Abstract
Background
Preeclampsia (PE) increases maternal and perinatal morbidity and mortality. Based on a multitude of data from randomized clinical trials, clinical practice guidelines endorse using ASA to prevent PE in women who are “at risk.” However, data are lacking about the level of absolute risk to warrant starting ASA prophylaxis.
Methods and Findings
We present two approaches for objectively determining the minimum absolute risk for PE at which ASA prophylaxis is justified. The first is a new approach—the minimum control event rate (CERmin). The second approach uses a pre-existing concept—the minimum event rate for treatment (MERT). Here we show how the CERmin is derived, and then use the CERmin and the MERT to guide us to a reasonable risk threshold for starting a woman on ASA prophylaxis against PE based on clinical risk assessment. We suggest that eligible women need not be at “high risk” for preeclampsia to warrant ASA, but rather at some modestly elevated absolute risk of 6–10%.
Conclusions
Given its very low cost, its widespread availability, ease of administration and its safety profile, ASA is a highly attractive agent for the prevention of maternal and perinatal morbidity worldwide.
Background
Preeclampsia (PE), defined as new-onset hypertension after 20 weeks gestation often with significant proteinuria [1, 2] affects between 2.5% and 7.6% of pregnancies in the general population Worldwide [3]. PE is associated with increased maternal and perinatal morbidity and mortality [4], cited as the leading cause of admission to the intensive care unit during the puerperal period [5, 6], and is the fourth leading cause of maternal death [7]. Additionally, women with PE have a higher risk of placental abruption, and their fetuses have a higher risk of stillbirth, preterm delivery, and intrauterine growth restriction [8–10].
Acetylsalicylic acid (ASA) effectively reduces the risk of PE and its aforementioned sequelae [11–14], without producing adverse effects on the mother or fetus [3, 15]. Meta-analyses of randomized controlled trials (RCTs) demonstrate a 17% relative risk reduction (RRR) for PE with ASA over placebo [11]. ASA prophylaxis started early in the pregnancy, at 12–16 weeks, confers a 53% RRR for PE and a 91% RRR for severe PE [12–14]. Important benefits are also seen for the fetus, such as a reduced risk of preterm birth, intrauterine growth restriction and neonatal death and morbidity [12–14] (S1 Table).
Current guidelines for obstetricians and midwives endorse using ASA for the prevention of PE in women at risk of this condition [1]. However, guidance about what level of PE risk warrants ASA prophylaxis is lacking. This uncertainty has resulted in inconsistencies in recommendations and possible underuse of ASA [16]. Without an agreed upon threshold of risk for PE, based upon valid algorithms, it is problematic for clinicians to identify which women should receive ASA.
Establishing a threshold level of risk for PE is needed by clinicians, since there is a growing body of evidence that details how different clinical risk factors can forecast PE risk. Based on this evidence, estimates of PE risk range from less than 4% for women with no risk factors up to around 25% for women with just one risk factor, such as chronic hypertension [17, 18]. Other clinical risk factors also heighten the risk. For example, the risk of developing PE is 10% in obese women with a body mass index of 30 kg/m2 or greater, increasing to 16% if her systolic blood pressure is also over 120 mm Hg [18].
We present two approaches for objectively determining the minimum risk for PE at which ASA prophylaxis is justified. The first is a new approach—the minimum control event rate (CER min). The second approach uses a pre-existing concept—the minimum event rate for treatment (MERT) [19]. Here we show how the CERmin is derived, and then use the CERmin and the MERT to guide us to a reasonable risk threshold for starting a woman on ASA prophylaxis against PE based on clinical risk assessment.
Methods and Results
Threshold Number Needed to Treat (NNTT)
A useful measure of ASA efficacy in this context is the number needed to treat (NNT)—the number of patients a clinician needs to treat in order to prevent one target outcome, such as a stroke [19]. The NNT has been widely adopted to communicate treatment effect sizes in a clinically meaningful way [20]. In 2001, Sinclair et al. introduced the idea of using a threshold for the NNT—the NNT T—which is the maximum number of patients a clinician would be willing to treat to prevent one target outcome [21]. The NNT T, which aims to guide treatment recommendations, is determined by estimating the clinical value of preventing one target event and identifying the number of patients that would need to be treated such that this benefit is equivalent to the negative consequences of treating the same number of patients—both in terms of adverse events and costs [21]. In the specific case of using ASA to prevent PE and related outcomes, we herein use the number needed to prevent (NNP) and the threshold NNP T.
The value of preventing one target event can be estimated in quality adjusted life years (QALY) gained. To solve for the NNP T, we assign a $50 000 value per QALY gained, in accordance with the conventional willingness to pay threshold accepted in cost-effectiveness analysis research [22, 23].
Deriving the Minimum Control Event Rate (CERmin)
The CERmin is the minimum disease event rate in the placebo control group(s) of one or more RCTs in which the intervention (e.g., ASA) is cost-effective.
To derive the CERmin for ASA prophylaxis, we consider both the NNPT and the known prevalence of PE in the ASA treatment groups within the RCTs—the treatment event rate (TER).
First, we isolate the CER, in terms of TER and NNP:
| (Equation 1) |
In the case of CERmin, we focus on the NNPT, which was derived using the equation by Shimbo et al. [24], a simplification of that by Sinclair et al. [21]:
where DC is the direct cost of the treatment for one patient, and QALYs gained is the number of quality adjusted life years gained by avoiding one target event.
Now, substituting the equation for NNPT provided by Shimbo et al. [24] into Equation 1, we can approximate the CERmin as follows:
Hence, we arrive at the final equation:
| (Equation 2) |
Application of the CERmin to preeclampsia prophylaxis with ASA
Using Equation 2 for the CERmin we fill in the known values for ASA prophylaxis, namely, the DC, QALYs gained, and TER.
The DC of taking 81 mg baby ASA daily for one year varies between $14 to $24 [25, 26], and ASA can be purchased at any drug store. Assuming that a woman needs to take ASA prophylaxis from 12 to 38 weeks gestation, she will pay a DC of $10.
Next, we consider QALYs gained per case of preeclampsia prevented. In the case of ASA prophylaxis, one analysis considered the benefits to both the mother and baby, arriving at a value of 0.52 QALYs gained per pregnancy [1]. Nonetheless, a value of 0.52 QALYs gained per pregnancy may be a generous estimate, so we determined NNPT values at various QALYs gained, starting at as low as 0.005 QALYs gained (Fig. 1).
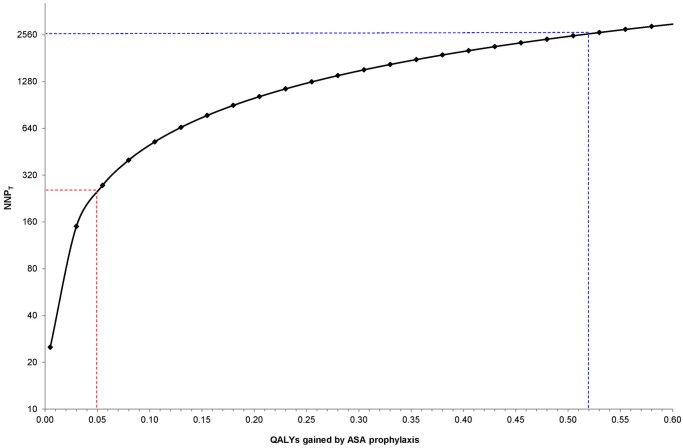
Fig 1. Threshold number needed to prevent (NNPT) at varying quality-adjusted life years (QALYs) gained per preeclampsia event avoided.
NNPT calculated as: (QALYs gained x $50000) / DC [24], assuming a direct cost (DC) of ASA of $10 per pregnancy. The dashed blue lines show that, at 0.52 QALYs gained by ASA prophylaxis [1], a clinician would be willing to give ASA prophylaxis to as many as 2600 women—the NNPT. For a more modest amount of 0.05 QALYs gained, the NNPT would be 250 (dashed red lines). The NNPT is presented on a log2 scale for ease of viewing.
In calculating the CERmin by Equation 2, we first fix the value for QALYs gained at 0.05 and 0.52, and observe how the CERmin changes with increasing TER (Fig. 2). We see that at a DC of $10, the TER very closely approximates the CERmin when QALYs gained is 0.52. At a QALY gain of 0.05, the CERmin is 0.4% higher than the TER.
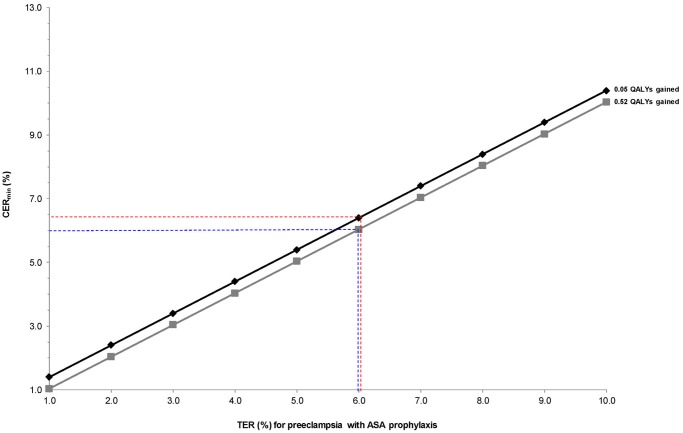
Fig 2. Minimum control event rate (CERmin) for preeclampsia with varying treatment event rates (TER) for preeclampsia, at fixed values of 0.05 (black) and 0.52 (grey) quality-adjusted life years (QALYs) gained with ASA prophylaxis in pregnancy [1].
CERmin calculated as: TER + [DC / (QALYs gained x $50 000)], assuming a direct cost (DC) of ASA of $10 per pregnancy. The dashed blue lines indicate that at a TER for preeclampsia of 6.0% and a QALY gain of 0.52 [1], the CERmin for preeclampsia is close to the TER (6.0%). At a smaller QALY gain of 0.05, a TER of 6.0% corresponds to a slightly higher CERmin of 6.4% (dashed red lines).
If we fix the TER at 6.0%—a rate observed in many ASA RCTs [11, 12, 14]—we see how the CERmin changes with varying QALYs gained (Fig. 3). The CERmin declines with increasing QALYs gained, but these changes become very minor above a QALY gain of 0.05.
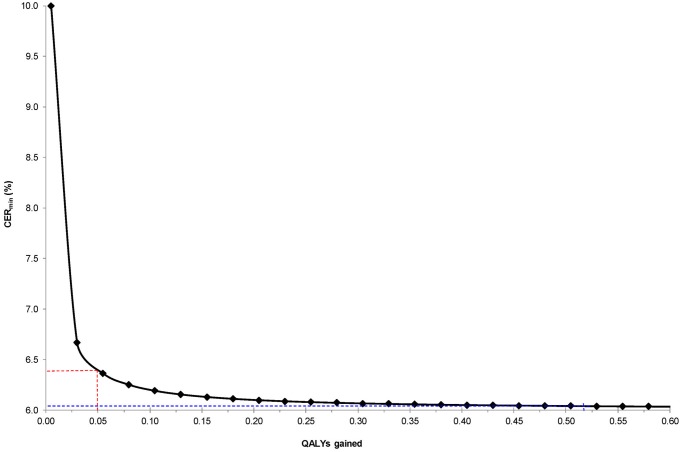
Fig 3. Minimum control event rate (CERmin) for preeclampsia with varying quality-adjusted life years (QALYs) gained, at a fixed treatment event rate (TER) for preeclampsia of 6.0%.
CERmin calculated as: TER + [DC / (QALYs gained x $50 000)], assuming a direct cost (DC) of ASA of $10 per pregnancy. The dashed blue lines indicate that at 0.52 QALYs gained [1], the CERmin for preeclampsia is close to the TER (6.0%). At a more modest gain of 0.05 QALYs, the CERmin increases slightly to 6.4% (dashed red lines).
We can also determine how the CERmin changes with DC. Under the reality that ASA is cheap, we see that the TER very closely approximates the CERmin for even a small QALY gain (Fig. 4). If ASA were much more expensive, then our threshold to treat would increase dramatically, and few women could achieve the required level of risk.
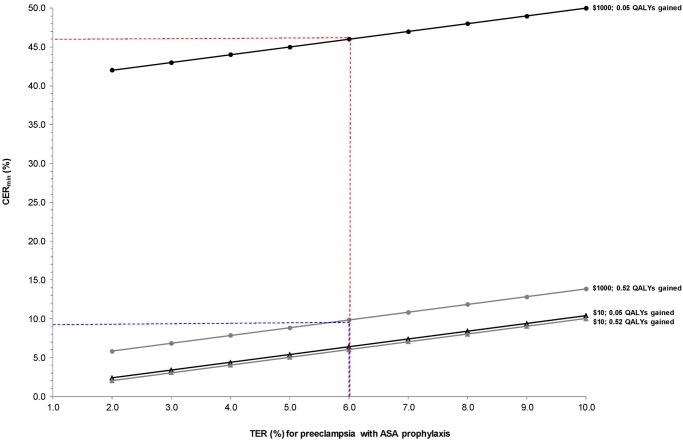
Fig 4. Minimum control event rate (CERmin) for preeclampsia with varying treatment event rates (TER) for preeclampsia at fixed values of 0.05 (black) and 0.52 (grey) quality-adjusted life years (QALYs) gained with ASA prophylaxis in pregnancy [1], and varied by two direct costs (DC) of ASA: $10 (actual cost, triangles) and $1 000 (circles).
CERmin calculated as: TER + [DC / (QALYs gained x $50 000)]. At a TER of 6.0% and a DC of ASA of $10, the CERmin for preeclampsia is essentially the same as the TER with 0.05 or 0.52 QALYs gained (6.4% and 6.0%, respectively). At a TER of 6.0%, but a DC of ASA of $1000, the CERmin increases to 9.8% with 0.52 QALYs gained (dashed blue lines) and 46.0% with 0.05 QALYS gained (dashed red lines).
Application of MERT to preeclampsia prophylaxis with ASA
Sinclair et al. developed the minimum event rate for treatment (MERT) in order to arrive at the event rate (i.e., absolute risk) to justify starting a specific treatment for a given disease [21]. The equation for the MERT is as follows:
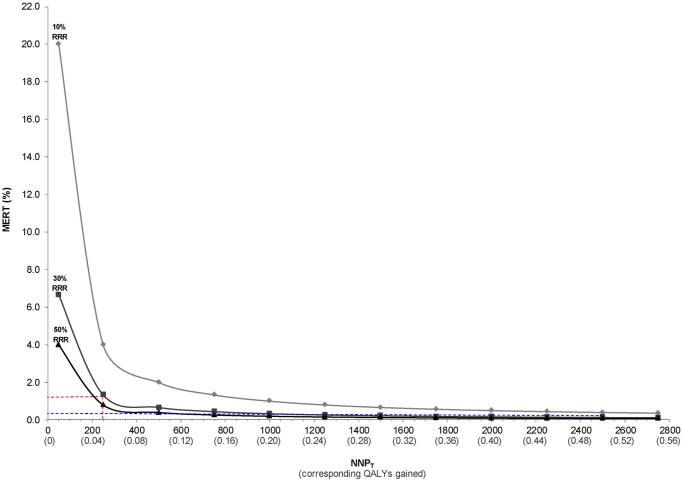
As expected, the MERT changes with varying NNPT [21], and is highest when the NNPT (and corresponding QALY gained) is lowest (Fig. 5). Not only does the MERT decline (i.e., improve) with increasing NNPT, but so too does the MERT decline with greater efficacy of ASA, indicated by a higher RRR against developing PE (Fig. 5).
Fig 5. Minimum event rate for treatment (MERT) for ASA to prevent preeclampsia at varying thresholds of number needed to prevent (NNPT) and at 10%, 30% and 50% relative risk reductions (RRR) for the efficacy of ASA.
NNPT calculated as: (QALYs gained x $50000) / DC [24], assuming a direct cost (DC) of ASA of $10 per pregnancy. MERT is calculated as: 1 / (NNPT x RRR) [21]. At a NNPT of 250 (corresponding to 0.05 QALYs gained), the MERT is 1.3% (dashed red lines), which declines to 0.13% when the NNPT is 2600 (corresponding to 0.52 QALYs gained, [dashed blue lines]).
Discussion
When initiated in the window between 12 to 16 weeks gestation, ASA effectively reduces the risk of PE and its related adverse perinatal outcomes, including intrauterine growth restriction, preterm birth, and neonatal death [11–14, 27]. To date, there is no objective estimate about the threshold of risk to warrant ASA prophylaxis in a given woman. This uncertainty may partly contribute to the variability in recommendations for ASA prophylaxis, or its uptake among practitioners, resulting in ASA being unnecessarily withheld in some women [16].
To help address this problem, we proposed using the CERmin, which, based on the TER and NNPT, arrives at the absolute risk (i.e., threshold rate) at which prophylaxis is indicated. The CERmin declines as a preventive therapy becomes more effective and less costly. The CERmin is conceptually similar to the MERT [21], but the former relies on knowing the TER and NNPT, and the latter on knowing the RRR and NNPT. Both the CERmin and the MERT are estimated with a degree of uncertainty [28]. An advantage of the MERT is that RRRs conveniently express the magnitude of treatment effect and are transportable across populations with various baseline risk [29]. However, RRRs conceal the underlying absolute risks, while TERs do not.
One concern about using the equation for NNPT by Shimbo et al. [24] is that it does not account for the costs saved by avoiding PE-related events, thereby overestimating the CERmin and the MERT. Additionally, it does not account for potential adverse effects (i.e., harm) conferred by a treatment and the resultant associated costs, in terms of DC and QALYs lost. However, in the specific case of ASA prophylaxis for PE, the NNPT formula by Shimbo et al. appears valid, as ASA has no demonstrable adverse effects on the pregnancy, delivery or the neonate [3, 15]. As an example, one RCT assigning 9364 pregnant women either ASA or matching placebo found that ASA was not associated with significant increases in placental haemorrhages or in bleeding during preparation for epidural anaesthesia [30]. In the EAGeR trial, which assigned women with prior spontaneous pregnancy loss to ASA 81 mg daily (615 women) or placebo (613 women), from preconception until 36 weeks’ gestation or completion of 6 menstrual cycles, the risk of side effects and adverse events was the same in both groups [31]. However, in those ASA RCTs with smaller sample sizes, large beneficial effect sizes are more likely to be seen, while the chance of detecting rare adverse outcomes is reduced [32, 33].
The NNPT values that we calculated for ASA prophylaxis were very high, and the subsequent CERmin and MERT values very low—a product of ASA’s extremely low cost and absence of known adverse effects. The MERT, even at conservative estimates of QALYs gained, was mostly under 1%, which is seemingly too low to truly reflect the absolute risk of PE that can justify ASA prophylaxis. The estimates of the CERmin, though not as dramatically low as those for the MERT, were often barely higher than the TER. At such low CERmin or MERT values, a clinician might be tempted to recommend ASA to a woman with no known risk factors—ASA can conceivably prevent PE with no associated risks and a minimal cost. However, the clinician and/or patient may decide to forgo ASA prophylaxis when her risk of PE is low, and there remains the potential to introduce a rare side effect from ASA, and/or there is a minor inconvenience of taking ASA. A future study should measure the preferences of a group of caregivers and parents, to see if the risk threshold for starting ASA differs from the CERmin or MERT.
Although the methods used herein may slightly underestimate the threshold risk for PE to warrant ASA prophylaxis, our results suggest that eligible women need not be at “high risk” for PE, but rather, at some modestly elevated risk. In the development of a clinical prediction rule that identifies women at risk of PE, the threshold adopted to consider ASA prophylaxis may only need to be slightly higher than that of the general population, perhaps somewhere between 6% and 10% [11–14]. Ideally, the threshold range we have provided can help physicians choose the right woman in whom ASA prophylaxis is warranted. Nonetheless, using this threshold as a guide would be enhanced by having more information on the contributions of common clinical risk factors for the development of PE, independently, and in combination; that is, we need a very simple and accurate tool for determining a woman’s absolute risk of PE in the first trimester of pregnancy, before 12 to 16 weeks gestation. Finally, it is worthwhile to further investigate the efficacy and QALYs gained from ASA prophylaxis separately for the mother and her baby.
Supporting Information
(DOCX)
Acknowledgments
We wish to thank Lisa Askie (NHMRC Clinical Trials Centre, University of Sydney, Sydney, Australia), Sally Lord (Principal Research Fellow, NHMRC Clinical Trials Centre, University of Sydney, Sydney, Australia) and Ziad Al-Rubaie (NHMRC Clinical Trials Centre, University of Sydney, Sydney, Australia) for their fantastic comments in revising this manuscript.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by a Knowledge Synthesis Grant from the Canadian Institutes for Health Research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. National Collaborating Centre for Women's and Children's Health (UK) (2010) Hypertension in Pregnancy: The Management of Hypertensive Disorders During Pregnancy. London: RCOG Press; 1194 p. [PubMed] [Google Scholar]
- 2. Hutcheon J, Lisonkova S, Joseph K (2011) Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol 25: 391–403. 10.1016/j.bpobgyn.2011.01.006 [DOI] [PubMed] [Google Scholar]
- 3. Caritis S, Sibai B, Hauth J, Lindheimer M, Klebanoff M, et al. (1998) Low-dose aspirin to prevent preeclampsia in women at high risk. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med 338: 701–705. [DOI] [PubMed] [Google Scholar]
- 4. Ananth C, Friedman A (2014) Ischemic placental disease and risks of perinatal mortality and morbidity and neurodevelopmental disorders. Semin Perinatol 38: 151–158. 10.1053/j.semperi.2014.03.007 [DOI] [PubMed] [Google Scholar]
- 5. Loverro G, Pansini V, Greco P, Vimercati A, Parisi A, et al. (2001) Indications and outcome for intensive care unit admission during puerperium. Arch Gynecol Obstet 265: 195–198. [DOI] [PubMed] [Google Scholar]
- 6. Ray J, Urquia M, Berger H, Vermeulen M (2012) Maternal and neonatal separation and mortality associated with concurrent admissions to intensive care units. CMAJ 184: E956–962. 10.1503/cmaj.121283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Public Health Agency of Canada (2013) Perinatal Health Indicators for Canada 2013. Available: http://www.aphp.ca/pdf/CAC%20report%202013%20EN.pdf. Accessed 03 August 2014.
- 8. Basso O, Rasmussen S, Weinberg C, Wilcox A, Irgens L, et al. (2006) Trends in fetal and infant survival following preeclampsia. JAMA 296: 1357–1362. [DOI] [PubMed] [Google Scholar]
- 9. Ananth C, Savitz D, Luther E, Bowes WJ (1997) Preeclampsia and preterm birth subtypes in Nova Scotia, 1986 to 1992. Am J Perinatol 14: 17–23. [DOI] [PubMed] [Google Scholar]
- 10. Odegard R, Vatten L, Nilsen S, Salvesen K, Austgulen R (2000) Preeclampsia and fetal growth. Obstet Gynecol 96: 950–955. [PubMed] [Google Scholar]
- 11. Duley L, Henderson-Smart D, Meher S, King J (2007) Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev 2:CD004659 [DOI] [PubMed] [Google Scholar]
- 12. Roberge S, Villa P, Nicolaides K, Giguere Y, Vainio M, et al. (2012) Early administration of low-dose aspirin for the prevention of preterm and term preeclampsia: a systematic review and meta-analysis. Fetal Diagn Ther 31: 141–146. 10.1159/000336662 [DOI] [PubMed] [Google Scholar]
- 13. Roberge S, Nicolaides K, Demers S, Villa P, Bujold E (2013) Prevention of perinatal death and adverse perinatal outcome using low-dose aspirin: a meta-analysis. Ultrasound Obstet Gynecol 41: 491–499. 10.1002/uog.12421 [DOI] [PubMed] [Google Scholar]
- 14. Bujold E, Roberge S, Lacasse Y, Bureau M, Audibert F, et al. (2010) Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol 116: 402–414. 10.1097/AOG.0b013e3181e9322a [DOI] [PubMed] [Google Scholar]
- 15. Imperiale T, Petrulis A (1991) A meta-analysis of low-dose aspirin for the prevention of pregnancy-induced hypertensive disease. JAMA 266: 260–264. [PubMed] [Google Scholar]
- 16. Chappell L, Seed P, Enye S, Briley A, Poston L, et al. (2010) Clinical and geographical variation in prophylactic and therapeutic treatments for pre-eclampsia in the UK. BJOG 117: 695–700. 10.1111/j.1471-0528.2010.02507.x [DOI] [PubMed] [Google Scholar]
- 17. Bramham K, Parnell B, Nelson-Piercy C, Seed PT, Poston L, et al. (2014) Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ 348: g2301 10.1136/bmj.g2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. North RA, McCowan LM, Dekker GA, Poston L, Chan EH, et al. (2011) Clinical risk prediction for pre-eclampsia in nulliparous women: development of model in international prospective cohort. BMJ 342: d1875 10.1136/bmj.d1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laupacis A, Sackett D, Roberts R (1988) An assessment of clinically useful measures of the consequences of treatment. N Engl J Med 318: 1728–1733. [DOI] [PubMed] [Google Scholar]
- 20. Cook RJ, Sackett DL (1995) The number needed to treat: a clinically useful measure of treatment effect. BMJ 310: 452–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sinclair J, Cook R, Guyatt G, Pauker S, Cook D (2001) When should an effective treatment be used? Derivation of the threshold number needed to treat and the minimum event rate for treatment. J Clin Epidemiol 54: 253–262. [DOI] [PubMed] [Google Scholar]
- 22. National Cholesterol Education Program (1994) Second report of the expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel II). Circulation 89: 1333–1445. [DOI] [PubMed] [Google Scholar]
- 23. Laupacis A, Feeny D, Detsky A, Tugwell P (1992) How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ 146: 473–481. [PMC free article] [PubMed] [Google Scholar]
- 24. Shimbo T, Nagata-Kobayashi S, Morimoto T, Fukui T (2002) Quick estimation of threshold "number needed to treat". J Clin Epidemiol 55: 531 [PubMed] [Google Scholar]
- 25. Earnshaw S, Scheiman J, Fendrick A, McDade C, Pignone M (2011) Cost-utility of aspirin and proton pump inhibitors for primary prevention. Arch Intern Med 171: 218–225. 10.1001/archinternmed.2010.525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li R, Zhang P, Barker L, Hoerger T (2010) Cost-effectiveness of aspirin use among persons with newly diagnosed type 2 diabetes. Diabetes Care 33: 1193–1199. 10.2337/dc09-1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xiong X, Mayes D, Demianczuk N, Olson D, Davidge S, et al. (1999) Impact of pregnancy-induced hypertension on fetal growth. Am J Obstet Gynecol 180: 207–213. [DOI] [PubMed] [Google Scholar]
- 28. Walter S, Sinclair J (2009) Uncertainty in the minimum event risk to justify treatment was evaluated. J Clin Epidemiol 62: 816–824. 10.1016/j.jclinepi.2008.09.017 [DOI] [PubMed] [Google Scholar]
- 29. Schwartz LM, Woloshin S, Dvorin EL, Welch HG (2006) Ratio measures in leading medical journals: structured review of accessibility of underlying absolute risks. BMJ 333: 1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. CLASP (Collaborative Low-dose Aspirin Study in Pregnancy) Collaborative Group (1994) CLASP: a randomised trial of low-dose aspirin for the prevention and treatment of pre-eclampsia among 9364 pregnant women. Lancet 343: 619–629. [PubMed] [Google Scholar]
- 31. Schisterman EF, Silver RM, Lesher LL, Faraggi D, Wactawski-Wende J, et al. (2014) Preconception low-dose aspirin and pregnancy outcomes: results from the EAGeR randomised trial. Lancet 384: 29–36. 10.1016/S0140-6736(14)60157-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henderson JT, Whitlock EP, O'Conner E, Senger CA, Thompson JH, et al. (2014) Low-Dose Aspirin for the Prevention of Morbidity and Mortality From Preeclampsia: A Systematic Evidence Review for the US Preventive Services Task Force. Agency for Healthcare Research and Quality (US) Report No.: 14–05207-EF-1. [PubMed]
- 33. Askie LM, Duley L, Henderson-Smart DJ, Stewart LA, PARIS Collaborative Group (2007) Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet 369: 1791–1798. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper.