Abstract
μ-Conotoxins block voltage-gated sodium channels (VGSCs) and compete with tetrodotoxin for binding to the sodium conductance pore. Early efforts identified μ-conotoxins that preferentially blocked the skeletal muscle subtype (NaV1.4). However, the last decade witnessed a significant increase in the number of μ-conotoxins and the range of VGSC subtypes inhibited (NaV1.2, NaV1.3 or NaV1.7). Twenty μ-conotoxin sequences have been identified to date and structure–activity relationship studies of several of these identified key residues responsible for interactions with VGSC subtypes. Efforts to engineer-in subtype specificity are driven by in vivo analgesic and neuromuscular blocking activities. This review summarizes structural and pharmacological studies of μ-conotoxins, which show promise for development of selective blockers of NaV1.2, and perhaps also NaV1.1,1.3 or 1.7.
Currently, there are approximately 128 peptide-derived drugs in various stages of clinical development [1]. In 2012 alone, six peptide drugs received FDA approval, making this class of compounds second only to small-molecule drugs in approvals granted during a given year [1]. Biologics such as peptides are rapidly gaining acceptance as viable therapeutic entities and, as the search to identify new drug leads continues [2], one source of bioactive peptides that has shown particular promise is the complex venom mixtures of predatory organisms. Venoms have evolved over millions of years as efficient mediators of defense, predation and competition. They are of interest to the pharmaceutical industry for their potential therapeutic benefits, resulting largely from the fact that the individual constituents are often highly potent ligands for specific subsets of key therapeutic targets (e.g., cell-surface receptors, ion channels and transporters). Out of the six FDA-approved drugs derived from venoms, four are of peptide origin: eptifibatide [3], bivalirudin [4], ziconotide [5] and exenatide [6]. Furthermore, approximately 20 additional venom-derived peptides are currently at various stages of clinical/preclinical development [7].
The venoms of marine snails of the genus Conus constitute an abundant source of neuroactive peptides [8–10]. Cone snails hunt by injection of a venom cocktail containing at least 100–200 bioactive peptides designed to rapidly immobilize prey or defend against predators [11,12]. The complexity of these venoms, combined with the large number of identified Conus species so far (500–700 species) [13], highlights the tremendous potential of these venoms as a source of pharmacological tools for the study or even treatment of numerous neurological disorders [14].
Conotoxins are broadly classified into 16 gene ‘superfamilies’ based on the endoplasmic reticulum (ER) signal peptide sequence [15]. Each superfamily is further subdivided according to disulfide bridging framework and/or pharmacological target [15]. The M-superfamily encompasses 10 distinct cysteine frameworks and at least four distinct molecular targets. Within this superfamily are two classes of peptides that inhibit voltage-gated sodium channels (VGSCs): the μ-conotoxins, which block Na+ conductance by direct occlusion of the VGSC pore [16], and the μO-conotoxins, which act as gating modifiers by binding to sites on the voltage-sensing domain on the extra-cellular surface of the VGSC [17,18]. This review will focus on the μ-conotoxins, thus far identified only in the venoms of piscivorous members of Conus. They are characterized by a Type III cysteine framework (i.e., CysI-CysIV, CysII-CysV, CysIII-CysVI) [16,19].
The sodium channel α-subunit (NaV1) is a large pore-forming protein complex comprised of four homologous domains arranged about a central pore region (D1–D4) [20]. Each domain comprises six membrane-spanning segments (S1–S6) with an extended extracellular looped region (P-loop) connecting segments 5 and 6 (Figure 1) [21]. To date, nine distinct isoforms of the α-subunit (NaV1.1 - NaV1.9) have been described in various excitable tissues of the CNS or PNS, where they have been shown to modulate signal transduction in neuronal, cardiac or skeletal muscle cell types (Table 1) [22]. The flux of sodium ions through VGSCs is responsible for both the initiation of action potentials and propagation of these signals along the length of the axon. Abnormal sodium conductance, through illness or injury, can lead to spontaneous firing of neurons and hyperexcitability, resulting in channelopathies associated with neuropathic pain [23] or other neurological disorders [24]. Channelopathies can also have the opposite effect and result in the inability to perceive painful stimuli in humans [25]. A 2006 Nature paper first described how a congenital loss-of-function mutation (i.e., nonsense-codon mutation) in the SCN9A gene that codes for the NaV1.7 VGSC subtype resulted in an individual’s inability to perceive pain [25].
Figure 1. Voltage-gated sodium channels structure.
(A) Crystal structure of the bacterial sodium channel NaVAb (PDB code 4EKW). Structure illustrates the four homologous domains of the channel (DI-DIV) arranged around the highly selective pore region through which Na+ permeates. (B) Individual domain comprising six membrane-spanning subunits (S1–S6) with the site of action (P-loop site 1) for μ-conotoxins discussed throughout this review [21]. (C) Cartoon of the VGSC α- and β-subunits. Selectivity filter is formed by the looped regions between S5 and S6 (i.e., P-loop). Approximate locations of neurotoxin-binding Sites 1-5 are shown on the α-subunit. Site 1, the location of μ-conotoxin binding, is emphasized. β-subunit crystal structure from Gilchrist et al. (PDB code 4MZ2) [26].
VGSC: Voltage-gated sodium channels.
Table 1.
Sodium channel subtypes and their distribution.
| NaV1-subtype† | Distribution | TTX sensitivity |
|---|---|---|
| NaV1.1 | DRG, CNS, Heart | × |
| NaV1.2 | DRG, CNS | × |
| NaV1.3‡ | DRG, CNS, PNS | × |
| NaV1.4 | Muscle | × |
| NaV1.5 | Heart | |
| NaV1.6‡ | DRG, CNS | × |
| NaV1.7 | DRG, SCG | × |
| NaV1.8 | DRG | |
| NaV1.9 | DRG |
To date, nine NaV1-subtypes have been identified in the central/peripheral nervous systems or in muscle tissues (skeletal or cardiac). Many subtypes have been observed in the DRG with six of these subtypes being sensitive to block by the alkaloid neurotoxin TTX [20,27].
In addition to NaV1.1 and 1.5 (as indicated above, NaV1.3 and 1.6 have also been observed in cardiac tissue).
DRG: Dorsal root ganglia; PNS: Peripheral nervous system; SCG: Superior cervical ganglion neurons; TTX: Tetrodotoxin.
Three VGSC subtypes have demonstrated roles in the transmission of pain signals: NaV1.3, NaV1.7 and NaV1.8 (Table 1) [28,29]. NaV1.3 expression is significantly increased in various neuropathic pain states, including nerve injury, spinal nerve ligation, postherpetic neuralgia and diabetic neuropathy [30–33]. NaV1.7 is an essential and nonredundant requirement for nociception in humans [25]. Selective NaV1.8 mRNA axonal transport and local upregulation may contribute to the hyperexcitability of peripheral nerves in some neuropathic pain states [34]. The NaV1.9 subtype has also been suggested as a potential target for pain. However, this subtype is localized to the PNS and NaV1.9 inhibitors may be more useful as treatments of inflammatory rather than neuropathic pain [28,35–36]. Taken together, these results provide support for the use of VGSC inhibitors that modulate neuronal excitability and could prove useful in the development of new analgesic compounds [22,37–40].
The alkaloid neurotoxins tetrodotoxin (TTX) and saxitoxin (STX) are potent inhibitors of VGSCs and were integral in defining neurotoxin binding site 1, a binding site deep inside the VGSC pore within the re-entrant P-loop region between S5 and S6 (Figure 1) [20,41]. However, TTX and STX have shown limited therapeutic potential due to their lack of selectivity among NaV1 subtypes (TTX-sensitive subtypes include NaV1.1–1.4, 1.6 and 1.7) [42–44]. Subtypes that are less sensitive to the effects of TTX are called ‘TTX resistant’ (NaV1.5, 1.8 and 1.9). To mitigate potential adverse side effects arising from the lack of subtype specificity of TTX (e.g., cognitive effects, paralysis, ataxia), clinical investigations of TTX have been limited to focal (intramuscular) administration [37,45]. Several small molecule inhibitors of NaV1-subtypes have also been described, but often lack target specificity leading to undesirable off-target effects (Figure 2). A potential advantage of peptide-based therapeutics is their intrinsic specificity for certain classes of molecular targets [27,46,47]. This specificity continues to be a key driver of studies of Conus venoms that have led to characterization of the μ-conotoxins.l.
Figure 2. Examples of small molecule inhibitors of voltage-gated sodium channels.
†Indicates clinically used voltage-gated sodium channels.
Data taken from [27].
Numerous venom-derived neurotoxins elicit their biological effects through interaction at discrete sites within the α-subunit of the VGSC [48] (Figure 1C). Venom peptides have been shown to act at Site 1 (μ-conotoxins and nonpeptidic guanidinium toxins), Site 3 (scorpion α-toxins and anemone toxins), Site 4 (scorpion β-toxins, spider β-toxins and μO-conotoxins) and Site 6 (δ-conotoxins), while Sites 2 and 5 are targeted predominantly by small organic neurotoxins such as the batrachotoxins and breve-toxins [49]. Peptide components of non-Conus origin have also been shown to block NaV1, though the site of action of many of these toxins has yet to be fully defined. Among these are Tx1, hainantoxin-I and ProTx-II (which is of interest because of its subtype selective block of NaV1.7) [39–40,50].
The μ-conotoxins are employed as paralytic tools by fish-hunting marine gastropods from the genus Conus. Over the nearly three decades since the first reports of μ-conotoxins isolated from the venom of Conus geographus (μ-GIIIA/B/C), which preferentially blocked muscle subtypes, 17 distinct μ-conotoxins have been identified (Figure 3). More recently, μ-conotoxins with preference for neuronal subtypes were reported [51]. These peptides modulate the activity of NaV1-subtypes by binding at the outer vestibule at Site 1 of the sodium channel pore, in many cases with submicromolar affinities [52,53]. This review summarizes the efforts to define the structural features of μ-conotoxins responsible for their potency and selectivity for NaV1- subtypes as a basis for the development of both novel pharmacological tools and potential therapeutics.
Figure 3. The μ-conotoxins as an emerging class of sodium channel blocking peptides.
Timeline illustrates the relative discoveries and/or characterization of the members of this family.
Data taken from [53].
Muscle-subtype preferring μ-conotoxins
μ-Conotoxins from Conus geographus
The first μ-conotoxins to be isolated and characterized were μ-GIIIA and its congeners μ-GIIIB and μ-GIIIC, from the venom of C. geographus (Figure 4) [54]. Early work showed, for the first time, that μ-conotoxin GIIIA could discriminate between sodium channels isolated from muscle (Kd= 25 nM) and nerve (Kd ~ 11,000 nM) preparations [54,55]. This information was among the first to suggest that discrete VGSC isoforms existed within different excitatory tissue types. Recent work by Wilson et al. [53] established the selectivity profile for μ-GIIIA in Xenopus oocytes expressing rat or mouse NaV1-subtypes (Figure 5). Consistent with previous results, μ-GIIIA showed the highest potency for the skeletal muscle subtype NaV1.4 (0.019 μM), followed by the neuronal NaV1.1 (0.26 μM), subtypes NaV1.6 (0.68 μM) and NaV1.2 (17.8 μM) [53]. μ-GIIIA is a highly basic, 22 amino acid residue peptide that is stabilized by three disulfide bridges (Cys3–Cys15; Cys4–Cys20; Cys10–Cys21). Ala-replacement studies showed that the most critical residues for VGSC blockade are localized in the C-terminal half of the peptide (Arg13, Arg19, Hyp17 and Lys16) (Figure 6) [56]. The solution structure of the μ-GIIIA[R13A] mutant showed that these residues were located on the same face of the molecule, suggesting that this region of the peptide may interact directly with the channel [57]. The structure of μ-GIIIA also revealed the close proximity of the hydroxyl group of Hyp17 to the guanidino group of Arg13, which led to the hypothesis the μ-GIIIA interacted with NaV1 subtypes in a similar manner to the guanidinium toxins at Site 1 [57]. Yanagawa et al. [58], showed that μ-GIIIA inhibited the VGSC binding of 3H-Lys-TTX, and conversely, that 3H-Pr-GIIIA prevented the binding of both TTX and STX, providing strong evidence that μ-GIIIA and the guanidinium toxins compete for the same binding site on muscle NaV1 [55,58].
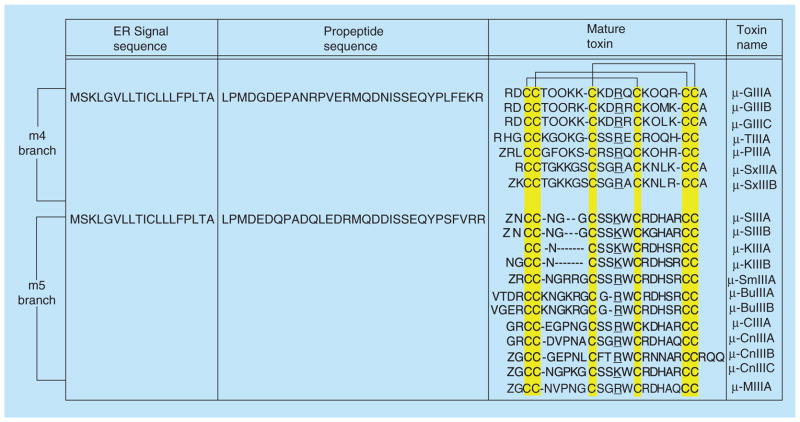
Figure 4. Summary of identified μ-conotoxins from m4 and m5 branches of the M-superfamily.
ER signal and propeptide sequences for μ-GIIIA and μ-SIIIA are illustrated as examples. ‘O’ denotes hydroxyproline; ‘Z’ denotes pyroglutamic acid. Arg13 in μ-GIIIA, or the residue in the equivalent position, is underlined to illustrate the sequence differences among conotoxins that preferentially block muscle versus neuronal subtypes.
ER: Endoplasmic reticulum
Figure 5. Selectivity profiles of μ-conotoxins against NaV1-subtypes.
Data were obtained from reported IC50values; *represents data obtained from pKd values [53]. Pain-relevant subtypes are highlighted in red. Broken bars represent values greater than 100 μM.
For color images please see online http://www.future-science.com/doi/full/10.4155/FMC.14.107
Figure 6. Structure–activity characterization of μ-conotoxins.
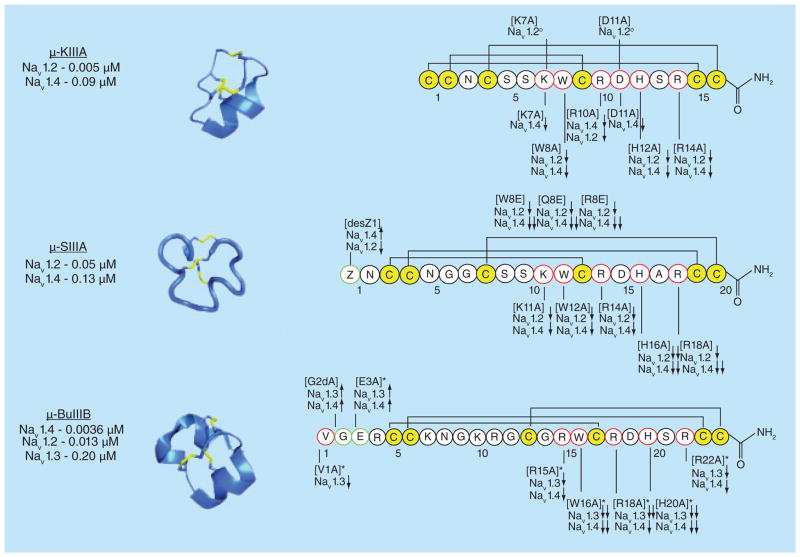
Potencies of characterized μ-conotoxins against skeletal (NaV1.4) and neuronal (NaV1.2) subtypes. Solution structures showing the folded peptide structures with disulfide connectivity. PDB ID# 1TCK (μ-GIIIA), 1R9I (μ-PIIIA), 1Q2J (μ-SmIIIA), 2LXG (μ-KIIIA) and 2LO9 (μ-BuIIIB). BMRB Entry# 20024 (μ-TIIIA) and 20023 (μ-SIIIA). Results of individual amino acid replacements on NaV1-subtype blockade. (↑) indicates mutations that improve potency against NaV1-subtypes, (↓) denotes decreased potency and (°) represents no change in potency. (desZ1) indicates deletion of pyroglutamic acid in position 1 of μ-SIIIA.
μ-Conotoxins from Conus purpurascens
More than a decade after the first report of μ-conotoxins from C. geographus, another 22-residue peptide sharing an identical Cys framework, μ-PIIIA, was identified from the venom of C. purpurascens (Figure 4) [60]. As with μ-GIIIA, this toxin showed a strong preference for muscle NaV1 subtypes (IC50= 44 nM) over those from brain (IC50 = 640 nM). In contrast to μ-GIIIA, μ-PIIIA exhibited nearly irreversible block of NaV1-subtypes [60]. The solution structure of μ-PIIIA confirmed the close proximity of the guanidino group of Arg14 to the hydroxyl group of Hyp18, providing further support for the proposed interactions between μ-GIIIA/PIIIA and Site 1 of the muscle VGSC subtype [61]. Like μ-GIIIA, μ-PIIIA exhibited the highest potency against NaV1.4 (0.036 μM), although affinities for other VGSC subtypes in the central (CNS) and peripheral (PNS) nervous systems were on average higher than those for μ-GIIIA (Figure 5) [53]. Because of the strong preference of μ-conotoxins μ-GIIIA/B/C and μ-PIIIA for muscle over neuronal subtypes, these peptides have also served as valuable tools for studying the VGSC isoform selectivity and function of skeletal muscle VGSCs [62].
μ-Conotoxins from Conus stercusmuscarum
μ-SmIIIA was predicted from the cDNA library from the venom of C. stercusmuscarum in 2002 [63]. μ-SmIIIA was shown to block Na+ current in sympathetic neurons (TTX-resistant NaV1 channels) with near irreversibility (for 0.5 μM μ-SmIIIA, kobs = 0.24 min−1) [63]. Notable differences between μ-SmIIIA and previously described μ-conotoxins included only modest noncysteine sequence homology and the presence of Trp15 in the second intercysteine loop (Figure 4). The solution structure of μ-SmIIIA showed distinct differences from the μ-conotoxins of C. geographus both in its topology and the positioning of certain amino acid residues; it was hypothesized that these structural differences contributed to the block of TTX-resistant NaV1-subtypes by μ-SmIIIA [64]. This was supported by SmIIIA-PIIIA chimeras, which retained activity against amphibian TTX-resistant channels [64]. Block of TTX-resistant channels in amphibian preparations did not, however, translate to similar activities in mammalian channels [63]. Despite low nanomolar potency for NaV1.4 (0.22 nM), μ-SmIIIA exhibited relatively poor selectivity among NaV1-subtypes in the CNS and PNS (NaV1.1, 3.8 nM, NaV1.2, 1.3 nM, NaV1.3, 35 nM) (Figure 5). Moreover, μ-SmIIIA exhibited low micromolar block of the cardiac subtype NaV1.5 [53], ruling it out as a viable candidate for therapeutic development.
μ-Conotoxins from Conus striolatus
The μ-conotoxins SxIIIA and SxIIIB, reported by Walewska et al. [65], also inhibited the skeletal muscle subtype preferentially (NaV1.4 = 0.007 μM; NaV1.2 = 1 μM) [53]. Among the conotoxins that preferentially target this subtype, there appears to be a pattern of hydroxyproline residues in the first and third intercysteine loops that is absent in μ-SxIIIA/B (Figure 4). Similarly, among conotoxins that block neuronal subtypes there is a high degree of sequence homology within the third intercysteine loop. These peptides also possess a Trp residue at an equivalent position, which is absent in μ-SxIIIA/B (Figure 4). Intraperitoneal or subcutaneous injection of μ-SxIIIA resulted in paralysis in mice [65]. Furthermore, SxIIIA was found to block NaV1.4, expressed in Xenopus oocytes, with low nanomolar potency (IC50 = 7 nM) [65]. The selectivity profile for μ-SxIIIA was later established as: NaV1.4 ≫ 1.1 > 1.6 > 1.2 ≫ all other subtypes (Figure 5) [53]. Notably, μ-SxIIIA was used as a model peptide for NMR-based mapping of disulfide connectivity [65]. Cysteines I–III (with respect to the disulfide framework) of μ-SxIIIA were labeled with 100% 15N/13C, while the remaining Cys-residues were labeled with a mixture of 70% (14N/12C):30% (15N/13C). This allowed for 3D triple-resonance NMR experiments to determine disulfide connectivity from Cys Hα/Hβ2/Hβ3 NOESY cross peaks.
μ-Conotoxins from Conus tulipa
μ-TIIIA from C. tulipa potently inhibited both rNaV1.2 and rNav1.4 expressed in Xenopus oocytes (Kd = 45 and 5 nM, respectively) [53,66] (Figure 5), but showed little effect on isolated neurons from rat dorsal root ganglion (DRG). Alanine-replacement studies on μ-TIIIA showed that mutation of His2, Glu15 or Gln19 increased block of NaV1 subtypes, whereas replacement of Lys6, Lys9, Arg17 and His20 reduced block (Figure 6). The μ-TIIIA[E15A] mutant not only improved block but also switched subtype preference in favor of the neuronal subtype [66]. It was suggested that the negative charge at this position created an unfavorable interaction with the binding site within the sodium channel [66]. More recent work has shown that addition of either a neutral residue (μ-TIIIA[E15A, 23A]) or basic residue (μ-TIIIA[E15A, 23K]) to the C-terminus decreased overall potency for all subtypes, but increased preference for NaV1.2 [67]. Based on these findings, further positional scanning of Glu15 would be of interest to explore whether the selectivity profiles of μ-TIIIA could be shifted in favor of pain-relevant NaV1-subtypes.
Neuronal-subtype preferring μ-conotoxins
μ-Conotoxins from Conus kinoshitai
μ-KIIIA and μ-KIIIB are the shortest members of the μ-conotoxin family reported to date (Figure 4). In contrast to previously described μ-conotoxins, μ-KIIIA/B, preferentially blocked neuronal NaV1 subtypes [51]. μ-KIIIA also showed significant differences in amino acid composition and intercysteine loop sizes compared with muscle subtype preferring μ-conotoxins (Figure 4). Another notable difference was the substitution of the typically conserved Arg residue in loop 2 with Lys. These toxins also have a Trp residue within the second intercysteine loop and more closely resembled μ-SmIIIA in the third loop. cDNA sequence data also identified a peptide with an extended N-terminus, μ-KIIIB [68]. The major folding isoforms for the short (μ-KIIIA) and extended (μ-KIIIB) peptides exhibited comparable activities when tested in Xenopus oocytes expressing NaV1.2 [68,69]. As all available structure–activity relationship (SAR) data were obtained from the N-terminally truncated peptide, this review will focus on analogs of μ-KIIIA rather than μ-KIIIB. μ-KIIIA exhibited nearly 17-fold preference for neuronal over skeletal muscle subtypes (Kd rNaV1.2 = 0.003 μM and Kd rNaV1.4 = 0.05 μM) [69], and Ala-walk studies showed that four residues within loops 2 and 3 contributed to its NaV1.2 preference, namely, Trp8, Arg10, His12 and Arg14 [69]. Furthermore, block of the muscle subtype was decreased by Ala replacement of Lys7, Trp8, Arg10, Asp11, His12 and Arg14 (Figure 6).
Arg13 in μ-GIIIA and Arg14 in μ-PIIIA were shown to be crucial for the inhibition of skeletal muscle NaV1-subtypes, with the conservative analog μ-GIIIA[R13K] showing 10-fold lower potency for skeletal muscle subtypes [70]. Intriguingly, the neuronal-preferring conotoxins such as μ-SIIIA or μ-KIIIA possess a lysine at the equivalent position [51], and SAR studies of μ-KIIIA showed that mutations at this position (Lys7) could influence NaV1.2 subtype preference [51]. μ-KIIIA[K7A] retained similar activity to μ-KIIIA against rNaV1.2, but showed reduced activity against rNaV1.4, thereby increasing the selectivity window [69]. This was also shown in the block of NaV1.2 by the μ-KIIIA[K7Nle] analog, which more closely mimicked the steric bulk of a lysine residue at position 7 (block = 83 vs 33%) [69]. McArthur et al. recently showed that Ala substitution of either Lys7 or Arg10 led to lower Kd values and decreased maximal block, but retained the wild-type selectivity profile (NaV1.2 > 1.4 > 1.7), while replacement of either His12 or Arg14 decreased potency and altered the NaV1 selectivity profiles in favor of pain-relevant subtypes (μ-KIIIA[H12A] – NaV1.2 > 1.7 > 1.4 and μ-KIIIA[R14A] – NaV1.7 > 1.2 > 1.4) [71]. Taken together, these results demonstrated that Lys7 was important for both potency and efficacy of block by μ-KIIIA.
Analogs of μ-KIIIA were also constructed that incorporated nonnatural N-substituted Gly monomers (e.g., N-methylglycine, N-butylglycine and N-octoylglycine) at position 7 to explore the effects on NaV1 affinity (Kd or IC50) and efficacy (% block) [72]. These analogs retained the ability to block NaV1.2, although both the kinetics of block and affinities were affected. The N-methylglycine substituted peptide was the most potent of these analogs tested. Importantly, this peptide also exhibited the largest residual Na+ current of the series demonstrating a correlation between the size of the R-group of the substituted monomer and efficacy [72].
Mutations at Trp8 led to increased discrimination between neuronal and muscle subtypes. Replacement by Ala or Leu decreased the maximal block of both NaV1.2 and NaV1.4 (50 and 19% decrease, respectively). These substitutions also affected the kinetics of block, where kobs for NaV1.2 was significantly increased and the rate of block of NaV1.4 was essentially unchanged [69]. Van Der Haegen et al. replaced Trp8 with Arg, Gln or Glu to explore the electronic effects of varying charge at this position on NaV1 inhibition [73]; in all instances, NaV1 potency was decreased. More importantly, mutation of Trp8 resulted in increased selectivity for neuronal sodium channel subtypes through greater reduction in rNaV1.4 potency. This work also showed that substitution of Trp8 with less hydrophobic amino acid residues, regardless of charge, increased the reversibility of blockade [73].
Ala-substitution of basic residues (Lys7, Arg10, His12 and Arg14) influenced the affinity of and/or block by μ-KIIIA (Figure 6) [71]. Ala-substitution of His12 further improved the selectivity profile in favor of NaV1.2, followed by NaV1.7, and both were significantly preferred over NaV1.4. A similar effect was observed in μ-KIIIA[R14A], with a complete shift in the selectivity profile (NaV1.7 > NaV1.2 > NaV1.4). Alignment of the sequences of NaV1.2, NaV1.4 and NaV1.7 subtypes showed that binding specificity was dependent upon interactions between Arg14 and Asp1241 of domain III of the outer ring of the P-loop. By mutating Arg14 to Ala, tight interactions with NaV1.2 and NaV1.4 were disrupted; it was hypothesized that the slow off-rates of μ-KIIIA (koff = 0.002 min−1) were a consequence of these interactions [71]. Inspection of the hNaV1.7 sequence revealed that the equivalent position within the P-loop of the channel contained Ile (Ile1410) rather than the Asp observed in the neuronal and skeletal muscle subtypes. Using this information, future analogs may offer better discrimination between NaV1 subtypes.
Disulfide-deficient KIIIA analogs were constructed based on the ‘canonical’ μ-conotoxin framework CysI–CysIV, CysII–CysV, CysIII–CysVI (Figure 4) [74]. Electrophysiology experiments revealed that the CysII–CysV and CysIII–CysVI disulfide pairs were most critical for NaV1-blockade [74]. The solution structures of these analogs showed that removal of the CysI–CysIV bridge resulted in greater N-terminal flexibility, but, did not disrupt the α-helical C-terminal region known to be important for biological activity [75]. Based on these findings, the [desC1]KIIIA[S3/4Aopn, C9A] analog was constructed, which lacked the CysI–CysIV bridge and replaced less-critical amino acid residues with an Aopn (5-amino-3-oxapentanoic acid) backbone spacer unit. This analog not only blocked NaV1.2 with low nanomolar potency, but also exhibited analgesic activity in a mouse model of inflammatory pain [74]. Recently, it has been shown that the disulfide connectivity for synthetic μ-KIIIA is different from that of previously described μ-conotoxins [68,76]. These results are discussed in greater detail below.
μ-Conotoxins from Conus striatus
μ-SIIIA and μ-SIIIB from the venom of C. striatus [51,77] differ from one another by only two amino acid residues within the third intercysteine loop (Arg14 and Asp15 in μ-SIIIA vs Lys14 and Gly15 in μ-SIIIB) (Figure 4). In Xenopus oocytes expressing NaV1-subtypes, μ-SIIIA displayed a threefold preference for NaV1.2 over NaV1.4 (0.05 vs 0.13 μM) [53]. Greater subtype discrimination was achieved through removal of the N-terminal pyroglutamate residue. For μ-SIIIA(2-20), affinity for NaV1.2 was increased while that for NaV1.4 was decreased, while the opposite was true for μ-SIIIB [77]. The importance of individual residues was assessed in μ-SIIIA(2-20) using an 125I-TIIIA displacement assay against NaV1.2 and NaV1.4. Similar to μ-KIIIA, residues in the C-terminal helix were found to be most important for VGSC blockade. Alanine replacement of Lys11, Trp12, Arg14, His16 or Arg18 leads to the most pronounced effects on VGSC binding, with the majority of these mutations retaining preference for the neuronal subtype (Figure 6). Importantly, μ-SIIIA[H16A] exhibited a significant decrease in binding affinity for both neuronal and muscle subtypes, suggesting that this residue contributes to general NaV1 binding.
Yao et al. undertook detailed structural analyses of μ-conotoxins that blocked neuronal VGSC subtypes (μ-SmIIIA, μ-SIIIA and μ-KIIIA) [78]. These peptides shared near sequence identity at the C-terminus but differed significantly from the C-termini of skeletal muscle preferring μ-conotoxins. In μ-SIIIA, residues 11–16 formed a well-defined helix, while residues 3–5 associated to form a 310-helix [78]. Dynamics measurements of μ-SIIIA made using 13C NMR relaxation experiments showed that the N-terminus and Ser9 had larger magnitude motions on the subnanosecond timescale, while the C-terminus was more rigid. These data were interesting in light of functional data which suggested that the N-terminus was important for subtype selectivity, while the C-terminus contributed mainly to neuronal subtype interactions [78]. Schroeder et al. explored the structural and functional consequences of N-terminal modification of μ-SIIIA/B [67]. Replacement of the N-terminal pyroglutamate (Pyr1 or single letter code Z) with charged residues (i.e., μ-SIIIA[Z1R] and μ-SIIIB[Z1E]) either decreased (μ-SIIIA) or increased (μ-SIIIB) preference for NaV1.2. Greater selectivity could also be obtained through extension of the N-terminus. The role of negatively-charged residues at the N-terminus for NaV1.2 subtype selectivity was further demonstrated by the μ-SIIIB analogs μ-SIIIB[E0, Z1R] and μ-SIIIB(22–0)[N2E], respectively. Extension of the C-terminus of μ-SIIIA[D15A], using charged amino acids, afforded greater selectivity for the neuronal subtype [67]; specifically, the μ-SIIIA analogs μ-SIIIA(2-21)[D15A, 21K] and μ-SIIIA(2-21) [D15A, 21D] showed improved discrimination between NaV1.2 and NaV1.4 [67].
The C-terminal regions of neuronal subtype preferring μ-conotoxins are highly homologous. However, the N-termini of these peptides have a decreasing number of residues in the first intercysteine loop across the series μ-SmIIIA > μ-SIIIA > μ-KIIIA. We previously investigated this region as a potential site for modification by flexible backbone spacer units such as 6-aminohexanoic acid (Ahx) and 3-oxapentanoic acid (PEG) with the goal of creating polymer-peptide hybrid analogs of μ-SIIIA with analgesic activity (e.g., polytides) [79]. At 25 μM, backbone spacer containing analogs of μ-SIIIA demonstrated approximately 80% block of TTX-sensitive sodium currents from mouse DRG neurons, compared with 65% block by wild-type μ-SIIIA. The μ-SIIIA polytide analogs produced not only increased block but also faster onset of action compared with the native peptide. These results provided further evidence that the μ-conotoxin scaffold could accommodate significant structural modifica-tions and lent support to the concept of peptide engineering to enhance pharmacological properties. This work also demonstrated that μ-SIIIA analogs were potent analgesics in an in vivo pain model, with the most promising of the analogs (PEG-SIIIA) having higher potency of 0.05 mg/kg (μ-SIIIA = 0.9 mg/kg) following intraperitoneal administration [79].
μ-Conotoxins from Conus consors, Conus catus & Conus magus
In addition to a high degree of homology among the C-termini of μ-CnIIIA, μ-CnIIIB, μ-CIIIA and μ-MIIIA, these peptides also possess a characteristic Pro-Asn dipeptide in the first intercysteine loop (Figure 4) [80]. These peptides blocked sodium conductance of TTX-resistant NaV1 in frog DRG neurons with varying potency and selectivity, with μ-CnIIIA (87% block – neuronal subtype selective) and μ-CIIIA (96% block – nonselective) being the most potent of this group. However, when tested in a mammalian system (mouse), μ-CnIIIA and μ-CIIIA blocked only weakly and lacked significant subtype selectivity [80]. μ-CnIIIA was nearly equipotent against neuronal and skeletal muscle subtypes (0.25 vs 0.27 μM). Likewise, μ-MIIIA showed poor discrimination between these two subtypes (0.45 vs 0.33 μM) (Figure 5) [53].
Recently, another μ-conotoxin from C. consors was reported by Favreau et al. [81]. This peptide was interesting in that the N-terminus of μ-CnIIIC was homologous to μ-TIIIA (loop 1), whereas the C-terminus resembled neuronal subtype-preferring cono-toxins such as μ-KIIIA and μ-SIIIA in that μ-CnIIIC possesses a lysine in the equivalent position to Arg14 of μ-GIIIA (Figure 4). This peptide blocked NaV1.2 (IC50 =1.3 nM) and NaV1.4 (100% block at 1 μM) with little or no effect on cardiac subtypes in HEK 293 cells. μ-CnIIIC also blocked the seemingly disparate nicotinic acetylcholine receptors (nAChR) α3β2 subtype and inhibited global nerve action potentials from sciatic and olfactory nerves with slow reversibility [81]. The authors suggested that the effects of μ-conotoxins on nAChR targets responsible for the modulation of pain signals may be more widespread than originally thought, perhaps offering a potential new molecular target for these potent analgesic compounds [81].
μ-Conotoxins from Conus bullatus
Three μ-conotoxins (μ-BuIIIA, μ-BuIIIB and μ-BuIIIC) from the venom of C. bullatus blocked NaV1.4 with near irreversibility [59]. These peptides had an extended N-terminus and a shorter second intercysteine loop compared with previously described μ-conotoxins (Figure 4). Wilson et al. determined the subtype-selectivity profiles for μ-BuIIIA and μ-BuIIIB and confirmed that NaV1.4 was the preferred target, [53]. followed by NaV1.2 and NaV1.3 (Figure 5)
Thus far, the μ-conotoxins in this review have been compared for their abilities to block either neuronal (NaV1.2) or skeletal muscle (NaV1.4) subtypes. However, μ-BuIIIB is also a potent inhibitor of NaV1.3 (Figure 5) [53]. The exact pharmacological role of this subtype remains to be confirmed, but studies by Haines et al. demonstrated upregulation of NaV1.3 following peripheral nerve axotomy, implicating this subtype as a potential target for the treatment of neuropathic pain [82,83]. Moreover, there are currently no selective probes of this channel subtype, making μ-BuIIIB an attractive lead compound to study NaV1.3. Recent studies have focused on determination of interactions between μ-BuIIIB and NaV1.3 [84,85]. The solution structure was determined by Kuang et al. [84] and it was hypothesized that the signature characteristics (an N-terminal extension and differences in intercysteine loop sizes) might contribute to the block of NaV1.3 [84].
Ala-replacement of the N-terminal residues of μ-BuIIIB influenced its potency but not its selectivity (Figure 6). Substitution of either Gly2 or Glu3 resulted in significant improvements to NaV1.3 potency. Importantly, substitution of Gly2 with D-Ala resulted in a μ-BuIIIB analog with greater than 40-fold higher potency for NaV1.3, presumably through stabilization of a type II β-turn at the N-terminus [84]. Interestingly, complete removal of the N-terminal extension still resulted in a fourfold improvement in the block of NaV1.3 over wild-type μ-BuIIIB [84]. Despite the gains in potency, selectivity for NaV1.3 was not generated. These observations suggested, however, that further SAR studies of μ-BuIIIB would be valuable in order to identify the key structural components involved in NaV1.3 blockade.
Detailed SAR of μ-BuIIIB has been largely inhibited by synthetic inaccessibility owing to its propensity to form numerous folding isoforms during oxidative folding. A strategy was therefore devised that directed folding toward a single bridging pattern through incorporation of a diselenide bridge and disulfide bridge removal (Figure 7). The rationale for this strategy lies in two previous reports on μ-conotoxin KIIIA [72,74]. A disulfide-deficient, diselenide-containing scaffold was constructed in which Cys5 and Cys17 were replaced with selenocysteine and the Cys6–Cys23 pairing was removed by alanine substitution (i.e., ddSecBuIIIB) [85]. These studies provided valuable insight into which amino acids contributed to block of the NaV1.3 subtype. Similar to the other μ-conotoxins, residues near the C-terminus of μ-BuIIIB were most important for NaV1 block (Figure 6). In ddSecBuIIIB, the aromatic residues Trp16 and His20 were shown crucial for NaV1.3 inhibition, with alanine-substituted analogs having Kd values of >30 μM each, compared with 0.2 μM for wild-type μ-BuIIIB [85].
Figure 7. Description of the ddSec strategy used to facilitate structure–activity relationship studies of μ-BuIIIB.
(A) Cartoon depiction of μ-BuIIIB showing disulfide connectivity between cysteine thiols. (B) Cartoon of ddSecBuIIIB scaffold with disulfide depletion by removal of the Cys6–Cys23 bridge and diselenide bridge formation by selenocysteine replacement of Cys5 and Cys17. (Panels C and D) Upper and lower chromatograms show linear and folded elution patterns of μ-BuIIIB (C) and ddSecBuIIIB (D), respectively. *Indicates the properly folded isoform. Positional scanning of all noncysteine residues was subsequently performed using the ddSecBuIIIB scaffold. Reproduced with permission from [85] © 2014 FEBS.
The highly basic character of μ-conotoxins is thought to contribute to their activity in the negatively charged pore of VGSCs. Early reports highlighted the critical role of a single basic residue near the C-terminus of μ-conotoxins (e.g., [μ-GIIIA[Arg13]) for VGSC blockade [56,57]. Ala substitution of Arg15, Arg18 or Arg22 in ddSecBuIIIB resulted in Kd values of 1.84, 15.7 and 3.81 μM, respectively [85]. Interestingly, the most ‘critical’ of these basic residues was not located at the position equivalent to Arg13 in μ-GIIIA. Instead, multiple basic residues were shown to be important for activity (Figure 6), consistent with models previously constructed by McArthur et al. using μ-PIIIA [71].
The ddSecBuIIIB analog also served to identify residues for which Ala-substitution did not greatly affect biological activity (e.g., Val1, Gly2, Asn8, Gly14 and Ser21), suggesting these positions as possible sites for chemical modification to achieve enhanced pharmacological/physicochemical properties [85]. The most striking outcome of these studies was that the substitution of Glu3 increased NaV1.3 potency (Kd = 0.07 μM). These findings were in agreement with the report by Kuang et al., which suggested that the negative charge of Glu3 may result in unfavorable interactions with NaV1.3 [84]. Current efforts are directed at modifying the above-mentioned positions, particularly position 3, with the goal of developing potent and selective inhibitors of NaV1.3.
Disulfide connectivity in μ-conotoxins
Disulfide bridges serve to stabilize the global structure of conotoxins [86]. With three disulfide pairs, μ-conotoxins have the potential to form up to 15 distinct structural isomers. Some of these conformations will be naturally selected against because they are energetically disfavored, but ‘noncanonical’ iso-forms do occur and may be observed in some abundance even upon reaching folding equilibrium. Following oxidation of the fully reduced peptide, the species found in highest abundance within the folding mixture has traditionally been assumed to be the native fold, although this has not been confirmed in most cases.
Until recently, the disulfide connectivity of all NaV1-targetting μ-conotoxins within the m4 and m5 branches of the M-superfamily (Figure 4) was assumed to be the same (CysI–CysIV; CysII–CysV; CysIII–CysVI). A recent report by Tietze et al. showed that three major folding isoforms, with differing disul-fide connectivities, resulted from the direct oxidation of synthetic μ-PIIIA, namely, μ-PIIIA-1 (CysI–CysV; CysII–CysVI; CysIII–CysIV), μ-PIIIA-2 (CysI–CysIV; CysII–CysV; CysIII–CysVI) and μ-PIIIA-3 (CysI–CysII; CysIII–CysIV; CysV–CysVI) [87]. All three isoforms were shown to block NaV1.4, albeit with different potencies: IC50μ-PIIIA-1 = 46.7 nM, IC50μ-PIIIA-2 = 103.2 nM and IC50μ-PIIIA-3 = 203.7 nM [87]. These results showed that the ‘canonical’ disulfide bridging pattern for μ-PIIIA did not result in the most potent folding isoform [87]. Molecular dynamics simulations of each isoform in this study showed that the side-chain of Arg14 in μ-PIIIA-1 penetrated deeper into the binding pore, presumably contributing to the increased potency of this isomer [87].
Another study re-examined the ‘canonical’ μ-framework of μ-KIIIA [76]. Poppe et al. noted that, because through-bond scalar couplings between the two sulfur atoms are undetectable by NMR, interactions are typically inferred based on interproton distance constraints. To circumvent this problem, they developed a method known as the pattern of disulfides from local constraints (PADLOC) and suggested an alternative connectivity for the native fold of μ-KIIIA (CysI–CysV; CysII–CysIV; CysIII–CysVI) [76]. This was recently confirmed by Khoo et al., who isolated the major folding oxidation products of synthetic μ-KIIIA and studied connectivity by collision-induced dissociation MS [68,88]. This confirmed the connectivity of the major folding isoform and determined the connectivity of the isomer in the next highest abundance as CysI–CysVI; CysII–CysIV; CysIII–CysV, neither of which matched the assumed connectivity for μ-conotoxins reported previously. Whether these connectivities are a consequence of the unique structural features of this peptide (μ-KIIIA has only a single residue between CysII and CysIII), or an indication of greater toxin diversity within the μ-conotoxin family remains to be determined. An intriguing possibility is that Conus produces multiple active folding isoforms to increase its toxin repertoire.
A recent study by Safavi-Hemami et al. [89] provided evidence for the presence of both the globular (CysI–CysIII; CysII–CysIV) and ribbon (CysI–CysIV; CysII–CysIII) forms of α-conotoxin ImI in the venoms of Conus imperialis [89]. Pharmacological studies have traditionally focused on the activities of the globular form, assuming this to be the native disulfide connectivity. However, the authors point out that in numerous cases (e.g., α-AuIB), it is actually the ribbon form that elicits the greatest biological effect [89,90]. Although these studies emphasized the presence of multiple folding isoforms of α-conotoxins, it is conceivable that multiple ‘noncanonical’ isomers of μ-conotoxins are also present in venom. In light of this, an in-depth re-examination of μ-conotoxin connectivities would be beneficial, particularly for the remaining members of the m4 branch and for toxins belonging to the more recently described m5 branch.
Influence of NaVβ subunit expression on μ-conotoxin activities
In vivo, VGSCs comprise both a pore-forming α-subunit (260 kDa) and one or more accessory sodium channel β-subunits (32–36 kDa) (Figure 1) [91]. To date, four β-subunit subtypes have been identified (NaVβ1-NaVβ4). The odd-numbered subtypes (β1 and β3) associate with the α-subunit via noncovalent interactions whereas β2 and β4 subtypes are covalently linked to the α-subunit via disulfide bridges [92]. Despite these interactions, the α-subunit, which possesses the minimal essential features of a fully functioning VGSC, has traditionally been used to measure the effects of sodium channel agonists or antagonists in electrophysiology assays. However, co-expression of the β-subunit with the α-subunits influenced the steady-state activation of NaV1.8-subtypes expressed in Xenopus oocytes, suggesting a more significant role of the β-subunit in channel activation [91]. Furthermore, the β-subunits are of interest because expression levels are altered following spinal cord injury in multiple animal models [91].
Wilson et al. showed that co-expression of NaV1.8 with any of the four β-subunits resulted in higher affinity block by μO-MrVIB, resulting from an increased kon and modest decreases in koff [93]. Most recently, Zhang et al. reported the importance of these accessory proteins with respect to the activities of a number of μ-conotoxins [94]. The binding kinetics of μ-conotoxins PIIIA, TIIIA, KIIIA and SmIIIA were examined by two-electrode voltage-clamp electrophysiology in Xenopus oocytes co-expressing NaV1 with one of the four β-subunits. The key findings of this work were that co-expression of the β1 or β3 subunits in the presence of the NaV1 α-subunit increased the on-rates (kon) of μ-conotoxins, whereas kon was dramatically decreased in the presence of either β2 or β4 with NaV1. Conversely, little or no effect was observed upon treatment of the same system with either of the alkaloid toxins TTX or STX [94]. These results highlight the importance of β-subunits in obtaining a complete and physiologically relevant assessment of engineered subtype-selective blockers based on μ-conotoxins.
Analgesic activities of μ-conotoxins
Discovery of so-called ‘neuronal-preferring’ μ-conotoxins encouraged exploration of these compounds in pain models. Three reports have documented the abilities of the neuronal subtype-preferring conotoxins μ-KIIIA and μ-SIIIA to suppress nociception in animal models [69,74,79]. In all instances, compounds were tested in the formalin-induced pain assay in mice following intraperitoneal administration. In this assay, μ-KIIIA was effective in suppressing paw-licking times during the Phase II nociceptive response (inflammatory pain) with a calculated ED50 of 0.1 mg/kg [69]. Similarly, μ-SIIIA and its analogs were shown to be analgesic in this model with an ED50 of 0.9 mg/kg. The analog in which noncritical residues were replaced with a PEG backbone spacer, possessed of 0.05 mg/kg [79]. These results exemplified an ED50 the relative ease by which peptidic scaffolds can be modified to engineer in enhanced pharmacological and physicochemical properties. Analgesic effects were also observed with the minimized peptide analog des[C1] KIIIA[S3/4Aopn, C9A], where intraperitoneal administration of 10 nmol resulted in a 54% decrease in the inflammatory pain response compared with the saline control [74]. Despite these encouraging results, further work is necessary to transform neuronal-subtype preferring μ-conotoxins into analgesic drug leads.
Future perspective
Accelerating the pace of μ-conotoxin discovery & development
A very small fraction (< 1%) of the total complement of neuroactive peptides in Conus venoms has been identified to date, let alone characterized [95]. The discovery of new conotoxins has traditionally relied on bioassay-directed fractionation of whole venoms, followed by sequencing of individual peptide components [96], and this approach has been responsible for the discovery of nearly all μ-conotoxins described to date. However, assay-driven identification of large numbers of unique toxin sequences is impractical because it is often labour intensive, time consuming and costly. New methodologies such as transcriptomics [12,96,97] and proteomics/peptidomics [98,99] are already accelerating the pace at which novel peptide sequences are discovered and characterized. Proteomic/peptidomic approaches can be effective when coupled to other methods such as de novo sequencing. However, transcriptomics alone is not a viable option for the identification of new μ-conotoxins because of a higher propensity for base call errors (compared with traditional sequencing methods) and an inability to identify post-translation-ally modified residues, which are common in Conus toxins [100]. The most effective means for identifying new toxins from Conus venoms lies in the combination of the above-mentioned technologies with high-resolution MS [101,102]. In a recent article, Prashanth et al. suggested that the combination of transcriptomic and peptidomic methods, in conjunction with bioinformatics, could lead to the construction of large, searchable repositories for important sequence data [100]. Recently, such an approach was employed to identify new toxins and toxin families in the venoms of C. consors [11], C. flavidus [103] and C. marmoreous [102].
Future research on μ-conotoxin research will increasingly involve a combination of transcriptomic/proteomic approaches and high-throughput screening methods for the correlation of biological activities with new sequence information. Traditionally, determination of the biological activity of a newly identified conotoxin relied on patch-clamp or voltage-clamp electrophysiology assays, the major drawback of which is the inability to screen large numbers of peptide against multiple molecular targets. Calcium imaging studies employing Ca2+-sensitive dyes to study the effects of conotoxins against their molecular targets [104,105] have proven to be highly amenable to implementation in high-throughput screening assays, as discussed in [100].
Another issue faced in the study and development of conotoxin-based drugs is one of production. Ideally, bacterial or yeast expression systems would help to solve this problem, but the abundance of post-translational modifications in these peptides has made these approaches particularly challenging [86,106]. Furthermore, the reducing environment of the bacterial cytoplasm commonly results in misfolded forms of the peptide or sequestration to inclusion bodies [107]. Peptides can be recovered from the insoluble fraction, but misfolded peptides must undergo reduction and in vitro oxidative refolding to obtain the final product [107]. Recent advances in recombinant methods have shown promise in addressing issues of misfolded conotoxin isoforms and have proven useful for sequences that draw from the naturally occurring 20 amino acid repertoire [107,108]. However, a major impediment to large-scale expression of conotoxins still remains, that being the inability of recombinant expression systems to ‘decorate’ the primary sequence with PTMs commonly found in conotoxins. Several enzymes responsible for such modifications have been identified in Conus and, in the future it may be feasible to modify recombinantly expressed peptide with such enzymes [109–111]. In the meantime, synthetic approaches such as solid-phase peptide synthesis remain the more practical approach to synthesis of conotoxins for structural or functional characterization.
Synthetic approaches for producing conotoxins have been largely dependent on solid-phase peptide synthesis using Fmoc or Boc chemistries. As new chemistries are developed, the pace at which new conotoxin sequences can be produced is expected to increase. A major issue in synthesizing conotoxins is ensuring proper cyclization patterns. Early efforts focused on stepwise oxidation of Cys thiols using trityl (Trt), acetamidomethyl (Acm) or other orthogonal protecting groups to direct folding to a major isoform [112]. The major drawback to this approach is the requirement for multiple purification steps, resulting in decreased yields of the fully folded product. Recently, proxies for disulfide bridges such as dicarba- [113,114], diselenide- [115–117] or thioether bridges [118] have been developed. A significant advantage of these methods is the independent formation of macrocycles either on-resin or following cleavage from solid support without additional purification steps.
An incomplete understanding of the interactions between μ-conotoxins and their molecular targets (i.e., VGSCs) has made the rational design of peptide analogs with improved pharmacological action challenging. Early studies of interactions between μ-conotoxins and NaV1-subtypes were almost entirely reliant on mutational studies [71,119]. However, computational studies and the emergence of bacterial sodium channel models are anticipated to increase the rate at which potent and selective blockers of VGSCs can be developed. Such studies can provide useful information about subtle interactions between the toxin and the channel that may lead to improved pharmacology. A recent example of how computational studies can aid in the design of potent peptide analogs can be found in a publication describing mutants of the Stichodactyla helianthus potassium channel-blocking toxin ShK, which were designed on the basis of modeling and docking studies and exhibited improved selectivity for KV1.3 [120]. Unfortunately, in the absence of accurate models of human VGSCs, similar studies with μ-conotoxins have not been possible to date. The earliest modeling studies docked μ-GIIIA to a skeletal muscle VGSC model based upon the potassium channel and available mutational data [119]. These studies were valuable in defining the binding orientation of μ-GIIIA in the pore region of Domain II and they highlighted the importance of interactions between positively-charged μ-conotoxins and the negatively charged outer ring of the pore vestibule [119].
Recent crystal structures of bacterial VGSCs have provided another opportunity to discover important interactions between VGSCs and their ligands. In 2005, Pavlov et al. confirmed the critical role of the pore region for channel blockade using NaChBac, a prokaryotic VGSC [121]. The NaChBac model was useful from the perspective of being a simplified VGSC structure that possessed multiple common features to eukaryotic VGSCs, but showed greater sequence similarity to calcium channels than sodium channels [121]. Recently, the crystal structures of a number of prokaryotic sodium channel orthologs have provided valuable insights into the structure and function of VGSCs [21,122]. These structures may eventually yield structural information to help in the design of μ-conotoxin analogs with improved VGSC potency and/or selectivity. Payandeh et al. reported the crystal structure of a bacterial sodium channel (NaVAb) in its closed conformation in 2011 [21,123]. This structure helped explain the intrinsic selectivity of VGSCs for Na+ ions and how these ions are translocated across the membrane. It also aided in the understanding and interpretation of previous SAR data since the pore regions of NaVAb and the corresponding regions in vertebrate VGSCs share a significant degree of sequence homology [21]. Shortly after the first studies of NaVAb, McCusker et al. reported the crystal structure of the pore domain of another prokaryotic VGSC, NaVMs [122], which differed from NaVAb in that it was in an open conformation. The open state of NaVMs resembled the closed conformation of NaVAb in that the selectivity filters appeared similar, but a major difference between the two structures was observed in the fenestrations within the transmembrane region. NaVMs exhibits a much wider opening, suggesting that it may be able to accommodate large, hydrophobic pore-blocking drugs in the open state [122]. These models also differed in their putative mechanisms of sodium conductance across the membrane. Although significant differences exist between prokaryotic and eukaryotic VGSC subtypes (i.e., lack of tetrameric symmetry, differences in amino acid composition and selectivity filter loop size), elucidation of bacterial sodium channel structures has served as a valuable starting point from which interactions of VGSC subtypes and their ligands (i.e., μ-conotoxins) can be understood [124]. Computational studies using these structures [124,125] are expected to result in more accurate depictions of channel–ligand interactions than those based on other ion channel families.
μ-conotoxins as pharmacological tools & drug leads for the treatment of pain
The μ-conotoxins are interesting not only from the perspective of identifying potent therapeutic leads, but also as scaffolds upon which novel functionalities may be presented. SAR studies on disulfide-rich peptides have identified single amino acids or short di- or tri-peptide segments between Cys-residues that can be removed or replaced without diminishing biological activity. Short peptide sequences have been ‘grafted’ into such regions of the ‘knottin’ peptides to generate powerful tumor imaging agents [126] or peptide analogs with additional therapeutic potential [127]. The μ-conotoxins are extremely hypervariable in these loop regions and, as such, are well suited for such studies. This hyper-variability also makes these molecules ideal candidates for directed evolution experiments using scaffold-constrained random libraries in a manner described in [128]. Another promising application of μ-conotoxins is bioconjugation with small molecule VGSC inhibitors for increased analgesic effect. Sata et al. have shown that neurosteroids such as allopregnanolone sulfate and endocannabinoids such as anandamide can inhibit pain-relevant NaV1-subtypes expressed in Xenopus oocytes [129,130]. Coupling of these molecules to μ-conotoxin scaffolds could conceivably generate peptide analogs with enhanced analgesic activity. Moreover, such peptides may display the target specificity that is often lacking in small molecule inhibitors.
The μ-conotoxins are clearly valuable pharmacological tools for identifying different NaV1-subtypes in neuronal membrane preparations [53]. The differences in selectivity profiles among this family have most recently allowed for examination of specific TTX-sensitive NaV1-subtypes in small and large DRG and superior cervical ganglion neurons [131]. It is anticipated that μ-conotoxins will continue to be used in these types of studies and that they will have prominent roles in probing the structure and function of VGSC subtypes in the future.
Several conotoxin-derived therapeutics are currently in various stages of preclinical or clinical evaluation (Figure 8). The development of μ-conotoxins has focused largely on identifying peptides that act as potent analgesics through inhibition of pain-relevant NaV1-subtypes in the periphery [71,85]. However, blockade of the muscle subtype NaV1.4, long viewed as an undesirable characteristic of many μ-conotoxins, may in fact have clinical applications, as suggested by a recent report on μ-CnIIIC highlighting such inhibitors as potential myorelaxant drugs [81].
Figure 8. Conotoxins at various stages of preclinical and clinical development.

n.a.: Not available
The intrinsic specificity of μ-conotoxins toward VGSCs, coupled with the fact that these peptides are amenable to chemical modification, makes these molecules an attractive alternative to currently used small molecule inhibitors of VGSCs. Modification of peptide scaffolds, either through amino acid substitution or by incorporation of nonnatural chemical motifs, has resulted in peptide analogs with improved pharmacological properties. These modifications have been underpinned by detailed structural and pharmacological studies. Furthermore, it has been estimated that fewer than 1% of all conotoxins have been pharmacologically characterized to date [133], which provides some perspective on the enormous potential of conotoxins, including the μ-conotoxins, as an emerging class of novel pharmaceutical tools.
Executive summary.
Background
Voltage-gated sodium channels (VGSCs) represent an attractive target for the treatment of neuropathic pain, epilepsy and a number of neurological disorders.
More than 20 μ-conotoxins have been described to date, many with distinct selectivity profiles for VGSC subtypes in the CNS or PNS.
μ-Conotoxin inhibitors of VGSCs
Early work identified μ-conotoxins that were selective for skeletal muscle subtypes and have been valuable in determining the structure and function of VGSCs.
Neuronal subtype preferring μ-conotoxins have been important for discrimination of VGSC subtypes and for exploration as potential analgesics.
Structure–activity relationship studies have identified critical residues in various μ-conotoxins as a basis for enhancing potency and/or selectivity.
Applications of μ-conotoxin inhibitors of VGSCs
Muscle-preferring μ-conotoxins have been important for the study of VGSCs; these peptides may also be useful as myorelaxant drugs when administered focally.
Neuronal subtype-preferring μ-conotoxins have shown analgesic activity and may be useful as treatments for pain, epilepsy or other neurological disorders.
Future perspectives
The complexity of the more than 50,000 Conus venom peptides highlights the potential for discovery of numerous therapeutic peptides, including new μ-conotoxins.
Differences in subtype-selectivity, in addition to the amenability of the μ-conotoxin scaffold to chemical modification, are expected to yield useful pharmacological probes in addition to potential drug leads.
Acknowledgments
The authors wish to thank D Yoshikami for numerous conversations integral to this work and BM Olivera for generous support and discussions.
Key terms
- Conotoxins
Neuroactive peptides isolated from the venoms of marine snails of the genus Conus, usually 10–30 amino acids in length and often cross-linked by one or more disulfide bridges.
- Voltage-gated sodium channel
Membrane-spanning ion channel responsible for initiating and propagating action potentials in excitable tissues
- Sodium channel α-subunit (NaV1)
NaV1-subtypes have four transmembrane spanning domains arranged around a central pore through which Na+ ions can permeate. The α-subunit is the minimum structure required for a functional voltage-gated sodium channel
- Selenocysteine
Often referred to as the 21st proteinogenic amino acid, selenocysteine is an isosteric replacement for cysteine. In selenocysteine, the sulfur-containing thiol of cysteine is replaced by a selenium-containing selenol
- Sodium channel β-subunit (NaVβ)
Accessory proteins associated with the α-subunit by either noncovalent (NaVβ1 or β3) or covalent (NaVβ2 or β4) interaction. These subunits modulate channel gating, regulate VGSC trafficking/expression and promote cell adhesion/migration; NaVβ-subtypes can also modulate biological activities of venom-derived toxins
- Two-electrode voltage-clamp electrophysiology
A method that assesses the effects of ligands on heterologously expressed VGSCs, typically in Xenopus oocytes. Kinetics of block are measured by changes in ionic current resulting from Na+ flow-through VGSCs
Footnotes
For reprint orders, please contact reprints@future-science.com
Financial & competing interests disclosure
RS Norton acknowledges fellowship support from the NHMRC. This work was also supported by the National Institutes of General Medical Sciences, National Institutes of Health [Grant GM48677]. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest;
•• of considerable interest
- 1.Kaspar AA, Reichert JM. Future directions for peptide therapeutics development. Drug Discov Today. 2013;18(171–178):8078–9017. doi: 10.1016/j.drudis.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Osborne R. Fresh from the biotech pipeline-2012. Nat Biotechnol. 2013;31(2):100–103. doi: 10.1038/nbt.2498. [DOI] [PubMed] [Google Scholar]
- 3.Tricoci P, Newby LK, Kandzari DE, Harrington RA. Present and evolving role of eptifibatide in the treatment of acute coronary syndromes. Expert Rev Cardiovasc Ther. 2007;5(3):401–412. doi: 10.1586/14779072.5.3.401. [DOI] [PubMed] [Google Scholar]
- 4.Warkentin TE, Koster A. Bivalirudin: a review. Expert Opin Pharmacother. 2005;6(8):1349–1371. doi: 10.1517/14656566.6.8.1349. [DOI] [PubMed] [Google Scholar]
- 5.Pope JE, Deer TR. Ziconotide: a clinical update and pharmacologic review. Expert Opin Pharmacother. 2013;14(7):957–966. doi: 10.1517/14656566.2013.784269. [DOI] [PubMed] [Google Scholar]
- 6.Bhavsar S, Mudaliar S, Cherrington A. Evolution of exenatide as a diabetes therapeutic. Curr Diabetes Rev. 2013;9(2):161–193. doi: 10.2174/1573399811309020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King GF. Venoms as a platform for human drugs: translating toxins into therapeutics. Expert Opin Biol Ther. 2011;11(11):1469–1484. doi: 10.1517/14712598.2011.621940. [DOI] [PubMed] [Google Scholar]
- 8.Norton RS, Olivera BM. Conotoxins down under. Toxicon. 2006;48(7):780–798. doi: 10.1016/j.toxicon.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 9.Han TS, Teichert RW, Olivera BM, Bulaj G. Conus venoms - a rich source of peptide-based therapeutics. Curr Pharm Des. 2008;14(24):2462–2479. doi: 10.2174/138161208785777469. [DOI] [PubMed] [Google Scholar]
- 10.Vetter I, Lewis RJ. Therapeutic potential of cone snail venom peptides (conopeptides) Curr Top Med Chem. 2012;12(14):1546–1552. doi: 10.2174/156802612802652457. [DOI] [PubMed] [Google Scholar]
- 11.Violette A, Biass D, Dutertre S, et al. Large-scale discovery of conopeptides and conoproteins in the injectable venom of a fish-hunting snail using a combined proteomic and transcriptomic approach. J Proteomics. 2012;75(17):5215–5225. doi: 10.1016/j.jprot.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Hu H, Bandyopadhyay PK, Olivera BM, Yandell M. Elucidation of the molecular envenomation strategy of the cone snail Conus geographus through transcriptome sequencing of its venom duct. BMC Genomics. 2012;13:1–12. doi: 10.1186/1471-2164-13-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olivera BM. Conus Snail Venom Peptides. In: Kastin AJ, editor. The Handbook of Biologically Active Peptides. Academic Press; Waltham, MA, USA: 2006. pp. 381–388. [Google Scholar]
- 14.Carstens BB, Clark RJ, Daly NL, Harvey PJ, Kaas Q, Craik DJ. Engineering of conotoxins for the treatment of pain. Curr Pharm Des. 2011;17(38):4242–4253. doi: 10.2174/138161211798999401. [DOI] [PubMed] [Google Scholar]
- 15.Puillandre N, Koua D, Favreau P, Olivera BM, Stöcklin R. Molecular phylogeny, classification and evolution of conopeptides. J Mol Evol. 2012;74(56):297–309. doi: 10.1007/s00239-012-9507-2. [DOI] [PubMed] [Google Scholar]
- 16.Norton RS. μ-conotoxins as leads in the development of new analgesics. Molecules. 2010;15(4):2825–2844. doi: 10.3390/molecules15042825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leipold E, Debie H, Zorn S, et al. μO-Conotoxins inhibit NaV channels by interfering with their voltage sensors in domain-2. Channels (Austin) 2007;1(4):253–262. doi: 10.4161/chan.4847. [DOI] [PubMed] [Google Scholar]
- 18.Gajewiak J, Azam L, Imperial J, et al. A disulfide tether stabilizes the block of sodium channels by the conotoxin μO§-GVIIJ. Proc Natl Acad Sci USA. 2014;111(7):2758–2763. doi: 10.1073/pnas.1324189111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19•.Knapp O, McArthur JR, Adams DJ. Conotoxins targetting neuronal voltage-gated sodium channel subtypes: potential analgesics? Toxins (Basel) 2012;4(11):1236–1260. doi: 10.3390/toxins4111236. A detailed review of the structure and function of voltage-gated sodium channels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20••.Catterall WA. Voltage-gated sodium channels at 60: structure, function and pathophysiology. J Physiol. 2012;590(Pt 11):2577–2589. doi: 10.1113/jphysiol.2011.224204. First report of the crystal structure of a voltage-gated sodium channel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Payandeh J, Scheuer T, Zheng N, Catterall WA. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475(7356):353–358. doi: 10.1038/nature10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu FH, Catterall WA. Overview of the voltage-gated sodium channel family. Genome Biol. 2003;4(3):207.201–207.207. doi: 10.1186/gb-2003-4-3-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waxman SG, Dib-Hajj S, Cummins TR, Black JA. Sodium channels and pain. Proc Natl Acad Sci USA. 1999;96(14):7635–7639. doi: 10.1073/pnas.96.14.7635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilchrist J, Dutton S, Diaz-Bustamante M, et al. NaV1.1 modulation by a novel triazole compound attenuates epileptic seizures in rodents. ACS Chem Biol. 2014;9(5):1204–1212. doi: 10.1021/cb500108p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cox JJ, Reimann F, Nicholas AK, et al. An SCN9A channelopathy causes congenital inability to experience pain. Nature. 2006;444(7121):894–898. doi: 10.1038/nature05413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilchrist J, Das S, Van Petegem F, Bosmans F. Crystallographic insights into sodium-channel modulation by the β4 subunit. Proc Natl Acad Sci USA. 2013;110(51):5016–5024. doi: 10.1073/pnas.1314557110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.England S. Voltage-gated sodium channels: the search for subtype-selective analgesics. Expert Opin Investig Drugs. 2008;17(12):1849–1864. doi: 10.1517/13543780802514559. [DOI] [PubMed] [Google Scholar]
- 28.Krafte DS, Bannon AW. Sodium channels and nociception: recent concepts and therapeutic opportunities. Curr Opin Pharmacol. 2008;8(1):50–56. doi: 10.1016/j.coph.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Priest BT. Future potential and status of selective sodium channel blockers for the treatment of pain. Curr Opin Drug Discov Devel. 2009;12(5):682–692. [PubMed] [Google Scholar]
- 30•.Waxman SG, Kocsis JD, Black JA. Type III sodium channel mRNA is expressed in embryonic but not adult spinal sensory neurons, and is reexpressed following axotomy. J Neurophysiol. 1994;72(1):466–470. doi: 10.1152/jn.1994.72.1.466. Describes the potential role of NaV1.3 in neuropathic pain states. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hains BC, Saab CY, Klein JP, Craner MJ, Waxman SG. Altered sodium channel expression in second-order spinal sensory neurons contributes to pain after peripheral nerve injury. J Neurosci. 2004;24(20):4832–4839. doi: 10.1523/JNEUROSCI.0300-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong S, Morrow TJ, Paulson PE, Isom LL, Wiley JW. Early painful diabetic neuropathy is associated with differential changes in tetrodotoxin-sensitive and -resistant sodium channels in dorsal root ganglion neurons in the rat. J Biol Chem. 2004;279(28):29341–29350. doi: 10.1074/jbc.M404167200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garry EM, Delaney A, Anderson HA, et al. Varicella zoster virus induces neuropathic changes in rat dorsal root ganglia and behavioral reflex sensitisation that is attenuated by gabapentin or sodium channel blocking drugs. Pain. 2005;118(12):97–111. doi: 10.1016/j.pain.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Thakor DK, Lin A, Matsuka Y, et al. Increased peripheral nerve excitability and local NaV1.8 mRNA up-regulation in painful neuropathy. Mol Pain. 2009;5(14) doi: 10.1186/1744-8069-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amaya F, Wang H, Costigan M, et al. The voltage-gated sodium channel NaV1.9 is an effector of peripheral inflammatory pain hypersensitivity. J Neurosci. 2006;26(50):12852–12860. doi: 10.1523/JNEUROSCI.4015-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strickland IT, Martindale JC, Woodhams PL, Reeve AJ, Chessell IP, Mcqueen DS. Changes in the expression of NaV1.7, NaV1.8 and NaV1.9 in a distinct population of dorsal root ganglia innervating the rat knee joint in a model of chronic inflammatory joint pain. Eur J Pain. 2007;12(5):564–572. doi: 10.1016/j.ejpain.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Nieto FR, Cobos EJ, Tejada MÁ, Sánchez-Fernández C, González-Cano R, Cendán CM. Tetrodotoxin (TTX) as a therapeutic agent for pain. Mar Drugs. 2012;10(2):281–305. doi: 10.3390/md10020281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ekberg J, Jayamanne A, Vaughan CW, et al. μO-conotoxin MrVIB selectively blocks NaV1.8 sensory neuron specific sodium channels and chronic pain behavior without motor deficits. Proc Natl Acad Sci USA. 2006;103(45):17030–17035. doi: 10.1073/pnas.0601819103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmalhofer WA, Calhoun J, Burrows R, et al. Pro Tx-II, a selective inhibitor of NaV1.7 sodium channels, blocks action potential propagation in nociceptors. Mol Pharmacol. 2008;74(5):1476–1484. doi: 10.1124/mol.108.047670. [DOI] [PubMed] [Google Scholar]
- 40.Yang S, Xiao Y, Kang D, et al. Discovery of a selective NaV1.7 inhibitor from centipede venom with analgesic efficacy exceeding morphine in rodent pain models. Proc Natl Acad Sci USA. 2013;110(43):17534–17539. doi: 10.1073/pnas.1306285110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cestèle S, Catterall WA. Molecular mechanisms of neurotoxin acton on voltage-gated sodium channels. Biochimie. 2000;82(9–10):883–892. doi: 10.1016/s0300-9084(00)01174-3. [DOI] [PubMed] [Google Scholar]
- 42.Rosker C, Lohberger B, Hofer D, Steinecker B, Quasthoff S, Schreibmayer W. The TTX metabolite 4,9-anhydro-TTX is a highly specific blocker of the NaV1.6 voltage-dependent sodium channel. Am J Physiol Cell Physiol. 2007;293(2):C783–C789. doi: 10.1152/ajpcell.00070.2007. [DOI] [PubMed] [Google Scholar]
- 43.Zhang MM, McArthur JR, Azam L, et al. Synergistic and antagonistic interactions between tetrodotoxin and μ-conotoxin in blocking voltage-gated sodium channels. Channels (Austin) 2009;3(1):32–38. doi: 10.4161/chan.3.1.7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang MM, Gruszczynski P, Walewska A, Bulaj G, Olivera BM, Yoshikami D. Cooccupancy of the outer vestibule of voltage-gated sodium channels by μ-conotoxin KIIIA and saxitoxin or tetrodotoxin. J Neurophysiol. 2010;104(1):88–97. doi: 10.1152/jn.00145.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hagen NA, Fisher KM, Lapointe B, et al. An open-label, multi-dose efficacy and safety study of intramuscular tetrodotoxin in patients with severe cancer-related pain. J Pain Symptom Manage. 2001;34(2):171–182. doi: 10.1016/j.jpainsymman.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 46.Craik DJ, Fairlie DP, Liras S, Price D. The future of peptide-based drugs. Chem Biol Drug Des. 2013;81(1):136–147. doi: 10.1111/cbdd.12055. [DOI] [PubMed] [Google Scholar]
- 47.Mccormack K, Santos S, Chapman ML. Voltage sensor interaction site for selective small molecule inhibitors of voltage-gated sodium channels. Proc Natl Acad Sci USA. 2013;110(29):E2724–E2732. doi: 10.1073/pnas.1220844110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Catterall WA, Cestèle S, Yarov-Yarovoy V, Yu FH, Konoki K, Scheuer T. Voltage-gated ion channels and gating modifier toxins. Toxicon. 2007;49(2):124–141. doi: 10.1016/j.toxicon.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 49.Stevens M, Peigneur S, Tytgat J. Neurotoxins and their binding areas on voltage-gated sodium channels. Front Pharmacol. 2011;2(71):1–13. doi: 10.3389/fphar.2011.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li D, Xiao Y, Hu W, et al. Function and solution structure of hainantoxin-I, a novel insect sodium channel inhibitor from the Chinese bird spider Selenocosmia hainana. FEBS Lett. 2003;555(3):616–622. doi: 10.1016/s0014-5793(03)01303-6. [DOI] [PubMed] [Google Scholar]
- 51.Bulaj G, West PJ, Garrett JE, et al. Novel conotoxins from Conus striatus and Conus kinoshitai selectively block TTX-resistant sodium channels. Biochemistry. 2005;44(19):7259–7265. doi: 10.1021/bi0473408. [DOI] [PubMed] [Google Scholar]
- 52••.French RJ, Terlau H. Sodium channel toxins - receptor targeting and therapeutic potential. Curr Med Chem. 2004;11(23):3053–3064. doi: 10.2174/0929867043363866. Pharmacological characterization of various μ-conotoxins against NaV1.1–NaV1.8. This work established the selectivity profiles for a number of sodium channel blocking conotoxins. [DOI] [PubMed] [Google Scholar]
- 53.Wilson MJ, Yoshikami D, Azam L, et al. μ-Conotoxins that differentially block sodium channels NaV1.1 through 1.8 identify those responsible for action potentials in sciatic nerve. Proc Natl Acad Sci USA. 2011;108(25):10302–10307. doi: 10.1073/pnas.1107027108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cruz LJ, Gray WR, Olivera BM, et al. Conus geographus toxins that discriminate between neuronal and muscle sodium channels. J Biol Chem. 1985;260(16):9280–9288. [PubMed] [Google Scholar]
- 55.Moczydlowski E, Olivera BM, Gray WR, Strichartz GR. Discrimination of muscle and neuronal Na-channel subtypes by binding competition between [3H]saxitoxin and μ-conotoxins. Proc Natl Acad Sci USA. 1986;83(14):5321–5325. doi: 10.1073/pnas.83.14.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sato K, Ishida Y, Wakamatsu K, et al. Active site of μ-conotoxin GIIIA, a peptide blocker of muscle sodium channels. J Biol Chem. 1991;266(26):16989–16991. [PubMed] [Google Scholar]
- 57•.Wakamatsu K, Kohda D, Hatanaka H, et al. Structure-activity relationships of μ-conotoxin GIIIA: structure determination of active and inactive sodium channel blocker peptides by NMR and simulated annealing calculations. Biochemistry. 1992;31(50):12577–12584. doi: 10.1021/bi00165a006. Established the discrete location for μ-conotoxin binding as neurotoxin binding site 1 in the pore region of the α-subunit. [DOI] [PubMed] [Google Scholar]
- 58.Yanagawa Y, Abe T, Satake M. μ-Conotoxins share a common binding site with tetrodotoxin/saxitoxin on eel electroplax Na channels. N Neurosci. 1987;7(5):1498–1505. doi: 10.1523/JNEUROSCI.07-05-01498.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holford M, Zhang MM, Gowd KH, et al. Pruning nature: biodiversity-derived discovery of novel sodium channel blocking conotoxins from Conus bullatus. Toxicon. 2009;53(1):90–98. doi: 10.1016/j.toxicon.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shon KJ, Olivera BM, Watkins M, et al. μ-Conotoxin PIIIA, a new peptide for discriminating among tetrodotoxin-sensitive Na channel subtypes. J Neurosci. 1998;18(12):4473–4481. doi: 10.1523/JNEUROSCI.18-12-04473.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nielsen KJ, Watson M, Adams DJ, et al. Solution structure of μ-conotoxin PIIIA, a preferential inhibitor of persistent tetrodotoxin-sensitive sodium channels. J Biol Chem. 2002;277(30):27247–27255. doi: 10.1074/jbc.M201611200. [DOI] [PubMed] [Google Scholar]
- 62.Li RA, Tomaselli GF. Using the deadly μ-conotoxins as probes of voltage-gated sodium channels. Toxicon. 2004;44(2):117–122. doi: 10.1016/j.toxicon.2004.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.West PJ, Bulaj G, Garrett JE, Olivera BM, Yoshikami D. μ-Conotoxin SmIIIA, a potent inhibitor of tetrodotoxin-resistant sodium channels in amphibian sympathetic and sensory neurons. Biochemistry. 2002;41(51):15388–15393. doi: 10.1021/bi0265628. [DOI] [PubMed] [Google Scholar]
- 64.Keizer DW, West PJ, Lee EF, et al. Structural basis for tetrodotoxin-resistant sodium channel binding by μ-notoxin SmIIIA. J Biol Chem. 2003;278(47):46805–46813. doi: 10.1074/jbc.M309222200. [DOI] [PubMed] [Google Scholar]
- 65.Walewska A, Skalicky JJ, Davis DR, et al. NMR-based mapping of disulfide bridges in cysteine-rich peptides: application to the μ-conotoxin SxIIIA. J Am Chem Soc. 2008;130(43):14280–14286. doi: 10.1021/ja804303p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lewis RJ, Schroeder CI, Ekberg J, et al. Isolation and structure-activity of μ-conotoxin TIIIA, a potent inhibitor of tetrodotoxin-sensitive voltage-gated sodium channels. Mol Pharmacol. 2007;71(3):676–685. doi: 10.1124/mol.106.028225. [DOI] [PubMed] [Google Scholar]
- 67.Schroeder CI, Adams D, Thomas L, Alewood PF, Lewis RJ. N- and C-terminal extensions of μ-conotoxins invcrease potency and selectivity for neuronal sodium channels. Biopolymers. 2012;98(2):161–165. doi: 10.1002/bip.22032. [DOI] [PubMed] [Google Scholar]
- 68.Khoo KK, Gupta K, Green BR, et al. Distinct disulfide isomers of μ-conotoxins KIIIA and KIIIB block voltage-gated sodium channels. Biochemistry. 2012;51(49):9826–9835. doi: 10.1021/bi301256s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang MM, Green BR, Catlin P, et al. Structure/function characterization of μ-conotoxin KIIIA, an analgesic, nearly irreversible blocker of mammalian neuronal sodium channels. J Biol Chem. 2007;282(42):30699–30706. doi: 10.1074/jbc.M704616200. [DOI] [PubMed] [Google Scholar]
- 70.Nakamura M, Niwa Y, Ishida Y, et al. Modification of Arg-13 of μ-conotoxin GIIIA with piperidinyl-Arg analogs and their relation to the inhibition of sodium channels. FEBS Lett. 2001;503(1):107–110. doi: 10.1016/s0014-5793(01)02714-4. [DOI] [PubMed] [Google Scholar]
- 71.McArthur JR, Singh G, McMaster D, Winkfein R, Tieleman DP, French RJ. Interactions of key charged residues contributing to selective block of neuronal sodium channels by μ-conotoxin KIIIA. Mol Pharmacol. 2011;80(4):573–584. doi: 10.1124/mol.111.073460. [DOI] [PubMed] [Google Scholar]
- 72.Walewska A, Han TS, Zhang MM, Yoshikami D, Bulaj G, Rolka K. Expanding chemical diversity of conotoxins: peptoid-peptide chimeras of the sodium channel blocker μ-KIIIA and its selenopeptide analogues. Eur J Med Chem. 2013;65:144–150. doi: 10.1016/j.ejmech.2013.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Van Der Haegen A, Peigneur S, Tytgat J. Importance of position 8 in μ-conotoxin KIIIA for voltage-gated sodium channel selectivity. FEBS J. 2011;278(18):3408–3418. doi: 10.1111/j.1742-4658.2011.08264.x. [DOI] [PubMed] [Google Scholar]
- 74.Han TS, Zhang MM, Walewska A, et al. Structurally minimized μ-conotoxin analogues as sodium channel blockers: implications for designing conopeptide-based therapeutics. ChemMedChem. 2009;4(3):406–414. doi: 10.1002/cmdc.200800292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75•.Khoo KK, Feng ZP, Smith BJ, et al. Structure of the analgesic μ-conotoxin KIIIA and effects on the structure and function of disulfide deletion. Biochemistry. 2009;48(6):1210–1219. doi: 10.1021/bi801998a. Showed that μ-conotoxins, specifically μ-KIIIA, could preferentially adopt disulfide frameworks that differed from the canonical μ-conotoxin connectivity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Poppe L, Hui JO, Ligutti J, Murray JK, Schnier PD. PADLOC: a powerful tool to assign disulfide bond connectivities in peptides and proteins by NMR spectroscopy. Anal Chem. 2012;84(1):262–266. doi: 10.1021/ac203078x. [DOI] [PubMed] [Google Scholar]
- 77.Schroeder CI, Ekberg J, Nielsen KJ, et al. Neuronally μ-conotoxins from Conus striatus utilize an alpha-helical motif to target mammalian sodium channels. J Biol Chem. 2008;283(31):21621–21628. doi: 10.1074/jbc.M802852200. [DOI] [PubMed] [Google Scholar]
- 78•.Yao S, Zhang MM, Yoshikami D, et al. Structure, dynamics, and selectivity of the sodium channel blocker μ-conotoxin SIIIA. Biochemistry. 2008;47:10940–10949. doi: 10.1021/bi801010u. Demonstrated the amenability of the μ-conotoxin scaffold for chemical modification. It also showed the analgesic activity of μ-SIIIA and its analogs following intraperitoneal (ip) administration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Green BR, Catlin P, Zhang MM, et al. Conotoxins containing nonnatural backbone spacers: cladistic-based design, chemical synthesis, and improved analgesic activity. Chem Biol. 2007;14(4):399–407. doi: 10.1016/j.chembiol.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 80.Zhang MM, Fiedler B, Green BR, et al. Structural and functional diversities among μ-conotoxins targeting TTX-resistant sodium channels. Biochemistry. 2006;45(11):3723–3732. doi: 10.1021/bi052162j. [DOI] [PubMed] [Google Scholar]
- 81.Favreau P, Benoit E, Hocking HG, et al. A novel μpeptide CnIIIC, exerts potent and preferential inhibition of NaV1.2/1.4 channels and blocks neuronal nicotinic acetylcholine receptors. Br J Pharmacol. 2012;166(5):1654–1668. doi: 10.1111/j.1476-5381.2012.01837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hains BC, Klein JP, Saab CY, Craner MJ, Black JA, Waxman SG. Upregulation of sodium channel NaV1.3 and functional involvement in neuronal hyperexcitability with central neuropathic pain after spinal cord injury. J Neurosci. 2003;23(26):8881–8892. doi: 10.1523/JNEUROSCI.23-26-08881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lindia JA, Köhler MG, Martin WJ, Abbadie C. Relationship between sodium channel NaV1.3 expression and neuropathic pain behavior in rats. Pain. 2005;117(1–2):145–153. doi: 10.1016/j.pain.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 84.Kuang Z, Zhang MM, Gupta K, et al. Mammalian neuronal sodium channel blocker μ-conotoxin BuIIIB has a structured N-terminus that influences potency. ACS Chem Biol. 2013;8:1344–1351. doi: 10.1021/cb300674x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Green BR, Zhang MM, Chhabra S, et al. Interactions of disulfide-deficient selenocysteine analogs of μ-BuIIIB with the voltage-gated sodium channel NaV1.3. FEBS J. 2014;281(13):2885–2898. doi: 10.1111/febs.12835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bulaj G, Olivera BM. Folding of conotoxins: formation of the native disulfide bridges during chemical synthesis and biosynthesis of Conus peptides. Antioxid Redox Signal. 2008;10(1):141–155. doi: 10.1089/ars.2007.1856. [DOI] [PubMed] [Google Scholar]
- 87.Tietze AA, Tietze D, Ohlenschläger O, et al. Structurally diverse μ-conotoxin PIIIA isomers block sodium channel 51(17), 4058–4061 NaV1.4. Angew Chem Int Ed Engl. 2012 doi: 10.1002/anie.201107011. [DOI] [PubMed] [Google Scholar]
- 88.Bhattacharyya M, Gupta K, Gowd KH, Balaram P. Rapid mass spectromeric determination of disulfide connectivity in peptides and proteins. Mol Biosyst. 2013;9(6):1340–1350. doi: 10.1039/c3mb25534d. [DOI] [PubMed] [Google Scholar]
- 89.Safavi-Hemami H, Gorasia DG, Steiner AM, et al. Modulation of conotoxin structure and function is achieved through a multienzyme complex in the venom glands of cone snails. J Biol Chem. 2012;287(41):34288–34303. doi: 10.1074/jbc.M112.366781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dutton JL, Bansal PS, Hogg RC, Adams DJ, Alewood PF, Craik DJ. A new level of conotoxin diversity, a non-native disulfide bond connectivity in α-conotoxin AuIB reduces structural definition but increases biological activity. J Biol Chem. 2002;277(50):48849–48857. doi: 10.1074/jbc.M208842200. [DOI] [PubMed] [Google Scholar]
- 91.Vijayaragavan K, Powell AJ, Kinghorn IJ, Chahine M. Role of auxiliary β1-, β2-, and β3-subunits and their interaction with NaV1.8 voltage-gated sodium channel. Biochem Biophys Res Commun. 2004;319(2):531–540. doi: 10.1016/j.bbrc.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 92.Chahine M, O’Leary ME. Regulatory role of voltage-gated Na channel β subunits in sensory neurons. Front Pharmacol. 2011;2(70):1–8. doi: 10.3389/fphar.2011.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93•.Wilson MJ, Zhang MM, Azam L, Olivera BM, Bulaj G, Yoshikami D. NaVβ subunits modulate the inhibition of O-conotoxin NaV1.8 by the analgesic gating modifier μMrVIB. J Pharmacol Exp Ther. 2011;338(2):687–693. doi: 10.1124/jpet.110.178343. Showed the influence of the β-subunit on voltage-gated sodium channels (VGSC) blockade by μ-conotoxins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang MM, Wilson MJ, Azam L, et al. Co-expression of NaVβ subunits alters the kinetics of inhibition of voltage-gated sodium channels ny pore-blocking μ-conotoxins. Br J Pharmacol. 2013;168(7):1597–1610. doi: 10.1111/bph.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lewis RJ, Dutertre S, Vetter I, Christie MJ. Conus venom pharmacology. Pharmacol Rev. 2012;64(2):259–298. doi: 10.1124/pr.111.005322. [DOI] [PubMed] [Google Scholar]
- 96.Robinson SD, Safavi-Hemami H, McIntosh LD, Purcell AW, Norton RS, Papenfuss AT. Diversity of conotoxin gene superfamilies in the venomous snail, Conus victoriae. PLoS One. 2014;9(2):e87648. doi: 10.1371/journal.pone.0087648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hu H, Bandyopadhyay PK, Olivera BM, Yandell M. Characterization of the Conus bullatus genome and tis venom-duct transcriptome. BMC Genomics. 2011;12(60):11–5. doi: 10.1186/1471-2164-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ueberheide B, Fenyö D, Alewood PF, Chait BT. Rapid sensitive analysis of cyseine rich peptide venom components. Proc Natl Acad Sci USA. 2009;106(17):6910–6915. doi: 10.1073/pnas.0900745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bhatia S, Kil YJ, Ueberheide B, et al. Constrained de novo sequencing of conotoxins. J Proteome Res. 2012;11(8):4191–4200. doi: 10.1021/pr300312h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Prashanth JR, Lewis RJ, Dutertre S. Towards an integrated approach for accelerated conopeptide discovery. Toxicon. 2012;60:470–477. doi: 10.1016/j.toxicon.2012.04.340. [DOI] [PubMed] [Google Scholar]
- 101.Terrat Y, Biass D, Dutertre S, et al. High-resolution picture of a venom gland transcriptome: case study with the marine snail Conus consors. Toxicon. 2012;59(1):34–46. doi: 10.1016/j.toxicon.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 102.Dutertre S, Jin AH, Kaas Q, Jones A, Alewood PF, Lewis RJ. Deep venomics reveals the mechanism for expanded peptide diversity in cone snail venom. Mol Cell Proteomics. 2013;12(2):312–329. doi: 10.1074/mcp.M112.021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lu A, Yang L, Xu S, Wang C. Various conotoxin diversifications revealed by a venomic study of Conus flavidus. Mol Cell Proteomics. 2014;13(1):105–118. doi: 10.1074/mcp.M113.028647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Teichert RW, Raghuraman S, Memon T, et al. Characterization of two neuronal subclasses through constellation pharmacology. Proc Natl Acad Sci USA. 2012;109(31):12578–12763. doi: 10.1073/pnas.1209759109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Imperial JS, Cabang AB, Song J, et al. A family of excitatory peptide toxins from venemous crassispirine snails: using constellation pharmacology to assess bioactivity. Toxicon. 2014;89:45–54. doi: 10.1016/j.toxicon.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Craig AG, Bandyopadhyay PK, Olivera BM. Post-translationally modified neuropeptides from Conus venoms. Eur J Biochem. 1999;264(2):271–275. doi: 10.1046/j.1432-1327.1999.00624.x. [DOI] [PubMed] [Google Scholar]
- 107.Klint JK, Senff S, Saez NJ, et al. Production of recombinant disulfide-rich venom peptides for structural and functional analysis via expression in the periplasm of E. coli. PLoS One. 2013;8(5):e63865. doi: 10.1371/journal.pone.0063865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nguyen VD, Hatahet F, Salo KEH, Enlund E, Zhang C, Ruddock LW. Pre-expression of a sulfhydryl oxidase significantly increases the yields of eukaryotic disulfide bond containing proteins expressed in the cytoplasm of E. coli. Microb Cell Fact. 2011;10:1–13. doi: 10.1186/1475-2859-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stanley TB, Stafford DW, Olivera BM, Bandyopadhyay PK. Identification of a vitamin K-dependent carboxylase in the venom duct of a Conus snail. FEBS Lett. 1997;407(1):85–88. doi: 10.1016/s0014-5793(97)00299-8. [DOI] [PubMed] [Google Scholar]
- 110.Bandyopadhyay PK, Garrett JE, Shetty RP, Keate T, Walker CS, Olivera BM. γ-Glutamyl carboxylation: an extracellular posttranslational modification that antedates the divergence of molluscs, arthropods, and chordates. Proc Natl Acad Sci USA. 2002;99(3):1264–1269. doi: 10.1073/pnas.022637099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ul-Hasan S, Burgess DM, Gajewiak J, et al. Characterization of the peptidylglycine α-amidating monooxygenase (PAM) from the venom ducts of neogastropods, Conus bullatus and Conus geographus. Toxicon. 2013;74:215–224. doi: 10.1016/j.toxicon.2013.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hargittai B, Barany G. Controlled syntheses of natural and disulfide-mispaired regioisomers of α-conotoxin S1. J Pept Res. 1999;54(6):468–479. doi: 10.1034/j.1399-3011.1999.00127.x. [DOI] [PubMed] [Google Scholar]
- 113.Macraild CA, Illesinghe J, Leirop BJV, et al. Structure and activity of (2,8)-dicarba-(3,12)-cystino α-ImI, an α-conotoxin containing a nonreducible cystine analogue. J Med Chem. 2009;52:755–762. doi: 10.1021/jm8011504. [DOI] [PubMed] [Google Scholar]
- 114.Van Leirop BJ, Robinson SD, Kompella SN, et al. Dicarba α-conotoxin Vc1.1 analogues with differential selectivity for nicotinic acetylcholine and GABAB receptors. ACS Chem Biol. 2013;8(8):1815–1821. doi: 10.1021/cb4002393. [DOI] [PubMed] [Google Scholar]
- 115.Walewska A, Zhang MM, Skalicky JJ, Yoshikami D, Olivera BM, Bulaj G. Integrated oxidative folding of cysteine/selenocysteine containing peptides: improving chemical synthesis of conotoxins. Angew Chem Int Ed Engl. 2009;48(12):2221–2224. doi: 10.1002/anie.200806085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Armishaw CJ, Daly NL, Nevin ST, Adams DJ, Craik DJ, Alewood PF. α-selenoconotoxins, a new class of potent α7 neuronal nicotinic receptor antagonists. J Biol Chem. 2006;281(20):14136–14143. doi: 10.1074/jbc.M512419200. [DOI] [PubMed] [Google Scholar]
- 117.Steiner AM, Woycechowsky KJ, Olivera BM, Bulaj G. Reagentless oxidative folding of disulfide-rich peptides catalyzed by an intramolecular diselenide. Angew Chem Int Ed Engl. 2012;51(23):5580–5584. doi: 10.1002/anie.201200062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.De Araujo AD, Mobli M, King GF, Alewood PF. Cyclization of peptides by using selenolanthionine bridges. Angew Chem Int Ed Engl. 2012;51(41):10298–10302. doi: 10.1002/anie.201204229. [DOI] [PubMed] [Google Scholar]
- 119.Choudhary G, Aliste MP, Tieleman DP, French RJ, Dudley SCJ. Docking of μ-conotoxin GIIIA in the sodium channel outer vestibule. Channels (Austin) 2007;1(5):344–352. doi: 10.4161/chan.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rashid MH, Heinzelmann G, Hug R, et al. A potent and selective peptide blocker of the K V1.3 channel: prediction from free-energy simulations and experimental confirmation. PLoS One. 2013;8(11):e78712. doi: 10.1371/journal.pone.0078712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pavlov E, Bladen C, Winkfein R, Diao C, Dhaliwal P, French RJ. The pore, not cytoplasmic domains, underlies inactivation in a prokaryotic sodium channel. Biophys J. 2005;89:232–242. doi: 10.1529/biophysj.104.056994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122•.McCusker EC, Bagnéris C, Naylor CE, et al. Structure of a bacterial voltage-gated sodium channel pore reveals mechanisms of opening and closing. Nat Commun. 2012;3:1102. doi: 10.1038/ncomms2077. Crystal structures of NaVAb in two potentially inactivated states, providing insight into VGSC gating. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Payandeh J, El-Din TMG, Scheuer T, Zheng N, Catterall WA. Crystal structure of a voltage-gated sodium channel in two potentially inactivated states. Nature. 2012;486:135–140. doi: 10.1038/nature11077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rashid MH, Mahdavi S, Kuyucak S. Computational studies of marine toxins targeting ion channels. Mar Drugs. 2013;11(3):848–869. doi: 10.3390/md11030848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen R, Chung SH. Binding modes of μ-conotoxin to 102(3), the bacterial sodium channel (NaVAb) Biophys J. 2012:483–488. doi: 10.1016/j.bpj.2011.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Moore SJ, Leung CL, Norton HK, Cochran JR. Engineering agatoxin, a cystine-knot peptide from spider venom, as a molecular probe for in vivo tumor imaging. PLoS One. 2013;8(4):e60498. doi: 10.1371/journal.pone.0060498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vita C, Roumestand C, Toma F, Ménez A. Scorpion toxins as natural scaffolds for protein engineering. Proc Natl Acad Sci USA. 1995;92(14):6404–6408. doi: 10.1073/pnas.92.14.6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kolmar H. Alternative binding proteins: biological activity and therapeutic potential of cysteine-knot miniproteins. FEBS J. 2008;275(11):2684–2690. doi: 10.1111/j.1742-4658.2008.06440.x. [DOI] [PubMed] [Google Scholar]
- 129.Horishita T, Yanagihara N, Ueno S, et al. Neurosteroids allopregnanolone sulfate and pregnanolone sulfate have diverse effect on the α subunit of the neuronal voltage-gated sodium channels NaV1.2, NaV1.6, NaV1.7, and NaV1.8 expressed in Xenopus oocytes. Anesthesiology. 2014;121(3):620–631. doi: 10.1097/ALN.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 130.Okura D, Horishita T, Ueno S, et al. The endocannabinoid anandamide inhibits voltage-gated sodium channels NaV1.2, NaV1.6, NaV1.7, and NaV1.8 in oocytes. Anesth Xenopus Analg. 2014;118(3):554–562. doi: 10.1213/ANE.0000000000000070. [DOI] [PubMed] [Google Scholar]
- 131.Zhang MM, Wilson MJ, Gajewiak J, et al. Pharmacological fractionation of tetrodotoxin-sensitive sodium currents in rat dorsal root ganglion neurons by μ-conotoxins. Br J Pharmacol. 2013;169(1):102–114. doi: 10.1111/bph.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Essack M, Bajic VB, Archer JA. Conotoxins that confer therapeutic possibilities. Mar Drugs. 2012;10(6):1244–1265. doi: 10.3390/md10061244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lewis RJ. Conotoxins: molecular and therapeutic targets. Prog Mol Subcell Biol. 2009;46:45–65. doi: 10.1007/978-3-540-87895-7_2. [DOI] [PubMed] [Google Scholar]