Abstract
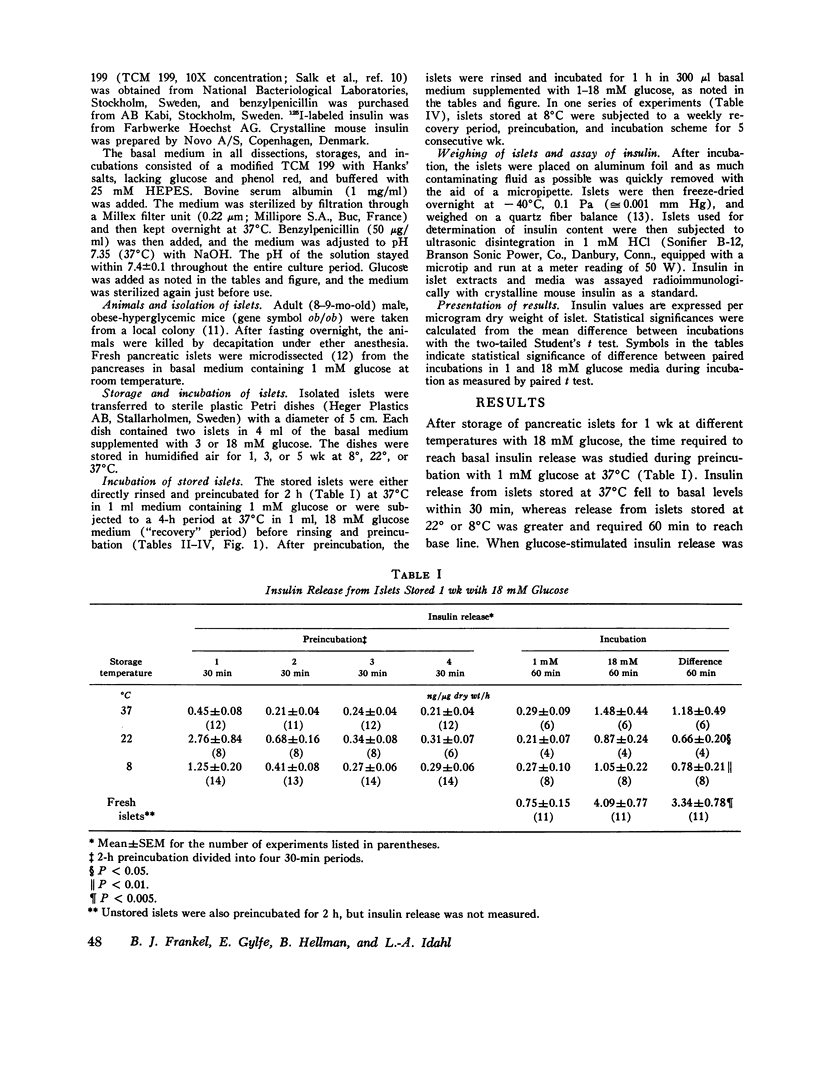
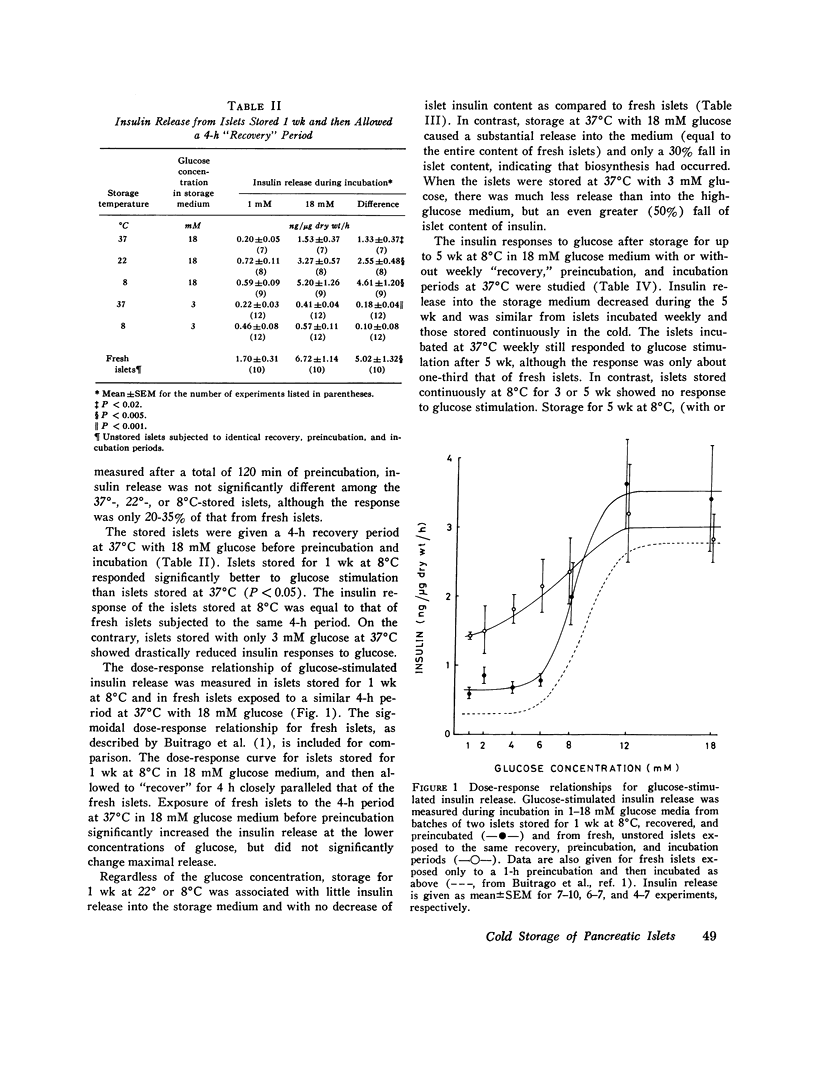
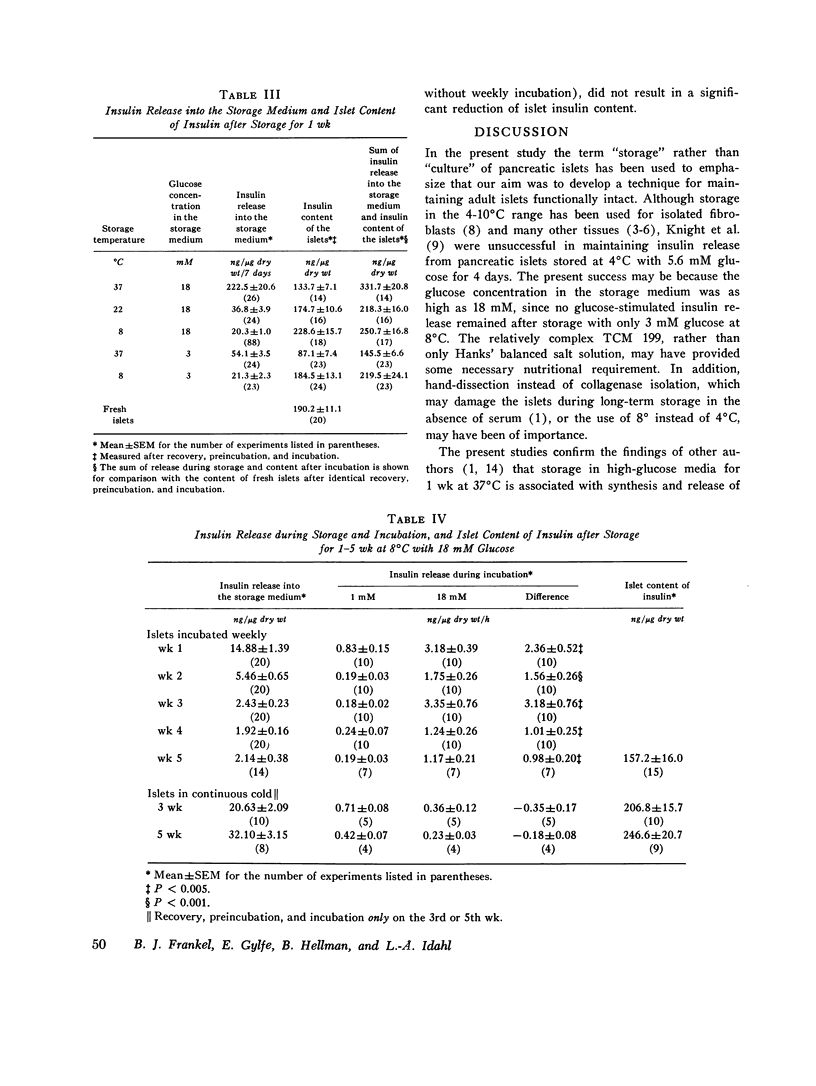
Insulin content and release were measured from hand-dissected pancreatic islets from noninbred ob/ob mice after 1-5 wk storage in tissue culture medium 199 at various temperatures and glucose concentrations. After storage of islets for 1 wk at 37 degrees, 22 degrees, or 8 degrees C in 18 mM glucose medium and preincubation with 1 mM glucose, glucose-stimulated insulin release during the subsequent incubation was only 20-35% of that of fresh islets. The addition of a 4-h period at 37 degrees C with 18 mM glucose between the cold storage and perincubation restored glucose-stimulated insulin release from 8 degrees C stored islets to fresh-islet levels. Release throughout the 1-18 mM glucose range was strikingly parallel to that of fresh islets. Exposure of fresh islets to the same 4-h period increased basal release but did not affect maximal release. When islets were stored at 8 degrees C with 18 mM glucose for more than 1 wk, a short period at 37 degrees C every week was necessary for maintenance of release. After 5 wk of this procedure, glucose-stimulated insulin release was one-third that of fresh islets, or similar to that of islets stored for only 1 wk at 37 degrees C. Storage at 8 degrees C for 1 wk with 3 mM glucose, or continuously for 3 or 5 wk with 18 mM glucose, maintained islet insulin content, whereas release was lost. Thus, glucose-stimulated insulin release is best maintained by storage of pancreatic islets in tissue culture medium with a high concentration of glucose at 8 degrees C with short weekly periods at 37 degrees C.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson A. Long-term effects of glucose on insulin release and glucose oxidation by mouse pancreatic islets maintained in tissue culture. Biochem J. 1974 Jun;140(3):377–382. doi: 10.1042/bj1400377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson A., Westman J., Hellerström C. Effects of glucose on the ultrastructure and insulin biosynthesis of isolated mouse pancreatic islets maintained in tissue culture. Diabetologia. 1974 Dec;10(6):743–753. doi: 10.1007/BF01219536. [DOI] [PubMed] [Google Scholar]
- Behounková E. Histologic examination of skin grafts stored at +4 degrees C. Acta Chir Plast. 1971;13(3):128–132. [PubMed] [Google Scholar]
- Belzer F. O. Renal preservation. N Engl J Med. 1974 Aug 22;291(8):402–404. doi: 10.1056/NEJM197408222910806. [DOI] [PubMed] [Google Scholar]
- Dragomir C. T., Păuşescu E. Theoretical considerations regarding the cell membrane function during cold-induced preservation of tissues and organs: new possibilities for optimizing the process. J Theor Biol. 1974 Oct;47(2):281–293. doi: 10.1016/0022-5193(74)90198-2. [DOI] [PubMed] [Google Scholar]
- Durst A. L. The present status of liver transplantation. Isr J Med Sci. 1973 Jan;9(1):98–101. [PubMed] [Google Scholar]
- HELLERSTROEM C. A METHOD FOR THE MICRODISSECTION OF INTACT PANCREATIC ISLETS OF MAMMALS. Acta Endocrinol (Copenh) 1964 Jan;45:122–132. [PubMed] [Google Scholar]
- Hellman B. Studies in obese-hyperglycemic mice. Ann N Y Acad Sci. 1965 Oct 8;131(1):541–558. doi: 10.1111/j.1749-6632.1965.tb34819.x. [DOI] [PubMed] [Google Scholar]
- Hoshi M., Shreeve W. W. Release and production of insulin by isolated, perifused rat pancreatic islets. Control by glucose. Diabetes. 1973 Jan;22(1):16–24. doi: 10.2337/diab.22.1.16. [DOI] [PubMed] [Google Scholar]
- Huang J. S., Downes G. L., Childress G. L., Felts J. M., Belzer F. O. Oxidation of 14C-labeled substrates by dog kidney cortex at 10 and 38 degrees C. Cryobiology. 1974 Oct;11(5):387–394. doi: 10.1016/0011-2240(74)90105-9. [DOI] [PubMed] [Google Scholar]
- Knight M. J., Scharp D. W., Kemp C. B., Ballinger W. F., Lacy P. E. Effects of cold storage on the function of isolated pancreatic islets. Cryobiology. 1973 Apr;10(1):89–90. doi: 10.1016/0011-2240(73)90013-8. [DOI] [PubMed] [Google Scholar]
- Kostianovsky M., Lacy P. E., Greider M. H., Still M. F. Long term (15 days) incubation of islets of Langerhans isolated from adult rats and mice. Lab Invest. 1972 Jul;27(1):53–61. [PubMed] [Google Scholar]
- LOWRY O. H. The quantitative histochemistry of the brain; histological sampling. J Histochem Cytochem. 1953 Nov;1(6):420–428. doi: 10.1177/1.6.420. [DOI] [PubMed] [Google Scholar]
- Matsumura T., Takaoka T., Katsuta H. Survival of cultured cells in the cold. A kinetic study with special reference to the effect of serum in culture media. Exp Cell Res. 1973 Feb;76(2):297–304. doi: 10.1016/0014-4827(73)90380-7. [DOI] [PubMed] [Google Scholar]
- SALK J. E., YOUNGNER J. S., WARD E. N. Use of color change of phenol red as the indicator in titrating poliomyelitis virus or its antibody in a tissue-culture system. Am J Hyg. 1954 Sep;60(2):214–230. doi: 10.1093/oxfordjournals.aje.a119714. [DOI] [PubMed] [Google Scholar]
- Veith F. J., Koerner S. K. The present status of lung transplantation. Arch Surg. 1974 Dec;109(6):734–740. doi: 10.1001/archsurg.1974.01360060004002. [DOI] [PubMed] [Google Scholar]



