Abstract
We investigated the neural basis of repetition priming (RP) during mathematical cognition. Previous studies of RP have focused on repetition suppression as the basis of behavioral facilitation, primarily using word and object identification and classification tasks. More recently, researchers have suggested associative stimulus-response learning as an alternate model for behavioral facilitation. We examined the neural basis of RP during mathematical problem solving in the context of these two models of learning. Brain imaging and behavioral data were acquired from 39 adults during novel and repeated presentation of three-operand mathematical equations. Despite widespread decreases in activation during repeat, compared with novel trials, there was no direct relation between behavioral facilitation and the degree of repetition suppression in any brain region. Rather, RT improvements were directly correlated with repetition enhancement in the hippocampus and the postero-medial cortex [posterior cingulate cortex, precuneus, and retro-splenial cortex; Brodmann’s areas (BAs) 23, 7, and 30, respectively], regions known to support memory formation and retrieval, and in the SMA (BA 6) and the dorsal midcingulate (“motor cingulate”) cortex (BA 24d), regions known to be important for motor learning. Furthermore, improvements in RT were also correlated with increased functional connectivity of the hippocampus with both the SMA and the dorsal midcingulate cortex. Our findings provide novel support for the hypothesis that repetition enhancement and associated stimulus-response learning may facilitate behavioral performance during problem solving.
INTRODUCTION
Repetition priming (RP) refers to facilitation in behavioral performance upon subsequent exposure to a stimulus (Henson, 2003; Henson, Shallice, & Dolan, 2000; Scarborough, Cortese, & Scarborough, 1977). RP has been widely used to investigate the neural and the behavioral mechanisms that underlie rapid learning. In conjunction with improvements in RT, stimulus repetition is often accompanied by attenuation of neural responses (repetition suppression; RS), which can be recorded either at the level of single cells (Rainer & Miller, 2000; Desimone, 1996; Miller, Li, & Desimone, 1991) or across multiple brain regions ranging from unimodal sensory to heteromodal association cortices (Maccotta & Buckner, 2004; Henson, 2003; Buckner & Koutstaal, 1998; Buckner et al., 1995; Demb et al., 1995; Raichle et al., 1994).
The precise location and extent of attenuation in neural activity depends on the level of information processing required by the task, and researchers frequently distinguish between perceptual and conceptual priming in this context. Perceptual priming is mainly related to the physical attributes of the stimulus, whereas conceptual priming is mainly related to semantic processing and decision making independent of the physical attributes. At the perceptual level, priming effects emerge in modality-specific cortical regions that are involved in extracting physical features of stimuli (Gilaie-Dotan, Nir, & Malach, 2008; Bergerbest, Ghahremani, & Gabrieli, 2004; Doniger et al., 2001; Grill-Spector et al., 1999). With greater cognitive demand, RS is also observed in “higher order” cortical regions, including temporal lobe areas specific to, for example, recognition of objects (Koutstaal et al., 2001), scenes (Bunzeck, Schutze, & Duzel, 2006; Blondin & Lepage, 2005), faces (Bunzeck et al., 2006; Eger, Schweinberger, Dolan, & Henson, 2005), or words (Orfanidou, Marslen-Wilson, & Davis, 2006). Most previous studies of RP have focused on perceptual and conceptual priming of objects and words (Roediger, 2003). With visually presented objects, participants are typically asked to decide whether images depict living or nonliving objects (Wig, Grafton, Demos, & Kelley, 2005), natural or manufactured objects (Zago, Fenske, Aminoff, & Bar, 2005), indoor or outdoor scenes (Bunzeck et al., 2006; Turk-Browne, Yi, & Chun, 2006), and possible or impossible objects (Habeck, Hilton, Zarahn, Brown, & Stern, 2006). With visually presented words, participants Journal of Cognitive Neuroscience 22:4, pp. 790–805 are typically asked to distinguish between living or nonliving items (Lustig & Buckner, 2004; Maccotta & Buckner, 2004), and with words presented aurally, participants are asked to decide whether words were real or pseudowords (Gagnepain et al., 2008; Orfanidou et al., 2006) and whether environmental sounds were made by an animal (Bergerbest et al., 2004). Tasks that involve semantic processing (e.g., retrieving conceptual information about words or objects) typically show RS effects in the PFC, primarily within the left inferior frontal cortex (IFC; Lustig & Buckner, 2004; Maccotta & Buckner, 2004; Wagner, Gabrieli, & Verfaellie, 1997; Buckner et al., 1995; Demb et al., 1995). Here, we examine the generalizability of findings from previous RP studies with a novel task involving mathematical problem solving and a control task involving number identification.
We investigate the neural basis of behavioral improvements upon repeated processing of mathematical information in the context of two current models. The more prominent of these models of RP, the “tuning” model (Wiggs & Martin, 1998; Desimone, 1996; Li, 1993), posits that performance improvements result from concurrent reductions in neural responses (Desimone, 1996; Morton, 1969). Neuronal populations that are not essential for processing the stimuli drop out of the initial cell assembly, yielding more efficient processing (Wiggs & Martin, 1998; Gupta & Cohen, 1990). Evidence for fine tuning of neuronal representations comes from the finding that brain areas that demonstrate RS are typically a subset of those that were originally involved in performing the task (Henson, 2003) and that RS increases with repeated stimulus presentations (Sayres & Grill-Spector, 2006; Grill-Spector & Malach, 2001; Henson et al., 2000).
Despite considerable evidence that repeated task performance results in both behavioral facilitation and RS, evidence supporting a link between the two has been generally weak (McMahon & Olson, 2007). In the first place, only a relatively small number of brain imaging studies of RP have reported examining the relation between behavioral changes and RS. Of these, the most consistent findings to date have been an association between behavioral facilitation and RS in the IFC (Gagnepain et al., 2008; Bunzeck et al., 2006; Orfanidou et al., 2006; Wig et al., 2005; Zago et al., 2005; Bergerbest et al., 2004; Lustig & Buckner, 2004; Maccotta & Buckner, 2004). Wig et al. (2005) found that disrupting activity by applying transcranial magnetic stimulation to the pars triangularis region of the left IFC [Brodmann’s area (BA) 45] during initial stimulus presentation interfered with RP. However, it is not clear what role, if any, the IFC plays in facilitating performance during repeated stimulus processing. Importantly, these same studies did not find a direct relationship between improvements in behavioral performance and other brain areas that had displayed RS, including the occipital cortex (Bunzeck et al., 2006; Wig et al., 2005), the cerebellum (Bunzeck et al., 2006; Orfanidou et al., 2006), the fusiform cortex (Orfanidou et al., 2006; Maccotta & Buckner, 2004), the parahippocampal gyrus (Turk-Browne et al., 2006), and the caudate nucleus (Bunzeck et al., 2006). Thus, the link between RS and behavioral facilitation has only been consistently demonstrated in the IFC. However, other studies have reported improvements in behavioral performance without any decreases in activity in the IFC (Eger, Henson, Driver, & Dolan, 2004; Henson, Shallice, Gorno-Tempini, & Dolan, 2002), and individuals with lesions in the IFC (Broca’s aphasia) nonetheless demonstrate intact lexical-semantic priming (Hagoort, 1997). A further challenge to the tuning model is the finding that RS is not always accompanied by behavioral improvements (Henson & Mouchlianitis, 2007; Lin & Ryan, 2007; Ryan & Schnyer, 2007).
An alternative, but not necessarily mutually exclusive, model has recently been proposed, which posits that performance enhancements during repeated stimulus presentation may arise from rapid stimulus-response learning (Schnyer, Dobbins, Nicholls, Schacter, & Verfaellie, 2006; Dobbins, Schnyer, Verfaellie, & Schacter, 2004; Logan, 1990). Under this model, an association is formed between a stimulus and a particular response during initial stimulus presentation; during repeated presentation of the stimulus, the appropriate response is cued, bypassing more elaborate semantic processing that accompanied initial stimulus presentation (Horner & Henson, 2008). In support of this model, Dobbins et al. (2004) found that despite using the same stimuli upon repeated presentation, changing the task rules diminished behavioral facilitation. This study demonstrated that it was not the repeated processing of the stimuli that was resulting in behavioral facilitation but rather the formation of an association between the stimulus and its correct response. Behavioral facilitation may therefore be more directly related to brain systems that support associative learning rather than RS. It would then be expected that associative learning would involve repetition enhancement as stimulus-response mappings are formed. Although increases in neural activity have been reported in conjunction with RP (Bunzeck et al., 2006; Orfanidou et al., 2006; Fiebach, Gruber, & Supp, 2005; Zago et al., 2005; Henson et al., 2000), surprisingly few studies have found a direct relation between behavioral facilitation and increased neural activity. A notable exception is the recent study by Horner and Henson (2008), which found that repetition enhancement within the posterior PFC (BA 44/6) reflected performance improvements, whereas RS within posterior perceptual regions reflected facilitation of perceptual processing independent of performance improvements.
The main goal of our study is to investigate the neural basis of RP during mathematical problem solving. We used fMRI to examine the neural basis of RP during a task involving three-operand addition and subtraction. There have been no previous brain imaging studies of RP using such tasks to our knowledge, although one behavioral study had demonstrated robust RP effects using both visual word and symbolic representations of simple addition problems (Sciama, Semenza, & Butterworth, 1999). The neural basis of these performance improvements is unknown. To address this question, we compared RP during two conditions, one involving mathematical calculation (MC condition) and a second involving number identification (NI condition). We aimed to compare the magnitude of RP in the two conditions and to identify brain regions that mediate RP in these two conditions. Accordingly, we examined both neural RS and repetition enhancement and their relation to performance improvements.
We predicted that the MC condition, which involves semantic fact retrieval and problem solving, would show a greater magnitude of behavioral improvements compared with the NI condition, which involves basic number recognition. We also predicted that changes in brain responses during the MC condition would be more widespread and involve the posterior parietal cortex (PPC), notably in the intraparietal sulcus (IPS), the pars triangularis region of the IFC, and the ventral premotor cortex— regions consistently implicated in many prior studies of mathematical cognition (Delazer, Benke, Trieb, Schocke, & Ischebeck, 2006; Delazer, Karner, Zamarian, Donnemiller, & Benke, 2006; Houde & Tzourio-Mazoyer, 2003; Menon, Mackenzie, Rivera, & Reiss, 2002; Rivera, Menon, White, Glaser, & Reiss, 2002; Gruber, Indefrey, Steinmetz, & Kleinschmidt, 2001; Menon, Rivera, White, Eliez, et al., 2000; Menon, Rivera, White, Glover, & Reiss, 2000; Rickard et al., 2000; Dehaene, Spelke, Pinel, Stanescu, & Tsivkin, 1999). We further hypothesized that under the tuning model of RP, the magnitude of behavioral priming would be positively correlated with RS in the prefrontal and the parietal cortex. If, on the other hand, RP arises from stimulus-response association, we would expect that performance improvements would be related to increased activity in brain regions implicated in associative memory formation, notably the hippocampus and other medial-temporal lobe (MTL) regions (Schnyer et al., 2006). Finally, within the context of these two models, we test the hypothesis that changes in functional connectivity may also contribute to improvements in performance. First, we examine whether regions that exhibit RS also demonstrate performance-related increases in functional connectivity. This would suggest that the formation of stronger interactions allows these regions to process information more efficiently, thus partially explaining the link between performance improvement and decreased activation. Secondly, we examine whether regions involved in mnemonic and motoric processes demonstrate performance-related increases in functional connectivity. This would suggest that stimulus-response association arises in part from increased crosstalk between such regions.
METHODS
Participants
Forty-one healthy adults (20 men and 21 women) between the ages of 18 and 35 years (M = 22.6 years, SD = 4.172 years) participated in the study. Two subjects had large movement artifacts in their fMRI data, and they were subsequently excluded from the study. Participants were recruited from the Stanford University community after giving written informed consent. All protocols were approved by the Human Subjects Committee at Stanford University School of Medicine, and participants were treated in accordance with the Americal Psychological Association “Code of Conduct.”
fMRI Task
The experiment consisted of four conditions, involving novel and repeated presentation of MC trials [MC novel (MC-N) and MC repeat (MC-R)] and NI trials [NI novel (NI-N) and NI repeat (NI-R)] tasks. The MC-N and the NI-N epochs consisted entirely of novel (previously unseen) stimuli, whereas the MC-R and the NI-R epochs consisted entirely of stimuli contained in the previous MC-N or NI-N epoch, respectively. Participants were presented with 16 alternating MC and NI epochs, each lasting 28 sec. Two counterbalanced versions were used, such that half of the participants began with MC and the other half began with NI. The repeated version of each novel MC (NI) epoch was presented in the subsequent MC (NI) epoch; for example, a subject who began with the MC epoch would see the sequence—MC-N, NI-N, MC-R, NI-R—repeated four times with different sets of novel stimuli each time. Repeated trials in the MC condition used the same problems and the same outcomes as in the novel trials. To ensure that responses were not dependent on simple two-operand fact retrieval (e.g., 2 + 4 = 6), we used three-operand trials to facilitate MC and subsequent response learning.
Each MC epoch consisted of seven 3-operand equations of the form a + b − c = d (e.g., 5 + 4 − 2 = 7), using only single-digit numerals and addition and subtraction operators. Participants pressed one of two keys to indicate whether the equation was correct or incorrect. Fifty percent of equations were correct, and equations were randomly presented. Incorrect equations had answers that were adjusted randomly by ±1 and ±2. Each NI epoch consisted of seven 7-symbol strings (e.g., 4 @ 3 & 2 o 5). Participants pressed one of two keys to indicate whether the string contained the numeral 5. Half the strings contained the numeral 5 and half did not, and the strings were randomly generated. Each equation or string was presented for 3.5 sec followed by a blank screen for 0.5 sec.
fMRI Data Acquisition
Images were acquired on a 3-T GE Signa scanner (GE Medical Systems, Milwaukee, WI) using a standard GE whole head coil (software Lx 8.3). Head movement was minimized during scanning by a comfortable custom-built restraint. A total of 28 axial slices (4.5-mm thickness) parallel to the AC-PC line and covering the whole brain were imaged using a T2*-weighted gradient-echo spiral pulse sequence (repetition time = 2000 msec, echo time = 30 msec, flip angle = 70°, 1 interleave). The field of view was 20 cm, and the matrix size was 64 × 64, providing an in-plane spatial resolution of 3.125 mm. To reduce blurring and signal loss arising from field inhomogeneities, we used an automated high-order shimming method based on spiral acquisitions before acquiring functional MRI scans (Speelman, Simpson, & Kirsner, 2002). To aid in localization of functional data, we used a high-resolution T1-weighted spoiled grass gradient recalled inversion recovery three-dimensional MRI sequence with the following parameters: inversion time = 300 msec, repetition time = 8 msec; echo time = 3.6 msec; flip angle = 15°; 22 cm field of view; 124 slices in coronal plane; 256 × 192 matrix; 2 number of excitations (NEX), acquired resolution = 1.5 × 0.9 × 1.1 mm. The images were reconstructed as a 124 × 256 × 256 matrix with a 1.5 × 0.9 × 0.9-mm spatial resolution. Structural and functional images were acquired in the same scan session.
fMRI Data Analysis
Preprocessing
The first five volumes were not analyzed to allow for signal equilibration effects. A linear shim correction was applied separately for each slice during reconstruction using a magnetic field map acquired automatically by the pulse sequence at the beginning of the scan. Functional MRI data were then analyzed using SPM5 analysis software (http://www.fil.ion.ucl.ac.uk/spm). Images were realigned to correct for motion, corrected for errors in slice timing, spatially transformed to standard stereotaxic space [based on the Montreal Neurological Institute (MNI) coordinate system], resampled every 2 mm using sinc interpolation, and smoothed with a 4-mm FWHM Gaussian kernel to decrease spatial noise prior to statistical analysis. Translational movement in millimeters (x, y, z) and rotational motion in degrees (pitch, roll, yaw) were calculated based on the SPM5 parameters for motion correction of the functional images in each subject. None of the participants had movement greater than 3 mm of translation or 3° of rotation.
Individual Subject Analyses
Task-related brain activation was identified using a general linear model. Individual subject analyses were first performed by modeling task-related conditions. For the mathematical cognition task, brain activity related to the four task conditions (MC-N, MC-R, NI-N, and NI-R) was modeled using boxcar functions with the SPM canonical hemodynamic response function and a temporal derivative to account for voxelwise latency differences in hemodynamic response. The boxcar function for a given task condition was equal to “1” throughout each of the four 28-sec epochs of the condition and “0” otherwise. Low-frequency drifts at each voxel were removed using a high-pass filter (0.5 cycles/min), and serial correlations were accounted for by modeling the fMRI time series as a first degree autoregressive process (Friston et al., 1997). The following comparisons were performed: (1) MC minus NI; (2) MC-N minus MC-R; and (3) NI-N minus NI-R. Voxelwise t statistics maps for each comparison were generated for each participant using ANOVA on the respective contrast images.
Group Analyses
A group-level analysis for each comparison was performed by entering the individual-subject contrast maps into a random-effects analysis. The resulting voxelwise t statistic maps were used to determine group-level activation for each comparison. Significant clusters of activation were determined using a voxelwise statistical height threshold of (p < .01), with corrections for multiple spatial comparisons at the cluster level (p < .01). In addition, two group-level covariate analyses were performed between brain activation and behavioral measures: (1) MC-N minus MC-R versus RT improvements in the MC condition, and (2) NI-N minus NI-R versus RT improvements in the NI condition. The “RT improvement” in the MC (NI) condition for each subject was defined as the difference between the subject’s average RT across novel MC (NI) trials and average RT across repeated MC (NI) trials.
Activation foci were superimposed on high-resolution T1-weighted images, and their locations were interpreted using known neuroanatomical landmarks (Duvernoy, Bourgouin, Cabanis, & Cattin, 1999). MNI coordinates were transformed to Talairach coordinates using a nonlinear transformation.
ROI Analysis
ROIs were defined based on a subset of significant (p < .01) activation clusters identified in the analysis described above (see also Tables 1 and 2). The percentage of signal change was extracted from the mean time series of each ROI and within each task condition, using the MarsBaR toolbox (http://marsbar.sourceforge.net/). An average percent signal change value for a given condition was computed by finding the average signal amplitude within a 12- to 30-sec time window of each epoch corresponding to that condition and normalizing that value to a percentage of the baseline (defined as the mean signal in the ROI across the entire scan). Details of the percent signal change calculation are provided in the MarsBaR documentation. A Wilcoxon sign-rank test was used to test for significant differences in percentage signal change across task conditions. Linear regression of percent signal change against RT within the MC-R minus MC-N and the NI-R minus NI-N conditions was performed to test whether RS or repetition-induced increases in neural activation were significantly correlated with behavioral improvements.
Table 1.
Brain Regions That Showed RS during the Calculation and Identification Conditions
| Regions | Brodmann’s area |
p Value (Corrected) |
No. of Voxels | Peak Z Score |
Peak MNI Coordinates (mm) |
|---|---|---|---|---|---|
| (A) Mathematical Calculation: Novel Minus Repeat | |||||
| L IFC, OFC, insula | 47, 45, 48 | <.01 | 363 | 3.78 | −40, 24, 4 |
| R IFC, insula, putamen, caudate, globus pallidum |
47, 11, 48 | <.01 | 326 | 4.17 | 18, 16, 6 |
| L putamen, caudate, ventral striatum | <.01 | 358 | 4.08 | −14, 12, −12 | |
| R SMA, midcingulate cortex, SFG | 6, 32 | <.01 | 403 | 3.70 | 24, 8, 62 |
| R rolandic operculum, precentral gyrus, postcentral gyrus |
6, 4 | <.01 | 819 | 4.15 | 54, −8, 14 |
| L/R thalamus | <.01 | 285 | 4.40 | 4, −16, 4 | |
| L supramarginal, postcentral gyrus | 40, 2, 4 | <.01 | 233 | 4.09 | −58, −28, 34 |
| L calcarine sulcus, middle occipital gyrus | 17, 19, 37 | <.01 | 242 | 4.26 | −18, −72, −10 |
| R lingual gyrus, fusiform gyrus | 17, 18, 19 | <.01 | 283 | 3.60 | 18, −72, −10 |
| R middle and inferior occipital gyrus, lateral fusiform gyrus |
19, 37 | <.01 | 240 | 4.00 | 38, −82, −2 |
| (B) Number Identification: Novel Minus Repeat | |||||
| R fusiform gyrus, inferior temporal gyrus | 19, 37 | <.01 | 227 | 4.37 | 36, −66, −16 |
| R superior parietal lobule | 7, 17 | <.01 | 157 | 3.81 | 22, −74, 50 |
SFG = superior frontal gyrus.
Table 2.
Brain Regions Where Reaction Time Improvements Were Correlated with Repetition Enhancement
| Regions | Brodmann’s areas |
p Value (Corrected) |
No. of Voxels | Peak Z Score |
Peak MNI Coordinates (mm) |
|---|---|---|---|---|---|
| (A) Mathematical Calculation: Novel Minus Repeat | |||||
| Positive correlation | |||||
| R hippocampus | 34 | <.01 | 155 | 4.24 | 20, −16, −14 |
| L/R dorsal midcingulate cortex, SMA | 24d, 6 | <.01 | 351 | 4.07 | 2, −22, 48 |
| L/R posteromedial cortex (PCC, precuneus retrosplenial cortex) and lingual gyrus |
23, 30, 7 | <.01 | 481 | 4.21 | −18, −54, 8 |
| Negative correlation | |||||
| ns | |||||
| (B) Number Identification: Novel Minus Repeat | |||||
| Positive correlation | |||||
| ns | |||||
| Negative correlation | |||||
| ns | |||||
PCC = posterior cingulate cortex.
Functional Connectivity Analysis
Functional connectivity between pairs of ROIs was assessed by finding the correlation between the two ROI time series for each participant. To examine the functional connectivity for each separate task condition, we extracted and used temporal segments from the relevant condition (accounting for hemodynamic delay) in the correlation. Significance was assessed by applying the Fisher transformation to the correlation coefficients and performing a sign-rank test on the resulting z-values. In addition, we tested for performance-related changes in functional connectivity between MC-N and MC-R conditions by regressing the change in z-values against the change in RT. Detailed procedures are described in a previous publication (Menon & Levitin, 2005).
RESULTS
Behavioral
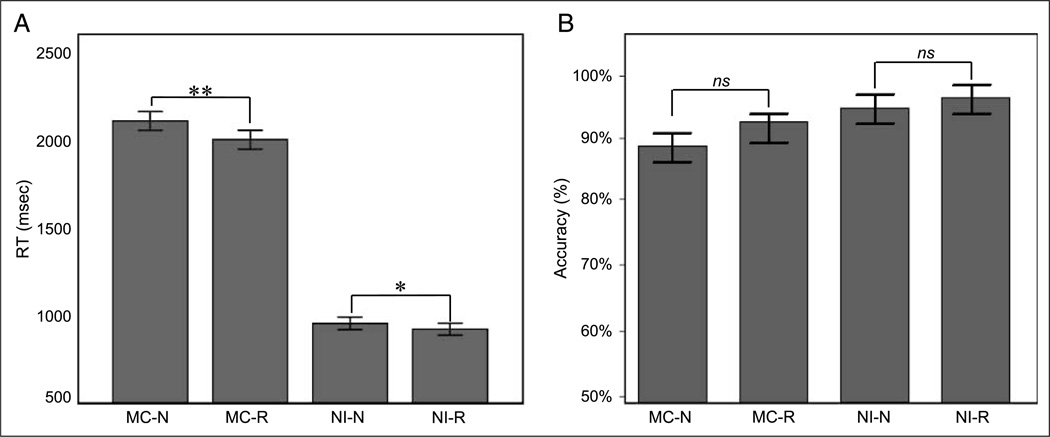
Participants were required to verify the accuracy of three-operand equations (e.g., 4 + 5 − 7 = 2) during the MC condition and indicate whether the number “5” was present in a string of symbols during the NI condition. The mean RT for the MC condition was 2121 msec (σ = 359 msec) during novel presentation of stimuli and 2021 msec (σ = 357 msec) during repeated presentation of stimuli. For the NI condition, the mean RT for novel presentation was 959 msec (σ = 243 msec) and the mean RT for repeated presentation was 926 msec (σ = 232 msec). A two-way repeated measures ANOVA with the factors stimulus type (MC vs. NI) and presentation type (novel vs. repeat) revealed a significant interaction, F(1,41) = 7.44, p < .05, partial η2 = .16. Post hoc simple effect contrasts showed that mean RTs for the MC conditions were significantly higher than mean RTs for the NI conditions, F(1,38) = 590.375, p < .001, partial η2 = .94, and mean RTs for the novel conditions were significantly higher than mean RTs for the repeated conditions, F(1,38) = 23.46, p < .001, partial η2 = .38. Differences between RTs for novel and repeated trials were significant in both the MC, F(1,38) = 23.468, partial η2 = .38, p < .001, and the NI conditions, F(1,41) = 7.447, partial η2 = .16, p < .05, as shown in Figure 1A. Changes in RT between novel and repeated trials, for both MC and NI conditions, are plotted for each subject in Supplementary Figure 1.
Figure 1.
Behavioral changes underlying RP during MC and NI. (A) RT during both the MC and the NI conditions were significant lower in the repeat compared with the novel trials. RP effects are larger for the MC compared with the NI conditions. (B) Accuracy differences between novel and repeated trials were not significant in both the MC and NI conditions. MC-N = mathematical calculation, novel; MC-R = mathematical calculation, repeat; NI-N = number identification, novel; NI-R = number identification, repeat.
Similar repeated measures ANOVA conducted using accuracy as the dependent variable did not reveal any significant interactions or main effects, as shown in Figure 1B.
Brain Activation in the MC Compared with the NI Condition
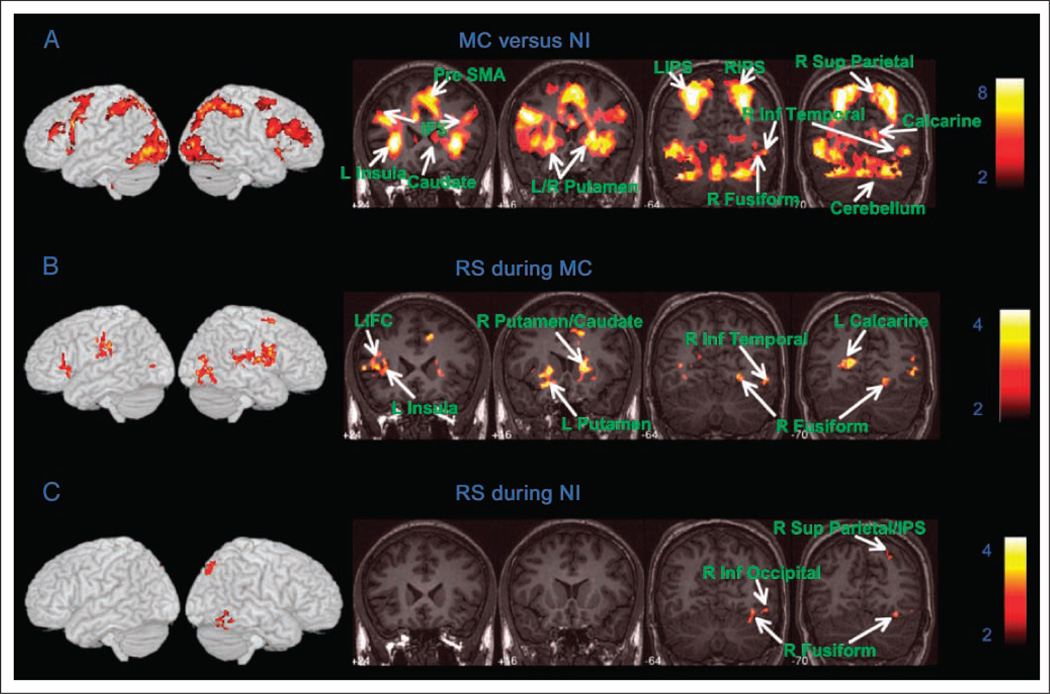
We demarcated brain regions that showed significant activation during MC beyond basic number processing and motor response. Compared with NI, the MC condition revealed greater activation bilaterally in the superior and inferior parietal cortex (BAs 7, 40, 39), the precuneus, the IFC (BA 47/12) and adjoining insula, the calcarine (BA 17), the lingual gyrus (BA 18), the right middle occipital (BA 19), the left superior occipital gyrus, the SMA (BA 6), the anterior/midcingulate cortex (BA 32/24), and the BG and thalamus, as shown in Figure 2A and Supplementary Table 1.
Figure 2.
Brain regions that showed task-related activation and RS. (A) Overall task-related activation during the MC compared with the NI condition. (B) In the MC condition, RS was detected bilaterally in the IFC, the middle and inferior occipital cortex, the inferior temporal cortex, the BG, and the thalamus. (C) In the NI condition, RS was detected only in the posterior regions of the right hemisphere, within the inferior temporal cortex and the superior parietal cortex.
Brain Regions Showing RS
MC Condition
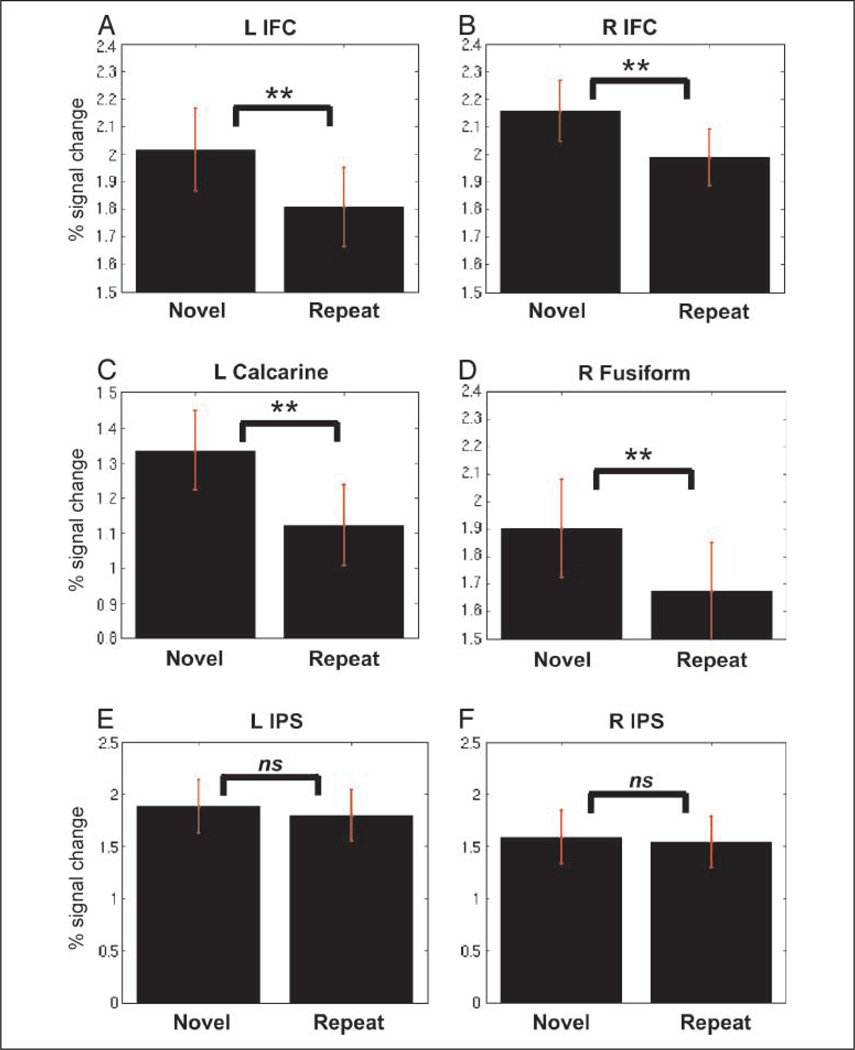
Repeated trials elicited reduced activation bilaterally in cortical regions in the middle and inferior occipital (BA 19), the inferior frontal gyrus (BA 47), the right superior frontal gyrus (BA 6), the middle and inferior temporal gyrus (BA 37), the precentral and postcentral gyri (BAs 4, 6), the left supramarginal (BA 40), the bilateral calcarine (BA 17), the dorsal midcingulate cortex (dMCC, BA 24d), the lingual (BA 18) and fusiform gyri (BA 37), and the thalamus and BG (Figure 2B, Table 1A). Analysis of regional signal change in clusters that showed significant RS (Table 1A) confirmed significant RS effects, as illustrated for responses in the left and right IFC, the left calcarine, and the right fusiform gyrus (Figure 3A – D). In contrast, when we examined the left and the right IPS clusters activated during the MC compared with the NI condition, no significant RS effects were found in either the left and the right IPS (Figure 3E – F).
Figure 3.
RS during mathematical problem solving. Significant reductions in brain activation during the repeated compared with the novel MC condition were found in the (A) left IFC, (B) right IFC, (C) left calcarine, and (D) right fusiform gyrus. No significant reductions were found in (E) the left or (F) the right IPS.
NI Condition
Repeated presentation of the number strings elicited reduced activation only in the posterior regions of the right hemisphere. RS was detected in the fusiform gyrus (BA 37), the superior occipital gyrus (BA 19), the superior parietal lobule (BA 7), and the cuneus (Figure 2C, Table 1B). No brain regions showed significantly greater activation during the repeat compared with the novel stimulus presentation.
Neural Correlates of Behavioral Facilitation
MC Condition
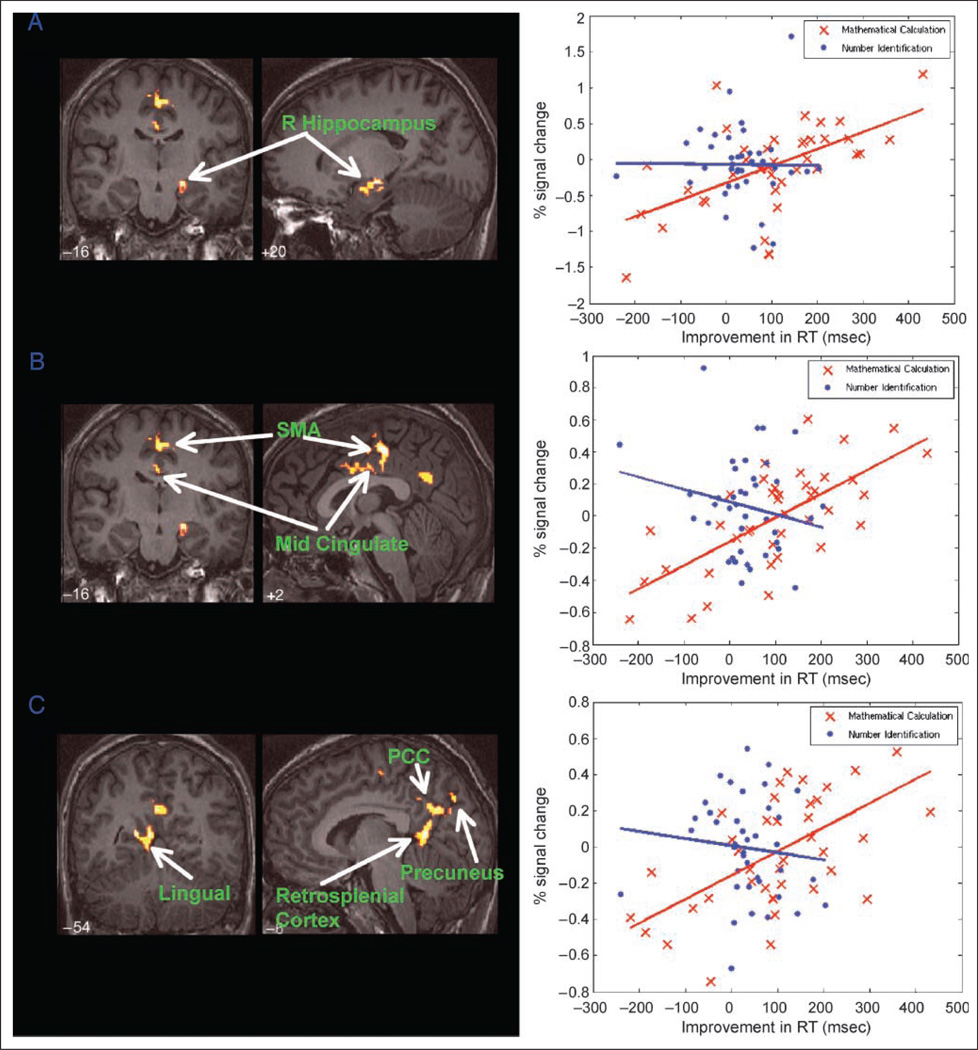
Regression analysis was used to assess the relationship between RT improvement and changes in neural response between novel and repeated MC trials. The magnitude of RS was not correlated with improvement in RT. The magnitude of increases in activation with repeated presentation was, however, correlated with improvement in RT in three distinct regions: (1) the right hippocampus, (2) the left and right SMA and the dMCC, and (3) the retrosplenial cortex, posterior cingulate cortex, precuneus, and anterior lingual gyrus (Figure 4, Table 2). Because RTs are variable across subjects, we performed additional analysis to examine changes in brain activation correlated with normalized changes in RT. Critically, we found that both hippocampus and midcingulate cortex responses were correlated with percent changes in RT (specifically, the percent decrease in RT from MC-N to MC-R). Supplementary Figure 2 shows results from this new analysis. In addition, we examined whether brain activation in the MC-N condition was predictive of RT improvement; no significant clusters were found.
Figure 4.
Brain areas where RT improvements were correlated with repetition enhancement during mathematical problem solving. Brain areas in which activation during the novel compared with the repeat trials was associated with RT improvements. Left column: Coronal and sagittal views of the (A) hippocampus, (B) dMCC and SMA, and (C) posterior medial cortex, including the retrosplenial cortex, posterior cingulate cortex. Right column: For all three cortical clusters, improvements in RT were significantly correlated with the percentage signal change between the novel and the repeat trials for the MC but not the NI condition.
NI Condition
No regions showed significant correlations between RT changes and either increases or decreases in brain activation from the novel to the repeated trials in the NI condition.
ROI analyses were used to further clarify the relationship between RT improvement and the observed RS effects. ROIs were created based on individual clusters demonstrating RS in the MC and the NI conditions and were separately analyzed for the presence of significant correlations between RT improvement and RS. There were no significant relations between RT improvement and RS within any of the individual clusters. Improved accuracy was not correlated with either increases or decreases in activation anywhere in the brain during either the MC or the NI conditions.
Changes in Functional Connectivity Associated with Repetition Enhancement
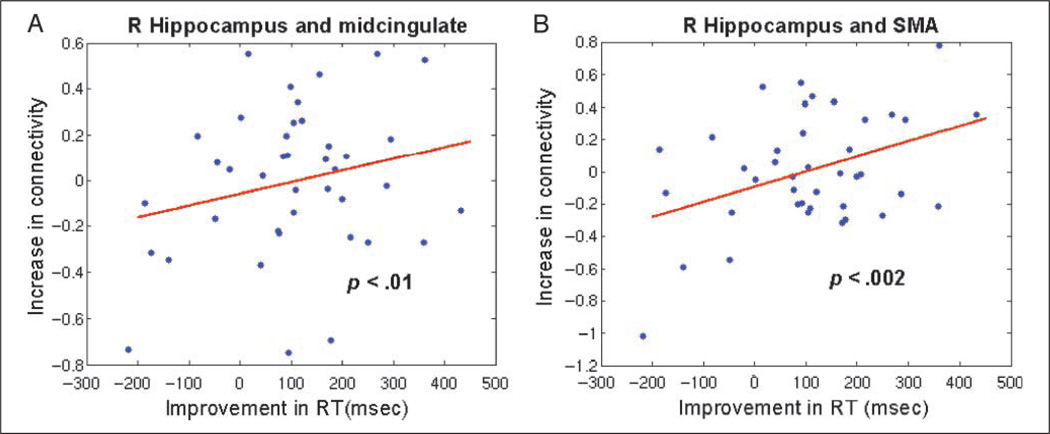
Within brain regions that showed repetition enhancement between the novel and the repeat MC conditions, we examined whether changes in interregional functional connectivity were predictive of RT improvement. Significant correlations between increases in functional connectivity and improvement in RT were found between the right hippocampus ROI and the dMCC/SMA ROI (p < .05 FDR corrected for multiple comparisons), as shown in Figure 5. We further clarified that the functional connectivity between these ROIs was significant during both the MC-N (r̄ = .21, Z = .24; p < .001) and the MC-R (r̄ = .26, Z= .29; p < .001) conditions. We then partitioned the combined dMCC/SMA cluster into separate dMCC and SMA ROIs and found that the right hippocampus was significantly correlated with both (p < .01 and p < .002, respectively). Finally, we examined whether functional connectivity in the MC-N condition was predictive of RT improvement; no significant relationships were obtained.
Figure 5.
RT improvements were correlated with increased functional connectivity of the hippocampus. Improvements in RT were significantly correlated with increase in function connectivity (A) between the right hippocampus and the dMCC and (B) between the right hippocampus and the SMA.
Changes in Functional Connectivity Associated with RS
Within brain regions that showed RS between the novel and the repeat MC conditions, we examined whether changes in interregional functional connectivity might be predictive of RT improvement. We focused on the functional connectivity of the left IFC and the nine other brain regions that showed significant RS effects (as shown in Table 1). Functional connectivity between these regions showed no increases between the novel and the repeat conditions (p > .05). Furthermore, RT improvement was not significantly correlated with changes in functional connectivity between these regions (p > .05). Similar results were found for all other regions taken pairwise.
DISCUSSION
The primary goals of our study were to investigate the neural mechanisms underlying RP of mathematical information and to examine behavioral and neural changes in the context of two models of RP. At the behavioral level, we observed robust RP effects in both the MC and the NI conditions. RTs were significantly faster during the repeated compared with the novel trials during both conditions, with mean RT decrements of 100 msec in the MC condition and 33 msec in the NI condition. There were no significant changes in accuracy, perhaps because performance levels were uniformly high and close to ceiling during the initial presentation. With repeated stimulus presentation, widespread decreases in brain response within the frontal, the temporal, and the occipital lobes were observed in the MC condition. However, performance improvements were not correlated with the degree of RS. Instead, we found that improvements in RT were associated with increased activation in three brain areas—(1) the hippocampus, (2) the SMA and the dMCC cortex, and (3) the posterior cingulate cortex, precuneus, and adjoining retrosplenial cortex. Our findings provide new information about the neural basis of RP during mathematical problem solving.
RS during Number Identification and Mathematical Problem Solving
RS was observed in both the NI and the MC conditions, with significantly stronger effects in the MC condition. In the NI condition, RS was restricted to the right posterior cortex, encompassing the fusiform gyrus and superior parietal lobule within dorsal and the ventral stream regions implicated in priming perceptual and abstract representations of numbers (Ansari, 2008; Hubbard, Piazza, Pinel, & Dehaene, 2005; Naccache & Dehaene, 2001). These findings are consistent with previous studies demonstrating RS in the same regions during word and object recognition (Henson, 2003), and they suggest that RS in these areas is related primarily to stimulus identification.
During the NI condition, RS was not observed in the IFC or any other region of PFC. In contrast, widespread and robust bilateral RS of the IFC, the extrastriate cortex and middle occipital gyrus, the fusiform gyrus, the dorsal striatum, and the thalamus accompanied repeated performance of MC trials. RS in some of these regions, most notably in the IFC, has been reported during semantic processing of objects and words (Henson et al., 2000; Buckner, Koutstaal, Schacter, Wagner, & Rosen, 1998; Blaxton et al., 1996; Hamann & Squire, 1996). This suggests that RS effects observed here reflect general cognitive processes, a view consistent with previous findings that the IFC and other nonparietal regions play a supportive role in MC (Menon, Rivera, White, Glover, et al., 2000). Practice-related reductions in the extent and magnitude of activity within a cortical network encompassing bilateral ventral and dorsal PFC, ACC, and fusiform gyrus have been consistently observed during arithmetic learning (Delazer et al., 2003, 2005; Qin et al., 2003) and also across a wide range of nonmathematical tasks (Hill & Schneider, 2006; Chein & Schneider, 2005). Although these changes arise from experimental manipulations where problems are repeated more than once, they overlap with several regions that showed RS in the MC condition. Whether this same constellation of brain regions shows single-trial RS in tasks unrelated to mathematical cognition is at present not clear. Nevertheless, our findings suggest a common role for the left IFC in RP of both mathematical and nonmathematical stimuli (Henson & Rugg, 2003; Sohn, Goode, Stenger, Carter, & Anderson, 2003). The left IFC has been widely implicated in verbal memory retrieval (Martin & Cheng, 2006; Thompson-Schill & Botvinick, 2006; Wagner, Pare-Blagoev, Clark, & Poldrack, 2001), and memory retrieval is critical in solving multistep mathematical problems such as those used in our study (Danker & Anderson, 2007). Collectively, these studies suggest that the IFC contributes to more efficient memory retrieval during repeated mathematical problem solving. However, IFC responses were not directly correlated with RP in our study, leaving unclear its precise role in facilitating the observed behavioral improvements.
Interestingly, RS was not observed in left and right PPC regions that are known to play a crucial role in mathematical cognition. Functional brain imaging studies have shown that the IPS region plays a crucial role in performing arithmetic calculations, independent of other processing demands such as working memory (Delazer et al., 2003; Zago & Tzourio-Mazoyer, 2002; Menon, Rivera, White, Glover, et al., 2000). Patients with damage to the PPC demonstrate significant deficits in performance of mental addition and subtraction tasks (Delazer, Benke, et al., 2006; Barnea-Goraly, Eliez, Menon, Bammer, & Reiss, 2005; Levin et al., 1996; Takayama, Sugishita, Akiguchi, & Kimura, 1994; Benton, 1987), and in normal healthy controls, transcranial magnetic stimulation over the IPS disrupts numerosity processing (Cappelletti, Barth, Fregni, Spelke, & Pascual-Leone, 2007). As expected, PPC regions, notably along the banks of the IPS, showed strong activation during the MC condition compared with the NI condition, and yet there was no evidence for RS in these regions even at a liberal threshold (p < .05, uncorrected). Critically, the observed effect sizes for RS in the left and the right IPS were extremely small (Cohen’s d = .05 and .03, respectively). This suggests that the PPC is fully recruited each time MC is performed, in contrast to the IFC and other frontal lobe regions. The invariant and obligatory nature of activation in these parietal regions further confirms the critical role of the IPS and surrounding cortex in processing mathematical information.
Performance Improvements during Mathematical Problem Solving Are Related to Repetition Enhancement, Not RS
We hypothesized that under the tuning model of RP, the magnitude of behavioral priming would be positively correlated with prefrontal and parietal regions that showed RS in the MC condition. On the other hand, under the stimulus-response learning model of RP, we predicted that performance improvements would be related to increased activity in brain regions associated with associative memory formation, notably the hippocampus and other MTL regions. We found that RT improvements during the MC condition were associated with increases, rather than decreases, in brain response during the processing of repeat compared with novel stimuli. Importantly, we found that responses in the hippocampus were positively correlated with the magnitude of RT improvements. Our findings are consistent with and extend previous findings concerning the role of the MTL in behavioral facilitation. For example, Schnyer et al. (2006) reported that, compared with healthy controls, patients with MTL lesions demonstrate decreased behavioral facilitation and also found that patients with amnesia failed to show a priming advantage for multiple repetitions; indeed, our findings suggest that the MTL can mediate performance enhancements even after a single repetition. Although the location and the extent of MTL lesions and its overlap across the amnesiacs studies were unspecified in the study of Schnyer et al., our study provides new information about the anatomical specificity of anterior hippocampal regions involved in rapid learning. Whereas most previous human neuroimaging studies of the hippocampus have focused on its role in accurate recall of recently encoded information (Henson, Hornberger, & Rugg, 2005), our study shows that the hippocampus can contribute to rapid improvements in RT, independent of accuracy.
Although several previous studies have reported that behavioral improvements were correlated with RS in the IFC (Gagnepain et al., 2008; Bunzeck et al., 2006; Orfanidou et al., 2006; Wig et al., 2005; Zago et al., 2005; Bergerbest et al., 2004; Lustig & Buckner, 2004; Maccotta & Buckner, 2004), we did not observe any such effects here in either the MC or the NI conditions. One explanation for this difference could be that, unlike the word and object classification tasks used in most prior studies, our tasks did not involve complex semantic processing.
The well-known role of the hippocampus in mediating episodic memory formation (Giovanello, Schnyer, & Verfaellie, 2004; Kirwan & Stark, 2004; Ranganath et al., 2004; Davachi, Mitchell, & Wagner, 2003; Cohen, Poldrack, & Eichenbaum, 1998) suggests the possibility that explicit retrieval may be in effect during mathematical problem solving. At one extreme end of this argument, one might posit that when presented with mathematical equations the second time, participants may be simply recalling their previous responses without recalculation of the problem. However, this is unlikely because mean RTs on repeated MC trials were still about 2 sec, significantly longer than RTs in the NI condition that involved more effortless recognition of number and symbol strings. Furthermore, brain regions such as the PPC, which are crucial for MC, were recruited equally during novel and repeated trials. We believe it is more likely that participants were still performing calculations on repeated trials and that there was facilitation at some stage in the process, perhaps because of faster recall of partial sums or emerging familiarity of the stimuli and the associated response. It is important to note that many tasks involving RP may involve some form of explicit recognition, unless the primes are presented subconsciously (Horner & Henson, 2008). In some cases, explicit recognition of the stimulus may occur after the response has been made (Henson & Rugg, 2003). Our finding that MTL activation was correlated with RT improvements raises the possibility that subjects may have recognized components of the problem solution prior to response, perhaps because of the temporally extended and sequential nature of information processing involved in mathematical problem solving.
Decreases in RT with repetition were also correlated with increased activation in a cluster encompassing the lingual gyrus extending dorsally along the banks of the isthmus of the cingulate gyrus to the posterior medial cortex. The lingual gyrus is part of a hierarchical pathway that provides processed visual input to the hippocampus (Van essen, Anderson, & Felleman, 1992), suggesting that some aspects of the stimulus-response associations observed in our study may be initiated upstream from the hippocampus. The posterior medial cortex, which includes the posterior cingulate cortex (BA 23), the precuneus (BA 7), and the adjoining retrosplenial cortex (BA 30), is tightly coupled to the hippocampal formation (Parvizi, Van hoesen, Buckwalter, & Damasio, 2006) and is also thought to be involved in directed recollection of previously seen items (Cavanna & Trimble, 2006; Vincent et al., 2006). It is noteworthy that RP in our study was also associated with neural changes in the SMA and the dMCC. The SMA has been consistently implicated in motor response learning (Lee & Quessy, 2003; Aizawa, Inase, Mushiake, Shima, & Tanji, 1991) and sensorimotor adaptation (Paz, Natan, Boraud, Bergman, & Vaadia, 2005). The dorsal motor cingulate area is tightly linked with the SMA and the primary motor cortex (Hatanaka et al., 2003). Anatomical connectivity analysis also indicates that this region is tightly coupled to the lateral parietal surface, including the inferior parietal cortex and much of IPS (Vogt, Berger, & Derbyshire, 2003), suggesting a mechanism by which the motor system can be primed to respond during MC. To our knowledge, this is the first time that a relationship has been established between behavioral facilitation during RP and repetition enhancements of the SMA and dMCC. Our findings are consistent with the suggestion that the dMCC plays a pivotal role in reorganizing activity in a host of motor structures to produce appropriate behavioral outputs (Vogt, Hof, & Vogt, 2004). The coactivation of brain regions important for memory and motor learning suggests that learning of stimulus-response associations may contribute to RP.
RT Improvements Are Associated with Increased Hippocampal Functional Connectivity
We further hypothesized that under the stimulus-response learning model of RP, performance improvements would be related to increased connectivity of brain regions associated with associative memory formation, notably the hippocampus. Our analysis focused on the functional connectivity of MTL with other brain regions that showed repetition enhancement. This analysis showed that increases in functional connectivity of the right hippocampus with both the dMCC and the SMA, but not the posteromedial cortex, were significantly correlated with improvements in RT. This finding suggests that connectivity of brain regions important for memory and motor learning plays an important role in mediating RP and further supports the hypothesis that stimulus-response associations may facilitate performance during mathematical problem solving.
We also examined the hypothesis that the magnitude of behavioral priming would be positively correlated with functional connectivity changes among prefrontal and parietal regions that showed RS in the MC condition. We were particularly interested in the IFC, based on its purported causal role in behavioral priming (Wig et al., 2005). However, in our data, there was no evidence for significant changes in functional connectivity in the MC compared with NI conditions between the left IFC and any of these brain regions, although each of these regions showed RS. Further, changes in RT were not related to changes in functional connectivity between the IFC and other brain regions or between any other pairs of brain regions. These findings further rule out the possibility that RS is the primary source of behavioral improvement during mathematical problem solving in our study.
Conclusion
Most previous studies of RP have focused on tasks involving word and object recognition. In these domains, attention has focused on RS as the mechanism underlying behavioral facilitation. Here, we took a different approach and examined the neural basis of RP during mathematical problem solving. We showed that mechanisms other than RS may play an important role in mediating RT improvements, even when RP is accompanied by widespread RS. In particular, we found that repetition enhancement in the hippocampus, the posteromedial cortex, and the SMA contributed significantly to performance improvements.
Our results are consistent with findings in amnesiacs that MTL damage can impair RP (Schnyer et al., 2006) and further suggest that the enhanced recruitment of brain regions responsible for STM formation and their increased functional connectivity with motor learning systems can contribute to behavioral improvements (Suzuki, 2008). Our results provide novel support for the stimulus-response learning hypothesis of RP and help to elucidate the neural mechanisms underlying rapid learning in the context of tasks involving mathematical problem solving. The assumption that RS underlies all behavioral improvements during RP is therefore an oversimplification (Busch, Groh-Bordin, Zimmer, & Herrmann, 2008; Schacter, Wig, & Stevens, 2007; Ganel et al., 2006; Fiebach et al., 2005). It is much more likely that the memory and the brain systems underlying facilitation of behavior during RP depend on both the cognitive processes and the knowledge structures evoked during a specific task (Roediger, 2003).
Supplementary Material
Acknowledgments
The authors thank Sarah Wu and Shanti Shanker for their assistance with the initial stages of data analysis. This research was supported by an NSF Graduate Fellowship and a Stanford Graduate Fellowship to C. C. and by grants from the NIH (HD047520 and HD059205) and the NSF (BCS/DRL-0750340) to V. M.
REFERENCES
- Aizawa H, Inase M, Mushiake H, Shima K, Tanji J. Reorganization of activity in the supplementary motor area associated with motor learning and functional recovery. Experimental Brain Research. 1991;84:668–671. doi: 10.1007/BF00230980. [DOI] [PubMed] [Google Scholar]
- Ansari D. Effects of development and enculturation on number representation in the brain. Nature Reviews Neuroscience. 2008;9:278–291. doi: 10.1038/nrn2334. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Eliez S, Menon V, Bammer R, Reiss AL. Arithmetic ability and parietal alterations: A diffusion tensor imaging study in velocardiofacial syndrome. Brain Research, Cognitive Brain Research. 2005;25:735–740. doi: 10.1016/j.cogbrainres.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Benton AL. Mathematical disability and the Gerstmann syndrome. In: Deloche G, Seron X, editors. Mathematical disabilities: A cognitive neuropsychological perspective. New Jersey: Lawrence Erlbaum; 1987. [Google Scholar]
- Bergerbest D, Ghahremani DG, Gabrieli JD. Neural correlates of auditory repetition priming: Reduced fMRI activation in the auditory cortex. Journal of Cognitive Neuroscience. 2004;16:966–977. doi: 10.1162/0898929041502760. [DOI] [PubMed] [Google Scholar]
- Blaxton TA, Zeffiro TA, Gabrieli JD, Bookheimer SY, Carrillo MC, Theodore WH, et al. Functional mapping of human learning: A positron emission tomography activation study of eyeblink conditioning. Journal of Neuroscience. 1996;16:4032–4040. doi: 10.1523/JNEUROSCI.16-12-04032.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondin F, Lepage M. Decrease and increase in brain activity during visual perceptual priming: An fMRI study on similar but perceptually different complex visual scenes. Neuropsychologia. 2005;43:1887–1900. doi: 10.1016/j.neuropsychologia.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Koutstaal W. Functional neuroimaging studies of encoding, priming, and explicit memory retrieval. Proceedings of the National Academy of Sciences, U.S.A. 1998;95:891–898. doi: 10.1073/pnas.95.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Koutstaal W, Schacter DL, Wagner AD, Rosen BR. Functional-anatomic study of episodic retrieval using fMRI: I. Retrieval effort versus retrieval success. Neuroimage. 1998;7:151–162. doi: 10.1006/nimg.1998.0327. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Petersen SE, Ojemann JG, Miezin FM, Squire LR, Raichle ME. Functional anatomical studies of explicit and implicit memory retrieval tasks. Journal of Neuroscience. 1995;15:12–29. doi: 10.1523/JNEUROSCI.15-01-00012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunzeck N, Schutze H, Duzel E. Category-specific organization of prefrontal response-facilitation during priming. Neuropsychologia. 2006;44:1765–1776. doi: 10.1016/j.neuropsychologia.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Busch NA, Groh-Bordin C, Zimmer HD, Herrmann CS. Modes of memory: Early electrophysiological markers of repetition suppression and recognition enhancement predict behavioral performance. Psychophysiology. 2008;45:25–35. doi: 10.1111/j.1469-8986.2007.00607.x. [DOI] [PubMed] [Google Scholar]
- Cappelletti M, Barth H, Fregni F, Spelke E, Pascual-Leone A. rTMS over the intraparietal sulcus disrupts numerosity processing. Experimental Brain Research. 2007;179:631–642. doi: 10.1007/s00221-006-0820-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: A review of its functional anatomy and behavioral correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chein JM, Schneider W. Neuroimaging studies of practice-related change: fMRI and meta-analytic evidence of a domain-general control network for learning. Brain Research, Cognitive Brain Research. 2005;25:607–623. doi: 10.1016/j.cogbrainres.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Poldrack RA, Eichenbaum H. Memory for items and memory for relations in the procedural/declarative memory framework. Memory. 1998;5:131–178. doi: 10.1080/741941149. [DOI] [PubMed] [Google Scholar]
- Danker JF, Anderson JR. The roles of prefrontal and posterior parietal cortex in algebra problem solving: A case of using cognitive modeling to inform neuroimaging data. Neuroimage. 2007;35:1365–1377. doi: 10.1016/j.neuroimage.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: Distinct medial temporal lobe processes build item and source memories. Proceedings of the National Academy of Sciences, U.S.A. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Spelke E, Pinel P, Stanescu R, Tsivkin S. Sources of mathematical thinking: Behavioral and brain-imaging evidence. Science. 1999;284:970–974. doi: 10.1126/science.284.5416.970. [DOI] [PubMed] [Google Scholar]
- Delazer M, Benke T, Trieb T, Schocke M, Ischebeck A. Isolated numerical skills in posterior cortical atrophy—An fMRI study. Neuropsychologia. 2006;44:1909–1913. doi: 10.1016/j.neuropsychologia.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Delazer M, Domahs F, Bartha L, Brenneis C, Lochy A, Trieb T, et al. Learning complex arithmetic—An fMRI study. Brain Research, Cognitive Brain Research. 2003;18:76–88. doi: 10.1016/j.cogbrainres.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Delazer M, Ischebeck A, Domahs F, Zamarian L, Koppelstaetter F, Siedentopf CM, et al. Learning by strategies and learning by drill-evidence from an fMRI study. Neuroimage. 2005;25:838–849. doi: 10.1016/j.neuroimage.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Delazer M, Karner E, Zamarian L, Donnemiller E, Benke T. Number processing in posterior cortical atrophy—A neuropsychological case study. Neuropsychologia. 2006;44:36–51. doi: 10.1016/j.neuropsychologia.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JD. Semantic encoding and retrieval in the left inferior prefrontal cortex: A functional MRI study of task difficulty and process specificity. Journal of Neuroscience. 1995;15:5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R. Neural mechanisms for visual memory and their role in attention. Proceedings of the National Academy of Sciences, U.S.A. 1996;93:13494–13499. doi: 10.1073/pnas.93.24.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins lG, Schnyer DM, Verfaellie M, Schacter DL. Cortical activity reductions during repetition priming can result from rapid response learning. Nature. 2004;428:316–319. doi: 10.1038/nature02400. [DOI] [PubMed] [Google Scholar]
- Doniger GM, Foxe JJ, Schroeder CE, Murray MM, Higgins BA, Javitt DC. Visual perceptual learning in human object recognition areas: A repetition priming study using high-density electrical mapping. Neuroimage. 2001;13:305–313. doi: 10.1006/nimg.2000.0684. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM, Bourgouin P, Cabanis EA, Cattin F. The human brain: Functional anatomy, vascularization and serial sections with MRI. New York: Springer; 1999. [Google Scholar]
- Eger E, Henson RN, Driver J, Dolan RJ. BOLD repetition decreases in object-responsive ventral visual areas depend on spatial attention. Journal of Neurophysiology. 2004;92:1241–1247. doi: 10.1152/jn.00206.2004. [DOI] [PubMed] [Google Scholar]
- Eger E, Schweinberger SR, Dolan RJ, Henson RN. Familiarity enhances invariance of face representations in human ventral visual cortex: fMRI evidence. Neuroimage. 2005;26:1128–1139. doi: 10.1016/j.neuroimage.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Gruber T, Supp GG. Neuronal mechanisms of repetition priming in occipitotemporal cortex: Spatiotemporal evidence from functional magnetic resonance imaging and electroencephalography. Journal of Neuroscience. 2005;25:3414–3422. doi: 10.1523/JNEUROSCI.4107-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Gagnepain P, Chetelat G, Landeau B, Dayan J, Eustache F, Lebreton K. Spoken word memory traces within the human auditory cortex revealed by repetition priming and functional magnetic resonance imaging. Journal of Neuroscience. 2008;28:5281–5289. doi: 10.1523/JNEUROSCI.0565-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganel T, Gonzalez CL, Valyear KF, Culham JC, Goodale MA, Kohler S. The relationship between fMRI adaptation and repetition priming. Neuroimage. 2006;32:1432–1440. doi: 10.1016/j.neuroimage.2006.05.039. [DOI] [PubMed] [Google Scholar]
- Gilaie-Dotan S, Nir Y, Malach R. Regionally-specific adaptation dynamics in human object areas. Neuroimage. 2008;39:1926–1937. doi: 10.1016/j.neuroimage.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Giovanello KS, Schnyer DM, Verfaellie M. A critical role for the anterior hippocampus in relational memory: Evidence from an fMRI study comparing associative and item recognition. Hippocampus. 2004;14:5–8. doi: 10.1002/hipo.10182. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Edelman S, Avidan G, Itzchak Y, Malach R. Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron. 1999;24:187–203. doi: 10.1016/s0896-6273(00)80832-6. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Malach R. fMR-adaptation: A tool for studying the functional properties of human cortical neurons. Acta Psychologica. 2001;107:293–321. doi: 10.1016/s0001-6918(01)00019-1. [DOI] [PubMed] [Google Scholar]
- Gruber O, Indefrey P, Steinmetz H, Kleinschmidt A. Dissociating neural correlates of cognitive components in mental calculation. Cerebral Cortex. 2001;11:350–359. doi: 10.1093/cercor/11.4.350. [DOI] [PubMed] [Google Scholar]
- Gupta P, Cohen NJ. Theoretical and computational analysis of skill learning, repetition priming, and procedural memory. Psychological Review. 1990;109:401–448. doi: 10.1037/0033-295x.109.2.401. [DOI] [PubMed] [Google Scholar]
- Habeck C, Hilton HJ, Zarahn E, Brown T, Stern Y. An event-related fMRI study of the neural networks underlying repetition suppression and reaction time priming in implicit visual memory. Brain Research. 2006;1075:133–141. doi: 10.1016/j.brainres.2005.11.102. [DOI] [PubMed] [Google Scholar]
- Hagoort P. Semantic priming in Broca’s aphasics at a short SOA: No support for an automatic access deficit. Brain and Language. 1997;56:287–300. doi: 10.1006/brln.1997.1849. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Squire LR. Level-of-processing effects in word-completion priming: A neuropsychological study. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1996;22:933–947. doi: 10.1037//0278-7393.22.4.933. [DOI] [PubMed] [Google Scholar]
- Hatanaka N, Tokuno H, Hamada I, Inase M, Ito Y, Imanishi M, et al. Thalamacotical and intracortical connections of monkey cingulate motor areas. Journal of Comparative Neurology. 2003;462:121–138. doi: 10.1002/cne.10720. [DOI] [PubMed] [Google Scholar]
- Henson R, Mouchlianitis E. Effect of spatial attention on stimulus-specific haemodynamic repetition effects. Neuroimage. 2007;35:1317–1329. doi: 10.1016/j.neuroimage.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Henson R, Shallice T, Dolan R. Neuroimaging evidence for dissociable forms of repetition priming. Science. 2000;287:1269–1272. doi: 10.1126/science.287.5456.1269. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rugg MD. Neural response suppression, haemodynamic repetition effects, and behavioural priming. Neuropsychologia. 2003;41:263–270. doi: 10.1016/s0028-3932(02)00159-8. [DOI] [PubMed] [Google Scholar]
- Henson RN. Neuroimaging studies of priming. Progress in Neurobiology. 2003;70:53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Henson RN, Hornberger M, Rugg MD. Further dissociating the processes involved in recognition memory: An fMRI study. Journal of Cognitive Neuroscience. 2005;17:1058–1073. doi: 10.1162/0898929054475208. [DOI] [PubMed] [Google Scholar]
- Henson RN, Shallice T, Gorno-Tempini ML, Dolan RJ. Face repetition effects in implicit and explicit memory tests as measured by fMRI. Cerebral Cortex. 2002;12:178–186. doi: 10.1093/cercor/12.2.178. [DOI] [PubMed] [Google Scholar]
- Hill NM, Schneider W. Brain changes in the development of expertise: Neurological evidence on skill-based adaptations. In: Ericsson KA, Charness N, Feltovich P, Hoffman R, editors. The Cambridge handbook of expertise and expert performance. Cambridge, MA: Cambridge University Press; 2006. pp. 653–682. [Google Scholar]
- Horner AJ, Henson RN. Priming, response learning and repetition suppression. Neuropsychologia. 2008;46:1979–1991. doi: 10.1016/j.neuropsychologia.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde O, Tzourio-Mazoyer N. Neural foundations of logical and mathematical cognition. Nature Reviews Neuroscience. 2003;4:507–514. doi: 10.1038/nrn1117. [DOI] [PubMed] [Google Scholar]
- Hubbard EM, Piazza M, Pinel P, Dehaene S. Interactions between number and space in parietal cortex. Nature Reviews Neuroscience. 2005;6:435–448. doi: 10.1038/nrn1684. [DOI] [PubMed] [Google Scholar]
- Kirwan CB, Stark CE. Medial temporal lobe activation during encoding and retrieval of novel face-name pairs. Hippocampus. 2004;14:919–930. doi: 10.1002/hipo.20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutstaal W, Wagner AD, Rotte M, Maril A, Buckner RL, Schacter DL. Perceptual specificity in visual object priming: Functional magnetic resonance imaging evidence for a laterality difference in fusiform cortex. Neuropsychologia. 2001;39:184–199. doi: 10.1016/s0028-3932(00)00087-7. [DOI] [PubMed] [Google Scholar]
- Lee D, Quessy S. Activity in the supplementary motor area related to learning and performance during a sequential visuomotor task. Journal of Neurophysiology. 2003;89:1039–1056. doi: 10.1152/jn.00638.2002. [DOI] [PubMed] [Google Scholar]
- Levin HS, Scheller J, Rickard T, Graftman J, Martinkowski K, Winslow M, et al. Dyscalculia and dyslexia after right hemisphere injury in infancy. Archives of Neurology. 1996;53:88–96. doi: 10.1001/archneur.1996.00550010108024. [DOI] [PubMed] [Google Scholar]
- Li L. The representation of stimulus familiarity in anterior inferior temporal cortex. Journal of Neurophysiology. 1993;69:1918–1929. doi: 10.1152/jn.1993.69.6.1918. [DOI] [PubMed] [Google Scholar]
- Lin CY, Ryan L. Repetition priming without identification of the primes: Evidence for a component process view of priming. Neuroimage. 2007;38:589–603. doi: 10.1016/j.neuroimage.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Logan GD. Repetition priming and automaticity: Common underlying mechanisms? Cognitive Psychology. 1990;22:1–35. [Google Scholar]
- Lustig C, Buckner RL. Preserved neural correlates of priming in old age and dementia. Neuron. 2004;42:865–875. doi: 10.1016/j.neuron.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Maccotta L, Buckner RL. Evidence for neural effects of repetition that directly correlate with behavioral priming. Journal of Cognitive Neuroscience. 2004;16:1625–1632. doi: 10.1162/0898929042568451. [DOI] [PubMed] [Google Scholar]
- Martin RC, Cheng Y. Selection demands versus association strength in the verb generation task. Psychonomic Bulletin & Review. 2006;13:396–401. doi: 10.3758/bf03193859. [DOI] [PubMed] [Google Scholar]
- McMahon DBT, Olson CR. Repetition suppression in monkey inferotemporal cortex: Relation to behavioral priming. Journal of Neurophysiology. 2007;97:3532–3543. doi: 10.1152/jn.01042.2006. [DOI] [PubMed] [Google Scholar]
- Menon V, Levitin DJ. The rewards of music listening: Response and physiological connectivity of the mesolimbic system. Neuroimage. 2005;28:175–184. doi: 10.1016/j.neuroimage.2005.05.053. [DOI] [PubMed] [Google Scholar]
- Menon V, Mackenzie K, Rivera SM, Reiss AL. Prefrontal cortex involvement in processing incorrect arithmetic equations: Evidence from event-related fMRI. Human Brain Mapping. 2002;16:119–130. doi: 10.1002/hbm.10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Rivera SM, White CD, Eliez S, Glover GH, Reiss AL. Functional optimization of arithmetic processing in perfect performers. Brain Research, Cognitive Brain Research. 2000;9:343–345. doi: 10.1016/s0926-6410(00)00010-0. [DOI] [PubMed] [Google Scholar]
- Menon V, Rivera SM, White CD, Glover GH, Reiss AL. Dissociating prefrontal and parietal cortex activation during arithmetic processing. Neuroimage. 2000;12:357–365. doi: 10.1006/nimg.2000.0613. [DOI] [PubMed] [Google Scholar]
- Miller EK, Li L, Desimone R. A neural mechanism for working and recognition memory in inferior temporal cortex. Science. 1991;254:5036. doi: 10.1126/science.1962197. [DOI] [PubMed] [Google Scholar]
- Morton J. The interaction of information in word recognition. Psychological Review. 1969;76:165–178. [Google Scholar]
- Naccache L, Dehaene S. The priming method: Imaging unconscious repetition priming reveals an abstract representation of number in the parietal lobes. Cerebral Cortex. 2001;11:966–974. doi: 10.1093/cercor/11.10.966. [DOI] [PubMed] [Google Scholar]
- Orfanidou E, Marslen-Wilson WD, Davis MH. Neural response suppression predicts repetition priming of spoken words and pseudowords. Journal of Cognitive Neuroscience. 2006;18:1237–1252. doi: 10.1162/jocn.2006.18.8.1237. [DOI] [PubMed] [Google Scholar]
- Parvizi J, Van hoesen GW, Buckwalter J, Damasio A. Neural connections of the posteriomedia cortex in the macaque. Proceedings of the National Academy of Sciences, U.S.A. 2006;103:1563–1568. doi: 10.1073/pnas.0507729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz R, Natan C, Boraud T, Bergman H, Vaadia E. Emerging patterns of neuronal responses in supplementary and primary motor areas during sensorimotor adaptation. Journal of Neuroscience. 2005;25:10941–10951. doi: 10.1523/JNEUROSCI.0164-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Sohn MH, Anderson JR, Stenger VA, Fissell K, Goode A, et al. Predicting the practice effects on the blood oxygenation level-dependent (BOLD) function of fMRI in a symbolic manipulation task. Proceedings of the National Academy of Sciences, U.S.A. 2003;100:4951–4956. doi: 10.1073/pnas.0431053100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Fiez JA, Videen TO, MacLeod AM, Pardo JV, Fox PT, et al. Practice-related changes in human brain functional anatomy during nonmotor learning. Cerebral Cortex. 1994;4:8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- Rainer G, Miller EK. Effects of visual experience on the representation of objects in the prefrontal cortex. Neuron. 2000;27:8–10. doi: 10.1016/s0896-6273(00)00019-2. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D’Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2004;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Rickard T, Romero SG, Basso G, Wharton CM, Filtman S, Grafman J. The calculating brain: An fMRI study. Neuropsychologia. 2000;38:325–335. doi: 10.1016/s0028-3932(99)00068-8. [DOI] [PubMed] [Google Scholar]
- Rivera SM, Menon V, White CD, Glaser B, Reiss AL. Functional brain activation during arithmetic processing in females with fragile X Syndrome is related to FMR1 protein expression. Human Brain Mapping. 2002;16:206–218. doi: 10.1002/hbm.10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger HL. Rethinking implicit memory. In: Bowers JS, Marsolek CJ, editors. Rethinking implicit memory. Oxford University Press; 2003. [Google Scholar]
- Ryan L, Schnyer DM. Regional specificity of format specific priming effects in word reading using functional magnetic resonance imaging. Cerebral Cortex. 2007;17:982–992. doi: 10.1093/cercor/bhl009. [DOI] [PubMed] [Google Scholar]
- Sayres R, Grill-Spector K. Object-selective cortex exhibits performance-independent repetition suppression. Journal of Neurophysiology. 2006;95:995–1007. doi: 10.1152/jn.00500.2005. [DOI] [PubMed] [Google Scholar]
- Scarborough DL, Cortese C, Scarborough H. Frequency and repetition effects in lexical memory. Journal of Experimental Psychology: Human Perception and Performance. 1977;3:1–17. [Google Scholar]
- Schacter DL, Wig GS, Stevens WD. Reductions in cortical activity during priming. Current Opinion in Neurobiology. 2007;17:171–176. doi: 10.1016/j.conb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Schnyer DM, Dobbins IG, Nicholls L, Schacter DL, Verfaellie M. Rapid response learning in amnesia: Delineating associative learning components in repetition priming. Neuropsychologia. 2006;44:140–149. doi: 10.1016/j.neuropsychologia.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Sciama SC, Semenza C, Butterworth B. Repetition priming in simple addition depends on surface form and typicality. Memory & Cognition. 1999;27:116–127. doi: 10.3758/bf03201218. [DOI] [PubMed] [Google Scholar]
- Sohn MH, Goode A, Stenger VA, Carter CS, Anderson JR. Competition and representation during memory retrieval: Roles of the prefrontal cortex and the posterior parietal cortex. Proceedings of the National Academy of Sciences, U.S.A. 2003;100:7412–7417. doi: 10.1073/pnas.0832374100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speelman CP, Simpson TA, Kirsner K. The unbearable lightness of priming. Acta Psychologica. 2002;111:191–204. doi: 10.1016/s0001-6918(02)00049-5. [DOI] [PubMed] [Google Scholar]
- Suzuki WA. Associative learning signals in the brain. Progress in Brain Research. 2008;169:305–320. doi: 10.1016/S0079-6123(07)00019-2. [DOI] [PubMed] [Google Scholar]
- Takayama Y, Sugishita M, Akiguchi I, Kimura J. Isolated acalculia due to left parietal lesion. Archives of Neurology. 1994;51:286–291. doi: 10.1001/archneur.1994.00540150084021. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Botvinick MM. Resolving conflict: A response to Martin and Cheng (2006) . Psychonomic Bulletin & Review. 2006;13:402–408. doi: 10.3758/bf03193860. discussion 409-411. [DOI] [PubMed] [Google Scholar]
- Turk-Browne NB, Yi DJ, Chun MM. Linking implicit and explicit memory: Common encoding factors and shared representations. Neuron. 2006;49:917–927. doi: 10.1016/j.neuron.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Van essen DC, Anderson CH, Felleman DJ. Information processing in the primate visual system: An integrated systems perspective. Science. 1992;255:419–423. doi: 10.1126/science.1734518. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, et al. Coherent spontaneous activity identifies a hippocampal-parietal memory network. Journal of Neurophysiology. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Berger GR, Derbyshire SW. Structural and functional dichotomy of human midcingulate cortex. European Journal of Neuroscience. 2003 doi: 10.1111/j.1460-9568.2003.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Hof PR, Vogt LJ. Cingulate gyrus. In: Paxinos G, Mai JK, editors. The human nervous system. San Diego: Elsevier; 2004. pp. 915–949. [Google Scholar]
- Wagner AD, Gabrieli JD, Verfaellie M. Dissociations between familiarity processes in explicit recognition and implicit perceptual memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1997;23:305–323. doi: 10.1037//0278-7393.23.2.305. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Pare-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: Left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Wig GS, Grafton ST, Demos KE, Kelley WM. Reductions in neural activity underlie behavioral components of repetition priming. Nature Neuroscience. 2005;8:1228–1233. doi: 10.1038/nn1515. [DOI] [PubMed] [Google Scholar]
- Wiggs CL, Martin A. Properties and mechanisms of perceptual priming. Current Opinion in Neurobiology. 1998;8:227–233. doi: 10.1016/s0959-4388(98)80144-x. [DOI] [PubMed] [Google Scholar]
- Zago L, Fenske MJ, Aminoff E, Bar M. The rise and fall of priming: How visual exposure shapes cortical representations of objects. Cerebral Cortex. 2005;15:1655–1665. doi: 10.1093/cercor/bhi060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zago L, Tzourio-Mazoyer N. Distinguishing visuospatial working memory and complex mental calculation areas within the parietal lobes. Neuroscience Letters. 2002;331:45–49. doi: 10.1016/s0304-3940(02)00833-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.