Abstract
Background
In vitro and animal studies have shown positive effects of resveratrol on lipid and lipoprotein metabolism, but human studies specifically designed to examine these effects are lacking.
Objective
The primary outcome parameter of this study in overweight and slightly obese subjects was the effect of resveratrol on apoA-I concentrations. Secondary outcome parameters were effects on other markers of lipid and lipoprotein metabolism, glucose metabolism, and markers for inflammation and endothelial function.
Design
This randomized, placebo-controlled crossover study was conducted in 45 overweight and slightly obese men (n = 25) and women (n = 20) with a mean age of 61 ± 7 years. Subjects received in random order resveratrol (150 mg per day) or placebo capsules for 4 weeks, separated by a 4-week wash-out period. Fasting blood samples were collected at baseline and at the end of each intervention period.
Results
Compliance was excellent as indicated by capsule count and changes in resveratrol and dihydroresveratrol concentrations. No difference between resveratrol and placebo was found in any of the fasting serum or plasma metabolic risk markers (mean ± SD for differences between day 28 values of resveratrol vs. placebo: apoA-I; 0.00 ± 0.12 g/L (P = 0.791), apoB100; -0.01 ± 0.11 g/L (P = 0.545), HDL cholesterol; 0.00 ± 0.09 mmol/L (P = 0.721), LDL cholesterol -0.03 ± 0.57 mmol/L (P = 0.718), triacylglycerol; 0.10 ± 0.54 mmol/L (P = 0.687), glucose; -0.08 ± 0.28 mmol/L (P = 0.064), insulin; -0.3 ± 2.5 mU/L (P = 0.516)). Also, no effects on plasma markers for inflammation and endothelial function were observed. No adverse events related to resveratrol intake were observed.
Conclusion
150 mg of daily resveratrol intake for 4 weeks does not change metabolic risk markers related to cardiovascular health in overweight and slightly obese men and women. Effects on glucose metabolism warrant further study.
Trial Registration
ClinicalTrials.gov NCT01364961
Introduction
The many approaches that exist to lower cardiovascular risk are mainly aimed at lowering LDL cholesterol concentrations. Based on epidemiological studies [1–4], increasing HDL cholesterol concentrations may also reduce cardiovascular risk, but this concept has been challenged by intervention studies [5–7]. However, a recent meta-analysis has suggested that increasing apolipoprotein A-I (apoA-I) concentrations is a more important target to reduce the risk of major cardiovascular events than increasing HDL cholesterol concentrations [8]. ApoA-I is the major protein constituent of the HDL particle, which plays a crucial role in many of the favorable effects attributed to HDL particles, including—next to their fundamental role in reverse cholesterol transport—anti-inflammatory, anti-thrombotic and anti-oxidative effects [9,10].
Epidemiological studies have shown a positive association between higher polyphenol intakes and reduced cardiovascular risk [11,12]. This association may be due to increased intakes of trans-resveratrol (3,5,4’-trihydroxystilbene), a polyphenol found in the skin of black grapes. Resveratrol may influence numerous mechanisms, which act on the progression as well as on the regression of atherosclerosis [13]. In fact, in vitro and animal studies have shown many positive effects of resveratrol on genes and proteins involved in lipid and glucose metabolism, including increases in the activities of peroxisome proliferator-activated receptor α (PPARα) and PPARγ [14,15].
While the results from in vitro and animal studies are promising, and an HDL cholesterol elevating effect of berries [16] and red wine [17], both of which contain resveratrol, has been reported, human studies with the primary aim to examine the effects of resveratrol on lipid and lipoprotein metabolism are scarce. Furthermore, human data onto the effect of resveratrol on markers for metabolic risk is limited and results are conflicting [18–20]. Therefore, the primary aim of the present study was to evaluate the effect of 4 wk resveratrol supplementation (150 mg/day, 99% pure trans-resveratrol [resVida provided by DSM Nutritional Products Ltd. (Kaiseraugst, Switzerland]) on apoA-I concentrations in overweight and slightly obese subjects with low HDL cholesterol concentrations. Secondary aims were to assess effects on other markers of lipid and lipoprotein metabolism, glucose metabolism, and markers for inflammation and endothelial function.
Subjects and Methods
Subjects
The protocol for this trial and supporting CONSORT checklist are available as supporting information; see S1 CONSORT Checklist and S1 Protocol.
Subjects were recruited via posters in university and hospital buildings and by advertisements in local newspapers. In addition, men and women who had participated in earlier studies at the Department of Human Biology (Maastricht University) were approached. Subjects came to university for two screening visits with an interval of ≥ 3 days. Fasting blood was sampled on both occasions for lipoprotein and glucose analyses. Also, height, weight and blood pressure were determined. Furthermore, subjects were asked to complete a general and medical questionnaire. Inclusion criteria were as follows: age between 45 and 70 y; mean high-density lipoprotein cholesterol concentration < 1.21 mmol/L for men and < 1.53 mmol/L for women, in order to include subjects with HDL cholesterol concentrations below mean values found in the PROCAM study [2]; mean serum total cholesterol concentration < 8.0 mmol/L; plasma glucose < 7.0 mmol/L; body mass index between 25–35 kg/m2; non-smoker; willing to abstain from resveratrol-rich products two weeks prior to the study and during the study; stable body weight (weight gain or loss < 3 kg in three months prior to screening visit); no indication for treatment with cholesterol-lowering drugs according to Dutch Cholesterol Consensus; no use of medication or a prescribed diet known to affect serum lipid or glucose metabolism; no active cardiovascular disease or recent (< 6 months) event such as acute myocardial infarction or cerebrovascular accident; willing to stop consumption of vitamin supplements, fish oil capsules or products rich in plant stanol or sterol esters two weeks prior to and during the study; self-reported high alcohol consumption (> 21 units / week for men or > 14 units / week for women); no abuse of drugs; no pregnant or breastfeeding women; no participation in another biomedical study within 1 month prior to the first screening visit; not having donated blood (as blood donor) within 1 month prior to the first screening visit, planning to donate blood during the study or within one month after finishing the study. Finally, subjects who were impossible or difficult to puncture as evidenced during the screening visits could not participate. Participants were informed about the aim of the study and nature and risk of the experimental procedures before their written informed consent was obtained. This study was conducted according to the guidelines laid down in the Declaration of Helsinki, approved by the Ethics Committee of the Maastricht University Medical Centre, and registered on 17 January 2011 at ClinicalTrials.gov as NCT01364961.
Study design and products
The study had a double-blind, randomized, placebo-controlled, cross-over design with two 4-wk intervention periods separated by a 4-wk wash-out period. Subjects were allocated to start with either resveratrol or placebo capsules according to a pre-established computer generated randomization scheme, and were asked to take two capsules daily during lunch and dinner. Resveratrol (150 mg/day; resVida, 99.9% trans-resveratrol) and placebo capsules were kindly provided by DSM Nutritional Products Ltd. (Kaiseraugst, Switzerland). Capsules were provided in weekly containers, coded with either ‘A’ or ‘B’ to blind subjects and investigators. Four containers were provided to the volunteers at the start of each intervention periods and subjects were asked to return all containers including any unused capsules, which were counted as a measure of compliance. Two weeks prior to the study and throughout the total study period, subjects were asked to abstain from resveratrol-containing products (such as grapes, wine, berries, peanuts, peanut butter, soy and soy products and pomegranate). At the end of both experimental periods, subjects completed a food-frequency questionnaire (FFQ) in which food intake from the previous 4 wk was recorded. A dietitian immediately checked the FFQs and calculated energy and nutrient intakes of the subjects using the Dutch food-composition tables. Subjects were asked to record any signs of illnesses, use of medication or alcohol consumption in a study diary.
Blood sampling
Fasting blood samples were taken by venipuncture at the start and at day 25 of each intervention period. At day 28 blood was sampled through an intravenous catheter using a Vacutainer system (Becton, Dickinson and Company, Franklin Lanes, NY, USA). On days preceding blood drawings, subjects were asked to avoid the intake of drinks containing alcohol or caffeine, and not to take part in any strenuous activity. Blood samples were taken from a forearm vein after an overnight fast (no food or drink after 8 PM, except for water), by the same person, and at the same location. Serum separator tubes (Becton, Dickinson and Company, Franklin Lanes, NY, USA) were used for the analysis of lipids and lipoproteins, insulin and hsCRP. Serum tubes were kept at room temperature for at least 30 minutes after sampling to allow clotting. Serum was obtained by centrifugation at 1300 × g for 15 minutes at 21°C. EDTA-coated tubes (Becton, Dickinson and Company, Franklin Lanes, NY, USA) were used for measurement of inflammatory markers, resveratrol and dihydroresveratrol, and clinical chemistry measurements. Sodium fluoride (NaF) tubes (Becton, Dickinson and Company, Franklin Lanes, NY, USA) were used for analysis of plasma glucose. After blood drawing, NaF and EDTA-coated tubes were kept on ice and centrifuged within 30 minutes. Plasma was obtained by centrifugation at 1300 × g for 15 minutes at 4°C. Serum and plasma samples were portioned into aliquots, snap-frozen in liquid N2, and stored at -80°C. Hemostatic and hematologic measurements were performed in whole blood from citrate-filled and EDTA-coated tubes respectively (Becton, Dickinson and Company, Franklin Lanes, NY, USA). These measurements were performed within 4 hours after blood drawing.
Clinical and laboratory measurements
All samples were analyzed for serum apoA-I (Horiba ABX, Montpellier, France), apolipoprotein B-100 (apoB-100; Horiba ABX, Montpellier, France), high-sensitive C-reactive protein (hsCRP; Horiba ABX, Montpellier, France), total cholesterol (CHOD-PAP method; Roche Diagnostics, Mannheim, Germany), HDL cholesterol (precipitation method; Roche Diagnostics, Mannheim, Germany) and triacylglycerol with correction for free glycerol (GPO Trinder; Sigma-Aldrich Corp., St. Louis, MO, USA). LDL cholesterol was calculated by using the Friedewald formula [21]. Furthermore, fasting EDTA-plasma samples were analyzed for insulin concentrations (RIA; Millipore, Billerica, MA, USA) and fasting Na-F plasma samples were analyzed for glucose concentrations (Horiba ABX, Montpellier, France). IL-6, TNFα, E-selectin, thrombomodulin, P-selectin, ICAM-3, sICAM-1 and sVCAM-1 were measured by commercially available Multi Spot ELISA kits (Meso Scale Discovery, Rockville, MD, USA).
Liver and kidney function, and general health parameters (ureum, creatinin, alkaline phosphatase (ALP), γ-glutamyl transpeptidase (GGT), aspartate aminotransferase (ASAT), alanine aminotransferase (ALAT), total bilirubin, total protein, albumin, sodium, potassium, calcium, phosphorus and chloride) were measured in serum on a Beckman Coulter Synchron LX20 PRO Clinical System (Beckman Coulter Inc., Fullerton, CA, USA). Hematologic parameters (erythrocyte-, thrombocyte-, and leucocyte count, haemoglobin, haematocrit, erythrocyte mean corpuscular volume, erythrocyte mean corpuscular haemoglobin (concentration), and erythrocyte relative distribution weight) were measured on a Beckman Coulter LH750 (Beckman Coulter Inc., Fullerton, CA, USA) until October 2011. From October 2011 onwards, measurements were performed on a Sysmex XE-5000 System (Sysmex Corporation, Kobe, Japan). Haemostatic measurements (prothrombin time (PT) and activated partial thromboplastin time (aPTT)) were performed on a Sysmex CA-7000 Analyzer (Sysmex Corporation, Kobe, Japan).
Body weight was measured at each visit in the morning, after an overnight fast. Seated blood pressure was measured each visit (Omron M7, Omron Healthcare Co., Ltd, Kyoto, Japan), after 5 minutes rest. Measurements were performed four times and the average of the last three measurements is reported.
The degree of insulin resistance was estimated by HOMAIR, as described [22].
Compliance was checked by capsule count and by measuring total resveratrol and dihydroresveratrol concentrations (both free and conjugated forms) in plasma by liquid chromatography-mass spectrometry at day 28 of both intervention periods by DSM Nutritional Products, Ltd. Kaiseraugst, Switzerland.
Statistical analyses
Before the start of the study, it was calculated that the statistical power to detect a true difference of at least 0.05 g/L in serum apo A-I concentrations between the experimental and control period was over 80%, when 45 subjects were included at P = 0.05. For the calculations, a within-subject variability of 0.12 g/L in serum apo A-I concentrations was used. As the expected drop-out rate was 10%, a total of 50 men and women were included.
A Shapiro-Wilk normality test was performed to examine if the changes between days 28 of the resveratrol and control periods were normally distributed. A student’s paired t-test was used to compare the values of the experimental and control periods from day 28 in normally distributed data. When data were not normally distributed, a Wilcoxon signed-rank test was used. Results, presented as means ± standard deviations (SD), were considered to be statistically significant if p < 0.05. Statistical analyses were performed using SPSS 19.0 for Mac Os X (SPSS Inc., Chicago, IL, USA).
Results
Subject characteristics and compliance
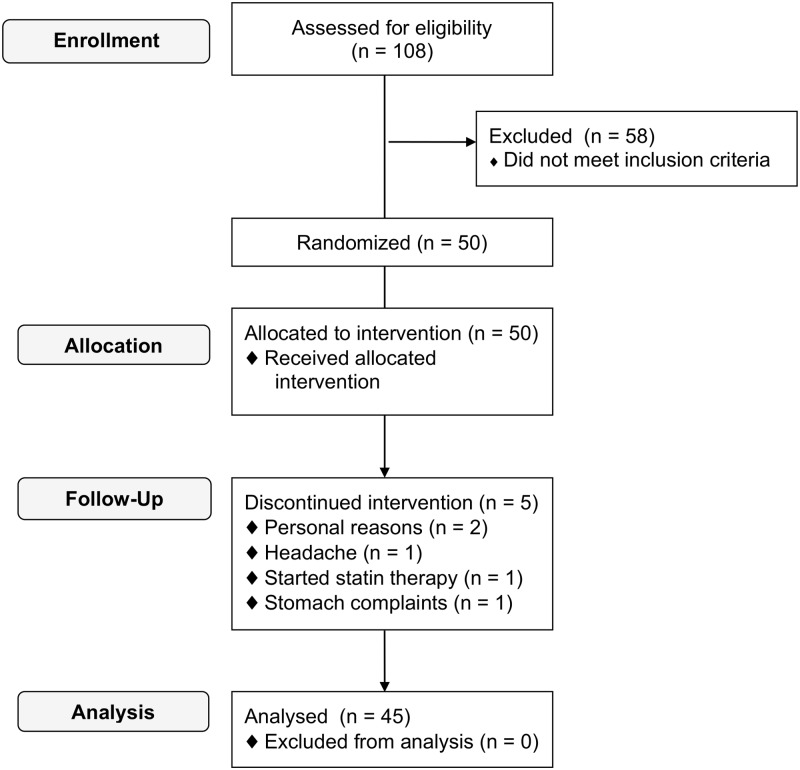
A flow diagram of participants throughout the study is shown in Fig. 1. After screening, 50 subjects were eligible for participation and started the study. Two female subjects discontinued intervention because of personal reasons, 1 male subject dropped out because of a headache, one female subject dropped out because of stomach complaints and another female subject started statin treatment after randomization. A total of five subjects dropped out either during or straight after the first intervention period. Three of these subjects dropped out during the control period and two subjects during the resveratrol period. A total of 45 subjects (25 men and 20 women) completed the study. Baseline characteristics of study participants are shown in Table 1. Fourteen subjects used medication such as antidepressants, antihypertensives or antacids throughout the study without a change in dosage.
Fig 1. Subject flow chart.
Table 1. Baseline characteristics of participants.1,2 .
| All (N = 45) | Males (N = 25) | Females (N = 20) | |
|---|---|---|---|
| Age (y) | 60 ± 7 | 62 ± 7 | 59 ± 7 |
| BMI (kg/m 2 ) | 28.8 ± 3.2 | 28.5 ± 2.9 | 29.2 ± 3.6 |
| Systolic BP (mmHg) | 136 ± 17 | 138 ± 16 | 135 ± 18 |
| Diastolic BP (mmHg) | 88 ± 9 | 88 ± 10 | 88 ± 9 |
| Heart rate (BPM) | 67 ± 8 | 67 ± 8 | 68 ± 7 |
| Glucose (mmol/L) | 5.66 ± 0.53 | 5.71 ± 0.57 | 5.59 ± 0.48 |
| Total cholesterol (mmol/L) | 6.38 ± 1.00 | 6.20 ± 1.01 | 6.60 ± 0.97 |
| HDL cholesterol (mmol/L) | 1.14 ± 0.23 | 1.00 ± 0.17 | 1.30 ± 0.18 |
1Values are means ± SD.
2BP, blood pressure; BPM: beats per minute.
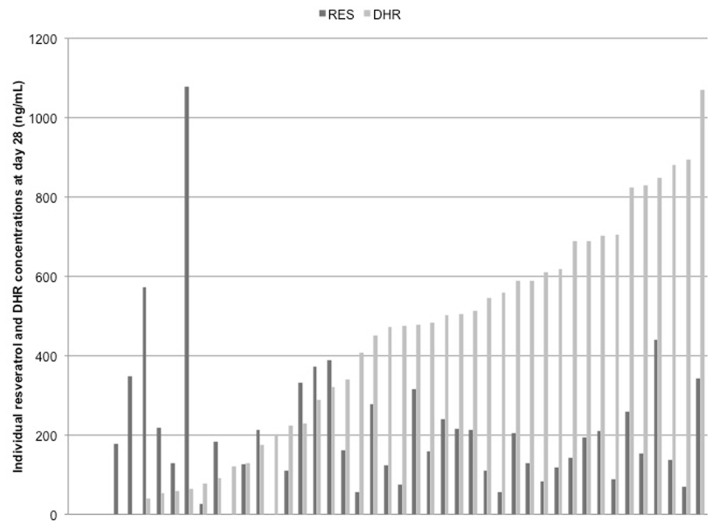
As evidenced from pill count, compliance ranged between 88% and 111% and was on average 99% during both the control and resveratrol period. To further check for compliance, free and total (sum of conjugated and free resveratrol) resveratrol and dihydroresveratrol (DHR) concentrations were measured in all plasma samples. Free resveratrol and free DHR were not detectable, except in all plasma samples from one subject. Total resveratrol and DHR were detectable in three subjects during the control period and in one subject at the start of the resveratrol period. At day 28 of the resveratrol period, mean total resveratrol concentrations were 197 ± 185 ng/mL and those of DHR 408 ± 298 ng/mL. During the entire experimental period, one subject had no measurable plasma concentrations of total resveratrol or total DHR. Two subjects had no measurable resveratrol and DHR concentrations at day 28, while these compounds were present at day 25. Another two subjects had no measurable DHR concentrations during the total study period. For all subjects, plasma total resveratrol concentrations ranged between 0 and 1080 ng/mL, while total DHR concentrations varied between 0 and 1070 ng/mL during the resveratrol period. Individual resveratrol and DHR concentrations are shown in Fig. 2.
Fig 2. Individual total resveratrol (RES) and total dihydroresveratrol (DHR) concentrations at day 28.
Composition of the diets between the periods was comparable (S1 Table). Body weight at the end of the control period was 84 ± 12 kg and at the end of the resveratrol period 84 ± 12 kg. These values were not significantly different (p = 0.910).
Metabolic risk markers
The effects of 4-wk resveratrol supplementation on serum lipid and lipoprotein concentrations are presented in Table 2. No statistically significant differences were observed between the resveratrol and placebo periods on serum apoA-I and apoB-100 concentrations. In addition, no effects were found on serum total-, LDL- and HDL cholesterol and ratio of total to HDL-cholesterol. Triacylglycerol concentrations were significantly higher (P < 0.05) after the resveratrol period compared to the control period.
Table 2. Effect of 4-wk resveratrol intake on serum lipids and lipoproteins. 1 .
| Placebo | Resveratrol | Difference | P value | |
|---|---|---|---|---|
| Total cholesterol (mmol/L) | 5.70 ± 0.90 | 5.69 ± 0.83 | -0.02 ± 0.62 | 0.870 |
| LDL cholesterol (mmol/L) | 3.83 ± 0.84 | 3.77 ± 0.75 | -0.06 ± 0.59 | 0.530 |
| HDL cholesterol (mmol/L) | 1.15 ± 0.23 | 1.15 ± 0.24 | -0.01 ± 0.09 | 0.696 |
| Total:HDL cholesterol ratio | 5.14 ± 1.31 | 5.16 ± 1.24 | 0.02 ± 0.58 | 0.863 |
| Triacylglycerol (mmol/L) 2 | 1.58 ± 0.72 | 1.68 ± 0.77 | 0.10 ± 0.54 | 0.048 |
| Apolipoprotein A-I (g/L) 2 | 1.28 ± 0.19 | 1.27 ± 0.20 | 0.00 ± 0.12 | 0.670 |
| Apolipoprotein B-100 (g/L) | 1.17 ± 0.20 | 1.16 ± 0.17 | -0.01 ± 0.11 | 0.545 |
Values are means ± SD.
1N = 45.
2These parameters were tested by a Wilcoxon signed-rank test for non-normal distributed data.
A significant increase in diastolic blood pressure and heart rate (P < 0.05 for both variables) was observed after the resveratrol period. Insulin concentrations, HOMAIR, systolic blood pressure and MAP were comparable between both periods (Table 3).
Table 3. Effect of 4-wk resveratrol intake on metabolic risk markers. 3 .
| BMI (kg/m 2 ) 1 | 28.3±3.1 | 28.4±3.1 | 0.0±0.5 | 0.760 |
| Glucose (mmol/L) 1,4 | 5.30±0.54 | 5.22±0.49 | -0.08±0.28 | 0.112 |
| Insulin (mU/L) 1 | 13.1±5.0 | 12.9±5.3 | -0.2±2.5 | 0.385 |
| HOMAIR 1, 4 | 3.13±1.35 | 3.03±1.44 | -0.10±0.69 | 0.208 |
| Systolic BP (mmHg) 2, 4 | 130±18 | 132±17 | 2±15 | 0.234 |
| Diastolic BP (mmHg) 2 | 84±9 | 86±9 | 2±7 | 0.044 |
| Heart rate (BPM) 2 | 64±8 | 67±8 | 3±7 | 0.025 |
| MAP (mmHg) 2, 4 | 99±11 | 102±11 | 2±9 | 0.064 |
Values are means ± SD.
1N = 45.
2N = 44.
3BP, blood pressure; BPM, beats per minute; MAP, mean arterial pressure.
4These parameters were tested by a Wilcoxon signed-rank test for non-normal distributed data.
Table 4 shows the effect of resveratrol supplementation on markers for inflammation and endothelial function. No significant differences were found for hsCRP concentrations between both interventions. Also, no differences between both periods were found for IL-6, TNFα, E-selectin, thrombomodulin, P-selectin, ICAM-3, sICAM-1, and sVCAM-1 concentrations. Ten subjects (6 men and 4 women) had hsCRP levels > 10 mg/L on one or more occasions, which were equally divided across intervention and time points of blood sampling. Conclusions did not change when these subjects were excluded from analyses.
Table 4. Effect of 4-wk resveratrol intake on markers for inflammation and endothelial function.1,2 .
| Placebo | Resveratrol | Difference | P value | |
|---|---|---|---|---|
| hsCRP (mg/L) 3 | 2.64 ± 2.93 | 4.51 ± 10.24 | 1.87 ± 10.02 | 0.326 |
| IL-6 (pg/mL) 3 | 1.63 ± 1.06 | 1.85 ± 1.42 | 0.23 ± 1.26 | 0.718 |
| TNFα (pg/mL) 3 | 4.00 ± 1.26 | 3.95 ± 0.74 | -0.05 ± 0.91 | 0.455 |
| E-Selectin (ng/mL) 3 | 9.86 ± 6.51 | 9.64 ± 6.72 | -0.22 ± 2.46 | 0.569 |
| Thrombomodulin (ng/mL) 3 | 3.32 ± 0.77 | 3.29 ± 0.77 | -0.03 ± 0.34 | 0.815 |
| P-Selectin (ng/mL) 3 | 75 ± 68 | 73 ± 52 | -2 ± 82 | 0.467 |
| ICAM-3 (ng/mL) 3 | 0.67 ± 0.29 | 0.67 ± 0.30 | 0.00 ± 0.15 | 0.480 |
| sICAM-1 (ng/mL) | 324 ± 86 | 322 ± 82 | -2 ± 64 | 0.829 |
| sVCAM-1 (ng/mL) | 561 ± 111 | 570 ± 130 | 8 ± 105 | 0.595 |
Values are means ± SD.
1N = 45.
2hsCRP, high-sensitivity C-reactive protein; ICAM-3, intercellular adhesion molecule-3; IL-6, interleukin-6; sICAM-1 soluble intercellular adhesion molecule-1; sVCAM-1, soluble vascular adhesion molecule-1.
3These parameters were tested by a Wilcoxon signed-rank test for non-normal distributed data.
Exploratory data analyses were performed to investigate the possible impact of subjects’ characteristics on the results. However, there were no indications that effects were different for men and women, or for obese (BMI ≥30 kg/m2; n = 16: 7 men and 9 women) and non-obese subjects (BMI <30 kg/m2; n = 29: 18 men and 11 women) separately. The same conclusion was drawn, when subgroups were formed based on median levels of day 0 of the control period for the HOMAIR index, serum triacylglycerol concentrations, or on DHR concentrations or DHR:resveratrol ratios of day 28 of the resveratrol period. When analyses were restricted to subjects that did not use any prescribed medication (n = 31), the decrease in plasma glucose concentrations during the resveratrol period became statistically significant (-0.14 mmol/L; P < 0.05)
General health parameters
Apart from statistically significant higher ALP values after resveratrol supplementation, no differences were found after 4-wk resveratrol treatment compared to control in any of the clinical chemistry, hematology and coagulation parameters (S2 Table).
Discussion
In this study with overweight and slightly obese men and women, we found no effects on serum apoA-I and other metabolic risk markers after 4-wk of daily supplementation with 150 mg trans-resveratrol. Compliance of the subjects was excellent, as indicated by capsule counts and total plasma resveratrol concentrations. A lack of effect on apoA-I concentrations does not support in vitro data, which suggests that resveratrol induces the expression of the apoA-I gene [14,23]. So far, however, no human studies have specifically addressed the effects of trans-resveratrol on apoA-I concentrations. Our finding that resveratrol supplementation also has no effect on HDL cholesterol agrees with the results of a very recent meta-analysis. In that study, no effects of resveratrol on HDL cholesterol concentrations were found, irrespective of dose, study duration, and cardiovascular risk status of the subjects [24]. A major limitation of this meta-analysis was that, in four out of the seven studies included, subjects used cholesterol-lowering and/or hypoglycemic drugs, while in none of the studies changes in serum lipid concentrations were the primary outcome parameter. In our study, however, subjects did not use these types of medication and were selected based on low HDL cholesterol concentrations. In addition, in only five of the seven studies, purified trans-resveratrol was used, whereas in the other studies resveratrol-enriched grape extract or Polygonum Cuspidatum extract containing trans-resveratrol was given. However, like in the recent meta-analysis, we also did not observe any effects on serum total cholesterol, LDL cholesterol and in addition apoB100 concentrations. Another recent double-blind cross over trial tested the effects of high-dose (e.g. 1000 mg during the first week and 2000 mg during the second week) trans-resveratrol intake on apoB100 production- and catabolic rate in overweight and obese, mildly hypertriglyceridemic males. Reductions of both apoB100 production and catabolic rates were found without accompanying changes in plasma triglyceride-rich lipoprotein (TRL) apoB100 concentrations [25]. This may agree with our results, although it should be noted that the major of the plasma apoB100 pool is transported by the triglyceride-poor LDL particles. To summarize, it is not very likely that the putative positive effects of resveratrol on cardiovascular health are mediated by changes in serum lipid or lipoprotein concentrations.
The question then remains whether resveratrol protects against cardiovascular disease through effects other than those on lipid and lipoprotein metabolism. Several studies have reported positive effects of resveratrol on insulin sensitivity, accompanied by a significant lowering of blood glucose levels. However, we did not observe a statistically significant change in HOMAIR and a tendency towards decreased fasting plasma glucose concentrations, which became statistically significant, when subjects who used medication were excluded from the statistical analyses. Results of resveratrol on plasma glucose concentrations are conflicting, and both positive and no-effect studies have been reported. Again, studies have been performed in diverse populations. Effects on fasting glucose concentrations have mainly been found in studies with type 2 diabetes mellitus (T2DM) patients [26,27], although part of these results have to be interpreted cautiously because of methodological constraints [27]. No effects have been found in a study that included obese, but otherwise healthy subjects [18]. Two other studies used the same type of trans-resveratrol as we did [19,20]. In one study, no effect on fasting glucose concentrations was found after 12 weeks of 75 mg/d resveratrol supplementation in non-obese women with normal glucose tolerance [20], whereas Timmers et al. reported a significant decrease of 0.18 mmol/L in glucose after 30-day resveratrol supplementation in obese, but healthy male subjects [19]. Although changes in glucose concentrations were a secondary outcome, our study had a statistical power of nearly 100% to detect a true change in glucose of 0.18 mmol/L at an alpha of 0.05. Thus, daily supplementation with 150 mg trans-resveratrol may improve glucose metabolism, but more well-designed studies are needed to answer this question.
In vitro and animal studies have suggested positive effects of resveratrol on endothelial function and low-grade systemic inflammation [28]. However, our results are in line with other human studies that did not show an effect on different markers for low-grade inflammation, such as CRP, IL1β, IL6, IL8, TNFα and monocyte chemotactic protein-1 [18–20,26,29]. In one study, a reduction in TNFα [19] was found, which we—and others [18]—could not reproduce. Regarding vascular and endothelial function, Agarwal et al. showed a lower expression of VCAM- and ICAM mRNA after treatment of human coronary artery endothelial cells with plasma from subjects treated with resveratrol [29]. However, we found no effects on circulating levels of ICAM-3, sICAM-1, sVCAM-1, E-Selectin and P-Selectin. Therefore, consistent evidence from human studies for beneficial effects of resveratrol intake on plasma markers for low-grade inflammation and endothelial function is missing.
Finally, it has been suggested that trans-resveratrol lowers systolic blood pressure (SBP) and mean arterial pressure (MAP) [19]. However, these effects could not be confirmed in other studies, which also found no effects on diastolic blood pressure (DBP) and heart rate [18,20,30]. In our study, SBP and MAP also remained unchanged, but DBP and heart rate increased after trans-resveratrol intake, for which we have no explanation.
It is possible that certain groups may benefit from resveratrol supplementation and other groups do not. Although our study was not powered to answer this question adequately, exploratory data analyses did not suggest that effects did depend on sex, BMI, triacylglycerol concentrations or HOMAIR. Interestingly, Bode et al. showed pronounced inter-individual differences in trans-resveratrol metabolism by human gut microbiota [31], which might also contribute to discrepancies between studies. Also, in our subjects we found large differences in plasma DHR concentrations. However, we found no indications that results did depend on DHR concentrations or DHR:resveratrol ratios.
In summary, we here show that 150 mg of daily resveratrol intake for four weeks does not change lipids, lipoproteins and other metabolic risk markers related to cardiovascular health in overweight and slightly obese men and women with low HDL cholesterol concentrations. Effects on glucose metabolism however warrant further study.
Supporting Information
(DOC)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We would like to thank Martine Hulsbosch and Maud Beckers for their technical support and Nina Wystyrk for her dietary assistance.
Data Availability
All relevant data necessary to replicate the results of this paper are contained within the paper and its Supporting Information files.
Funding Statement
The project is funded by TI Food and Nutrition, a public-private partnership on precompetitive research in food and nutrition. The public partners are responsible for the study design, data collection and analysis, decision to publish, and preparation of the manuscript. Trans-resveratrol capsules were provided by DSM Nutritional Products Ltd. (Kaiseraugst, Switzerland).
References
- 1. Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA. 1986;256: 2835–2838. [PubMed] [Google Scholar]
- 2. Assmann G, Schulte H, von Eckardstein A, Huang Y. High-density lipoprotein cholesterol as a predictor of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis. 1996;124 Suppl: S11–20. [DOI] [PubMed] [Google Scholar]
- 3. Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62: 707–714. [DOI] [PubMed] [Google Scholar]
- 4. Sharrett AR, Ballantyne CM, Coady SA, Heiss G, Sorlie PD, Catellier D, et al. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2001;104: 1108–1113. [DOI] [PubMed] [Google Scholar]
- 5. Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357: 2109–2122. [DOI] [PubMed] [Google Scholar]
- 6. Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365: 2255–2267. 10.1056/NEJMoa1107579 [DOI] [PubMed] [Google Scholar]
- 7. Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367: 2089–2099. 10.1056/NEJMoa1206797 [DOI] [PubMed] [Google Scholar]
- 8. Boekholdt SM, Arsenault BJ, Hovingh GK, Mora S, Pedersen TR, Larosa JC, et al. Levels and changes of HDL cholesterol and apolipoprotein A-I in relation to risk of cardiovascular events among statin-treated patients: a meta-analysis. Circulation. 2013;128: 1504–1512. 10.1161/CIRCULATIONAHA.113.002670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nicholls SJ. Apo a-I modulating therapies. Curr Cardiol Rep. 2011;13: 537–543. 10.1007/s11886-011-0223-0 [DOI] [PubMed] [Google Scholar]
- 10. Hovingh GK, Bochem AE, Kastelein JJ. Apolipoprotein A-I mimetic peptides. Curr Opin Lipidol. 2010;21: 481–486. 10.1097/MOL.0b013e3283404507 [DOI] [PubMed] [Google Scholar]
- 11. Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342: 1007–1011. [DOI] [PubMed] [Google Scholar]
- 12. Manach C, Mazur A, Scalbert A. Polyphenols and prevention of cardiovascular diseases. Curr Opin Lipidol. 2005;16: 77–84. [DOI] [PubMed] [Google Scholar]
- 13. Fan E, Zhang L, Jiang S, Bai Y. Beneficial effects of resveratrol on atherosclerosis. J Med Food. 2008;11: 610–614. 10.1089/jmf.2007.0091 [DOI] [PubMed] [Google Scholar]
- 14. Iannelli P, Zarrilli V, Varricchio E, Tramontano D, Mancini FP. The dietary antioxidant resveratrol affects redox changes of PPARalpha activity. Nutr Metab Cardiovasc Dis. 2007;17: 247–256. [DOI] [PubMed] [Google Scholar]
- 15. Inoue H, Jiang XF, Katayama T, Osada S, Umesono K, Namura S. Brain protection by resveratrol and fenofibrate against stroke requires peroxisome proliferator-activated receptor alpha in mice. Neurosci Lett. 2003;352: 203–206. [DOI] [PubMed] [Google Scholar]
- 16. Erlund I, Koli R, Alfthan G, Marniemi J, Puukka P, Mustonen P, et al. Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. Am J Clin Nutr. 2008;87: 323–331. [DOI] [PubMed] [Google Scholar]
- 17. Sacanella E, Vazquez-Agell M, Mena MP, Antunez E, Fernandez-Sola J, Nicolas JM, et al. Down-regulation of adhesion molecules and other inflammatory biomarkers after moderate wine consumption in healthy women: a randomized trial. Am J Clin Nutr. 2007;86: 1463–1469. [DOI] [PubMed] [Google Scholar]
- 18. Poulsen MM, Vestergaard PF, Clasen BF, Radko Y, Christensen LP, Stodkilde-Jorgensen H, et al. High-dose resveratrol supplementation in obese men: an investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes. 2013;62: 1186–1195. 10.2337/db12-0975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14: 612–622. 10.1016/j.cmet.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yoshino J, Conte C, Fontana L, Mittendorfer B, Imai S, Schechtman KB, et al. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab. 2012;16: 658–664. 10.1016/j.cmet.2012.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18: 499–502. [PubMed] [Google Scholar]
- 22. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28: 412–419. [DOI] [PubMed] [Google Scholar]
- 23. Hansen HC, Chiacchia FS, Patel R, Wong NC, Khlebnikov V, Jankowska R, et al. Stilbene analogs as inducers of apolipoprotein-I transcription. Eur J Med Chem. 2010;45: 2018–2023. 10.1016/j.ejmech.2009.12.076 [DOI] [PubMed] [Google Scholar]
- 24. Sahebkar A. Effects of resveratrol supplementation on plasma lipids: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2013. [DOI] [PubMed] [Google Scholar]
- 25. Dash S, Xiao C, Morgantini C, Szeto L, Lewis GF. High-dose resveratrol treatment for 2 weeks inhibits intestinal and hepatic lipoprotein production in overweight/obese men. Arterioscler Thromb Vasc Biol. 2013;33: 2895–2901. 10.1161/ATVBAHA.113.302342 [DOI] [PubMed] [Google Scholar]
- 26. Brasnyo P, Molnar GA, Mohas M, Marko L, Laczy B, Cseh J, et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br J Nutr. 2011;106: 383–389. 10.1017/S0007114511000316 [DOI] [PubMed] [Google Scholar]
- 27. Bhatt JK, Thomas S, Nanjan MJ. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr Res. 2012;32: 537–541. 10.1016/j.nutres.2012.06.003 [DOI] [PubMed] [Google Scholar]
- 28. Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z. Resveratrol attenuates TNF-alpha-induced activation of coronary arterial endothelial cells: role of NF-kappaB inhibition. Am J Physiol Heart Circ Physiol. 2006;291: H1694–1699. [DOI] [PubMed] [Google Scholar]
- 29. Agarwal B, Campen MJ, Channell MM, Wherry SJ, Varamini B, Davis JG, et al. Resveratrol for primary prevention of atherosclerosis: clinical trial evidence for improved gene expression in vascular endothelium. Int J Cardiol. 2013;166: 246–248. 10.1016/j.ijcard.2012.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wong RH, Berry NM, Coates AM, Buckley JD, Bryan J, Kunz I, et al. Chronic resveratrol consumption improves brachial flow-mediated dilatation in healthy obese adults. J Hypertens. 2013;31: 1819–1827. 10.1097/HJH.0b013e328362b9d6 [DOI] [PubMed] [Google Scholar]
- 31. Bode LM, Bunzel D, Huch M, Cho GS, Ruhland D, Bunzel M, et al. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am J Clin Nutr. 2013;97: 295–309. 10.3945/ajcn.112.049379 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data necessary to replicate the results of this paper are contained within the paper and its Supporting Information files.