Abstract
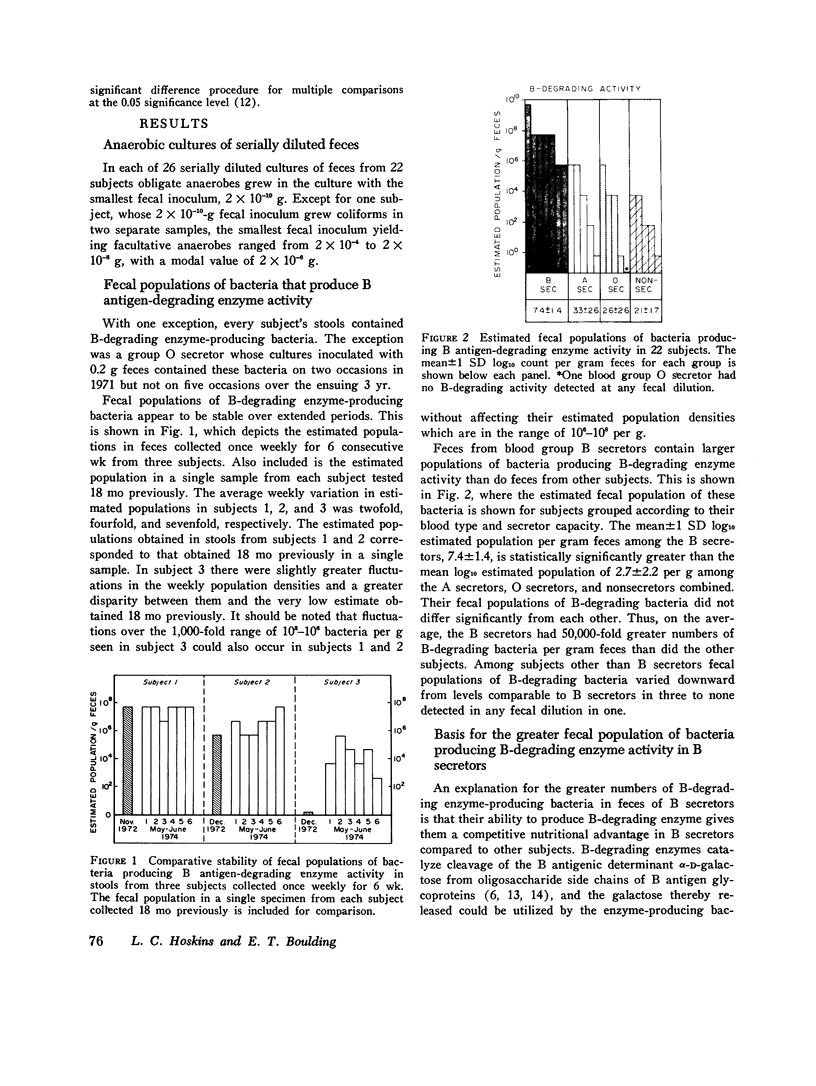
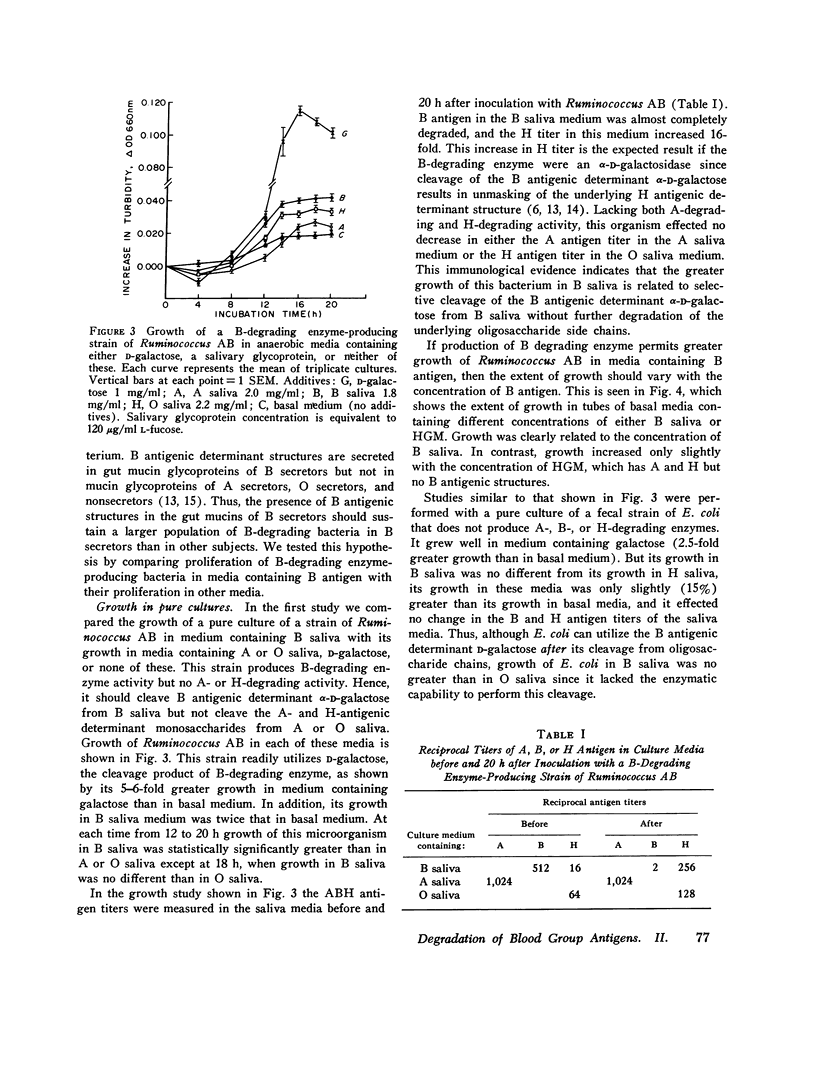
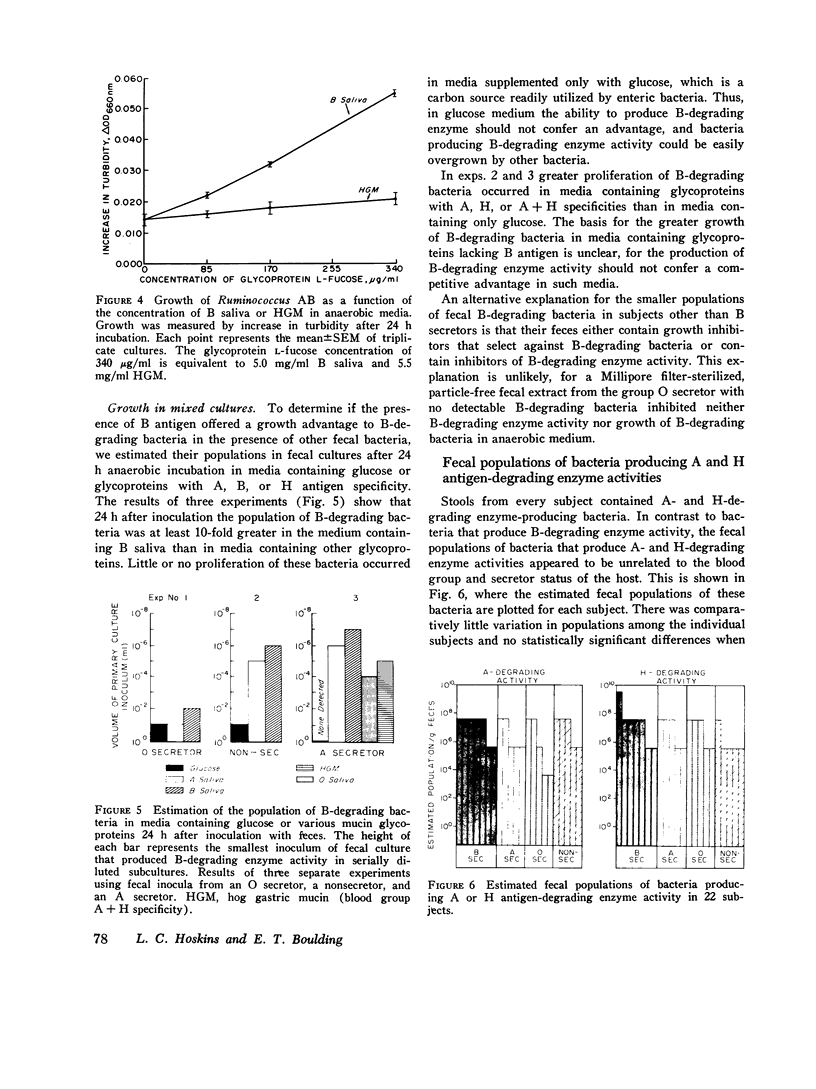
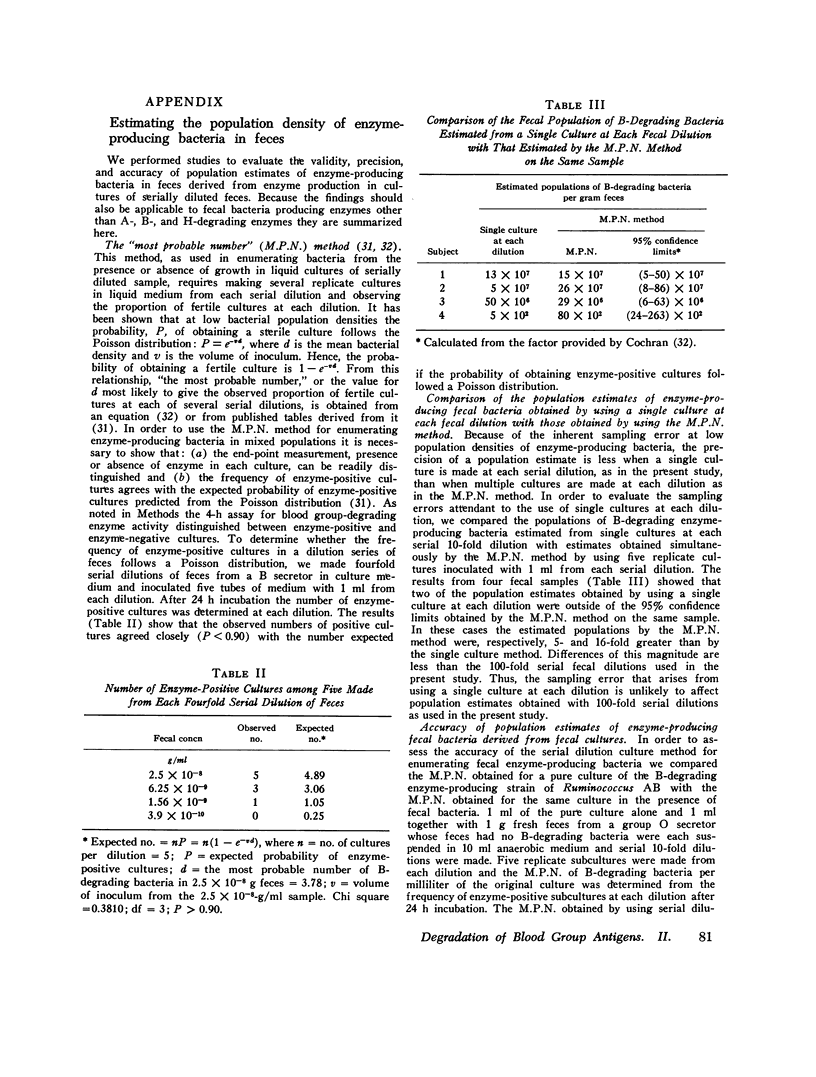
The autosomal dominant ABH secretor gene together with the ABO blood type gene control the presence and specificity of A, B, and H blood group antigens in human gut mucin glycoproteins. Certain obligate anaerobes in feces produce extracellular antigen-specific glycoside structures. We estimated the populations of these bacteria in feces of 22 healthy subjects by determining the greatest dilution of feces that yielded A, B, or H blood group-degrading enzyme activity after 24 h incubation in anaerobic cultures. Comparatively small populations of fecal bacteria produce blood group-degrading enzymes; their estimated populations were 10(8) per g or less in 21 subjects. Fecal populations of B-degrading bacteria were stable over time, and their population density averaged 50,000-fold greater in blood group B secretros than in other subjects. We present evidence that the greater fecal populations of B-degrading bacteria in B secretors is due in part to a competitive nutritional advantage gained by their ability to enzymatically cleave the B antigenic determinant alpha-D-galactose from gut mucins of B secretors. Fecal populations of bacteria producing A and H antigen-degrading enzyme activities were comparable in all subjects to the fecal population of B-degrading bacteria in B secretors. The large populations of fecal anaerobes may be an additional source of A antigen substrate for A-degrading bacteria; thus, antigens cross-reacting with A antigen were detected on cell walls of anaerobic bacteria from 3 of 10 cultures inoculated with 10(-10) g feces. Bacteria producing B-degrading activity likely represent a separate population from those producing A- or H-degrading activity since their fecal populations differed numerically in 14 subjects. These findings suggest that adaptation of blood group-degrading enzymes to mucin structures in human colon ecosystems is chiefly by mutation-selection of comparatively small populations of constitutive enzyme-producing strains rather than by substrate induced enzyme synthesis in many strains.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COCHRAN W. G. Estimation of bacterial densities by means of the "most probable number". Biometrics. 1950 Jun;6(2):105–116. [PubMed] [Google Scholar]
- Gall L. S. Normal fecal flora of man. Am J Clin Nutr. 1970 Nov;23(11):1457–1465. doi: 10.1093/ajcn/23.11.1457. [DOI] [PubMed] [Google Scholar]
- Gorbach S. L., Nahas L., Lerner P. I., Weinstein L. Studies of intestinal microflora. I. Effects of diet, age, and periodic sampling on numbers of fecal microorganisms in man. Gastroenterology. 1967 Dec;53(6):845–855. [PubMed] [Google Scholar]
- HAENEL H., MULLER-BEUTHOW W., SCHEUNERT A. Der Einfluss extremer Kostformen auf die faecale Flora des Menschen. II. Zentralbl Bakteriol Orig. 1957 Jul;169(1-2):45–65. [PubMed] [Google Scholar]
- HORIUCHI T., TOMIZAWA J. I., NOVICK A. Isolation and properties of bacteria capable of high rates of beta-galactosidase synthesis. Biochim Biophys Acta. 1962 Jan 22;55:152–163. doi: 10.1016/0006-3002(62)90941-1. [DOI] [PubMed] [Google Scholar]
- Hoskins L. C. Bacterial degradation of gastrointestinal mucins. II. Bacterial origin of fecal ABH(O) blood group antigen-destroying enzymes. Gastroenterology. 1968 Feb;54(2):218–224. [PubMed] [Google Scholar]
- Hoskins L. C., Boulding E. T. Degradation of blood group antigens in human colon ecosystems. I. In vitro production of ABH blood group-degrading enzymes by enteric bacteria. J Clin Invest. 1976 Jan;57(1):63–73. doi: 10.1172/JCI108270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins L. C. Ecological studies of intestinal bacteria. Relation between the specificity of fecal ABO blood group antigen-degrading enzymes from enteric bacteria and the ABO blood group of the human host. J Clin Invest. 1969 Apr;48(4):664–673. doi: 10.1172/JCI106024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins L. C., Zamcheck N. Bacterial degradation of gastrointestinal mucins. I. Comparison of mucus constituents in the stools of germ-free and conventional rats. Gastroenterology. 1968 Feb;54(2):210–217. [PubMed] [Google Scholar]
- Lewis I. M. Bacterial Variation with Special Reference to Behavior of Some Mutabile Strains of Colon Bacteria in Synthetic Media. J Bacteriol. 1934 Dec;28(6):619–639. doi: 10.1128/jb.28.6.619-639.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol. 1974 May;27(5):961–979. doi: 10.1128/am.27.5.961-979.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul D., Hoskins L. C. Effect of oral lactobacillus feedings on fecal lactobacillus counts. Am J Clin Nutr. 1972 Aug;25(8):763–765. doi: 10.1093/ajcn/25.8.763. [DOI] [PubMed] [Google Scholar]
- SPRINGER G. F. Relation of blood group active plant substances to human blood groups. Acta Haematol. 1958 Jul-Oct;20(1-4):147–155. doi: 10.1159/000205477. [DOI] [PubMed] [Google Scholar]
- SZULMAN A. E. The histological distribution of blood group substances A and B in man. J Exp Med. 1960 Jun 1;111:785–800. doi: 10.1084/jem.111.6.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter R. G., Huizenga K. A., Spencer R. J. A working hypothesis for the etiology and pathogenesis of nonspecific inflammatory bowel disease. Am J Dig Dis. 1972 Nov;17(11):1024–1032. doi: 10.1007/BF02239143. [DOI] [PubMed] [Google Scholar]
- TOMARELLI R. M., HASSINEN J. B., ECKHARDT E. R., CLARK R. H., BERNHART F. W. The isolation of a crystalline growth factor for a strain of Lactobacillus bifidus. Arch Biochem Biophys. 1954 Jan;48(1):225–232. doi: 10.1016/0003-9861(54)90327-6. [DOI] [PubMed] [Google Scholar]
- ZILLIKEN F., SMITH P. N., TOMARELLI R. M., GYORGY P. 4-O-beta-D-Galactopyranosyl-N-acetyl-D-Glucosamine in hog mucin. Arch Biochem Biophys. 1955 Feb;54(2):398–405. doi: 10.1016/0003-9861(55)90053-9. [DOI] [PubMed] [Google Scholar]







