Abstract
Background
Research suggests that altered interregional connectivity in specific networks, such as the default mode network (DMN), is associated with cognitive and psychotic symptoms in schizophrenia. In addition, frontal and limbic connectivity alterations have been associated with trauma, drug use and urban upbringing, though these environmental exposures have never been examined in relation to DMN functional connectivity in psychotic disorder.
Methods
Resting-state functional MRI scans were obtained from 73 patients with psychotic disorder, 83 non-psychotic siblings of patients with psychotic disorder and 72 healthy controls. Posterior cingulate cortex (PCC) seed-based correlation analysis was used to estimate functional connectivity within the DMN. DMN functional connectivity was examined in relation to group (familial risk), group × environmental exposure (to cannabis, developmental trauma and urbanicity) and symptomatology.
Results
There was a significant association between group and PCC connectivity with the inferior parietal lobule (IPL), the precuneus (PCu) and the medial prefrontal cortex (MPFC). Compared to controls, patients and siblings had increased PCC connectivity with the IPL, PCu and MPFC. In the IPL and PCu, the functional connectivity of siblings was intermediate to that of controls and patients. No significant associations were found between DMN connectivity and (subclinical) psychotic/cognitive symptoms. In addition, there were no significant interactions between group and environmental exposures in the model of PCC functional connectivity.
Discussion
Increased functional connectivity in individuals with (increased risk for) psychotic disorder may reflect trait-related network alterations. The within-network “connectivity at rest” intermediate phenotype was not associated with (subclinical) psychotic or cognitive symptoms. The association between familial risk and DMN connectivity was not conditional on environmental exposure.
Introduction
The disconnection hypothesis postulates that both cognitive and pathophysiological alterations contribute to dysfunctional integration of a distributed network of brain regions in schizophrenia [1,2]. Dysfunctional integration is often addressed with the concept of functional connectivity, which refers to the temporal correlation between two (or more) spatially distinct brain regions [3]. Functional connectivity can be examined in diverse networks. The default mode network (DMN) is active during rest and deactivated when goal-directed behavior is required and is thought to play a role in appraising external and internal stimuli, self-referential and reflective processes. Regions representing the DMN consist of the medial prefrontal cortex (MPFC), the posterior cingulate cortex (PCC) extending into the precuneus (PCu), the lateral parietal cortices, lateral temporal cortex, hippocampus (HC) and parahippocampal gyrus (PHG) [4,5]. Structural and functional alterations in these regions have been associated with schizophrenia [6]. In addition, the DMN has been implicated in self-referential processing [7,8], perspective-taking, self-other judgments [9,10], processing of agency [11] and memory functions [12], all of which appear to be altered in individuals with psychotic disorder. Misinterpretations in some of these processes may contribute to the formation of positive symptoms [8,13].
Studies on DMN connectivity in schizophrenia have shown conflicting results as to the direction of associations. Both decreased, increased and mixed patterns of functional connectivity [14–20], or no significant alterations in patients with schizophrenia [21] have been reported. Similarly, in individuals at higher than average risk for psychotic disorder (first-degree relatives) both increased (in the MPFC, bilateral inferior temporal gyrus (ITG), PCu) [22–24], and reduced functional connectivity (in prefrontal areas, PCC, PCu, ITG) [20,25,26] as well as an absence of significant differences with respect to controls [16,21] have been reported. Taken together, most studies have shown increased connectivity in patients with schizophrenia and first-degree relatives, though the larger studies (n = 258 and n = 799) suggest that patients have reduced DMN connectivity and that relatives have reduced [20] or no differences [16] in DMN connectivity with respect to controls.
(Subclinical) psychotic experiences [27] may arise from impaired monitoring or attribution of agency, which has been associated with posterior lateral parts of the DMN [11]. DMN resting-state studies using seed-based correlation analysis found that increased connectivity between the PCC and respectively the MPFC, other PCC regions, and temporal lobe areas including language areas [22,28] as well as decreased connectivity between the PCC and the temporal gyrus was associated with positive symptoms [28]. Moreover, two resting-state studies using independent component analysis (ICA) found that increased medial and superior frontal gyrus connectivity and decreased hippocampal and inferior parietal cortex connectivity was associated with positive symptoms [1,29]. Areas of the DMN have also been implicated in cognitive functions such as social cognition [7] and working memory (WM) capacities [12,30], and alterations therein have been associated with (the vulnerability for) schizophrenia [7,31–33]. As DMN activity is suppressed during cognitive tasks, altered connectivity in rest may lead to compromised suppression and decreased cognitive performance [1]. Indeed, studies have shown that DMN hyperconnectivity in patients with schizophrenia and their first-degree relatives was associated with reduced WM performance [22]. The relation between social cognition (e.g., Theory of Mind) and DMN resting-state functional connectivity in psychotic disorder has not been examined thus far.
Altered DMN connectivity may not only be conditional on genetic risk for psychotic disorder, but also on established environmental risk factors for schizophrenia such as cannabis use [34], childhood trauma [35] and developmental urbanicity [36]. To date, no resting-state functional connectivity studies have examined gene-environment interaction (G×E) in psychotic disorder. Nevertheless, resting-state fMRI studies have shown that altered stress-anticipation in individuals with a history of childhood poverty [37] and posttraumatic stress disorder (based on early life stress) [38] was associated with reduced DMN connectivity (PCC, PCu, PFC), whereas a task-based fMRI study in individuals showed a positive association between urban upbringing and perigenual anterior cingulate cortex (pACC) activity [39]. In addition, chronic cannabis use has been associated with altered resting-state PCC connectivity, but only in subjects without psychopathology [40].
The current study tested three hypotheses. First, it was hypothesized that individuals with (increased risk for) psychotic disorder would reveal aberrant connectivity (decreased and increased) within the DMN compared to healthy controls. Second, altered DMN connectivity, especially between the PCC and MPFC, in individuals with (increased risk for) psychotic disorder was expected to be associated with positive symptoms (i.e., hallucinations and delusions) and decreased (social) cognitive functioning. Third, it was examined whether DMN functional connectivity reflects a cerebral phenotype that is the outcome of G×E interaction in psychotic disorder.
Methods
Participants
Data pertain to baseline measurements of a longitudinal MRI study in Maastricht, the Netherlands. For recruitment and inclusion criteria of patients, their siblings and healthy controls, see [41].
The original sample comprised 89 patients with psychotic disorder, 97 siblings of patients with psychotic disorder and 88 controls. Forty-six participants were excluded from the analyses based on: high schizotypy (n = 3), movement (n = 8) or scanner artifacts (n = 14), smoking cannabis prior to scanning (n = 1) and experimental issues (n = 20). This resulted in a final sample comprising 73 patients with psychotic disorder, 83 siblings of patients with psychotic disorder and 72 controls. The sample comprised 46 families: 25 families with one patient and one sibling, three families with one patient and two siblings. One family with two patients, six families with two siblings, and two families with one patient and three siblings. In the control group, there were nine families with two siblings. In addition, 41 independent patients, 34 independent siblings, and 54 independent controls were included.
Diagnosis was based on the Diagnostic and Statistical Manual of Mental Disorder-IV (DSM-IV) criteria [42], assessed with the Comprehensive Assessment of Symptoms and History (CASH) interview [43]. Patients were diagnosed with: schizophrenia (n = 47), schizoaffective disorder (n = 9), schizophreniform disorder (n = 4), brief psychotic disorder (n = 2), and psychotic disorder not otherwise specified (n = 11). The CASH was also used to confirm the absence of a diagnosis of nonaffective psychosis in the siblings and absence of a lifetime diagnosis of any psychotic disorder or current affective disorder in the healthy controls. The occurrence of any psychotic disorder in first-degree family members also constituted an exclusion criterion for the controls. Schizotypy was assessed with the Structured Interview for Schizotypy-revised (SIS-r) [44]. Ten controls and 16 siblings were diagnosed (lifetime) with major depressive disorder, but none of them presented in a current depressive state.
Before MRI acquisition, participants were screened for the following exclusion criteria: 1) brain injury with unconsciousness of greater than 1 hour, 2) meningitis or other neurological diseases that might have affected brain structure or function, 3) cardiac arrhythmia requiring medical treatment, and 4) severe claustrophobia. In addition, participants with metal corpora aliena were excluded from the study, as were women with intrauterine device status and (suspected) pregnancy.
Ethics statement
The standing ethics committee of Maastricht University approved the study, and all the participants gave written informed consent in accordance with the committee’s guidelines and with the Declaration of Helsinki [38]. All participants understood the information given to them and could make an informed decision, which was verified by an experienced psychologist. Therefore, all participants included in the study were able to give informed consent without the use of a legal representative or guardian.
Behavioral Measures
Psychotic symptom assessment was carried out using the Positive and Negative Syndrome Scale (PANSS) [45]. The five factor model by van der Gaag (2006) was used, dividing the PANSS in positive symptoms, negative symptoms, disorganization symptoms, excitement, and emotional distress [46].
Theory of Mind (ToM) was assessed using the raw scores of the hinting task. This is a simple ToM test in which the participants must infer the intention behind indirect speech. The task has a maximum score of 20 [47].
WM was assessed using the raw scores of the arithmetic test of the Wechsler Adult Intelligence Scale-III (WAIS-III) [48]. This test consists of 20 timed arithmetic problems that address verbal comprehension and arithmetic skills.
Educational level was defined as highest accomplished level of education. Handedness was assessed using the Annett Handedness Scale [49].
Antipsychotic (AP) medication use was determined by patient report and verified with the treating consultant psychiatrist. Best estimate lifetime (cumulative) AP use was determined by multiplying the number of days of AP use with the corresponding haloperidol equivalents and summing these scores for all periods of AP use (including the exposure period between baseline assessment for the G.R.O.U.P. study and the moment of baseline MRI scanning), using the converting formulas for AP dose equivalents described in Andreasen and colleagues [50].
Substance use
Substance use was measured with the Composite International Diagnostic Interview (CIDI) sections B, J and L [51]. Use of cannabis and other drugs was based on the lifetime number of instances of drug use. CIDI frequency data on lifetime cannabis use were available for 220 participants (4% missing data). In addition, cannabis was tested in urine (18% missing data). The two measures were combined into one variable, which was coded as follows: never used cannabis = 0, ever used cannabis = 1 (0% missing data). Data on other drug use were available for 223 participants (2% missing data). Data on cigarette smoking and alcohol use were available for 212 participants (7% missing data) and 206 participants (9% missing data), respectively.
Childhood trauma
Childhood trauma was assessed with the Dutch version of the Childhood Trauma Questionnaire Short Form (CTQ). The short CTQ consists of 25 items rated on a 5-point Likert scale (1 = never true to 5 = very often true) inquiring about traumatic experiences in childhood. Five types of childhood maltreatment were assessed: emotional, physical and sexual abuse and emotional and physical neglect, with five questions covering each type of trauma [52]. A general measure of childhood trauma was created by calculating the mean of the 25 items. The CTQ data were missing for two participants (1% missing data).
Level of developmental urbanicity
A historical population density record for each municipality was generated from 1930 onwards using the Dutch Central Bureau of Statistics (CBS) and equivalent Belgium database [53]. It was determined where the subject lived at birth, between ages 0–4 years; between 5–9 years; 10–14 years; 15–19 years; 20–39 years; 40–59 years; and 60+ up to the actual age. For each of these records, the average population density was computed (by square kilometer, excluding water) of the municipality. Average population density was categorized in accordance with the Dutch CBS urbanicity rating (1=<500/km2; 2 = 500–1000/km2; 3 = 1000–1500/km2; 4 = 1500–2500/km2; 5 = 2500+/km2). The periods 0–4 years, 5–9 years and 10–14 years were merged to average urbanicity exposure between 0–14 years. The latter was used as the primary variable reflecting developmental urbanicity exposure in the analyses. This variable was collapsed a priori into 5 intervals (1 to 1.49 = 1; 1.5 to 2.49 = 2; 2.5 to 3.49 = 3; 3.5 to 4.49 = 4; 4.5 to 5 = 5) to reflect the same categories as used by the Dutch CBS [53]. Data on developmental urbanicity were available for all participants (0% missing data).
MRI acquisition
Functional and anatomical MRI images were acquired using a 3T Siemens scanner. The functional resting-state data were acquired using an Echo-Planar Imaging (EPI) sequence: number of volumes: 200; TE: 30 ms; TR: 1500 ms; voxel size: 3.5x3.5x4.0 mm3; flip angle 90°; total acquisition time: 5 min. During the scan, participants were instructed to lie with their eyes closed, think of nothing in particular, and not fall asleep. In addition, anatomical MRI scans had the following acquisition parameters: (1) Modified Driven Equilibrium Fourier Transform (MDEFT) sequence: number of slices: 176; voxel size: 1 mm isotropic; TE: 2.4 ms; TR: 7.92 ms; inversion time: 910 ms; flip angle: 15°; total acquisition time: 12 min 51 s; (2) Magnetization Prepared Rapid Acquisition Gradient-Echo (MPRAGE; Alzheimer’s Disease Neuroimaging Initiative) sequence: number of slices: 192; voxel size: 1 mm isotropic; TE: 2.6 ms; TR: 2250 ms; inversion time: 900 ms; flip angle 9°, total acquisition time: 7 min 23 s. For both anatomical scans the matrix size was 256x256 and field of view was 256x256 mm2. Two sequences were used because of a scanner update during data collection. The MPRAGE and MDEFT are very similar, but to prevent any systematic bias, the total proportion of MPRAGE scans (44%) was balanced between the groups.
Data preprocessing and analysis
Imaging data were preprocessed to account for head motion, as described by Patel et al. (2014) [54] and Jo et al. (2013) [55] using Analysis of Functional NeuroImages (AFNI, version 2011_12_21_1014) [56] as well as the Oxford Centre for Functional MRI of the Brain Software Library (FSL, version 5.0.4) [57,58]. The first four volumes of each resting-state data set were removed to eliminate the non-equilibrium effects of magnetization. Preprocessing steps included slice-time correction, motion correction, despiking of the functional data (removing artifactual outliers in voxelwise time series), temporal bandpass filtering (0.02–0.1 Hz), co-registration to structural scan, spatial normalization to standard space and spatial smoothing (6-mm full width at half maximum Gaussian kernel). Several sources of spurious variance (nuisance variables) were removed from the data through linear regression: six motion correction parameters and their first temporal derivatives, and cerebrospinal fluid (CSF) signal from ventricular regions of interest.
Functional connectivity analysis
BrainVoyager QX [59] and routines in Matlab (The Mathworks, Natick, MA, U.S.A.) were used (NeuroElf toolbox [www.neuroelf.net] and custom routines) to estimate functional connectivity for each participant using seed-based correlation analysis. First, whole brain signal intensity averaged across all brain voxels and white matter signal (derived from extracting the BOLD time course signal from a manually defined white matter ROI) were removed from the resting-state data via linear regression. Then, a correlation map was computed using an initiating seed region with a 6-mm radius in the posterior cingulate (PCC, MNI coordinate: 1, -55, 17) based on a previously described method [60]. In the current analysis the PCC was chosen as seed in a seed-based correlation analysis because of its central role in functions of the DMN (e.g. self-referential mental thoughts, WM). Furthermore, it is suggested that the PCC is the only region in the DMN that directly interacts with all other regions within this network [61] and has the highest metabolic activity compared to all other regions during rest [5,62].
Pearson’s correlation coefficients were computed between the time courses of the PCC seed and all other brain voxels and normalized using the Fisher’s r-to-z transformation. Visualization of group effects was restricted to those voxels that empirically were associated with the DMN in all participants. For this purpose, we created a DMN mask by thresholding a one-sample t-test map of the PCC connectivity across all participants, using a false-discovery rate FDR of q = 0.05 [63]. We then performed an ANCOVA with group as between-subject factor, controlling for the subject-level confounders sex, age, handedness and level of education. Significant group effects were visualized using a statistical (p = 0.05, uncorrected) and cluster-size threshold (52 voxels (i.e., 1404 mm3)). The cluster-size threshold was estimated using a simulation procedure that incorporates the spatial smoothness of the statistical map (1,000 Monte Carlo simulations [59,64]). The simulated maps were thresholded at the same voxel threshold as the statistical map and surviving clusters were tabulated. The minimum cluster size was selected by taking a false positive rate of 5%.
Group differences in DMN connectivity
As selection of regions with a significant between-subject (group) effect was performed using a voxel-level ANCOVA, which assumes independency of groups. Post-hoc analyses on mean individual functional connectivity coefficients of the voxel clusters were performed using multiple linear regression analyses in STATA (corrected for the same confounders) [65]. This was done using the REGRESS command in STATA with regional functional connectivity measures as dependent variables and group as independent variable. Group was entered as dummy variables (controls = 0, siblings = 1, patients = 2). Because of the non-independency of the groups (familial relationships) analyses were repeated with a multilevel random regression model using the XTREG command in STATA. In addition, the voxel-level ANCOVA and subsequent post-hoc tests were performed with full correction for the subject-level confounders tobacco, alcohol, cannabis and other drugs, as DMN connectivity may be influenced by these substances [66–70]. Lastly, since aberrant (increased and decreased) DMN connectivity has also been found in patients with major depression [71], a priori planned sensitivity analyses were carried out excluding all individuals with a history of affective disorder.
Associations between DMN connectivity and psychopathology ratings
The associations between DMN functional connectivity (independent variable) and (subclinical) positive symptoms / (social) cognitive performance (dependent variable) were examined with multiple linear regression analyses on the ANCOVA selected regions with a significant between-subject (group) effect. In patients, the association between DMN functional connectivity and symptoms was corrected for age, sex, lifetime AP medication and illness duration (analyses of PANSS positive symptoms). In siblings and controls, the subclinical symptom analyses were corrected for group, age and sex and the (social) cognition analyses were additionally corrected for handedness and level of education. Associations with (social) cognitive performance were investigated in the combined group (of patients, siblings and controls) and corrected for group, age, sex, handedness and level of education.
In order to examine whether the association between DMN functional connectivity and subclinical positive symptoms / (social) cognitive performance would be conditional on group, interactions were tested between group and DMN connectivity, in the ANCOVA selected regions. In case of significant interactions, stratified effect sizes for DMN connectivity were calculated for each group by using the STATA MARGINS routine. Analyses with subclinical positive symptoms were corrected for age and sex, whereas (social) cognitive performance was corrected for age, sex, handedness and level of education.
Associations between environmental exposure and DMN connectivity
Main effects of the three a priori hypothesized environmental exposures (cannabis, childhood trauma and developmental urbanicity) on functional connectivity were examined with multiple linear regression analyses using the ANCOVA selected regions with a significant between-subject (group) effect. The environmental exposures were entered as linear variables and as dummy variables (never or ever used cannabis; the childhood trauma score divided by its tertiles (low, medium, high trauma scores); five levels of population density).
In order to study whether altered DMN functional connectivity was the outcome of differential sensitivity to these environmental exposures, two-way interactions between group and environmental exposure (GxE) were examined and evaluated by Wald test [72]. In case of significant interactions, stratified effect sizes for all levels of environmental exposure per group were calculated by linear combination of effects from the model containing the interactions using the STATA MARGINS routine. Analyses were adjusted for the a priori hypothesized confounders age, sex, handedness and level of education.
In addition, associations between AP medication and functional connectivity were analyzed in patients only, with AP medication as independent variable and age, sex and illness duration as confounders.
To control for type I error, significant p-values were subjected to correction for multiple testing using the Simes method [73]. The Simes method avoids overcorrection associated with the Bonferroni correction in case the statistical tests are not independent, as was the case in the present study.
Results
Participant characteristics
There were more men than women in the patient group, whereas the opposite held for the control group. Patients had lower educational level than controls and siblings. Patients smoked more cigarettes and used more cannabis and other drugs (lifetime) than siblings and controls. Siblings used more alcohol than patients and controls. Patients, being in stable remission, had relatively low PANSS scores, and performed worse on WM and ToM indices compared to siblings and controls. Childhood trauma was more frequently experienced in patients than in siblings and controls, with no differences between the latter two. The three groups did not differ in developmental level of urban upbringing (Table 1). Out of 73 patients, 64 used AP medication at the time of scanning (second generation: n = 60; first generation: n = 4). The mean current dosage of AP medication in terms of standard haloperidol equivalents was 5.3 mg (SD = 4.8 mg). Furthermore, twelve patients used antidepressants, three used benzodiazepines, five used anticonvulsants and one used lithium. Two siblings and two controls used antidepressants, and one control used benzodiazepines.
Table 1. Participant characteristics.
| Patients (n = 73) | Siblings (n = 83) | Controls (n = 72) | |
|---|---|---|---|
| mean (SD) | mean (SD) | mean (SD) | |
| Age at scan | 27.8 (6.6) | 29.6 (9.1) | 30.0 (10.8) |
| Sex n(%) male | 49 (65%) | 45 (54%) | 26 (36%) |
| Handedness | 72.1 (63.9) | 80.1 (53.8) | 73.5 (61.2) |
| Level of education | 4.2 (2.0) | 5.2 (1.9) | 5.4 (1.8) |
| Cannabis use 1 | 51.7 (47.6) | 18.1 (36.0) | 8.4 (22.8) |
| Cigarettes use 2 | 11.4 (11.0) | 2.6 (6.2) | 1.9 (6.1) |
| Alcohol use 3 | 6.7 (13.0) | 10.1 (17.7) | 5.1 (7.2) |
| Other drug use 4 | 44.4 (87.5) | 6.4 (33.0) | 2.4 (12.8) |
| PANSS positive | 9.7 (4.1) | 7.4 (1.5) | 7.3 (1.2) |
| PANSS negative | 11.9 (6.0) | 8.5 (2.2) | 8.2 (1.0) |
| PANSS disorganization | 12.0 (3.3) | 10.4 (1.0) | 10.2 (1.2) |
| PANSS excitement | 9.9 (2.9) | 8.6 (1.4) | 8.3 (1.1) |
| PANSS emotional distress | 12.7 (5.1) | 9.9 (2.7) | 9.3 (2.1) |
| SIS-r positive subscale | 0.6 (0.4) | 0.5 (0.5) | |
| Hinting task (social cognition) | 18.0 (2.9) | 19.2 (1.3) | 19.3 (1.1) |
| WAIS-III Arithmetic (WM) | 12.5 (4.2) | 15.5 (3.7) | 15.5 (4.1) |
| CTQ total | 7.3 (2.9) | 5.8 (1.6) | 5.6 (1.8) |
| Level of developmental urbanicity | 2.3 (1.3) | 2.3 (1.4) | 2.6 (1.5) |
| Age of onset (yrs) | 21.4 (6.8) | ||
| Illness duration (yrs) | 6.4 (3.7) | ||
| Lifetime exposure to AP 5 | 7022.9 (6711.3) |
Abbreviations: SD = standard deviation, PANSS = Positive and Negative Syndrome Scale; SIS-r = Structured Interview for Schizotypy-revised; WAIS = Wechsler Adult Intelligence Scale; WM = Working Memory; CTQ = Childhood Trauma Questionnaire; AP = Anti-Psychotics.
(1) Lifetime number of instances of cannabis use
(2) Number of daily consumptions over the last 12 months
(3) Number of weekly consumptions over the last 12 months
(4) Lifetime number of times of hard drug use
(5) Lifetime number of days of AP use
Group differences in DMN connectivity
In all three groups, the PCC connectivity map showed significant positive correlations with other DMN regions, including medial frontal, lateral prefrontal, parietal and temporal areas (i.e., the hippocampal complex). The voxel-level ANCOVA revealed a between-subject (group) effect in three DMN regions: the left inferior parietal lobule (IPL), the left precuneus (PCu) and the right medial prefrontal cortex (MPFC) (Fig. 1, Table 2). Post-hoc analyses revealed that patients and siblings had increased connectivity between the PCC seed and left IPL, left PCu and right MPFC (Fig. 2, Table 3). No significant differences were observed between patients and siblings. Multilevel random regression analyses with XTREG did not influence the results. All significant findings were upheld after Simes correction (pSimes: p<0.033) (Table 3). Repeating the voxel-level ANCOVA and post-hoc analyses with additional confounders (tobacco, alcohol, cannabis and other drugs) did not affect the pattern of results, as did the exclusion of siblings and controls with a history of affective disorder (S1 and S2 Table).
Fig 1. Areas showing significant between-subject (group) effect in PCC connectivity.
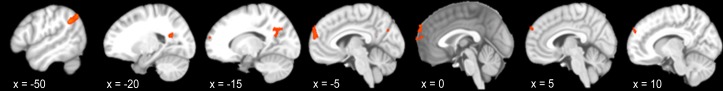
Results from voxel-level ANCOVA analysis.
Table 2. Regions of the default mode network with a significant between-subject (group) effect.
| Anatomical region | Hemisphere | Peak coordinates (MNI) | Cluster size (voxels) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Inferior parietal lobule | L | -48 | -64 | 43 | 82 |
| Precuneus | L | -15 | -59 | 33 | 63 |
| Medial prefrontal cortex | R | 9 | 56 | 31 | 89 |
Results from voxel-level ANCOVA analysis. Voxel size equals 3x3x3 mm3. Results represent regions with significant group differences using a statistical threshold p = 0.05 (uncorrected) and cluster threshold (52 voxels). Abbreviations: R, right; L, left.
Fig 2. Mean functional connectivity with 95% confidence interval for each region of the DMN that showed significant differences between the groups.
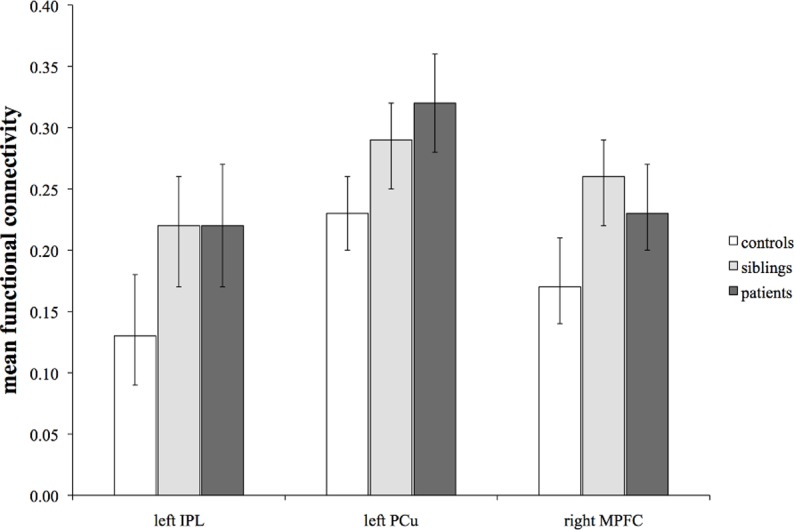
There was significantly higher PCC connectivity with the left IPL, left PCu and right MPFC in siblings and patients than in controls, with no significant differences between patients and siblings.
Table 3. Associations between familial risk of psychotic disorder (group) and functional connectivity.
| Regions of Interest | Functional Connectivity N = 228 | Group differences in functional connectivitymultiple linear regression analyses | Group differences in functional connectivitymultilevel random regression analyses | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Siblings | Controls | P vs. C | S vs. C | P vs. S | P vs. C | S vs. C | P vs. S | |||||||
| mean (SD) | mean (SD) | mean (SD) | B | p | B | p | B | p | B | p | B | p | B | p | |
| Left inferior parietal lobule | 0.22 (0.21) | 0.22 (0.22) | 0.13 (0.21) | 0.13 | 0.001* | 0.10 | 0.004* | 0.03 | 0.385 | 0.13 | 0.000* | 0.10 | 0.003* | 0.03 | 0.377 |
| Left precuneus | 0.32 (0.16) | 0.29 (0.16) | 0.23 (0.13) | 0.10 | 0.000* | 0.07 | 0.007* | 0.03 | 0.161 | 0.10 | 0.000* | 0.07 | 0.003* | 0.03 | 0.180 |
| Right medial prefrontal cortex | 0.23 (0.15) | 0.26 (0.17) | 0.17 (0.14) | 0.08 | 0.004* | 0.09 | 0.000* | -0.01 | 0.593 | 0.08 | 0.003* | 0.09 | 0.000* | -0.01 | 0.586 |
The Bs represent the regression coefficients from multiple linear regression and multilevel random regression analyses in STATA corrected for age, sex, handedness and level of education. Abbreviations: P = patients; S = siblings, C = controls; SD = standard deviation; the asterisks
(*) represent areas which are significant after Simes correction (PSimes<0.033).
Association between DMN connectivity and positive symptoms
There was no significant association between PANSS positive symptoms and DMN connectivity in the patients (left IPL: B = 1.37, P = 0.630; left PCu: B = -0.83, P = 0.829 and right MPFC: B = 0.54, P = 0.889). Repeating the analyses in patients with the 50% highest positive symptom scores (mean score = 12.24, SD = 4.36, range: 8 to 24) did not change the results. In the combined sibling and control group, SIS-r positive subscale scores and DMN functional connectivity were not significantly associated (left IPL: B = -0.02, P = 0.926; left PCu: B = 0.11, P = 0.637 and right MPFC: B = 0.02, P = 0.946). In addition, no significant group×DMN connectivity interactions in the model of subclinical positive symptoms were found (left IPL (F = 0.69, P = 0.408), left PCu (F = 0.01, P = 0.909) and right MPFC (F = 0.15, P = 0.699)).
Association between DMN connectivity and cognitive symptoms
In the total group, there was a significant association between WM performance and PCC connectivity with the left PCu (B = -3.63, P = 0.033), but not with the left IPL (B = -1.22, P = 0.309) and right MPFC (B = -0.67, P = 0.677). As the distribution of WM scores in the patient group was not Gaussian, a log transformation was performed which did not affect the results. The significant finding for WM in the whole group was not upheld after Simes correction (pSimes: p<0.006). There were no significant group×DMN connectivity interactions in the model of WM (left IPL (F = 0.39, P = 0.679), left PCu (F = 1.72, P = 0.181) and right MFPC (F = 0.16, P = 0.849)). Similarly, with regard to ToM, no significant associations with DMN connectivity were found in whole group analyses (i.e., PCC connectivity with the left PCu (B = -0.69, P = 0.264), left IPL (B = -1.08, P = 0.217), and right MPFC (B = 0.63, P = 0.445)), neither were their significant group×DMN connectivity interactions (left IPL (F = 0.01, P = 0.994), left PCu (F = 0.41, P = 0.665) and right MPFC (F = 0.51, P = 0.600)).
Exploratory analyses on DMN connectivity and other PANSS symptom dimensions
Exploratory post-hoc multiple linear regression analyses were performed with the remaining 4 symptom clusters (i.e., negative symptoms, disorganization, excitement and emotional distress) (corrected for group, age and sex). In the total group, no associations were found between the remaining symptom clusters and DMN connectivity. In patients, a positive association was found between emotional distress and PCC connectivity with the left IPL (B = 8.41, P = 0.016) and right MPFC (B = 10.44, P = 0.029).
Environmental exposure and DMN functional connectivity: main and interaction effects
Childhood trauma, cannabis use, and developmental urbanicity were not significantly associated with DMN functional connectivity in the whole group. There were no significant G×E interactions in the model of DMN functional connectivity (Table 4).
Table 4. Interactions between environmental risk and group for the regions that are functionally connected to the PCC seed.
| Environmental risk x group interactions | ||||||
|---|---|---|---|---|---|---|
| Childhood trauma | Cannabis use | Developmental urbanicity | ||||
| F | P | F | p | F | p | |
| Left inferior parietal lobule | 0.65 | 0.525 | 1.28 | 0.280 | 0.80 | 0.450 |
| Left precuneus | 0.25 | 0.780 | 0.21 | 0.812 | 0.21 | 0.814 |
| Right medial prefrontal cortex | 0.06 | 0.945 | 1.19 | 0.307 | 1.54 | 0.218 |
The F and P-values represent the results of the Wald test. No interactions were significant after Simes correction (PSimes<0.006).
Main effect of AP medication on functional connectivity
There was no significant association between lifetime AP use and functional connectivity between the PCC seed and left IPL (B = 0.00, P = 0.434), left PCu (B = -0.00, P = 0.370) or right MPFC (B = 0.00, P = 0.820).
Discussion
The objective of the current study was to examine functional connectivity within the DMN in patients with (increased risk for) psychotic disorder. The main finding was that patients and siblings had a similar pattern of increased connectivity between the PCC seed and other regions of the DMN (i.e., left IPL, left PCu and right MPFC) compared to controls. DMN connectivity was not associated with (subclinical) psychotic or cognitive symptoms. The association between familial risk and DMN connectivity was not conditional on environmental exposure.
DMN connectivity in patients with psychotic disorder
This study adds to the notion of altered interregional functional connectivity in psychotic disorder. Research to date has produced conflicting results as to whether connectivity within the DMN is increased or decreased in schizophrenia. For example, Liu and colleagues have found increased DMN connectivity between the MPFC and parietal regions and between the PCC and temporal regions in schizophrenia during resting-state [24]. Increased DMN connectivity has been described in most of the studies on schizophrenia [19,22,74,75] using either seed-based correlation analysis or ICA. In contrast, a handful of studies have reported decreased DMN connectivity [16,19,76], for example between the PCC and the lateral parietal/medial PFC and PCu [28]. Possible explanations for these inconsistencies may be that the DMN does not comprise a single network, but instead may include several networks, some of which may be altered in schizophrenia [8,77]. The interaction between different networks may lead to a dynamic pattern of dysconnectivity and could contribute to the discrepancy in findings. In addition, conflicting observations across resting-state fMRI studies may also be attributed to several other factors, including differences in patient population, sample size, cohort characteristics, confounding factors (e.g., psychotropic medication), and analytical procedures. Interestingly, the studies reporting decreased DMN connectivity predominantly used ICA analysis [16,19,76], whereas studies demonstrating increased DMN connectivity have used both ICA and seed-based methods [15,19,75]. The two largest studies, one using ICA analysis (n = 258 [16]) and the other seed-based analysis (n = 103 [60]) displayed respectively decreased and increased DMN connectivity. Of note, although univariate (e.g., seed-based correlation) and multivariate (e.g., ICA) statistical analyses may be of influence on the connectivity measurements, a direct comparison of both methods yielded equal results [78,79].
The present study used a relatively large sample size. Nevertheless, the statistical effects were comparatively small, which in combination with adequate statistical power suggests that true DMN effect sizes in psychotic disorder may be less strong than previously stated in smaller studies. However, it has to be noted that a recent study by Khadka et al. (2013) examined the posterior DMN (i.e., cingulate gyrus and PCu) with ICA in a comparably large sample (n = 258) as the current sample and found decreased DMN connectivity. In conclusion, the current evidence indicates that functional integration across regions of the DMN is altered in psychotic disorder although methodological differences across studies preclude definite conclusions. Therefore, using more standardized methods across symptom-based and/or intermediate phenotypes may help to improve the level of evidence.
Regions of the DMN are involved in mental functions such as the responsiveness to salient environmental events, awareness of the environment (IPL), self-referential or introspectively oriented mental activity, decision making (MPFC), self-processing, consciousness and memory processes (PCu) [5,80]. An overactive DMN as found in the current study could mediate distorted boundaries between imagination and perceptions from the external world, and between self and others. Thus, the DMN may underlie formation of psychotic symptoms and social and neurocognitive dysfunction [62,81] (see below).
DMN connectivity and familial risk for psychotic disorder
The siblings in this study exhibited increased DMN connectivity in similar parts of the DMN network as the patients. Siblings showed intermediate PCC connectivity with the left IPL and left PCu compared to patients and controls, whereas PCC connectivity with the MPFC was slightly higher compared to that of patients. This overlap suggests that DMN connectivity is associated with familial (and possibly genetic) factors. Thus, increased connectivity between the PCC and the left IPL, left PCu and right MPFC may represent trait-related intrinsic network alterations. The current findings replicate other seed-based studies that demonstrated a similar increased DMN functional connectivity ‘intermediate phenotype’ for psychotic disorder [22–24] Contrary to our findings, in two studies there was no evidence for altered connectivity within the DMN of first-degree relatives [16,21], and in one study there was evidence for a decreased DMN functional connectivity ‘intermediate phenotype’ [16]. Resting-state fMRI studies in individuals at ultra-high risk for psychosis [82] and first-episode schizophrenia [75,83] also revealed increased DMN functional connectivity, especially in frontal and parietal regions. Reported findings, in combination with the present results, suggest that DMN abnormalities in patients with psychotic disorder are associated with pre-existing vulnerability and persist over the course of the illness.
Clinical correlates of altered DMN functional connectivity
There was no significant association between DMN connectivity and positive symptoms in the patients. These results are in line with a recent large study in which correlations with PANSS positive scores did not survive Bonferroni correction [20] and contradictory to previous resting-state fMRI studies that suggest that severity of positive symptoms is associated with either increased [1,22,28] or decreased [28,29] DMN connectivity, depending on the location in the brain (i.e., frontal, parietal or temporal). A possible explanation for the absence of an association between positive symptoms and DMN functional connectivity in the current study is that most patients were in clinical remission, as reflected by relatively low PANSS scores with little variance.
In addition to an absence of associations with positive symptoms, there was also no significant association between WM and DMN connectivity in the present study. An explanation for the absence of an association between DMN connectivity and WM in patients with psychotic disorder may be that the more severe cognitive impairments in schizophrenia are the result of impaired between-network interactions, rather than altered within-network connectivity [21]. To further clarify the “connectivity at rest” (endo)phenotype and its differential relationships with cognitive functioning in patients and siblings, studies on network interactions are warranted.
Increased DMN connectivity was not associated with altered social cognition in individuals with (risk for) psychotic disorder. To our knowledge, only four fMRI studies have investigated the association between social cognition and resting-state DMN connectivity in individuals (at risk of) mental disorder, focusing on psychotic disorder and autism spectrum disorder (ASD). A study in unaffected first-degree relatives of individuals with schizophrenia and healthy controls found that for all participants connectivity between temporal regions and frontal-temporal regions predicted social functioning, empathy and perspective-taking [33]. Studies in ASD showed that social cognitive deficits in ASD were associated with decreased DMN connectivity between the PCC and superior frontal gyrus and between the precuneus and MPFC/anterior cingulate cortex. In addition, social cognitive deficits were associated with increased connectivity between the PCC and the temporal lobe [84–86].
Exploratory post-hoc symptom analyses showed that (altered) DMN connectivity may be associated with emotional distress in patients with psychotic disorder. A task-based fMRI study investigating neural circuits underling emotional distress in healthy individuals found an association between this mental state and brain activation in several regions, including the MPFC and PCC [87]. However, to our knowledge no resting-state fMRI studies have been conducted in patients with psychotic disorder examining the association between emotional distress and DMN functional connectivity. Therefore, future studies are warranted to further investigate this issue.
Association with environmental exposure
The present study did not provide evidence for a differential impact of environmental exposures on DMN functional connectivity in individuals with (risk) for psychotic disorder. Both familial liability and exposure to environmental risk factors have previously been associated with functional DMN alterations [17,37–40]. However, the studies providing evidence for an association between DMN alterations and environmental factors were conducted in traumatized and healthy populations, whereas no such studies have been carried out in psychotic disorder, let alone studies on GxE. It may be that DMN functional connectivity is not a sensitive enough outcome measure to investigate specific G×E interactions. For example, there is evidence to suggest that these environmental risk factors act through a final common pathway of dopamine (DA) dysregulation in regions of the mesolimbic circuit [88]. Therefore, the functional connectivity in mesocorticolimbic circuits [89] may be a good candidate for future resting-state fMRI research examining this type of interactions.
Methodological considerations
Advantages of the current study were the large sample size, the use of a representative population of patients with psychotic disorder (and siblings), and the correction for several potential confounding factors. Cannabis and other drugs, as well as alcohol and tobacco may have an effect on brain connectivity [66–70], but previous studies (e.g.,[21,26]) have not always corrected for these confounders and sample sizes were generally much smaller, likely contributing to the variance in study results.
Most of the patients in this study were receiving second generation AP medication at the time of scanning. The effect of AP medication on intrinsic networks is still unclear, although some studies suggest that AP medication normalizes aberrant connectivity [90,91]. However, in the current study there was no main effect of AP on DMN functional connectivity. Furthermore, both medicated patients and non-medicated siblings showed similar patterns of altered connectivity as compared to controls, which argues against this interpretation.
In seed-based analysis, variations in the seed positioning could have impacted the pattern of functional connectivity observed [92]. Nonetheless, the present study has face validity as previous resting-state fMRI studies of smaller sample sizes, and analysed with both seed-based and ICA techniques, have shown increased connectivity in similar regions of the DMN.
The physiological state (i.e., emotional state and arousal level) of the participants was not measured which could lead to altered functional connectivity. That is, studies have shown that a reduced arousal level was associated with reduced functional connectivity during rest [93]. In future studies, it would be valuable to include self-report questionnaires, administered after scanning, on for example wakefulness during the scan session.
The present study comprised cross-sectional data-analyses, precluding any causal and sequential inferences.
Supporting Information
(DOCX)
(DOCX)
Acknowledgments
We wish to thank all participants and contributing staff of the participating mental health care centers. We thank Truda Driesen and Inge Crolla for their coordinating roles in the data collection, as well as the G.R.O.U.P. investigators: Rene Kahn, Don Linszen, Jim van Os, Durk Wiersma, Richard Bruggeman, Wiepke Cahn, Lieuwe de Haan, Lydia Krabbendam and Inez Myin-Germeys.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was sponsored by the Dutch organization for scientific research NWO (Genetic Risk and Outcome of Psychosis [G.R.O.U.P.]) and the European Community’s Seventh Framework Programme under Grant Agreement No. HEALTH-F2-2009-241909 (European Network of National Schizophrenia Networks Studying Gene-Environment Interactions Consortium). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Camchong J, MacDonald AW, Bell C, Mueller BA, Lim KO. Altered functional and anatomical connectivity in schizophrenia. Schizophr Bull. 2011;37: 640–650. 10.1093/schbul/sbp131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Friston KJ. The disconnection hypothesis. Schizophr Res. 1998;30: 115–125. [DOI] [PubMed] [Google Scholar]
- 3. Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3: 89–97. [PubMed] [Google Scholar]
- 4. Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant "default mode" functional connectivity in schizophrenia. Am J Psychiatry. 2007;164: 450–457. [DOI] [PubMed] [Google Scholar]
- 5. Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2: 685–694. [DOI] [PubMed] [Google Scholar]
- 6. Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49: 1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schilbach L, Eickhoff SB, Rotarska-Jagiela A, Fink GR, Vogeley K. Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the "default system" of the brain. Conscious Cogn. 2008;17: 457–467. 10.1016/j.concog.2008.03.013 [DOI] [PubMed] [Google Scholar]
- 8. van de Ven V. Brain function when the voices are silent: aberrant default modes in auditory verbal hallucinations In: Jardri R, Pins A, Cachia A, Thomas T, editors. Neuroscience of Hallucinations. New York, NY: Springer; 2012. pp. 393–414. [Google Scholar]
- 9. Beer JS. The default self: feeling good or being right? Trends Cogn Sci. 2007;11: 187–189. [DOI] [PubMed] [Google Scholar]
- 10. Lindner M, Hundhammer T, Ciaramidaro A, Linden DE, Mussweiler T. The neural substrates of person comparison--an fMRI study. Neuroimage 2008;40: 963–971. 10.1016/j.neuroimage.2007.12.022 [DOI] [PubMed] [Google Scholar]
- 11. Farrer C, Franck N, Georgieff N, Frith CD, Decety J, Jeannerod M. Modulating the experience of agency: a positron emission tomography study. Neuroimage 2003;18: 324–333. [DOI] [PubMed] [Google Scholar]
- 12. Esposito F, Aragri A, Latorre V, Popolizio T, Scarabino T, Cirillo S, et al. Does the default-mode functional connectivity of the brain correlate with working-memory performances? Arch Ital Biol. 2009;147: 11–20. [PubMed] [Google Scholar]
- 13. Wible CG, Preus AP, Hashimoto R. A Cognitive Neuroscience View of Schizophrenic Symptoms: Abnormal Activation of a System for Social Perception and Communication. Brain Imaging Behav. 2009;3: 85–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33: 279–296. 10.1016/j.neubiorev.2008.09.002 [DOI] [PubMed] [Google Scholar]
- 15. Chang X, Shen H, Wang L, Liu Z, Xin W, Hu D, et al. Altered default mode and fronto-parietal network subsystems in patients with schizophrenia and their unaffected siblings. Brain Res. 2014;1562: 87–99. 10.1016/j.brainres.2014.03.024 [DOI] [PubMed] [Google Scholar]
- 16. Khadka S, Meda SA, Stevens MC, Glahn DC, Calhoun VD, Sweeney JA, et al. Is aberrant functional connectivity a psychosis endophenotype? A resting state functional magnetic resonance imaging study. Biol Psychiatry 2013;74: 458–466. 10.1016/j.biopsych.2013.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8: 49–76. 10.1146/annurev-clinpsy-032511-143049 [DOI] [PubMed] [Google Scholar]
- 18.Wotruba D, Michels L, Buechler R, Metzler S, Theodoridou A, Gerstenberg M, et al. Aberrant Coupling Within and Across the Default Mode, Task-Positive, and Salience Network in Subjects at Risk for Psychosis. Schizophr Bull. 2013 Nov 16. [DOI] [PMC free article] [PubMed]
- 19. Karbasforoushan H, Woodward ND. Resting-state networks in schizophrenia. Curr Top Med Chem. 2012;12: 2404–2414. [DOI] [PubMed] [Google Scholar]
- 20. Meda SA, Ruano G, Windemuth A, O'Neil K, Berwise C, Dunn SM, et al. Multivariate analysis reveals genetic associations of the resting default mode network in psychotic bipolar disorder and schizophrenia. Proc Natl Acad Sci USA 2014;111: E2066–2075. 10.1073/pnas.1313093111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Repovs G, Csernansky JG, Barch DM. Brain network connectivity in individuals with schizophrenia and their siblings. Biol Psychiatry 2011;69: 967–973. 10.1016/j.biopsych.2010.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106: 1279–1284. 10.1073/pnas.0809141106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Buuren M, Vink M, Kahn RS. Default-mode network dysfunction and self-referential processing in healthy siblings of schizophrenia patients. Schizophr Res. 2012;142: 237–243. 10.1016/j.schres.2012.09.017 [DOI] [PubMed] [Google Scholar]
- 24. Liu H, Kaneko Y, Ouyang X, Li L, Hao Y, Chen EY, et al. Schizophrenic patients and their unaffected siblings share increased resting-state connectivity in the task-negative network but not its anticorrelated task-positive network. Schizophr Bull. 2012;38: 285–294. 10.1093/schbul/sbq074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guo W, Su Q, Yao D, Jiang J, Zhang J, Zhang Z, et al. Decreased regional activity of default-mode network in unaffected siblings of schizophrenia patients at rest. Eur Neuropsychopharmacol. 2014;24: 545–552. 10.1016/j.euroneuro.2014.01.004 [DOI] [PubMed] [Google Scholar]
- 26. Jang JH, Jung WH, Choi JS, Choi CH, Kang DH, Shin NY, et al. Reduced prefrontal functional connectivity in the default mode network is related to greater psychopathology in subjects with high genetic loading for schizophrenia. Schizophr Res. 2011;127: 58–65. 10.1016/j.schres.2010.12.022 [DOI] [PubMed] [Google Scholar]
- 27. Schurhoff F, Szoke A, Meary A, Bellivier F, Rouillon F, Pauls D, et al. Familial aggregation of delusional proneness in schizophrenia and bipolar pedigrees. Am J Psychiatry 2003;160: 1313–1319. [DOI] [PubMed] [Google Scholar]
- 28. Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RW, et al. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007;33: 1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rotarska-Jagiela A, van de Ven V, Oertel-Knochel V, Uhlhaas PJ, Vogeley K, Linden DE. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr Res. 2010;117: 21–30. 10.1016/j.schres.2010.01.001 [DOI] [PubMed] [Google Scholar]
- 30. Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26: 13338–13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anticevic A, Repovs G, Barch DM. Working memory encoding and maintenance deficits in schizophrenia: neural evidence for activation and deactivation abnormalities. Schizophr Bull. 2013;39: 168–178. 10.1093/schbul/sbr107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Metzak PD, Riley JD, Wang L, Whitman JC, Ngan ET, Woodward TS. Decreased efficiency of task-positive and task-negative networks during working memory in schizophrenia. Schizophr Bull. 2012;38: 803–813. 10.1093/schbul/sbq154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dodell-Feder D, Delisi LE, Hooker CI. The relationship between default mode network connectivity and social functioning in individuals at familial high-risk for schizophrenia. Schizophr Res. 2014;156: 87–95. 10.1016/j.schres.2014.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tienari P, Wynne LC, Sorri A, Lahti I, Laksy K, Moring J, et al. Genotype-environment interaction in schizophrenia-spectrum disorder. Long-term follow-up study of Finnish adoptees. Br J Psychiatry 2004;184: 216–222. [DOI] [PubMed] [Google Scholar]
- 35. Geoffroy PA, Etain B, Houenou J. Gene X Environment Interactions in Schizophrenia and Bipolar Disorder: Evidence from Neuroimaging. Front Psychiatry 2013;4: 136 10.3389/fpsyt.2013.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van Os J, Pedersen CB, Mortensen PB. Confirmation of synergy between urbanicity and familial liability in the causation of psychosis. Am J Psychiatry 2004;161: 2312–2314. [DOI] [PubMed] [Google Scholar]
- 37. Sripada RK, Swain JE, Evans GW, Welsh RC, Liberzon I. Childhood Poverty and Stress Reactivity Are Associated with Aberrant Functional Connectivity in Default Mode Network. Neuropsychopharmacology 2014; 39: 2244–2251. 10.1038/npp.2014.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bluhm R, Williamson PC, Osuch EA, Frewen PA, Stevens TK, Boksman K, et al. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J Psychiatry Neurosci. 2009;34: 187–194. [PMC free article] [PubMed] [Google Scholar]
- 39. Lederbogen F, Kirsch P, Haddad L, Streit F, Tost H, Schuch P, et al. City living and urban upbringing affect neural social stress processing in humans. Nature 2011;474: 498–501. 10.1038/nature10190 [DOI] [PubMed] [Google Scholar]
- 40. Pujol J, Blanco-Hinojo L, Batalla A, Lopez-Sola M, Harrison BJ, Soriano-Mas C, et al. Functional connectivity alterations in brain networks relevant to self-awareness in chronic cannabis users. J Psychiatr Res. 2014;51: 68–78. 10.1016/j.jpsychires.2013.12.008 [DOI] [PubMed] [Google Scholar]
- 41. Habets P, Marcelis M, Gronenschild E, Drukker M, van Os J. Reduced cortical thickness as an outcome of differential sensitivity to environmental risks in schizophrenia. Biol Psychiatry 2011;69: 487–494. 10.1016/j.biopsych.2010.08.010 [DOI] [PubMed] [Google Scholar]
- 42. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 43. Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry 1992;49: 615–623. [DOI] [PubMed] [Google Scholar]
- 44. Vollema MG, Ormel J. The reliability of the structured interview for schizotypy-revised. Schizophr Bull. 2000;26: 619–629. [DOI] [PubMed] [Google Scholar]
- 45. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13: 261–276. [DOI] [PubMed] [Google Scholar]
- 46. van der Gaag M, Hoffman T, Remijsen M, Hijman R, de Haan L, van Meijel B, et al. The five-factor model of the Positive and Negative Syndrome Scale II: a ten-fold cross-validation of a revised model. Schizophr Res. 2006;85: 280–287. [DOI] [PubMed] [Google Scholar]
- 47. Corcoran R, Mercer G, Frith CD. Schizophrenia, symptomatology and social inference: investigating "theory of mind" in people with schizophrenia. Schizophr Res. 1995;17: 5–13. [DOI] [PubMed] [Google Scholar]
- 48. Wechsler D. Wechsler Adult Intelligence Scale—Third Revision. San Antonia, Texas: The Psychological Corporation; 1997. [Google Scholar]
- 49. Annett M. A classification of hand preference by association analysis. Br J Psychol. 1970;61: 303–321. [DOI] [PubMed] [Google Scholar]
- 50. Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry 2010;67: 255–262. 10.1016/j.biopsych.2009.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. WHO. Composite International Diagnostic Interview (CIDI). Geneva: World Health Organization; 1990. [Google Scholar]
- 52. Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. J Am Acad Child Adolesc Psychiatry 1997;36: 340–348. [DOI] [PubMed] [Google Scholar]
- 53. Central Bureau of Statistics. Bevolking der Gemeenten van Nederland. The Hague, the Netherlands: CBS Publications; 1993. [Google Scholar]
- 54. Patel AX, Kundu P, Rubinov M, Jones PS, Vertes PE, Ersche KD, et al. A wavelet method for modeling and despiking motion artifacts from resting-state fMRI time series. Neuroimage 2014;95: 287–304. 10.1016/j.neuroimage.2014.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jo HJ, Gotts SJ, Reynolds RC, Bandettini PA, Martin A, Cox RW, et al. Effective Preprocessing Procedures Virtually Eliminate Distance-Dependent Motion Artifacts in Resting State FMRI. J Appl Math. 2013. 10.1155/2013/935154 [DOI] [PMC free article] [PubMed]
- 56. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29: 162–173. [DOI] [PubMed] [Google Scholar]
- 57. Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002;17: 825–841. [DOI] [PubMed] [Google Scholar]
- 58. Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5: 143–156. [DOI] [PubMed] [Google Scholar]
- 59. Goebel R, Esposito F, Formisano E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: From single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum Brain Mapp. 2006;27: 392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Woodward ND, Rogers B, Heckers S. Functional resting-state networks are differentially affected in schizophrenia. Schizophr Res. 2011;130: 86–93. 10.1016/j.schres.2011.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage 2008;42: 1178–1184. 10.1016/j.neuroimage.2008.05.059 [DOI] [PubMed] [Google Scholar]
- 62. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124: 1–38. 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- 63. Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 2002;15: 870–878. [DOI] [PubMed] [Google Scholar]
- 64. Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33: 636–647. [DOI] [PubMed] [Google Scholar]
- 65. StataCorp. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP; 2011. [Google Scholar]
- 66. Ma N, Liu Y, Fu XM, Li N, Wang CX, Zhang H, et al. Abnormal brain default-mode network functional connectivity in drug addicts. PLoS One 2011;6: e16560 10.1371/journal.pone.0016560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Roberts GM, Garavan H. Evidence of increased activation underlying cognitive control in ecstasy and cannabis users. Neuroimage 2010;52: 429–435. 10.1016/j.neuroimage.2010.04.192 [DOI] [PubMed] [Google Scholar]
- 68. Tomasi D, Volkow ND, Wang R, Carrillo JH, Maloney T, Alia-Klein N, et al. Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers. PLoS One 2010;5: e10815 10.1371/journal.pone.0010815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Volkow ND, Ma Y, Zhu W, Fowler JS, Li J, Rao M, et al. Moderate doses of alcohol disrupt the functional organization of the human brain. Psychiatry Res. 2008;162: 205–213. 10.1016/j.pscychresns.2007.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ding X, Lee SW. Changes of functional and effective connectivity in smoking replenishment on deprived heavy smokers: a resting-state FMRI study. PLoS One 2013;8: e59331 10.1371/journal.pone.0059331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Veer IM, Beckmann CF, van Tol MJ, Ferrarini L, Milles J, et al. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Front Syst Neurosci. 2010 Sept 20. [DOI] [PMC free article] [PubMed]
- 72. Clayton D, Hills M. Statistical Models in Epidemiology: Oxford: Oxford University Press; 1993. [Google Scholar]
- 73. Simes R. An improved Bonferroni procedure for multiple tests of significance. Biometrika 1986;73: 751–754. [Google Scholar]
- 74.Kindler J, Jann K, Homan P, Hauf M, Walther S, Strik W, et al. Static and Dynamic Characteristics of Cerebral Blood Flow During the Resting State in Schizophrenia. Schizophr Bull. 2013 Dec 10. [DOI] [PMC free article] [PubMed]
- 75. Tang J, Liao Y, Song M, Gao JH, Zhou B, Tan C, et al. Aberrant default mode functional connectivity in early onset schizophrenia. PLoS One 2013;8: e71061 10.1371/journal.pone.0071061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Orliac F, Naveau M, Joliot M, Delcroix N, Razafimandimby A, Brazo P, et al. Links among resting-state default-mode network, salience network, and symptomatology in schizophrenia. Schizophr Res. 2013;148: 74–80. 10.1016/j.schres.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 77. Williamson PC, Allman JM. A framework for interpreting functional networks in schizophrenia. Front Hum Neurosci. 2012;6: 184 10.3389/fnhum.2012.00184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mannell MV, Franco AR, Calhoun VD, Canive JM, Thoma RJ, Mayer AR. Resting state and task-induced deactivation: A methodological comparison in patients with schizophrenia and healthy controls. Hum Brain Mapp. 2010;31: 424–437. 10.1002/hbm.20876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lee MH, Smyser CD, Shimony JS. Resting-state fMRI: a review of methods and clinical applications. AJNR Am J Neuroradiol. 2013;34: 1866–1872. 10.3174/ajnr.A3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain 2006;129: 564–583. [DOI] [PubMed] [Google Scholar]
- 81. Green MF, Leitman DI. Social cognition in schizophrenia. Schizophr Bull. 2008;34: 670–672. 10.1093/schbul/sbn045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Shim G, Oh JS, Jung WH, Jang JH, Choi CH, Kim E, et al. Altered resting-state connectivity in subjects at ultra-high risk for psychosis: an fMRI study. Behav Brain Funct. 2010;6: 58 10.1186/1744-9081-6-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Guo W, Liu F, Zhang J, Zhang Z, Yu L, Liu J, et al. Abnormal default-mode network homogeneity in first-episode, drug-naive major depressive disorder. PLoS One 2014;9: e91102 10.1371/journal.pone.0091102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Assaf M, Jagannathan K, Calhoun VD, Miller L, Stevens MC, Sahl R, et al. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. Neuroimage 2010;53: 247–256. 10.1016/j.neuroimage.2010.05.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage 2008;39: 1877–1885. [DOI] [PubMed] [Google Scholar]
- 86. Monk CS, Peltier SJ, Wiggins JL, Weng SJ, Carrasco M, Risi S, et al. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage 2009;47: 764–772. 10.1016/j.neuroimage.2009.04.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sinha R, Lacadie C, Skudlarski P, Wexler BE. Neural circuits underlying emotional distress in humans. Ann N Y Acad Sci. 2004;1032: 254–257. [DOI] [PubMed] [Google Scholar]
- 88. van Winkel R, Stefanis NC, Myin-Germeys I. Psychosocial stress and psychosis. A review of the neurobiological mechanisms and the evidence for gene-stress interaction. Schizophr Bull. 2008;34: 1095–1105. 10.1093/schbul/sbn101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature 2010;468: 203–212. 10.1038/nature09563 [DOI] [PubMed] [Google Scholar]
- 90. Schlosser R, Gesierich T, Kaufmann B, Vucurevic G, Hunsche S, Gawehn J, et al. Altered effective connectivity during working memory performance in schizophrenia: a study with fMRI and structural equation modeling. Neuroimage 2003;19: 751–763. [DOI] [PubMed] [Google Scholar]
- 91. Stephan KE, Magnotta VA, White T, Arndt S, Flaum M, O’Leary DS, et al. Effects of olanzapine on cerebellar functional connectivity in schizophrenia measured by fMRI during a simple motor task. Psychological Medicine 2001;31: 1065–1078. [DOI] [PubMed] [Google Scholar]
- 92. Cole DM, Smith SM, Beckmann CF. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front Syst Neurosci. 2010;4: 8 10.3389/fnsys.2010.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Duncan NW, Northoff G. Overview of potential procedural and participant-related confounds for neuroimaging of the resting state. J Psychiatry Neurosci. 2013;38: 84–96. 10.1503/jpn.120059 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper.


