086/652530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Drazin D, Hanna G, Shweikeh F, et al. Varicella-Zoster-Mediated Radiculitis Reactivation following Cervical Spine Surgery: Case Report and Review of the Literature. Case Rep Infect Dis. 2013;2013:647486. doi: 10.1155/2013/647486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Anderson MD, Tummala S. Herpes myelitis after thoracic spine surgery. J Neurosurg Spine. 2013;18:519–523. doi: 10.3171/2013.1.SPINE12960. [DOI] [PubMed] [Google Scholar]
- 146.Grauvogel J, Vougioukas VI. Herpes radiculitis following surgery for symptomatic cervical foraminal stenosis. Can J Neurol Sci. 2008;35:661–663. doi: 10.1017/s0317167100009501. [DOI] [PubMed] [Google Scholar]
- 147.Weiss R. Herpes zoster following spinal surgery. Clin Exp Dermatol. 1989;14:56–57. doi: 10.1111/j.1365-2230.1989.tb00884.x. [DOI] [PubMed] [Google Scholar]
- 148.Miller AE. Selective decline in cellular immune response to varicella-zoster in the elderly. Neurology. 1980;30:582–587. doi: 10.1212/wnl.30.6.582. [DOI] [PubMed] [Google Scholar]
- 149.Cohen JI, Brunell PA, Straus SE, et al. Recent advances in varicella-zoster virus infection. Ann Intern Med. 1999;130:922–932. doi: 10.7326/0003-4819-130-11-199906010-00017. [DOI] [PubMed] [Google Scholar]
- 150.Razonable RR, Fanning C, Brown RA, et al. Selective reactivation of human herpesvirus 6 variant a occurs in critically ill immunocompetent hosts.[see comment] Journal of Infectious Diseases. 2002;185:110–113. doi: 10.1086/324772. [DOI] [PubMed] [Google Scholar]
- 151.Tang Y-W, Johnson JE, Browning PJ, et al. Herpesvirus DNA Is Consistently Detected in Lungs of Patients with Idiopathic Pulmonary Fibrosis. J Clin Microbiol. 2003;41:2633–2640. doi: 10.1128/JCM.41.6.2633-2640.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Machens A, Bloechle C, Achilles EG, et al. Toxic megacolon caused by cytomegalovirus colitis in a multiply injured patient. J Trauma. 1996;40:644–646. doi: 10.1097/00005373-199604000-00023. [DOI] [PubMed] [Google Scholar]
- 153.Heininger A, Vogel U, Aepinus C, et al. Disseminated fatal human cytomegalovirus disease after severe trauma. Critical Care Medicine. 2000;28:563–566. doi: 10.1097/00003246-200002000-00046. [DOI] [PubMed] [Google Scholar]
- 154.Wildenauer R, Suttorp AC, Kobbe P. Cytomegalovirus colitis in an elderly polytraumatised patient. Dtsch Med Wochenschr. 2008;133:2383–2386. doi: 10.1055/s-0028-1100929. Cytomegalievirus-Kolitis bei einer polytraumatisierten alteren Patientin. [DOI] [PubMed] [Google Scholar]
- 155.Cheung AN, Ng IO. Cytomegalovirus infection of the gastrointestinal tract in non-AIDS patients. American Journal of Gastroenterology. 1993;88:1882–1886. [PubMed] [Google Scholar]
- 156.Chamberlain RS, Atkins S, Saini N, et al. Ileal perforation caused by cytomegalovirus infection in a critically ill adult. J Clin Gastroenterol. 2000;30:432–435. doi: 10.1097/00004836-200006000-00016. [DOI] [PubMed] [Google Scholar]
- 157.Page MJ, Dreese JC, Poritz LS, et al. Cytomegalovirus enteritis: a highly lethal condition requiring early detection and intervention. Dis Colon Rectum. 1998;41:619–623. doi: 10.1007/BF02235271. [DOI] [PubMed] [Google Scholar]
- 158.Murray RN, Parker A, Kadakia SC, et al. Cytomegalovirus in upper gastrointestinal ulcers. J Clin Gastroenterol. 1994;19:198–201. doi: 10.1097/00004836-199410000-00005. [DOI] [PubMed] [Google Scholar]
- 159.Blackman E, Vimadalal S, Nash G. Significance of gastrointestinal cytomegalovirus infection in homosexual males. Am J Gastroenterol. 1984;79:935–940. [PubMed] [Google Scholar]
- 160.Katzoli P, Sakellaris G, Ergazaki M, et al. Detection of herpes viruses in children with acute appendicitis. Journal of Clinical Virology. 2009;44:282–286. doi: 10.1016/j.jcv.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 161.Cook C, Zhang X, McGuinness B, et al. Intra-abdominal Bacterial Infection Reactivates Latent Pulmonary Cytomegalovirus in Immunocompetent Mice. Journal of Infectious Diseases. 2002;185:1395–1400. doi: 10.1086/340508. [DOI] [PubMed] [Google Scholar]
- 162.Frecentese DF, Cogbill TH. Spontaneous splenic rupture in infectious mononucleosis. Am Surg. 1987;53:521–523. [PubMed] [Google Scholar]
- 163.Rogues AM, Dupon M, Cales V, et al. Spontaneous splenic rupture: an uncommon complication of cytomegalovirus infection. J Infect. 1994;29:83–85. doi: 10.1016/s0163-4453(94)95195-0. [DOI] [PubMed] [Google Scholar]
- 164.Alliot C, Beets C, Besson M, et al. Spontaneous splenic rupture associated with CMV infection: report of a case and review. Scand J Infect Dis. 2001;33:875–877. doi: 10.1080/00365540110027114. [DOI] [PubMed] [Google Scholar]
- 165.Horwitz CA, Henle W, Henle G, et al. Clinical and laboratory evaluation of cytomegalovirus-induced mononucleosis in previously healthy individuals. Report of 82 cases. Medicine (Baltimore) 1986;65:124–134. doi: 10.1097/00005792-198603000-00005. [DOI] [PubMed] [Google Scholar]
- 166.Al-Tawfiq JA, Abed MS, Al-Yami N, et al. Promoting and sustaining a hospital-wide, multifaceted hand hygiene program resulted in significant reduction in health care-associated infections. Am J Infect Control. 2012 doi: 10.1016/j.ajic.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 167.Baumgartner JD, Glauser MP, Burgo-Black AL, et al. Severe cytomegalovirus infection in multiply transfused, splenectomized, trauma patients. Lancet. 1982;2:63–66. doi: 10.1016/s0140-6736(82)91688-9. [DOI] [PubMed] [Google Scholar]
- 168.Okun DB, Tanaka KR. Profound leukemoid reaction in cytomegalovirus mononucleosis. JAMA. 1978;240:1888–1889. [PubMed] [Google Scholar]
- 169.Langenhuijsen MM, van Toorn DW. Splenectomy and the severity of cytomegalovirus infection. Lancet. 1982;2:820. doi: 10.1016/s0140-6736(82)92707-6. [DOI] [PubMed] [Google Scholar]
- 170.Assy N, Gefen H, Schlesinger S, et al. Reactivation versus Primary CMV Infection after Splenectomy in Immunocompetent Patients. Digestive Diseases and Sciences. 2007;52:3477–3480. doi: 10.1007/s10620-006-9712-1. [DOI] [PubMed] [Google Scholar]
- 171.Forster MR, Trgovcich J, Zimmerman P, et al. Antiviral prevention of sepsis induced cytomegalovirus reactivation in immunocompetent mice. Antiviral Research. 2010;85:496–503. doi: 10.1016/j.antiviral.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cook C. Cytomegalovirus reactivation and mortality during critical illness: A $64,000 question. Critical Care Medicine. 2009;37:2475–2476. doi: 10.1097/CCM.0b013e3181ad932e. [DOI] [PubMed] [Google Scholar]
- 173.Cook CH, Trgovcich J. Cytomegalovirus reactivation in critically ill immunocompetent hosts: A decade of progress and remaining challenges. Antiviral Research. 2011;90:151–159. doi: 10.1016/j.antiviral.2011.03.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Camps, Camps K, Jorens, et al. Clinical Significance of Herpes Simplex Virus in the Lower Respiratory Tract of Critically Ill Patients. European Journal of Clinical Microbiology & Infectious Diseases. 2002;21:758–759. doi: 10.1007/s10096-002-0809-y. [DOI] [PubMed] [Google Scholar]
- 175.Tuxen DV. Prevention of lower respiratory herpes simplex virus infection with acyclovir in patients with adult respiratory distress syndrome. Chest. 1994;106:28S–33S. doi: 10.1378/chest.106.1_supplement.28s. [DOI] [PubMed] [Google Scholar]
- 176.Haik J, Weissman O, Stavrou D, et al. Is prophylactic acyclovir treatment warranted for prevention of herpes simplex virus infections in facial burns? A review of the literature. J Burn Care Res. 2011;32:358–362. doi: 10.1097/BCR.0b013e318217f6de. [DOI] [PubMed] [Google Scholar]
- 177.Bourdarias B, Perro G, Cutillas M, et al. Herpes simplex virus infection in burned patients: epidemiology of 11 cases. Burns. 1996;22:287–290. doi: 10.1016/0305-4179(95)00146-8. [DOI] [PubMed] [Google Scholar]


